Abstract
Background:
This study was performed to compare the effect of different doses of intrathecal meperidine on the incidence and intensity of shivering and other side-effects after spinal anesthesia for cesarean delivery.
Methods:
One hundred and fifty-six parturient women scheduled for elective cesarean delivery were enrolled in four groups. Spinal anesthesia consisted of heavy bupivacaine 0.5% (10 mg) in the standard group (Group I), heavy bupivacaine 0.5% (10 mg) plus meperidine (0.2 mg per kg) in Group II, heavy bupivacaine 0.5% (10 mg) plus meperidine (0.3 mg per kg) in Group III, heavy bupivacaine 0.5% (10 mg) plus meperidine (0.4 mg per kg) in Group IV. The signs and symptoms were recorded by an observer unaware of the study groups. Data were analyzed using analysis of variance, Kruskal–Wallis H-test and chi-square. A P value less than 0.05 was considered to be significant.
Results:
The systolic blood pressure, amount of bleeding, Pulse Rate, O2 saturation, neonatal apgar scores, core temperatures and sensory level revealed no difference between groups (P>0.05). The incidence (47.5%, 37.5%, 27.5% and 15.0%, respectively) and intensity of shivering decreased as the dose of meperidine increased (P=0.002) but the incidence of nausea and vomiting (8.0%, 15.4%, 25.9% and 35.8%, respectively) (P=0.000) and pruritis (25.64, 28.21, 38.46, and 48.72 respectively) increased as the dose of meperidine increased (P=0.000).
Conclusion:
The high dose of intrathecal meperidine is effective in reducing the incidence and intensity of shivering associated with spinal anesthesia for cesarean delivery but the high incidence of nausea and vomiting is unpleasant for the patient and can be a major problem with a high dose of meperdine.
Keywords: Bupivacaine 0.5%, delivery, intrathecal, meperidine, shivering
INTRODUCTION
Shivering associated with neuraxial anesthesia is a frequent complication; occurring in up to 55% of patients.[1] Shivering is uncomfortable for the patient and may interfere with monitoring of electrocardiogram, blood pressure (BP), and oxygen saturation.[1,2] The metabolic and hemodynamic consequences of shivering include increased disbursement of cardiac and systemic energy, increased oxygen consumption and carbon dioxide production, and increased cardiac work.[2] Those effects are particularly bothersome in the obstetrical population.[3] The mechanisms chiefly responsible for shivering in patients undergoing surgery are intraoperative temperature loss, increased sympathetic tone, pain and systemic release of pyrogens.[1,2] Spinal anesthesia for cesarean section still continues to be a popular technique because it provides many advantages such as rapid onset, high success rate, minimal maternal and fetal drug exposure and minimal maternal aspiration.[4] in spite of several investigations used to treat shivering after spinal anesthesia[3,5] little is known about the exact etiology of shivering and the best way of prevention.[2,3] Meperidine has been used for a long time to treat and prevent shivering, with somecontroversy of its optimal dose without producing undesired side-effects.[5,6] Roy et al.[5] recommended use of intrathecal meperidine to decrease the incidence of shivering without considerable side-effects in the obstetrical population but in another study Khan et al. found that intrathecal meperidine cannot be recommended in caesarean section to prevent shivering as its use is associated with increased incidence of nausea and vomiting.[6] However, these controversy and lack of meta-analysis study to clear the risk and benefit of injecting meperidine into intratechal spaceforced us to do this study.
The purpose of this study was to compare the effect of adding different doses of intrathecal meperdine on the incidence, intensity and other side-effects during delivery under spinal anesthesia.
METHODS
This randomized double-blinded clinical trial was conducted between August 2009 and August 2010 in the educational hospitals of Busheher University of Medical Sciences, Bushehr, Iran. This study was approved by the ethics committee of Busheher University of Medical Sciences and is registered at the clinicaltrials.gov database (reference no. IRCT138904101936N3). This study was performed according to the requirements of the declaration of Helsinki.
After attaining informed consent 156 parturient women (ASA physical status I or II) scheduled for elective cesarean delivery under spinal anesthesia were enrolled in this study. Parturient women with contraindications to regional anesthesia, hypersensitivity to amide local anesthetics or meperidine, history of headache, smoking, previous postoperative nausea and vomiting, opioid use or severe preeclampsia were excluded.
Patients were randomly assigned to four equal groups for spinal anesthesia according to numbers inserted in sealed envelopes. The temperature of the operating room was kept instantly using a calibrated temperature in cases and control groups. Before the spinal anesthesia was administered, patients were placed under standard monitoring (blood pressure, electrocardiogram, heart rate and oxygen saturation) and received IV lactated Ringer's solution 10mL/kg. During anesthesia, oxygen was given, and patients were covered with drapes but not activelywarmed.[4] All fluids were warmed to 37°C.[4] The patients were divided equally into four groups (n=39). Standard group received only 2 CC heavy bupivacaine 0.5% (10 mg) (Group I) and we added free preservative meperidine in three experimental groups. Heavy bupivacaine 0.5% (10 mg) plus 0/2 mg/kg free preservative meperidine (Group II), heavy bupivacaine 0.5% (10 mg) plus 0/3 mg/kg free preservative meperidine (Group III), heavy bupivacaine 0.5% (10 mg) plus 0/4 mg/ kg free preservative meperidine (Group IV). All groups were compared with standard group. Spinal anesthesia was administered in sitting position at the L4-5 interspace with a midline approach by using a 25-gauge Quincke needle. Solutions were prepared by another anesthesiologist so that the anesthesiologist performing the spinal block was blind to which drug was injected. Parturient woman was placed supine with left uterine displacement. Active support of ventilation was provided if there was any sign of respiratory depression (SpO2 less than 95%) in the case and control groups.
The time at the end of the injection was defined as T0. Sensory anesthesia was evaluated by pinprick at one-minute intervals for 10 min, five-minute intervals for 35 min, and then at ten-minute intervals until regression to L4. Once patients were in the post-anesthesia care unit, motor blockade was assessed with the Bromage scale[7]:1, unable to move feet; 2, able to move feet only; 3, just able to move knees; and 4, full flexion of knees and feet. The blood pressure was measured simultaneously with sensory levels and shivering intensity. Hypotension was defined as a decrease in systolic blood pressure to <90 mm Hg or 30% less than baseline value. It was treated with 5-10 mg of ephedrine IV and bradycardia (heart rate<50) was treated with intravenous atropine 0.5 mg. Pruritus was treated with diphenhydramine 25 mg IV. Vomiting was scored yes or no; nausea was scored none, mild, moderate or severe on a verbal patient/examiner/scale and metoclopramide 10 mg IV was administered for moderate to severe nausea and vomiting. Supplemental intraoperative analgesia was limited to IV fentanyl (1-2 μg/kg), which was standardized and used as a rescue dose if necessary. The tympanic temperature was monitored every 20 min from one side (right ear). The operating room temperature was maintained at 21°C–23°C for all patients using a calibrated temperature. Shivering was graded with a scale described by Crossley and Mahajan[8]: 0, no shivering; 1, piloerection or peripheral vasoconstriction but no visible shivering; 2, muscular activity in only one muscle group; 3, muscular activity in more than one muscle group but not generalized shivering; and 4, shivering involving the whole body. Pruritus, nausea, and vomiting were noted as they occurred. Apgar scores were recorded at 1, 5, and 10 min. All patients were asked on the first and second postoperative day about occurrence of headache, backache, paraesthesia, pain in thighs, buttocks or leg etc.
Duration of the surgery and weeks of pregnancy were all recorded precisely. Demographic data was collected by an observer unaware of the study groups; and demographic data except vomiting and maximal intensity of shivering were compared using Kruskal Wallis H. The maximal intensity of shivering and vomiting were compared using chi-square. To obtain at least 50% reduction in expected incidence, with α error of 0.05 and a β error of 0.2, a sample size of 39 patients per group was needed. P< 0.05 was considered statistically significant in the analysis.
RESULTS
There was no significant difference between groups in terms of demographic and surgical data [Table 1]. Time to highest sensory level and maximum number of segments blocked also Regression of sensory and motor blocks showed no difference between the groups.
Table 1.
Demographic and surgical data

Vomiting was increased with higher dose of meperidine and was most common in Group IV. There was more need to treatment in groups II, III, and IV. Finally, the usage of methoclopramide as antiemetic drug was more frequently in experimental groups. The systolic BP was similar between groups for each time interval, as was patient temperature.
We lost one patient in the control group and two patients in the experimental groups because of failure of spinal block. There was no need for rescue dose of fentanyl in the case and experimental groups. First need forpostoperative analgesia in Groups II, III, IV compared to standard group was (median time to need analgesic drug):130,140,140 versus 80 min,(P<0.05). Need for analgesic drugs was more in the standard group (17.6%)(P = 0.000) but was not significantly different between experimental groups (4.6%, 4.8% and 4.3%, respectively (P = 0.641). Bradycardia and signs of respiratory depression were not seen in all groups.
The incidence and intensity of shivering between the groups of study decreased as the dose of meperidine increased (incidence: 47.5%, 37.5%, 27.5% and 15.0%, respectively) (P=0.002) [Figure 1]. The incidence of pruritis also increased to 25.64, 28.21, 38.46, and 48.72 respectively) (P=0.000).
Figure 1.
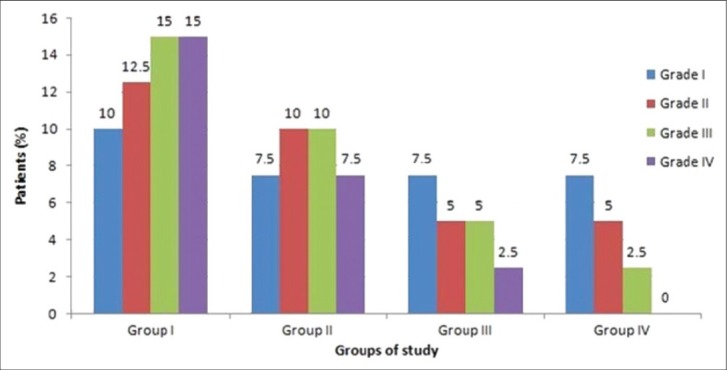
The intensity of shivering between groups of participants
DISCUSSION
According to the obtained results, adding meperidine, in increasing small dosages, to the intrathecal mixture anesthesia for cesarean delivery reduces the incidence and intensity of shivering but increases undesirable side-effects that bother the patients.
Although a lot of IV drugs were used for the treatment of postoperative shivering IV meperidine is the gold standard.[9] It is generally administered to treat shivering that has already begun.[9] The main consequences of postoperative shivering are an increase of oxygen consumption and of CO2 production.[10] Otherwise, it also produces an increase in intracranial pressure, interferes with electrocardiographic monitoring, and causes general discomfort, including a sensation of feeling cold.[1,2,10] Postoperative shivering produces physiological changes and constitutes a significant risk during the early postoperative period in high-risk patients.[11]
Effective treatment for these patients is necessary. The prevention of shivering is more important than its treatment and has not been well-investigated. When we compare treatment of shivering with intrathecal route and IV route we might find two advantages in the former: First, it is a preventive treatment, therefore, patients are spared the negative experience associated with shivering. Second, the administration of IV drug is associated with unwanted side-effects which can be harmful, especially in obstetrics women.[4] Although the etiology of post-spinal shivering is inadequately understood, various risk factors have been evaluated.[10,11] Among these, hypothermia, stress, uncontrolled pain, uninhibited spinal reflexes, and decreased sympathetic activity are frequently mentioned.[2,11] There are different ways to treat shivering but opioids have been more extensively evaluated.[4,9] Meperidine, which binds to both mu and k-opioid receptors, is frequently recommended for the treatment of postoperative shivering and the anti-shivering action of meperidine has previously been attributed to its action on κ-opioid receptors.[11–15]
Patel et al. compared intrathecal meperidine and lidocaine in 42 ASA physical status II or III patients who were candidates for endoscopic urological procedures and found that mean arterial blood pressure decreased significantly in the lidocaine group but not in the meperidine group.[16] Motor block was absent in ten patients in the meperidine group but was present in all the patients in the lidocaine group. Complications such as nausea, vomiting, itching, drowsiness and respiratory depression were similar in the two groups. It is concluded that low-dose meperidine, 0.5 mg kg–1 is effective as a spinal anesthetic agent and has few complications. Some of the findings of this study are against those of our study. In ours there is no BP change between groups and the incidence of side-effects is related to the dose of meperidine and is least in the control group.
Roy et al. studied the effect of a low dose of intrathecal meperidine on shivering in 40 parturient women scheduled for no emergent cesarean delivery.[5] Spinal anesthesia consisted of hyperbaric bupivacaine (0.75%; 10.5 mg), morphine 0.15 mg, and, in the experimental group, meperidine (0.2 mg/ kg) or, in the control group, normal saline. Time to highest sensory level, maximum number of blocked segments, sensory and motor blockade regression, and systolic blood pressure showed no difference between groups. The incidence of shivering was less in the meperidine group, as was its intensity. They found no difference between doses of diphenhydramine, and metoclopramide administered to the patient. Finally, they recommended use of intrathecal meperidine to decrease the incidence of shivering without considerable side-effects in the obstetrical population. Intrathecal meperidine (0.2 mg/kg) was effective in reducing the incidence and intensity of shivering associated with spinal anesthesia for cesarean delivery. Some findings of this study confirm our findings but we found a higher incidence of post operative nausea and vomiting and pruritis in the meperdine group that was dose-dependent.
Although, meperidine readily cross the placenta and block performed just a few minutes before delivery of fetus but none of newborns was depressed at the time of delivery because a highly lipid-soluble drug like meperidine is readily absorbed by lipid tissues and reabsorption into capillaries of the spinal cord is very slow.[17]
In one study[18] the effect of intrathecal meperidine on the incidence of shivering after spinal anesthesia was studied. Sixty patients with ASA Class I-II were divided into intrathecal meperidine group (case group) and control group (Group II). Case group received spinal tetracaine with meperidine 0.2 mg/kg and control group received spinal tetracaine without meperidine. Close observation for side-effects was done postoperatively. There was a significant reduction in the incidence of shivering in the case group (16.7%) when compared with the control group (56.7%). The nausea observed was higher in the case group. All findings of this study confirm us expect pruritis.
In one prospective randomized double-blinded study 72 parturient women, scheduled for elective caesarean section under spinal anesthesia, were enrolled in three different groups.[6] Spinal anesthesia consisted of bupivacaine 0.5% (10 mg) for the control group, and the same dose of bupivacaine with meperidine 12.5 or 25 mg for the experimental groups. Shivering was more intense in the control group than in the experimental groups. The intensity of shivering was comparable in the experimental groups. They found the highest incidence of shivering in the control group (16.7%) in comparison with the experimental groups (0/24). Nausea and vomiting was more frequent with higher doses of meperidine. Other complications were comparable. They concluded that intrathecal meperidine cannot be recommended for the prevention of shivering during spinal anesthesia for caesarean section as its use is associated with increased incidence of nausea and vomiting..Some result of this study confirm our findings. In our study the intensity of shivering was also dose-dependent. and we found a higher incidence of pruritis that was also dose-dependent
In another study the effect of adding meperidine 0.5% hyperbaric bupivacaine was investigated in 50 patient candidates for elective transurethral resection operations under spinal anesthesia.[19] The incidence and intensity of shivering was significantly less in the meperidine group. Pruritus was more common in the meperidine group. All findings of this study confirm us expect vomiting. All result of this study confirm our findings. expect vomiting
The single serious adverse effect of intrathecal opioid is respiratory-depressant but reported with much larger doses (50 mg) of intrathecal meperidine.[20] There has been no report of delayed respiratory depression with intrathecal meperidine, also some investigations confirm early respiratory depression in higher dosages than we used.[20] We found no respiratory depression and need for mechanical ventilation in any of the groups.
Finally, we had some limitations in our study. First: in our hospital it was impossible to determine meperidine level of cerebrospinal fluid. It can help us. to know more about our results. Second: it was impossible to monitor the temperature of CSF and compare it with the tympanic temperature obtained from the right ear and intensity of shivering. It could help us to know more about the relation between the temperature of the spinal cord, core temperature, intensity, incidence of shivering and add to our knowledge about the etiology of shivering.
CONCLUSION
According to this study, shivering continues to be a common problem after spinal anesthesia for cesarean delivery, with unknown etiology and no definite treatment. Although increasing the dose of intrathecal meperidine can decrease the incidence and intensity of shivering it can also increase the incidence of nausea and vomiting that can be a major problem in these patients. Finally, the use of intrathecal meperidine cannot be recommended for the prevention of shivering during spinal anesthesia for caesarean section as its use is associated with increased incidence of nausea and vomiting and we need to treat shivering with safer drugs.
ACKNOWLEDGEMENT
We would hereby like to acknowledge surgeons, Technician anesthesia, surgical technicians and other colleagues of the operating room and ward of Bentolhoda Hospital, Bushehr University of Medical Sciences who helped us sincerely.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Crowley LJ, Buggy DJ. Shivering and neuraxialanaesthesia. RegAnesth Pain Med. 2008;33:241–52. doi: 10.1016/j.rapm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.De Witte J, Sessler DI. Perioperative shivering: Physiology and pharmacology. Anesthesiology. 2002;96:467–84. doi: 10.1097/00000542-200202000-00036. [DOI] [PubMed] [Google Scholar]
- 3.Han JW, Kang HS, Choi SK, Park SJ, Park HJ, Lim TH. Comparison of the effects of intrathecal fentanyl and meperidine on shivering after cesarean delivery under spinal anaesthesia. Korean J Anesthesiol. 2007;52:657–62. [Google Scholar]
- 4.Chaney M. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903. doi: 10.1007/BF03011037. [DOI] [PubMed] [Google Scholar]
- 5.Roy J-D, Girard M, Drolet P. Intrathecalmeperidine decreases shivering during cesarean delivery under spinal anesthesia. AnesthAnalg. 2004;98:230–4. doi: 10.1213/01.ANE.0000093251.42341.74. [DOI] [PubMed] [Google Scholar]
- 6.Khan ZH, Zanjani AP, Makarem J, Samadi S. Antishivering effects of two different doses of intrathecalmeperidine in caesarean section: a prospective randomised blinded study. Eur J Anaesthesiol. 2011;28:202–6. doi: 10.1097/EJA.0b013e3283430802. [DOI] [PubMed] [Google Scholar]
- 7.Bromage PR. Continuous lumbar epidural analgesia for obstetrics. Can Med Assoc J. 1961;85:1136–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Crossley AWA, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. 1994;49:205–7. doi: 10.1111/j.1365-2044.1994.tb03422.x. [DOI] [PubMed] [Google Scholar]
- 9.Pauca AL, Savage RT, Simpson S, Roy RC. Effect of pethidine, fentanyl and morphine on post-operative shivering in man. ActaAnaesthesiolScand. 1984;28:138–43. doi: 10.1111/j.1399-6576.1984.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 10.Macintyre PE, Pavlin EG, Dwersteg JF. Effect of meperidine on oxygen consumption, carbon dioxide production, and respiratory gas exchange in post anesthesia shivering. AnesthAnalg. 1987;66:751–5. [PubMed] [Google Scholar]
- 11.Yu SC, NganKee WD, Kwan AS. Intrathecalmeperidine and shivering in obstetric anaesthesia. AnesthAnalg. 2004;99:1272–3. doi: 10.1213/01.ANE.0000133960.70591.EC. [DOI] [PubMed] [Google Scholar]
- 12.Kurz A, Sessler DI, Schroeder M, Kurz M. Thermoregulatory response thresholds during spinal anesthesia. AnesthAnalg. 1993;77:721–6. doi: 10.1213/00000539-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Alfonsi P, Sessler DI, Du Manoir B, Levron JC, Le Moing JP, Chauvin M. The effects of meperidine and sufentanil on the shivering threshold in postoperative patients. Anesthesiology. 1998;89:43–8. doi: 10.1097/00000542-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kranke P, Eberhart LH, Roewer N, Tramèr MR. Pharmacological treatment of postoperative shivering: A quantitative systematic review of randomized controlled trials. AnesthAnalg. 2002;94:453–60. doi: 10.1097/00000539-200202000-00043. [DOI] [PubMed] [Google Scholar]
- 15.Takada K, Clark DJ, Davies MF, Tonner PH, Krause TK, Bertaccini E, et al. Meperidineexerts agonist activity at the α2B-adrenoceptor subtype. Anesthesiology. 2002;96:1420–6. doi: 10.1097/00000542-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Patel D, Janardhan Y, Merai B, Robalino J, Shevde K. Comparison of intrathecalmeperidine and lidocaine in endoscopic urological procedures. Can J Anaesth. 1990;37:567–70. doi: 10.1007/BF03006327. [DOI] [PubMed] [Google Scholar]
- 17.Naguib M, Famewo C, Absood A. Pharmacokinetics of meperidine in spinal anaesthesia. CanAnaesthSoc J. 1986;33:162–6. doi: 10.1007/BF03010826. [DOI] [PubMed] [Google Scholar]
- 18.Chen JC, Hsu SW, Hu LH, Hong YJ, Tsai PS, Lin TC, et al. Intrathecalmeperidine attenuates shivering induced by spinal anesthesia. Ma ZuiXueZaZhi. 1993;31:19–24. [PubMed] [Google Scholar]
- 19.Chun DH, Kil HK, Kim HJ, Park C, Chung KH. Intrathecalmeperidinereducesintraoperativeshivering during transurethralprostatectomy in elderlypatients. Korean J Anesthesiol. 2010;59:389–93. doi: 10.4097/kjae.2010.59.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong B, Segstro R. Respiratory depression associated with meperidine spinal anaesthesia. Can J Anaesth. 1994;41:725–7. doi: 10.1007/BF03015628. [DOI] [PubMed] [Google Scholar]


