Abstract
MicroRNA (miRNA) influences carcinogenesis at multiple stages and it can effectively control tumor radiosensitivity by affecting DNA damage repair, cell cycle checkpoint, apoptosis, radio-related signal transduction pathways and tumor microenvironment. MiRNA also efficiently modulates tumor radiosensitivity at multiple levels by blocking the two essential non-homologous end-joining repair and homologous recombination repair pathways in the DNA damage response. It interferes with four radio-related pathways in ionizing radiation, including the PI3-K/Akt, NF-κB, MAPK and TGFβ signaling pathways. Moreover, the regulatory effect of miRNA in radiosensitivity can be enhanced when interacting with various key molecules, including H2AX, BRCA1, ATM, DNA-PK, RAD51, Chk1, Cdc25A, p53, PLK1, HIF-1 and VEGF, which are involved in these processes. Therefore, thoroughly understanding the mechanism of miRNA in tumor radiosensitivity could assist in finding novel targets to improve the radiotherapeutic effects and provide new clinical perspectives and insights for developing effective cancer treatments.
Introduction
Radiotherapy is an important modality in tumor combinational treatment and is used for treating multiple tumors with good therapeutic effects [1]. Moreover, when radiotherapy is combined with chemotherapy, surgery or other targeted therapies, treatment efficiency is improved and recurrence and cancer death rates are reduced [2]. Conversely, many tumors exhibit characteristics of radioresistance, which will affect radiotherapy efficacy. Thus, the question of how to reduce tumor radioresistance and improve tumor radiosensitivity is a hot topic in the tumor radiotherapeutic field.
Tumor radiosensitivity is associated with multiple factors and many diverse approaches are needed to optimize radiosensitivity. A total strategy for successful tumor radiotherapy is probably linked with approaches for increasing the radiosensitivity of tumor tissue. This means obtaining a maximal killing of tumor cells, while promoting an optimal reduction of acute and chronic normal tissue damage and decreasing adverse side effects [3–4]. Three characteristics of tumor tissue affect the consequences of radiotherapy. These characteristics include the degree of tumor tissue hypoxia, the survival ability of remaining tumor cells at 6–7 weeks post-radiotherapy and the capability of tumor cells to develop radioresistance [5]. Other factors, such as infiltration of inflammatory cells and generation of bone marrow derived cells, have a pronounced effect on tumor angiogenesis and tumor microenvironment (TME), which also affect tumor radiosensitivity [6]. Therefore, a thorough investigation and elucidation of factors involved in tumor radiosensitivity will increase our understanding in the emerging field of tumor radiotherapy.
A microRNA (miRNA) is defined as a small regulatory RNA molecule that consists of non-coding small RNA of about 22 nucleotides in length. MiRNA binds to the 3′-untranslated regions (3′-UTR) of target genes in a complete or in an incomplete complementary manner through its ‘seed sequence’ in the 5′-region and controls expression of target genes at the post-transcriptional level. The miRNA coding gene is first transcribed into a pri-miRNA, which is a double-strand stem-loop RNA about 300–1000 nucleotides long. Dorsha, a Class 2 RNase III enzyme, catalyzes the conversion of pri-miRNA into pre-miRNA of about 70–90 nucleotides long [7]. The Exportin-5/Ran–guanosine triphosphate complex promotes the transfer of pre-miRNA through the nucleus to the cytoplasm and then the Dicer enzyme catalyzes the removal of the stem-loop structure. Finally, the helicase degrades one of the complementary strands leaving a mature single-strand to exert biological functions [8]. The mature miRNA 5′-end includes a phosphoric acid and the 3′-end contains a hydroxyl group, which makes miRNAs different from the degraded small cytoplasmic RNA fragments. As mature miRNA is generated, it enters a nuclear protein complex and forms the RNA-induced silencing complex. In this way, it functions by targeting the messenger RNA (mRNA) of target genes to regulate gene expression at the post-transcriptional level and ultimately influences target gene translation and protein expression [9].
Multiple steps are involved in the regulation of target genes by miRNA. Notably, one miRNA can regulate the expression of many genes, and one gene can also be regulated by multiple miRNAs. Thus, miRNA and its target genes comprise a complicated interactive network, accompanied by various transcription factors and signaling molecules, which are all involved in carcinogenesis [10]. The latest research findings indicate that miRNAs affect various biological processes in carcinogenesis and play an important role in tumor development by influencing tumor cell growth, differentiation, apoptosis and cell cycle. Additionally, a promising role of miRNA in carcinogenesis is also emerging and miRNA was shown to be closely related to the process of epithelial–mesenchymal transition, properties of cancer stem cells, the initiation of tumor invasion and metastasis, and the therapeutic response to chemo- or radiotherapy [11–13].
In this review, we primarily illustrate the detailed regulatory mechanisms of miRNA in tumor radiosensitivity from diverse aspects, including the modulation of DNA damage repair, cell cycle checkpoint, apoptosis, radio-related signal transduction pathways and TME. We also highlight the clinical perspectives of miRNA in the future diagnosis and treatment of tumors and further present the significance of exploring new mechanisms and discovering novel targets to improve the therapeutic effects of radiotherapy.
Regulatory mechanism of miRNA in DNA damage repair
The tumor genome is characterized by genetic instability and defects in DNA damage repair ability. Concurrently, cancer cells initiate other backup signaling pathways to repair DNA damage induced by radiation [14]. Blocking these pathways causes a tumor to become radiosensitive, whereas normal tissue surrounding the tumor can become relatively resistant to radiotherapy [15]. Thus, controlling cellular reactions to radiotherapy by inhibiting DNA damage repair is a major focus in the translational radiotherapeutic research field.
Radiotherapy- or ionizing radiation-induced DNA damage in tumors triggers the DNA damage response (DDR) and activates multiple intracellular signaling transduction pathways involved in post-transcriptional regulation. Activation of DDR also determines whether cells repair DNA damage or undergo apoptosis when too much damage has occurred [16]. DNA damage repair includes base excision repair, single-strand break repair and double-strand break repair [17–18]. Tumor cells utilize two major pathways to repair double-strand breaks (DSBs), including the non-homologous end-joining (NHEJ) repair pathway, a fast but error-prone process in the G0/G1 cell cycle phase, and the homologous recombination (HR) repair pathway, a slow and error-free process occurring in the S/G2 phase [19–21]. During the DNA double-strand break repair process, multiple molecules, including the DNA damage sensors H2AX, MDC1, BRCA1, RAD50, NBS1, RNF8, the transducers ATM, ATR, and the effectors DNA-PK, Ku70/80, XRCC4, LIG4, RAD52, RAD51, BRCA1 and BRCA2, function in the DDR pathway [22–26].
MiRNA is involved in regulating the expression of important targets in the DDR pathway at the post-transcriptional level [27–28]. For example, miRNA-24 (miR-24) downregulates the expression of histone H2AX and suppresses DNA damage repair. Terminally, differentiated cells reduce the capability to repair DNA DSBs. MiR-24 is upregulated in differentiated blood cells, but a target of miR-24, H2AX, exhibits downregulation of its mRNA and protein levels. When DNA double-strand damage occurs, miR-24 reduces genomic stability and DNA damage repair ability by regulating H2AX expression [29]. Moreover, miR-24-mediated downregulation of H2AX increases cell death after DNA damage. Overall, suppressing miR-24 expression in differentiated tumor cells promotes DNA double-strand break repair and reduces cellular sensitivity to DDR [29–30]. MiR-421 regulates the ATM gene and the N-myc oncogene acts as a transcription factor on the miR-421 promoter region to upregulate miR-421 expression [31]. In this way, a new linear signaling pathway (i.e. N-Myc/miR-421/ATM) is established to play a role in regulating DNA synthesis in cell cycle S phase and in promoting tumor radiosensitivity. These findings provide new potential therapeutic targets for regulating the ATM-dependent DDR.
MiR-101 reportedly targets both DNA-PKcs and ATM to sensitize tumors to radiation. Thus, miR-101 will probably become a therapeutic agent to target DNA repair genes and enhance the effects of radiation mediated through multiple targets and pathways [32]. MiR-210 and miR-373 are upregulated in hypoxic cells, which contain high levels of hypoxia inducible factor-1 (HIF-1)α, and also regulate the expression of multiple factors in DNA damage repair pathways. Overexpression of miR-210 suppresses RAD52 expression, which is a crucial factor in DNA HR repair. Forced expression of miR-373 reduces expression of the nucleotide excision repair protein RAD23B and RAD52 [33]. Luciferase reporter assays demonstrate that miR-210 and miR-373 bind to the 3′-UTR of the RAD52 and RAD23B genes, respectively, indicating that these miRNAs expressed in hypoxia play a part in regulating proteins in the DNA HR and nucleotide excision repair pathways [34]. Thoroughly elucidating the regulatory mechanisms of miRNA in the DNA damage repair process will provide new insights into tumor radiosensitivity.
Regulatory mechanism of miRNA in cell cycle checkpoint and apoptosis
Tumor cells often exhibit at least one cell cycle checkpoint defect and especially at the G1/S phase checkpoint. Therefore, inhibiting the transition of other remaining checkpoints should prevent cell cycle progression and reduce DNA damage repair time, resulting in more tumor cells killed by radiotherapy [35]. Thus, using checkpoint inhibitors (e.g.) Chk1 and Chk2, to block cell cycle progression, could impact tumor radiosensitivity. Currently, this method was developed for clinical trials and is intended to enhance the cytotoxicity of antitumor drugs and radiotherapy efficacy [36–37]. In addition, inhibiting ATM, ATR and downstream proteins, such as Cdc25A, Chk1, Chk2, Cdk2, p53, p21, PLK1 or WEE1, can improve tumor radiosensitivity and hinder the DNA damage repair process [38]. Apoptosis is controlled by ATM and ATR and altering the function of apoptosis-associated proteins, such as p53, FAS, PUMA and Bax, could promote apoptosis and enhance radiotherapeutic effects [39–40].
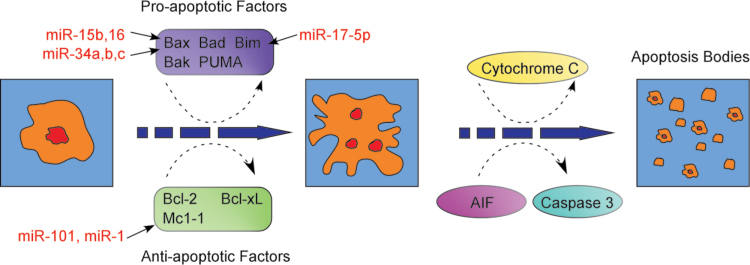
MiRNA participates in regulating cell cycle checkpoint and apoptosis. In the G1/S phase, many molecules, including Chk1, Chk2, p53, MDM2, p21, cyclin E, Cdk2 and Cdc25A, are controlled by miRNAs [41–42]. In the intra-S phase, miRNA regulates the expression of Chk1, Chk2, cyclin E, Cdk2, Cdc25A and SMC1. In the G2/M phase, the expression of Chk1, Chk2, p53, p21, cyclin B, Cdk1, Cdc25A, Cdc25B, Cdc25C, PLK1 and WEE1 are influenced by miRNAs [43–44]. During tumor cell apoptosis, miRNA modulates the expression of p53, Fas, NOXA and the Bcl-2 family [45–46], which contains proapoptotic factors (Bax, Bad, Bak, Bim and PUMA) and antiapoptotic factors (Bcl-2, Bcl-xL and Mcl-1). Downregulation of miR-17-5p upregulates the expression of Bim, which leads to the inhibition of Bax expression [47]. Upregulation of miR-101 and miR-1 represses Mcl-1 expression, whereas increasing the expression of miR-15b, miR-16 or miR-34a,b,c, accompanied by decreased miR-21 expression, contributes to Bax inhibition [48]. Moreover, suppression of Bax by proapoptotic factor Bim and antiapoptotic factors Mcl-1 and Bcl-2 enhances the permeability of mitochondrial membranes and induces cytochrome C and apoptosis-induced factor release, culminating in apoptosis [49].
MiR-372 acts as a tumor suppressor and targets cdk2 and cyclin A1 gene expression and regulates cell cycle progression and inhibits tumorigenesis. When miR-372 is downregulated, it not only promotes tumor cell proliferation but also speeds up S/G2 cell cycle phase progression. Thus, miR-372 contributes to initiation and development of cancer [50]. Overexpression of miR-29c suppresses cyclin E expression by binding to its 3′-UTR, inducing G1/G0 phase arrest and inhibiting tumor cell proliferation. In squamous cell carcinomas, miR-29c is usually expressed at a level that is too low to induce G1/G0 phase arrest, resulting in the growth and proliferation of tumor cells [51]. MiR-504 binds to two sites of the 3′-UTR in the p53 gene and negatively regulates p53 expression. Overexpression of miR-504 decreases p53 protein level in tumor cells and affects p53 transcriptional activity and apoptosis and cell cycle arrest mediated by p53 in response to stress. All of these effects induced by miR-504 ultimately promote carcinogenesis [52].
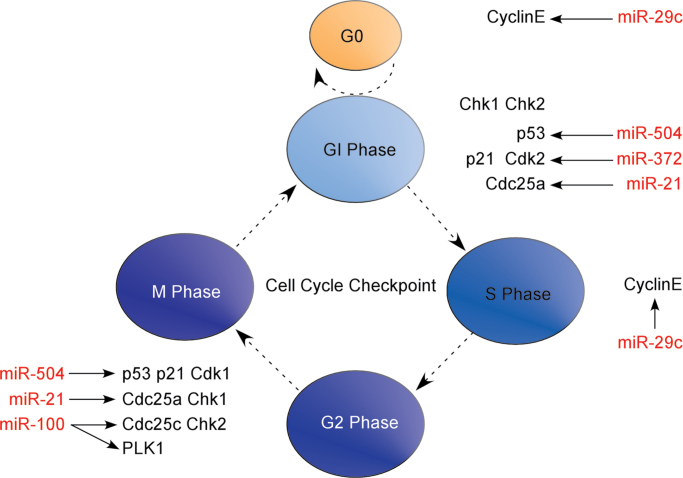
MiR-21 negatively regulates Cdc25A expression and cell cycle progression. By targeting Cdc25A, miR-21 delays the transition of the G1/S phase, inhibiting tumor cell proliferation. Also, the downregulation of Cdc25A expression induced by miR-21 activates changes in the G2/M checkpoint induced by DDR and affects the radiosensitivity of tumor cells [53]. In addition, miR-100 downregulates the expression of PLK1, which controls many stages of mitosis, and the overexpression of PLK1 corresponds with tumor radioresistance and poor clinical prognosis. MiR-100 suppresses PLK1 mRNA and protein levels and leads to decreased Cdc25C expression. When combined with radiotherapy, miR-100 induces G2/M phase arrest, activates caspases 3 and 7 and increases DNA DSBs and apoptosis. Concurrently, G2/M phase arrest is associated with aberrant spindle formation, which further contributes mitosis arrest [54]. Thus, low expression of miR-100 causes overexpression of PLK1, which in turn speeds up tumor progressios. Combining chemotherapeutic targeting of PLK1 with radiotherapy should promote mitotic catastrophe, increase cytotoxicity and offer an opportunity to effectively treat more tumors. Thoroughly understanding this regulatory mechanism of miRNA in cell cycle checkpoint and apoptosis should help improve radiotherapeutic effects by adding additional strategies to block or interfere with cell cycle progression (Figure 1.).
Fig. 1.
MiRNA is involved in the regulation of cell cycle checkpoint and apoptosis. a) MiRNA regulates cell cycle checkpoint of G1/G0 phase, G1/S phase, Intra-S phase and G2/M phase and prevents cell cycle progression to reduce DNA damage repair time, resulting in more tumor cells killed by radiotherapy. In this process, many important molecules, such as Chk1, Chk2, p53, p21, CyclinE, Cdk1, Cdk2, Cdc25a, Cdc25c, and PLK1, involved in cell cycle progression are under the regulation of miRNA. b) During apoptosis, miRNA regulates the expression of pro-apoptotic factors, including Bax, Bad, Bak, Bim and PUMA, and anti-apoptotic factors, including Bcl-2, Bcl-xL and Mcl-1, to enhance mitochondrial membrane permeability, induce cytochrome C and apoptosis induced factor (AIF) release, and activation of caspase 3, resulting in tumor cell apoptosis.
Regulatory mechanism of miRNA in radio-related signal transduction pathways
Four well-studied pathways are confirmed to play a role in radiotherapy and are closely associated with radiosensitivity. Three pathways, PI3-K/Akt, nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK), are regarded as survival pathways for ionizing radiation. The fourth pathway, transforming growth factor-β (TGFβ), indirectly affects tumor radioresistance by activating the expression of the ATM gene [55–61]. All four signaling pathways could have a major impact on tumor radioresistance just by their effect on apoptosis and DNA damage repair processes. The specific regulatory mechanism begins when tumor cells are inflicted with ionizing radiation or when intracellular receptor tyrosine kinases are activated by epidermal growth factor or insulin-like growth factor and the PI3-K/Akt, MAPK/extracellular signal-regulated kinases (ERKs) and NF-κB pathways are subsequently activated as cascades [62–63]. Activation of the PI3-K/Akt and MAPK/ERKs pathways suppresses expression of downstream target genes, including proapoptotic genes Bad and Bim. In contrast to these pathways, the NF-κB pathway enhances expression of the antiapoptotic protein, Mcl-1 [64–65]. Furthermore, changes in the expression of Bad, Bim and Mcl-1 impact apoptosis and ultimately contribute to tumor radioresistance.
Another possible regulatory mechanism might occur when the PI3-K/Akt and MAPK/ERKs pathways are activated by radiation, causing them to impact the DNA damage repair pathways in the nucleus. They especially influence the NHEJ pathway and the activities of DNA-PKcs, thereby modulating tumor radioresistance [66–67]. Moreover, epidermal growth factor receptor or insulin-like growth factor receptor are directly involved in the process of NHEJ after translocation to the nucleus and affect DNA-PKcs activities also contributing to tumor radioresistance [68–69]. In addition to participating in DDR, the TGFβ pathway is necessary for activating the ATM gene, which participates in two major repair pathways, including the NHEJ and HR pathways, during the occurrence of DNA DSBs, corresponding with tumor radioresistance [70–71].
Because of the intense focus on regulatory mechanisms of radio-related signal transduction pathways, many therapeutic methods are emerging to improve tumor radiosensitivity and reduce tumor radioresistance. One idea is to use small molecule inhibitors to block the activity of proteins in numerous signal transduction pathways. Representative approaches include using antibodies or kinase inhibitors to interfere with the function of epidermal growth factor receptor or insulin-like growth factor receptor kinase activity [72–73], or combining small molecule inhibitors, siRNAs or miRNAs to suppress the function of crucial signaling pathways, such as PI3-K, Akt, MAPK, NF-κB or TGFβ [74]. Adopting these methods should promote apoptosis, reduce DNA damage repair, improve the hypoxic state of the TME, increase perfusion and concentration of oxygen in tumor tissues and enhance tumor radiosensitivity and radiotherapeutic effects.
Studies show that miRNA is involved in the regulation of the four classical radio-related signaling pathways as indicated earlier. Specifically, miRNAs participate in the control of Akt activation and miR-21, miR-26, miR-221/222, miR-216a/217 and miR-486 jointly regulate the expression of PTEN, a tumor suppressor gene upstream of Akt. Furthermore, miR-155, miR-205 and miR-375 separately regulate the expression of the SHIP and PDK1 genes, which closely correlates with Akt activation. Moreover, miR-126 and miR-320 control PI3-K expression, affect the downstream activities of PIP3 and influence total and phosphorylated Akt protein levels [75–76]. MyoD and MRTF-A bind to the promoter region of miR-486 and further activate transcription of this miRNA. Mature miR-486 directly inhibits the translation of two crucial negative regulators, PTEN and Foxo1a, in the PI3-K/Akt pathway, and contributes to Akt phosphorylation and activation of this pathway. In addition, Akt activation promotes the phosphorylation of the negative regulator, GSK3β, and restrains the activity of Foxo1a, ensuring a constant active state of the PI3-K/Akt pathway [77]. MiR-221 and miR-222 target the PTEN gene and regulate PTEN protein expression, thus modulating growth, proliferation, apoptosis, invasion, metastasis and radiosensitivity of tumor cells. Thus, inhibiting miR-221 and miR-222 expression effectively blocks downstream signaling pathways and should be a promising therapy against cancer [78].
MiR-17-5p is overexpressed and acts as an oncogene to facilitate tumor cell proliferation and metastasis. The regulatory mechanism of miR-17-5p is associated with p38 MAPK activation and increased phosphorylation of heat shock protein 27. Furthermore, the signal transduction pathway of miR-17-5p–p38–heat shock protein 27 has been established, and the p38 MAPK pathway was confirmed to play a role in the phosphorylation of heat shock protein 27 induced by miR-17-5p, which all promote tumor invasion and metastasis. The crucial role of miR-17-5p in tumorigenesis indicates that miR-17-5p can act as a potential therapeutic target to enhance cancer treatment [79]. Multiple miRNAs are involved in regulating NF-κB signaling. Upstream of NF-κB, the subunit IκB is negatively regulated by IKKε, IKKβ, IKKα and IKKζ. In turn, IKKε is under the negative control of miR-155 and IKKβ is negatively controlled by miR-520h and miR-199a. IKKα is negatively controlled by miR-223, miR-15 and miR-16, and IKKζ is under the negative control of miR-124a. Meanwhile, the subunit p50 of NF-κB is negatively regulated by miR-9 and miR-218 and miR-301a indirectly controls the expression of p50 by targeting NKRF (NF-κB repressing factor). Activation of subunits p50 and p65 initiates the expression of various downstream miRNAs, including miR-301a, miR-28, miR-21, miR-29b, miR-146 and miR-143. Overall, the interactions between miRNA and the network of the NF-κB pathway demonstrate that miRNA plays an essential role in the activation and function of NF-κB, and the interplay and crosstalk among these molecules promote tumor initiation and progression [80].
Regulatory mechanism of miRNA in the tumor microenvironment
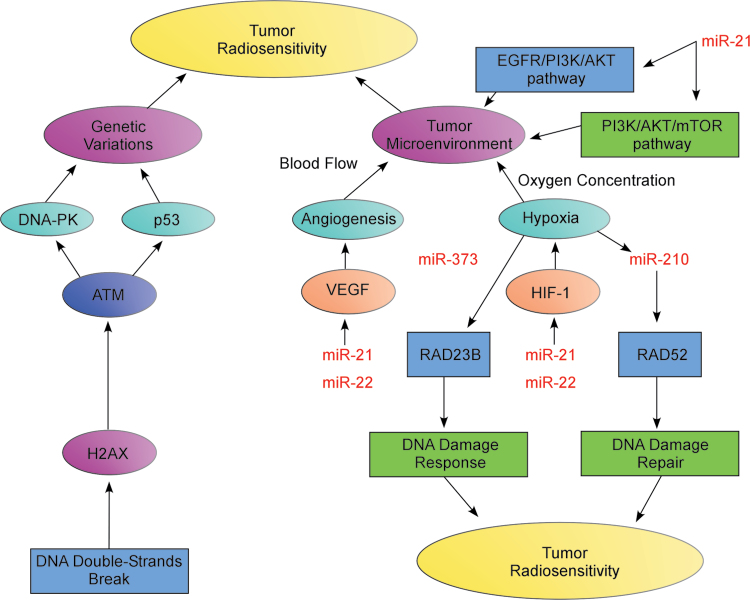
Tumor radiosensitivity is influenced by intrinsic factors like genetic variations and extrinsic factors like TME, in which hypoxia and angiogenesis are two factors that determine whether cancer cells are radiosensitive. Severely hypoxic tumor cells require a 2–3-fold higher dose of radiation compared with normal oxygenated cells to achieve the same killing effect [81–82]. In the TME, vascular endothelial growth factor (VEGF) and HIF-1 are two crucial factors that play a role in tumor radiosensitivity [83–84]. VEGF expression leads to blood vessel hyperproliferation, which improves tumor oxygenation. However, VEGF also increases vascular permeability. Thus, even though VEGF expression is high, tumor tissues still have regions of hypoxia and, therefore, inhibition of VEGF expression controls tumor cell proliferation after radiotherapy [85].
Hypoxia-induced signal transduction pathways are commonly activated and hypoxia modulates the activities of HIF-1, resulting in regulation of >100 target genes involved in tumor metabolism, proliferation, apoptosis and angiogenesis [86–87]. HIF-1 expression impacts tumor radiosensitivity, but the degree of influence varies by tumor type and other factors. Close interplay occurs between the HIF-1 activities and tumor radiosensitivity. Radiotherapy can lead to the activation of the HIF-1 pathway, and HIF-1 expression conversely affects the tumor radiation response and tumor clonogenicity capacity [88–89]. Additionally, inhibiting tumor angiogenesis with therapeutic drugs targeting VEGF, adopting anti-HIF-1 therapy or repressing the function of TME-related signaling pathways like EGFR/PI3-K/Akt or PI3-K/Akt/mTOR, will increase blood flow and oxygen concentration of tumor tissues, improve the state of the TME and elevate tumor radiosensitivity [90–92].
MiRNA plays a key role in the regulation of TME. MiR-210 acts as a unique and pleiotropic hypoxia-related ‘hypoxamir’ influencing numerous processes in hypoxia, including tissue ischemia, inflammation and carcinogenesis, proliferation and cell death. Notably, miR-210 facilitates tumor proliferation by activating cell cycle checkpoint and inhibits tumor cell death by decreasing the activity of caspase-8 or lowering the level of reactive oxygen species encouraging tumor cell immortality [93–95]. MiR-210 may also control the DNA damage repair capacity of tumor cells during hypoxia because hypoxia can boost the genomic instability of tumor cells and miR-210 targets DNA damage repair factor RAD52 to assist the repair of DNA DSBs. Another HIF-dependent miRNA, miR-373, downregulates the expression of RAD23B, affecting the recognition role of the XPC/RAD23B complex during DDR [34,93,96].
Additional findings confirm that miR-21 is associated with tumor growth and metastasis. By targeting the PTEN gene, miR-21 activates the Akt and ERK1/2 signaling pathways and leads to increased HIF-1α and VEGF expression, thereby facilitating tumor angiogenesis. Using inhibitors aimed at the Akt or ERK pathways suppresses angiogenesis and inhibits HIF-1α and VEGF expression. Overall, HIF-1α acts as a key regulator downstream of miR-21 playing a role in tumor angiogenesis and metastasis [97]. Meanwhile, miR-22 exhibits a low level of expression and upregulates HIF-1α expression and hypoxia-induced signal transduction pathways to promote tumor angiogenesis. Conversely, increasing miR-22 expression represses HIF-1α and VEGF expression under hypoxic conditions and leads to inhibition of angiogenesis. Thus, miR-22 alters blood flow and oxygen concentration around the tumor tissue and impacts the radiosensitivity of tumor cells [98]. Understanding the regulatory mechanisms of miRNA in tumor angiogenesis and hypoxia in the TME could lead to enhanced tumor radiosensitivity (Figure 2).
Fig. 2.
MiRNA is involved in the regulation of tumor microenvironment (TME). Tumor radiosensitivity is influenced by intrinsic factors like genetic variations and extrinsic factors like TME, in which hypoxia and angiogenesis are two essential factors that determine whether cancer cells are radiosensitive. In the TME, vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1 (HIF-1) are two crucial factors that play an important role in tumor radiosensitivity by changing blood flow and oxygen concentration of tumor tissues. TME-related signaling pathways like the EGFR/PI3-K/Akt and PI3-K/Akt/mTOR pathways can also improve the TME and elevate tumor radiosensitivity. Moreover, miRNA regulates VEGF and HIF-1 expression, and affects the function of TME-related signal transduction pathways. Conversely, in hypoxia, the expression of some hypoxia-related microRNAs is induced, activating their downstream genes involved in DDR and repair influencing radiosensitivity.
MiRNA also plays an active role in uncontrollable inflammation and the transition between inflammation and carcinogenesis and tumor metabolism, including aerobic glycolysis and oxidative phosphorylation, which are closely associated with tumor radiosensitivity or radioresistance [99–101]. Consequently, radio-related miRNAs modulate tumor radiosensitivity from a variety of aspects utilizing multiple approaches in complex layers with numerous targets.
Clinical perspective of miRNA in tumor radiotherapy
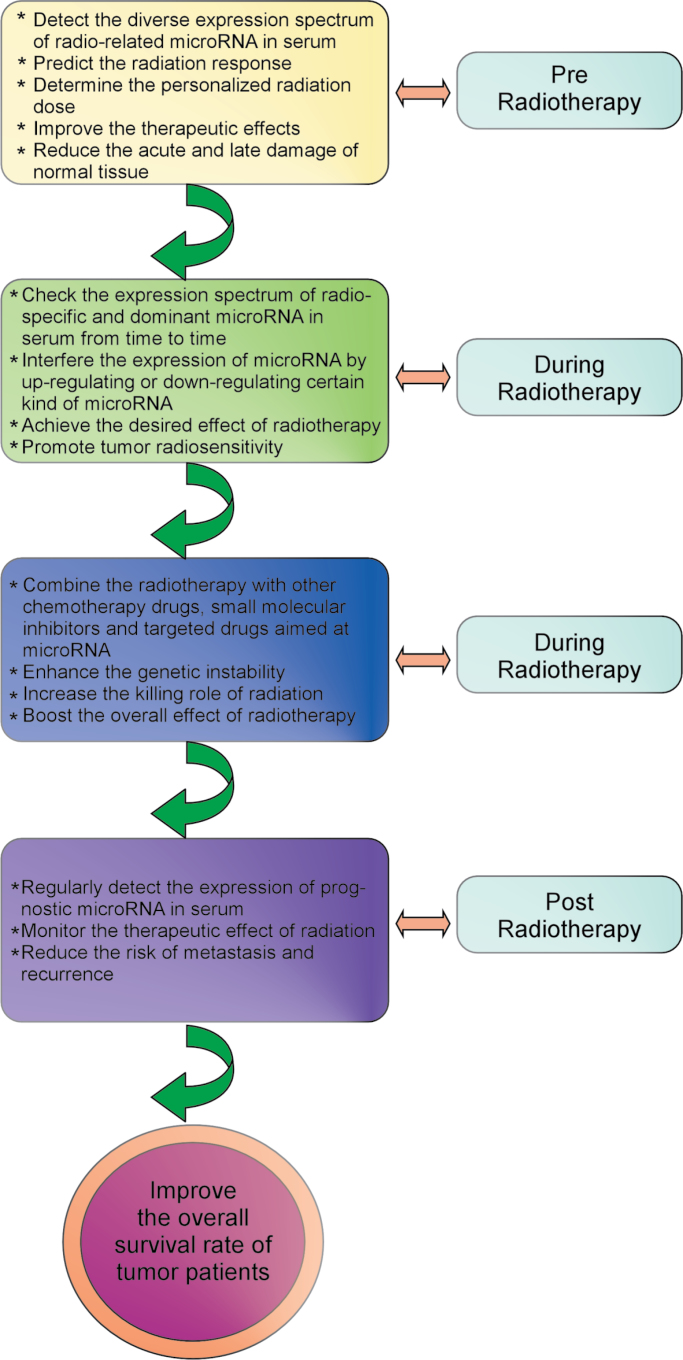
The regulatory role of miRNA in tumor radiosensitivity will probably be exploited in clinical therapies in the near future. The imaginative framework might include numerous steps. First, before tumor patients are treated with radiotherapy, the expression spectrum of radio-related miRNA in serum would be evaluated to (i) predict the radiation response of each patient, (ii) determine the personalized radiation dose for optimizing the therapeutic effects, and (iii) maximally reduce the acute and latent damage of normal tissues. Second, during the process of radiotherapy, checking the expression spectrum of radio-specific and dominant miRNAs in serum and altering the expression of specific miRNAs could help to efficiently achieve the desired effect of radiotherapy and further promote tumor radiosensitivity. Third, during the therapeutic period of radiation, radiotherapy can be combined with other chemotherapy drugs, small molecule inhibitors and drugs aimed at specific miRNAs in order to enhance the genetic instability of tumor cells, increase the killing rate of radiation and boost the overall effect of radiotherapy. Finally, when radiotherapy is complete, regularly detecting the expression of prognostic miRNAs in serum could help to monitor the therapeutic effect of radiation and reduce the risk of metastasis and cancer recurrence (Figure 3).
Fig. 3.
Clinical perspective of miRNA in tumor radiotherapy. For future clinical radiotherapy, the diverse expression spectrum of radio-related miRNAs in serum can be assessed and monitored before radiotherapy, during radiotherapy and after radiotherapy to boost the therapeutic effect of radiotherapy and improve the overall survival rate of tumor patients.
Discovering the role of miRNA in regulating tumor radiosensitivity, promotes the possibility that miRNA will be a promising target for clinical diagnosis and treatment. Achieving non-invasive detection of tumor radiosensitivity by using radio-specific miRNAs as biomarkers in serum is highly possible. In addition, the potential possibility to improve the radiotherapeutic effect by activating or inhibiting the expression of certain miRNAs and downstream target genes is extremely promising.
Conclusions
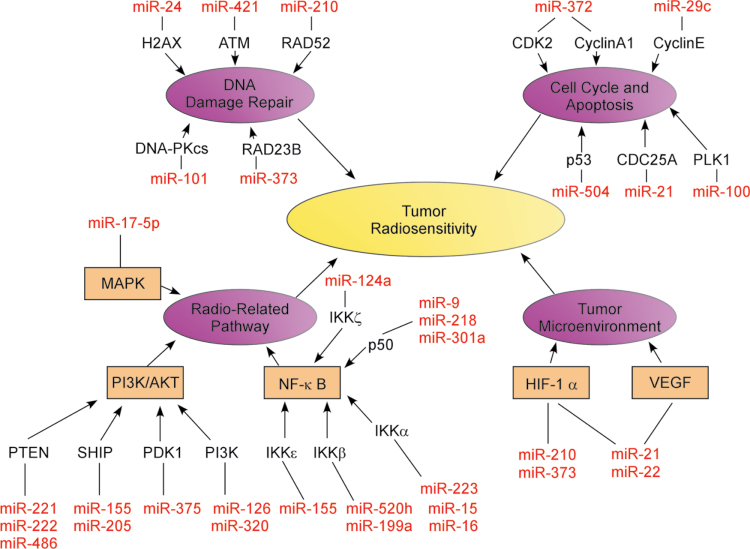
The regulatory mechanisms and role of radio-related miRNAs in tumor radiosensitivity are illustrated (Figure 4 and Supplementary Table 1, available at Carcinogenesis Online). Studies focusing on the role and regulation of miRNAs have become a hot topic in the cancer research field. Notably, miRNAs play a crucial role in various biological processes and in the initiation and development of cancer. Research studies focusing on miRNA cover many aspects of carcinogenesis, especially in the tumor therapeutic field, where more and more attention and insight have been centered on the regulatory mechanisms of miRNA in tumor radiotherapy. A thorough understanding of tumor radiosensitivity and the regulatory mechanisms of miRNA will not only provide new directions and insights to ultimately improve the radiotherapeutic effect but also bring new hope to more cancer patients.
Fig. 4.
A regulatory mechanism map of miRNA in tumor radiosensitivity. MiRNA regulates every aspect of tumor radiotherapy and tumor radiosensitivity by affecting the function of multiple key factors involved in DNA damage repair, cell cycle checkpoint, apoptosis, radio-related signal transduction pathways and TME.
Supplementary Material
Acknowledgments
Supplementary material
Supplementary Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Key Basic Research Program of China (2011CB504300); the Key Project of National Natural Science Foundation of China (30930101); the Co-Research Science Foundation of the Oversea and Hong Kong-Marco in China (81028012); the Hunan Provincial Innovation Foundation For Postgraduate in China (CX2012A005); the National Institutes of Health (USA) (R37 CA081064, CA027502, ES016548); The Hormel Foundation (USA).
Conflict of Interest Statement: None declared.
Glossary
| Abbreviation | Expansion |
|---|---|
| DDR | DNA damage response |
| DSB | double-strand breaks |
| ERK | extracellular signal-regulated kinases |
| HIF | hypoxia inducible factor-1 |
| HR | homologous recombination |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor-kappa B |
| NHEJ | non-homologous end-joining |
| TGF | transforming growth factor |
| TME | tumor microenvironment |
| UTR | untranslated region |
| VEGF | vascular endothelial growth factor. |
Footnotes
Precis: MicroRNAs regulate many aspects of tumor radiotherapy and impact tumor radiosensitivity by regulating DNA damage repair, cell cycle checkpoint, apoptosis, radio-related signal transduction pathways and tumor microenvironment, all of which determine the therapeutic effects of radiotherapy.
References
- 1. Chatterjee S., et al. (2011). Dosimetric and radiobiological comparison of helical tomotherapy, forward-planned intensity-modulated radiotherapy and two-phase conformal plans for radical radiotherapy treatmentr of head and neck squamous cell carcinomas. Br. J. Radiol. 84 1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Ruysscher D., et al. (2012). Individualised isotoxic accelerated radiotherapy and chemotherapy are associated with improved long-term survival of patients with stage III NSCLC: a prospective population-based study. Radiother. Oncol. 102 228–233 [DOI] [PubMed] [Google Scholar]
- 3. Boomsma M.J., et al. (2011). Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother. Oncol. 99 1–5 [DOI] [PubMed] [Google Scholar]
- 4. Begg A.C., et al. (2011). Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 11 239–253 [DOI] [PubMed] [Google Scholar]
- 5. Joubert A., et al. (2011). [Radiation biology: major advances and perspectives for radiotherapy]. Cancer Radiother. 15 348–354 [DOI] [PubMed] [Google Scholar]
- 6. Jamal M., et al. (2010). Microenvironmental regulation of glioblastoma radioresponse. Clin. Cancer Res. 16 6049–6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janga S.C., et al. (2011). MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv. Exp. Med. Biol. 722 59–74 [DOI] [PubMed] [Google Scholar]
- 8. Yang J.S., et al. (2011). Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell 43 892–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Kouwenhove M., et al. (2011). MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat. Rev. Cancer 11 644–656 [DOI] [PubMed] [Google Scholar]
- 10. Sayed D., et al. (2011). MicroRNAs in development and disease. Physiol. Rev. 91 827–887 [DOI] [PubMed] [Google Scholar]
- 11. Liu J., et al. (2011). MicroRNAs, an active and versatile group in cancers. Int. J. Oral Sci. 3 165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao L., et al. (2011). New role of microRNA: carcinogenesis and clinical application in cancer. Acta Biochim. Biophys. 43 831–839 [DOI] [PubMed] [Google Scholar]
- 13. Kasinski A.L., et al. (2011). Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 11 849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seviour E.G., et al. (2010). The DNA damage response: Balancing the scale between cancer and ageing. Aging 2 900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcu L.G. (2010). Altered fractionation in radiotherapy: from radiobiological rationale to therapeutic gain. Cancer Treat. Rev. 36 606–614 [DOI] [PubMed] [Google Scholar]
- 16. Thoms J., et al. (2010). DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin. Radiat. Oncol. 20 217–222 [DOI] [PubMed] [Google Scholar]
- 17. Vens C., et al. (2010). Targeting base excision repair as a sensitization strategy in radiotherapy. Semin. Radiat. Oncol. 20 241–249 [DOI] [PubMed] [Google Scholar]
- 18. Guo G.S., et al. (2011). DNA repair and synthetic lethality. Int. J. Oral Sci. 3 176–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukherjee B., et al. (2010). Targeting nonhomologous end-joining through epidermal growth factor receptor inhibition: rationale and strategies for radiosensitization. Semin. Radiat. Oncol. 20 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mladenov E., et al. (2011). Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutat. Res. 711 61–72 [DOI] [PubMed] [Google Scholar]
- 21. Holthausen J.T., et al. (2010). Regulation of DNA strand exchange in homologous recombination. DNA Repair 9 1264–1272 [DOI] [PubMed] [Google Scholar]
- 22. Wu J., et al. (2010). The role of BRCA1 in DNA damage response. Protein Cell 1 117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sak A, et al. (2010). Use of gammaH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin Radiat Oncol., 20 223–231 [DOI] [PubMed] [Google Scholar]
- 24. Bensimon A., et al. (2011). Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett. 585 1625–1639 [DOI] [PubMed] [Google Scholar]
- 25. Holloman W.K. (2011). Unraveling the mechanism of BRCA2 in homologous recombination. Nat. Struct. Mol. Biol. 18 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guirouilh-Barbat J.K., et al. (2010). AKT1/BRCA1 in the control of homologous recombination and genetic stability: the missing link between hereditary and sporadic breast cancers. Oncotarget 1 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu H., et al. (2011). MicroRNAs: new players in the DNA damage response. J. Mol. Cell Biol. 3 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landau D.A., et al. (2011). MicroRNAs in mutagenesis, genomic instability, and DNA repair. Semin. Oncol. 38 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lal A., et al. (2009). miR-24-mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat. Struct. Mol. Biol. 16 492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava N., et al. miR-24-2 controls H2AFX expression regardless of gene copy number alteration and induces apoptosis by targeting antiapoptotic gene BCL-2: a potential for therapeutic intervention. Breast Cancer Res. (2011);13:R39. doi: 10.1186/bcr2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu H., et al. (2010). ATM is down-regulated by N-Myc-regulated microRNA-421. Proc. Natl. Acad. Sci. U.S.A. 107 1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan D., et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS ONE. (2010);5:e11397. doi: 10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arai N., et al. (2011). Vital roles of the second DNA-binding site of Rad52 protein in yeast homologous recombination. J. Biol. Chem. 286 17607–17617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crosby M.E., et al. (2009). MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 69 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langerak P., et al. (2011). Regulatory networks integrating cell cycle control with DNA damage checkpoints and double-strand break repair. Philos. Trans.R. Soc. Lond., B, Biol. Sci. 366 3562–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garrett M.D., et al. (2011). Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol. Sci. 32 308–316 [DOI] [PubMed] [Google Scholar]
- 37. Lountos G.T., et al. (2011). Structural characterization of inhibitor complexes with checkpoint kinase 2 (Chk2), a drug target for cancer therapy. J. Struct. Biol. 176 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith J., et al. (2010). The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 108 73–112 [DOI] [PubMed] [Google Scholar]
- 39. Imreh G, et al. (2011). Chromosomal breaks during mitotic catastrophe trigger gammaH2AX-ATM-p53-mediated apoptosis. J. Cell Sci. 124 2951–2963 [DOI] [PubMed] [Google Scholar]
- 40. Stagni V., et al. (2008). ATM kinase activity modulates Fas sensitivity through the regulation of FLIP in lymphoid cells. Blood 111 829–837 [DOI] [PubMed] [Google Scholar]
- 41. Yang X., et al. (2009). miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 23 2388–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Oliveira P.E., et al. (2009). Hypoxia-mediated regulation of Cdc25A phosphatase by p21 and miR-21. Cell Cycle 8 3157–3164 [DOI] [PubMed] [Google Scholar]
- 43. Chien W.W., et al. (2011). Cyclin-dependent kinase 1 expression is inhibited by p16(INK4a) at the post-transcriptional level through the microRNA pathway. Oncogene 30 1880–1891 [DOI] [PubMed] [Google Scholar]
- 44. Legesse-Miller A., et al. (2009). let-7 Overexpression leads to an increased fraction of cells in G2/M, direct down-regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. J. Biol. Chem. 284 6605–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nie J., et al. (2012). microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis 33 220–225 [DOI] [PubMed] [Google Scholar]
- 46. Qin B., et al. (2011). MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem. Biophys. Res. Commun. 410 127–133 [DOI] [PubMed] [Google Scholar]
- 47.Fontana L., et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS ONE. (2008);3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zenz T., et al. (2009). miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood 113 3801–3808 [DOI] [PubMed] [Google Scholar]
- 49. Mérino D., et al. (2012). Bim must be able to engage all pro-survival Bcl-2 family members for efficient tumor suppression. Oncogene 31 3392–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian R.Q., et al. (2011). MicroRNA-372 is down-regulated and targets cyclin-dependent kinase 2 (CDK2) and cyclin A1 in human cervical cancer, which may contribute to tumorigenesis. J. Biol. Chem. 286 25556–25563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ding D.P., et al. (2011). miR-29c induces cell cycle arrest in esophageal squamous cell carcinoma by modulating cyclin E expression. Carcinogenesis 32 1025–1032 [DOI] [PubMed] [Google Scholar]
- 52. Hu W., et al. (2010). Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol. Cell 38 689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang P., et al. (2009). microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 69 8157–8165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shi W., et al. (2010). Significance of Plk1 regulation by miR-100 in human nasopharyngeal cancer. Int. J. Cancer 126 2036–2048 [DOI] [PubMed] [Google Scholar]
- 55. Xia S., et al. (2010). Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother. Radiopharm. 25 317–323 [DOI] [PubMed] [Google Scholar]
- 56.Ma X., et al. Down-regulation of EBV-LMP1 radio-sensitizes nasal pharyngeal carcinoma cells via NF-?B regulated ATM expression. PLoS ONE. (2011);6:e24647. doi: 10.1371/journal.pone.0024647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veuger S.J., et al. (2011). Persistence of unrepaired DNA double strand breaks caused by inhibition of ATM does not lead to radio-sensitisation in the absence of NF-?B activation. DNA Repair 10 235–244 [DOI] [PubMed] [Google Scholar]
- 58. Staples C.J., et al. (2010). Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J. Biol. Chem. 285 25928–25940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chou C.H., et al. (2009). Radiation-induced interleukin-6 expression through MAPK/p38/NF-kappaB signaling pathway and the resultant antiapoptotic effect on endothelial cells through Mcl-1 expression with sIL6-Ralpha. Int. J. Radiat. Oncol. Biol. Phys. 75 1553–1561 [DOI] [PubMed] [Google Scholar]
- 60. Bouquet F., et al. (2011). TGFß1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. 17 6754–6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zschenker O., et al. (2010). Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother. Oncol. 97 26–32 [DOI] [PubMed] [Google Scholar]
- 62. Lee K.M., et al. (2011). MicroRNA-7 increases radiosensitivity of human cancer cells with activated EGFR-associated signaling. Radiother. Oncol. 101 171–176 [DOI] [PubMed] [Google Scholar]
- 63. Kasten-Pisula U., et al. (2011). Cellular and tumor radiosensitivity is correlated to epidermal growth factor receptor protein expression level in tumors without EGFR amplification. Int. J. Radiat. Oncol. Biol. Phys. 80 1181–1188 [DOI] [PubMed] [Google Scholar]
- 64. Zeng K.W., et al. (2011). Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid ß-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 672 45–55 [DOI] [PubMed] [Google Scholar]
- 65.Bharadwaj U, et al. Mesothelin confers pancreatic cancer cell resistance to TNF-alpha-induced apoptosis through Akt/PI3K/NF-kappaB activation and IL-6/Mcl-1 overexpression. Mol. Canc. (2011);10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Toulany M., et al. (2008). PI3K-Akt signaling regulates basal, but MAP-kinase signaling regulates radiation-induced XRCC1 expression in human tumor cells in vitro. DNA Repair 7 1746–1756 [DOI] [PubMed] [Google Scholar]
- 67. Florczak U., et al. (2009). 2-Methoxyestradiol-induced radiosensitization is independent of SOD but depends on inhibition of Akt and DNA-PKcs activities. Radiother. Oncol. 92 334–338 [DOI] [PubMed] [Google Scholar]
- 68. Minjgee M., et al. (2011). K-RAS(V12) induces autocrine production of EGFR ligands and mediates radioresistance through EGFR-dependent Akt signaling and activation of DNA-PKcs. Int. J. Radiat. Oncol. Biol. Phys. 81 1506–1514 [DOI] [PubMed] [Google Scholar]
- 69. Liccardi G., et al. (2011). EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 71 1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Y., et al. (2011). Transforming growth factor-ß regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 30 1470–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Z., et al. (2011). Differential epithelium DNA damage response to ATM and DNA-PK pathway inhibition in human prostate tissue culture. Cell Cycle 10 3545–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu Y., et al. (2011). EGFR inhibitors with concurrent thoracic radiation therapy for locally advanced non-small cell lung cancer. Lung Cancer 73 249–255 [DOI] [PubMed] [Google Scholar]
- 73. Bergkvist G.T., et al. (2011). Studies on the inhibition of feline EGFR in squamous cell carcinoma: enhancement of radiosensitivity and rescue of resistance to small molecule inhibitors. Cancer Biol. Ther. 11 927–937 [DOI] [PubMed] [Google Scholar]
- 74. Lim W.K., et al. (2011). MicroRNAs dysregulated in breast cancer preferentially target key oncogenic pathways. Mol. Biosyst. 7 2571–2576 [DOI] [PubMed] [Google Scholar]
- 75. Sayed D., et al. (2010). AKT-ing via microRNA. Cell Cycle 9 3213–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sayed D., et al. (2010). MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 285 20281–20290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Small E.M., et al. (2010). Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl. Acad. Sci. USA 107 4218–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chun-Zhi Z., et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. (2010);10:367. doi: 10.1186/1471-2407-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang F., et al. (2010). MiR-17-5p promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology 51 1614–1623 [DOI] [PubMed] [Google Scholar]
- 80. Ma X., et al. (2011). MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 3 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Glazer P.M., et al. (2011). New translational possibilities for microenvironmental modulation of radiosensitivity. Radiat. Res. 176 412–414 [DOI] [PubMed] [Google Scholar]
- 82. Sheehan J.P., et al. (2010). Improving the radiosensitivity of radioresistant and hypoxic glioblastoma. Future Oncol. 6 1591–1601 [DOI] [PubMed] [Google Scholar]
- 83. Shinohara E.T., et al. (2009). Increasing sensitivity to radiotherapy and chemotherapy by using novel biological agents that alter the tumor microenvironment. Curr. Mol. Med. 9 1034–1045 [DOI] [PubMed] [Google Scholar]
- 84. Karar J., et al. (2009). Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol. Ther. 8 1994–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Solberg T.D., et al. (2008). Correlation between tumor growth delay and expression of cancer and host VEGF, VEGFR2, and osteopontin in response to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 72 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rezvani H.R., et al. (2011). HIF-1a in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J. Invest. Dermatol. 131 1793–1805 [DOI] [PubMed] [Google Scholar]
- 87. Cassavaugh J., et al. (2011). Hypoxia-mediated biological control. J. Cell. Biochem. 112 735–744 [DOI] [PubMed] [Google Scholar]
- 88. Yang W., et al. (2011). Hypoxia-inducible factor-1a downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and enhances radiosensitivity in chemical hypoxic human hepatoma SMMC-7721 cells. Cancer Biother. Radiopharm. 26 565–571 [DOI] [PubMed] [Google Scholar]
- 89. Harada H., et al. (2009). Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Br. J. Cancer 100 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Amini A., et al. (2012). The critical role of vascular endothelial growth factor in tumor angiogenesis. Curr. Cancer Drug Targets 12 23–43 [DOI] [PubMed] [Google Scholar]
- 91. Horsman M.R., et al. (2010). Vascular targeting therapy: potential benefit depends on tumor and host related effects. Exp. Oncol. 32 143–148 [PubMed] [Google Scholar]
- 92. Wang R., et al. (2011). Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr. Med. Chem. 18 3168–3189 [DOI] [PubMed] [Google Scholar]
- 93. Chan S.Y., et al. (2010). MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9 1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huang X., et al. (2010). MiR-210–micromanager of the hypoxia pathway. Trends Mol. Med. 16 230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Devlin C., et al. (2011). miR-210: more than a silent player in hypoxia. IUBMB Life 63 94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chan N., et al. (2009). Tumor hypoxia as a modifier of DNA strand break and cross-link repair. Curr. Mol. Med. 9 401–410 [DOI] [PubMed] [Google Scholar]
- 97.Liu L.Z., et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1a expression. PLoS ONE. (2011);6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamakuchi M., et al. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS ONE. (2011);6:e20291. doi: 10.1371/journal.pone.0020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deorukhkar A., et al. (2010). Targeting inflammatory pathways for tumor radiosensitization. Biochem. Pharmacol. 80 1904–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Das U.N. (2012). Radiation resistance, invasiveness and metastasis are inflammatory events that could be suppressed by lipoxin A4. Prostaglandins Leukot. Essent. Fatty Acids 86 3–11 [DOI] [PubMed] [Google Scholar]
- 101. Cosentino C., et al. (2011). ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 30 546–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.