Abstract
Peripheral nerve repair across long gaps remains clinically challenging despite progress made with autograft transplantation. While scaffolds that present trophic factors and extracellular matrix molecules have been designed, matching the performance of autograft-induced repair has been challenging. In this study, we explored the effect of cytokine mediated ‘biasing’ of macrophage phenotypes on Schwann cell (SC) migration and axonal regeneration in vitro and in vivo. Macrophage phenotype was successfully modulated by local delivery of either Interferon-gamma (IFN-γ) or Interleukin-4 (IL-4) within polymeric nerve guidance channels, polarizing them toward pro-inflammatory (M1) or pro-healing (M2a and M2c) phenotypes, respectively. The initial polarization of macrophages to M2a and M2c phenotype results in enhanced SC infiltration and substantially faster axonal growth in a critically-sized rat sciatic nerve gap model (15 mm). The ratio of pro-healing to pro-inflammatory population of macrophages (CD206+/CCR7+), defined as regenerative bias, demonstrates a linear relationship with the number of axons at the distal end of the nerve scaffolds. The present results clearly suggest that rather than the extent of macrophage presence, their specific phenotype at the site of injury regulates the regenerative outcomes.
Keywords: Immunomodulation, Nerve regeneration, Macrophage, Cytokine, Scaffold
1. Introduction
Peripheral nerve defects are caused due to trauma or in the course of surgery [1]; however, bridging long gaps remains a clinical challenge [2, 3]. The current clinical approach for repairing long nerve deficits (15 mm or longer) is to use nerve autografts; however, the paucity of donor nerves, the disparity of donor nerve sizes with the recipient sites and the modality mismatch has compelled a search for alternatives that can match or exceed autograft performance [1-4].
Several approaches to enhance regeneration have been explored including the design of novel nerve guidance channels [2], fillers within nerve guidance channels [5], local delivery of neurotrophic factors [6, 7], transplantation of cells [8, 9], and/or application of topographical cues [4, 10, 11]. While these approaches are promising, the overall success rate in matching autograft performance has been limited. Current approaches focus on enhancing axon growth by direct action on nerves, or glial cells, and here we investigate an alternative approach to influencing regenerative outcomes by modulating the initial inflammatory sequence via macrophages [12]. Since the sequence of cellular and molecular events associated with nerve regeneration is influenced by immune cells [13, 14], we hypothesize that modulating immune cells upstream of action on nerves or Schwann cells (SC) triggers endogenous repair mechanisms that can stimulate nerve repair across long gaps [12].
It is evident that the immune response plays an important role during regeneration in many tissues [15-17]. Macrophages are quite abundant and phenotypically diverse immune cell populations presented during nerve degeneration and regeneration [12, 13, 18]. Macrophages arrive at the site of injury within 24 h and their numbers at the site peak within 14-21 days [19], whereas it takes at least one week for lymphocyte influx to occur [13]. Macrophages, which are mainly recruited from circulation, account for the bulk of phagocytosis within days of peripheral nerve injury and play a critical role in debris removal, growth factor production, and remodeling of the extracellular matrix (ECM) of the distal nerve[20, 21]. Thus macrophages might represent an upstream ‘lever’ to influence downstream axon and SC regeneration (Fig. 1a).
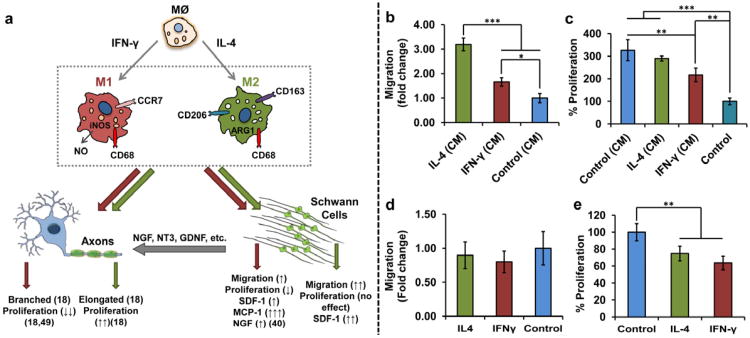
Figure 1. Polarizing macrophages to M1 and M2 phenotypes indirectly affects Schwann cells (SC) and axons, in vitro.
(a) Schematic diagram of effect of pro-inflammatory (M1) and anti-inflammatory (M2a and M2c) macrophages on SC and axons represented by red (dark) and green (light) arrows, respectively. M1 and M2 macrophages can modulate proliferation and morphology of axons as well as proliferation and secreting products of SCs. (b) effect of SC migration in CM from macrophages (72 h). Mean ± s.d. n = 4. ***P < 0.001. *P < 0.05 (one-way ANOVA followed by a Tukey's post-hoc) (c) SC proliferation in CM from macrophages. Mean ± s.d. n = 4. ***P < 0.001. **P < 0.01 (one-way ANOVA followed by a Tukey's post-hoc) (72h). Direct effect of the cytokines on the Schwann cell, (d) proliferation and (e) migration. Mean ± s.d. n = 4. **P < 0.01 (Student's t-test). Asterisks denote a significant difference. CM from IL-4 treated macrophages increases the SC migration almost 3 times without affecting the SC proliferation in comparison to the control. Direct addition of cytokines to SC does not have any effect on their migration but decreases their proliferation.
Recently, macrophages have been demonstrated to have a spectrum of activation states/phenotypes which has led to categorizing them broadly as classically activated (M1) and alternatively activated (M2) macrophages [22-24]. M1 macrophages, which are activated by injury-triggered endogenous inflammatory signals, such as T-helper 1 (Th1) cytokine IFN-γ, considered to be inflammatory, microbicidal, and tumor destructive [23, 24]. M1 macrophages produce high levels of oxidative metabolites (e.g. nitric oxide and superoxide) as well as pro-inflammatory cytokines. On the other hand, M2 macrophages, which can be activated by Th2 cytokines such as IL-4, IL-10 and IL-13, support tissue repair by producing anti-inflammatory cytokines which mediate angiogenesis, cell replacement and matrix remodeling while suppressing destructive immunity [24, 25]. The M2 phenotype has also been shown to have its own subtypes (M2a, M2b and M2c) [26, 27]. While the exact role of each M2 subtype is not well defined, it has been shown that M2a and M2c enhance tissue repair and pro-healing functionality, but the M2b subtype possesses regulatory function with some characteristics of both anti-inflammatory and pro-inflammatory macrophages [26, 27].
Despite the fact that macrophages constitute a substantial fraction of the post-injury cellular milieu, very little attention has been devoted to understanding the contribution from different phenotypes of macrophages and the effect of their modulation on regeneration after injury, especially in the context of the peripheral nervous system (PNS) [13, 28]. Here, we modulate the phenotype of macrophages by employing two well-known cytokines IFN-γ or IL-4, polarizing them toward pro-inflammatory (M1) or pro-healing (M2a and M2c) phenotypes, respectively. Then, we investigate the influence of macrophage phenotype on the peripheral nerve regeneration in a critically-sized, non-healing 15 mm rat sciatic nerve gap.
2. Materials and Methods
2.1 SC Proliferation Study
Alveolar rat macrophage cells (NR8383)(2 × 105) were placed in serum-free medium (F12K with 1% P/S;1% L-glutamine) and incubated, allowing attachment for 4 h (37°C, 5% CO2). After 4 h, IL-4 or IFN-γ (50 ng/mL in 1 mL) was added and cells were incubated for 24 h (n=4 per group). Then LPS (100 ng/mL) was added to IFN-γ containing medium and all cells were incubated for an additional 7 h. Then medium for all cells was changed to fresh serum-free medium and cells were incubated for another 24 h (37°C, 5% CO2). After 24 h, medium was collected and frozen at −80°C. 2 × 105 Schwann cells (RSC96) were added to the complete medium (DMEM with 10% FBS;1% P/S;1% l-glutamine) and incubated for 24 h (37°C, 5% CO2). After 24 h, the medium was removed upon process extension from RSC96 cells, and 250 μL of serum-free medium (DMEM with 1% P/S;1% l-glutamine) and 250 μL of CM from macrophages treated with IL-4, IFN-γ, or no cytokine (warmed to 37°C before adding) was added to the cells. Serum-free DMEM medium (250 μL) was added as the control (n=4 per group). The cells were incubated (37°C, 5% CO2) for 72 h. After incubation, medium was removed from top without disturbing cells and CCK8 assay (10% CCK8 in 500 μL serum-free DMEM medium; 450 nm) was performed on cells attached with a standard (31.25K to 500K cells).
2.2 SC Migration Study
Similar protocol to proliferation study was used to activate the macrophages except after activating macrophages with the cytokines, initial media was changed with fresh serum-free media and 24-well Boyden chambers (BD Falcon FluoroBlok, 8 μm) were inserted into activated macrophage wells and 5 × 104 Schwann cells (RSC96;expressing Green Fluoroscent Protein (see SI text for further details)) in 200 μL serum-free medium (F12K with 1% P/S;1% l-glutamine) were added to the top insert which does not have any coating. After 72 h incubation, the migrating cells at the bottom of the filters were fixed with paraformaldehyde and counted at five random fields per filter (n=4 per group).
2.3 In vivo implantation
Polysulfone tubes filled with 0.7% agarose (SeaPrep Lonza) mixed with 1 μg/ml rat recombinant cytokine IFN-γ, IL-4 (Antigenix America Inc.) or agarose only were prepared (n=4 per group). The Scaffolds were kept cooled in 4°C for 1 h. Adult Lewis male rats (250 – 300 g) were induced to anesthetic depth with inhaled isoflurane at 3-4%. The procedure for fabrication of PAN-MA nano-fiber film scaffolds (n=4) were explained elsewhere [4]. 17 mm nerve autografts, which were resected from the rat tibial nerve being bridged were used as our positive control (n=4). Throughout surgery, the animals were maintained at 1.5-2% isoflurane. Microscissors were used to transect the tibial nerve branch, and the nerve stumps were pulled 1 mm into each end of the 17 mm (agarose with cytokine) guidance scaffolds (leaving a 15 mm gap) or autografts and fixed into place with a single 10-0 nylon suture (Ethicon). The muscles were reapposed with 4-0 vicryl sutures (Ethicon) and the skin incision was clamped shut with wound clips (Braintree Scientific). After the surgery, the rats were placed under a warm light until sternal, and then housed separately with access to food and water ad libitum at constant temperature (19-22°C) and humidity (40-50%) on a 12:12 h light/dark cycle. To prevent toe chewing, a bitter solution (Grannick's Bitter Apple) was applied twice daily to the affected foot. Marcaine (0.25% w/v) was administered subcutaneously for post-surgical pain relief (0.3 ml/animal). Animals were maintained in facilities approved by the Institutional Animal Care and Use Committee (IACUC) at the Georgia Institute of Technology and in accordance with the current United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health regulations and standards.
2.4 Scaffold explantation and analysis
After three weeks, scaffolds or autografts were explanted for histological analysis of nerve regeneration. Explants were fixed in 4% paraformaldehyde in PBS (Sigma-Aldrich), washed, and stored in 30% sucrose in PBS for 24 h. Samples were embedded in O.C.T. gel (Tissue Tek) and frozen for cryosectioning (CM30505, Leica). Scaffold or autografts were sectioned transversely or longitudinally at a thickness of 16μm and reacted with immunofluorescent markers to quantify the different cell types, using techniques previously described [4]. Briefly, sections were incubated for 1 h at room temperature in a blocking solution of goat serum (Gibco) in PBS, incubated overnight at 4°C in a mixture of primary antibody and blocking solution, washed and incubated for 1 h at room temperature in a solution of secondary antibody mixed in 0.5% triton in PBS. Slides were washed twice more with PBS, incubated with DAPI for 10 min and then dried and cover slipped for evaluation. Primary antibodies NF160 (1:250, mouse IgG1, Sigma), S-100 (1:250, rabbit, IgG, DakoCytomation), CD68 (1:100, mouse IgG1, AbD Serotec (ED1)), CD163 (1:200, mouse IgG1, AbD Serotec (ED2)), CCR7 (1:200, rabbit, IgG, Cell Application), and CD206 (1:100, rabbit, IgG, Santa Cruz Biotechnology) were used. The following secondary antibodies were used: goat anti-rabbit IgG Alexa 488/594 (1:220, Invitrogen), goat anti-mouse IgG1 Alexa 488/594 (1:220, Invitrogen).
2.5 Statistical Analysis
Data were analyzed using the Student's t-test for unpaired data where appropriate or one-way ANOVA followed by a Tukey's least significant difference post-hoc test where warranted to determine significant differences between groups with 95% confidence; A p value < 0.05 was considered to represent a statistically significant difference.
3. Results
3.1 Effect of polarized macrophages on SC proliferation and migration in vitro
Using a Boyden Chamber (BC), the effect of M1 or M2 macrophage phenotype on SC migration was explored in vitro. Because some of the secretory products of macrophages are labile, with short half-lives (e. g., oxygen and nitrogen free radicals), media transfer assays may underestimate the migratory potential of conditioned media (CM) from macrophages. Therefore, SCs grown on the trans-well of the BC were placed in the wells of pro-inflammatory and anti-inflammatory plated macrophages. The phenotype of macrophages was confirmed by measuring the amount of nitrite production and the ratio of IL10 to IL-12 gene expression in cells after being exposed to IFN-γ and IL-4 cytokines (see Supplementary Fig. S1). After being primed overnight, these macrophages retained their activation state even after being washed to remove residual stimulating cytokines (see Supplementary Fig. S1). Both the M1 (treated with IFN-γ) and M2 (treated with IL-4) macrophages increased the rate of SC migration by ∼170% and ∼300%, respectively, in comparison to the control (macrophages treated with no cytokines) (Fig. 1b).
To confirm that enhanced SC migration was not due to enhanced SC proliferation, CCK8 assays were performed using the same number of cells treated with the CM from M1 and M2 macrophages at 72 h time point. Although CM from M2 macrophages did not have any significant effect on the rate of SC proliferation, CM from M1 macrophages marginally decreased proliferation rate of the SC compared to the CM from the control (n = 4, **P < 0.01, one-way ANOVA followed by a Tukey's post-hoc) (Fig. 1c). Further, direct addition of IFN-γ and IL-4 to the SC cultures did not have any effect on the SC migration (Fig. 1d) but both of the cytokines significantly decreased the SC proliferation (n = 4, P < 0.01, Student's t-test) (Fig. 1e).
3.2 Effect of immunomodulatory cytokines, IFN-γ or IL-4, on the macrophages within the nerve scaffold, in vivo
The influence of delivering either IFN-γ or IL-4 on the phenotypes of macrophages and the subsequent impact on the rate of peripheral nerve regeneration in rodents was assessed in vivo. IFN-γ or IL-4 (1000 ng/mL) mixed with an agarose hydrogel was injected inside a 17 mm, semi-permeable, hollow polysulfone tube that was in turn used to bridge a 15mm sciatic nerve gap in rats (Fig. 2a). Based on the in vitro study, these cytokines diffuse out from the hydrogel within 24 h (Supplementary Fig. S2).
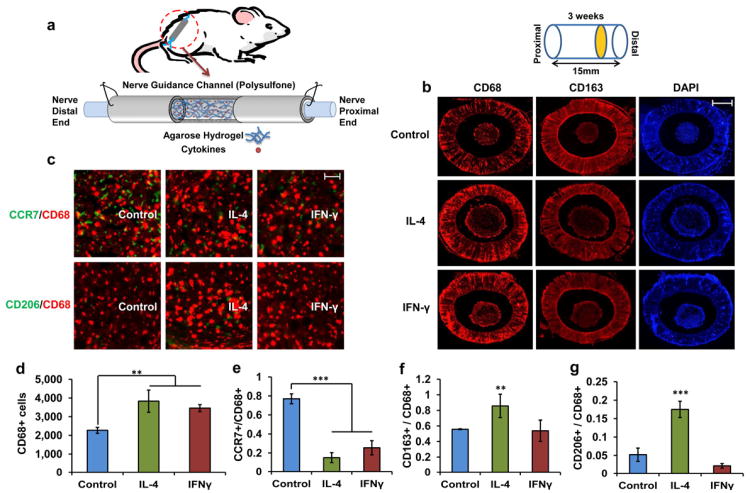
Figure 2. Effect of IFN-γ and IL-4 on the number of macrophages and their phenotypes within the nerve guidance scaffold.
(a) Schematic of rat sciatic nerve model and the nerve guidance channel structure. (b) Cross-sectional images of CD68+ cells and CD163+ cells representing number of macrophages and alternatively activated macrophages (M2) at the distal end, respectively. Blue represents the DAPI. Scale bar = 500 μm (c) magnified merged images of CCR7/CCD68 (green/red) and CD206/CD68 (green/red). Scale bar = 10 μm (d) Quantitative analysis of number of macrophages. Mean ± s.d. n = 4. **P < 0.01 (Student's t-test). (e) Ratio of the CCR7+ cells to the CD68+ cells represents the ratio of classically activated macrophages. Mean ± s.d. n = 4. ***P < 0.001 (Student's t-test). (M1) (f) Ratio of the CD163+ cells to the CD68+ cells represents the ratio of alternatively activated macrophages (M2). Mean ± s.d. n = 4. **P < 0.01 (Student's t-test) (g) Ratio of the CD206+ cells to the CD68+ cells represents the ratio of pro-healing macrophages (M2a and M2c). Mean ± s.d. n = 4. ***P < 0.001 (Student's t-test) . Asterisks denote a significant difference compared with non-cytokine treated control scaffolds. Both IL-4 and IFN-γ increases the number of macrophages at the distal end of the scaffold. Higher ratio of M2 macrophages indicated by CD163 and CD206 markers in comparison to the control and IFN-γ treated scaffold demonstrates the effective delivery of cytokines.
After three weeks, animals were euthanized, followed by immunohistochemical staining with CD68, CD163, CCR7 and CD206 to identify total number of activated macrophages, alternatively activated macrophages (M2), pro-inflammatory macrophages (M1) and anti-inflammatory/pro-healing macrophages (M2a and M2c), respectively. Previously established immunohistochemistry techniques for quantifying fluorescent pixel intensity of aforementioned antibodies were used to quantify macrophages and their subtype [4, 29-31]. Majority of macrophages stayed on the outer layer of cell regenerative cable (nerve bundle) and their density decreased approaching the center of the cable (Fig. 2b). Although both IFN-γ and IL-4 increased the total number of macrophages in comparison to non-cytokine treated scaffold (Fig. 2d), this increase was not significantly different between IFN-γ and IL-4 samples. However, the numbers of M1 (CCR7+) and M2 (CD163+) macrophages as well as M2 subtypes, M2a and M2c (CD206+) macrophages, were significantly different between IFN-γ and IL-4 treated scaffolds (Fig. 2c and e-g). The ratios of CCR7+ cells were significantly lower in both IFN-γ and IL-4 containing scaffold (n = 4, P < 0.001, Student's t-test) (Fig. 2e). However, the ratios of CD163+ and CD206+ indicating different subtype of M2 macrophages were higher in IL-4 containing scaffold in comparison to control (n = 4, P < 0.01 and P < 0.001, Student's t-test) while in IFN-γ containing scaffolds, these numbers were significantly different from the control scaffold (Fig. 2f, g).
3.3 Effect of immunomodulatory cytokines, IFN-γ or IL-4, on the SC infiltration and axonal growth, in vivo
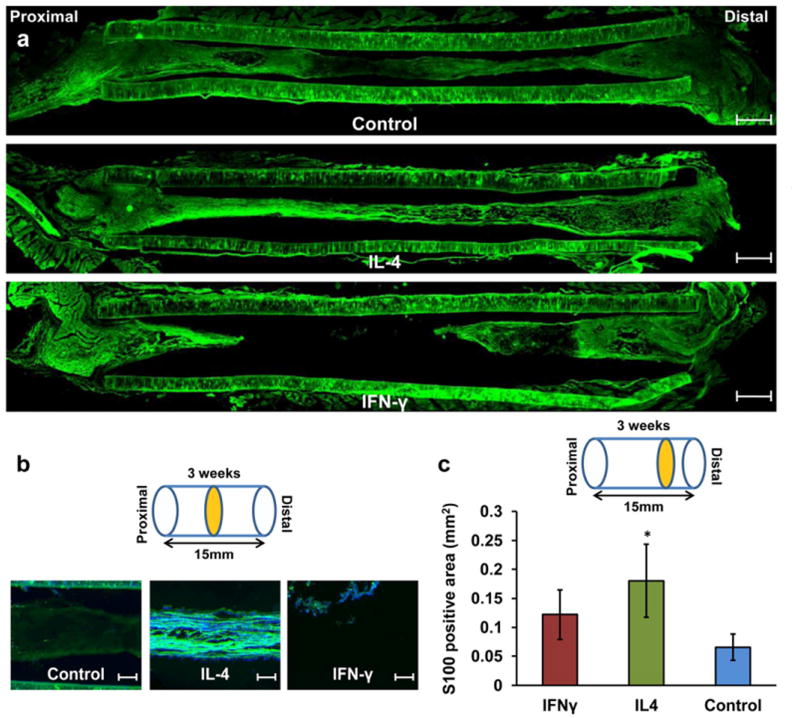
SC infiltration into the nerve gap was investigated by using immunostaining for SC protein marker (S100) (Fig. 3.). IL-4 treatment considerably enhanced the SC migration toward the middle of the hydrogel as shown in the longitudinally sectioned samples (Fig. 3a). On the other hand, both IFN-γ and non-cytokine treatment resulted in less SC infiltration and thinner regenerative cable (Fig. 3a, b). Even in the case of the non-cytokine treated control, where the cable formation was usually complete, SC rarely reached the midpoint (Fig. 3b). Quantitative analysis of area occupied by SC at the distal end of the scaffold using fluorescent pixel intensity of S100 demonstrated the similar trend (Fig. 3c).
Figure 3. Effect of IFN-γ and IL-4 on the Schwann cell infiltration within the nerve guidance scaffold.
(a) Longitudinal section of S100 staining for Schwann cell. Scale bar = 1 mm (b) Merged magnified images with DAPI at the mid-section. Scale bar = 250 μm (c) Quantitative analysis of SCs at the distal end by using S100. Mean ± s.d. n = 3. *P < 0.05 (Student's t-test). Asterisks denote a significant difference compared with non-cytokine treated control scaffold. IL-4 treated scaffold significantly enhances the SC infiltration inside the scaffold in comparison to the non-treated control scaffold.
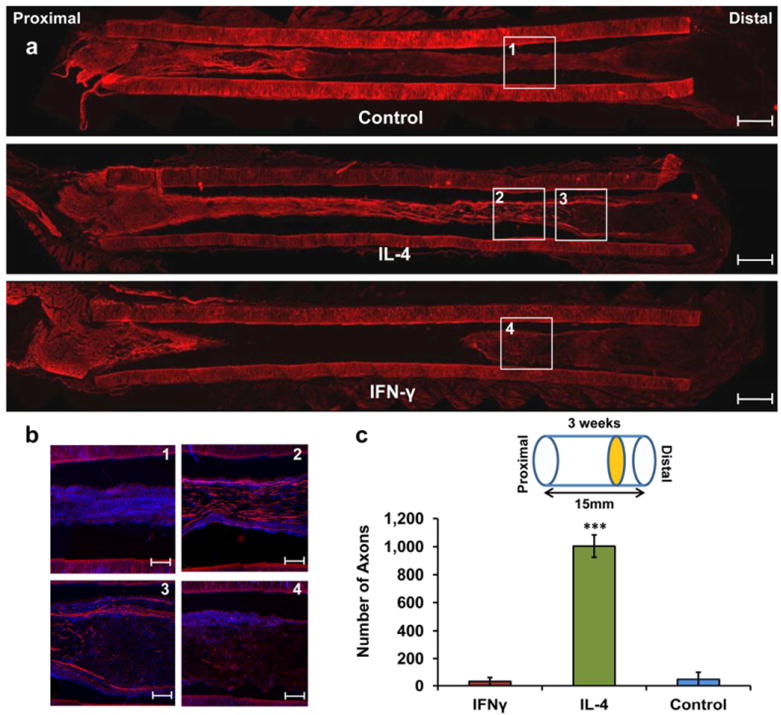
Axons were also visualized by using immunostaining for Neurofilament-160 (NF160) (Fig. 4). In contrast to the SC which infiltrate from the both ends of the scaffold, axons grow only from the proximal toward the distal end (Fig. 4a). In the case of IL-4 treated samples, axons reached almost the distal end after 3 weeks (Fig. 4b). Quantitative analysis using cross sectional samples at the distal end of the scaffold showed that number of axons (positively stained for NF160) in the IL-4 samples were ∼20 times higher in comparison to the non-cytokine treated control (n = 4, P < 0.001, Student's t-test) (Fig. 4c). This number was not significantly different from the control in IFN-γ treated scaffold.
Figure 4. Effect of IFN-γ and IL-4 on the axonal growth within the nerve guidance scaffold.
(a) Longitudinal section of NF160 staining for axons. Scale bar = 1 mm (b) Merged magnified images with DAPI magnified images. Scale bar = 250 μm (c) Quantitative analysis of NF160 at the distal ends. Mean ± s.d. n = 4. ***P < 0.001 (Student's t-test). Asterisks denote a significant difference compared with non-cytokine treated control scaffold. Number of axons at the distal end of the IL-4 treated scaffold is ∼20 times higher in comparison to the non-treated control scaffold.
3.4 Correlation between ratio of CD206+ to CCR7+ macrophages and number of axons in vivo
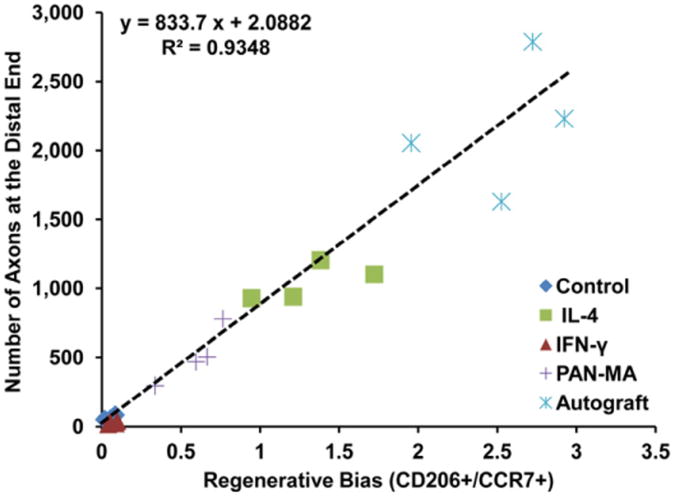
CD206+ and CCR7+ cells represent the population of macrophages that have pro-healing (M2a and M2c) and pro-inflammatory (M1) functions, respectively [26, 30]. We define the ratio of pro-healing to pro-inflammatory population of macrophages (CD206+/CCR7+) as the regenerative bias. In the IL-4 containing scaffold, the regenerative bias is approximately twenty times higher in comparison to the both non-cytokine treated control and IFN-γ containing scaffolds (Fig. 5 and Table S3). We calculated the regenerative bias as well as number of axons at the distal ends for two completely separate conditions too: autograft and polysulfone scaffold filled with a layer of Poly(acrylonitrile-co-methyl acrylate) (PAN-MA) nano fiber film [4, 32] (Fig. 5 and Table S3). Combined with this new set of data, we derived a relationship between the regenerative bias and axonal growth (Fig. 5):
Figure 5. Regenerative bias correlates with axonal growth.
At the distal end of the nerve stump, the ratio of pro-healing macrophages (CD206+) to pro-inflammatory macrophages (CCR7+), i.e. regenerative bias, directly correlates to (predicts) the final regenerative outcome (axonal growth) with a linear relationship at three weeks post-injury time point. The fact that data from autograft and PAN-MA nano-fiber scaffolds also fit the regenerative bias/axonal growth correlation demonstrates that this model can be universally applied across other nerve regeneration approaches.
Where A and R represent the number of axons and the regenerative bias (the ratio of CD206+ to CCR7+ cells) at the distal, respectively. Figure 5 shows that there is a direct correlation between ratio of pro-healing to pro-inflammatory macrophages and number of axons at the distal end of the nerve scaffold in this model. Nevertheless, samples with regenerative bias higher than 1.5, especially autografts, start to deviate from this linear relationship (Fig. 5).
4. Discussion
Although there is strong evidence to support the notion that macrophages modulate regeneration in the nervous system [12, 13, 18], it is unclear if they support [17, 33, 34] or hinder [35, 36] regenerative biochemical cascades after injury [37]. This apparent lack of clarity might stem from the ‘double-edged’ characteristics of macrophages [12, 38, 39]. On one hand macrophages, as the primary phagocytes of the innate immune system, release inflammatory neurotoxin and cytokines leading to degeneration and necrosis. On the other hand, macrophages scavenge debris, stimulate trophic factors such as nerve growth factor (NGF) and IL-6 [25, 40], and help remodeling the ECM [29]. These seemingly opposing effects may be explained by the existence of macrophages that are phenotypically and functionally heterogeneous.
It is not known if phenotype-committed macrophages are selectively recruited to the site of injury, or if the phenotype of non-committed macrophages is determined after recruitment by locally-present cytokines [41]. Regardless, macrophage phenotypes can be dynamically regulated by biological cues from the tissue microenvironment [42]. During the course of wound healing in some tissues, such as cutaneous or myocardial tissue, there is a well defined shift in macrophage activation states: from M1 to M2 and subsequent secreted products [43, 44]. However, in other tissues such as the spinal cord, this shift does not occur after injury [18] and hence, it is postulated that this results in impaired recovery of those tissues. Although macrophage phenotypes and their roles in the course of regeneration after traumatically injured central nervous system (CNS) have been explored [18, 27], the effect of macrophage phenotype on PNS regeneration and its associated cells (such as SC and axons) remains to be characterized [13, 28].
To address this gap in our understanding, the effects of modulating macrophage phenotype on nerve regeneration were evaluated in a rat sciatic nerve model using polysulfone/agarose hydrogel scaffolds mixed with two immunomodulatory cytokines, IL-4 or IFN-γ. Although agarose hydrogels have previously been demonstrated to support regeneration of peripheral nerves [45, 46], our study revealed that short-term release of anti-inflammatory cytokine (IL-4) in the PS/agarose nerve guidance scaffold substantially accelerates downstream axon regeneration.
Our results strongly suggest that rather than the extent of macrophage presence, their specific phenotype at the site of injury biases the regenerative outcomes. It can be postulated that these results stem from the indirect effect of immunomodulatory cytokines, IL-4 or IFN-γ, on SC and axons via modulation of macrophages phenotype since neither of these two cytokines have any significant direct effect on axonal growth [47] and SC migration (Fig. 1d). We established that modulating the initial inflammatory sequence via macrophages can bias the regenerative biochemical cascade in a significant manner that obviates the need for the downstream modulation of multiple factors after peripheral nerve injury.
In fact, we defined a new term, regenerative bias, based on the ratio of CD206+/CCR7+ ((M2a+M2c)/M1) macrophages and found a direct correlation between this number and the rate of SC infiltration and axonal growth in vivo (Fig. 3b, Fig. 5). Our data showed that the number of CD163+ cells (M2 macrophages) and CD206+ cells (M2a and M2c macrophages) were both increased by IL-4 treatment; however this increase was more drastic in the number of CD206+ cells in comparison to CD163+ cells; for this reason, we chose CD206+/CCR7+ ratio to represent inflammatory status of the scaffold as the regenerative bias instead of other potential options such as CD163+/CCR7+ (M2/M1) or CD206+/CD68+ (M2/MØ) ratios. Moreover, IL-4 polarizes macrophages toward the M2a subtype, directly, and toward the M2c subtype via M2a secretory products such as IL-10 or TGFβ, indirectly [22, 27]. CD163 is a common surface marker among all the subtype of M2 macrophages while CD206 is the surface marker only for M2a and M2c macrophages [27]. Therefore, compared to other potential aforementioned options, the ratio of the CD206+/CCR7+ cells better indicates the effectiveness of the immunomodulatory cytokine delivery at the site of nerve injury. Both CD206 and CCR7 are widely used to characterize the phenotype of macrophages in animal and human models [26, 29-31, 48]. CCR7 is also known to be expressed by certain subtypes of dendritic and T-cells; however, the level of this expression is much smaller as compared to macrophages [29-31].
It has already been shown that axons can also be directly affected by secretory products of different phenotypes of macrophages in the CNS [18, 49]. Although CM from the unstimulated macrophages has a minimal effect on axonal growth, M1 macrophages are neurotoxic [18, 49]. In contrast, M2 macrophages not only have no adverse effect on cortical neurons, but also promote the regenerative response [18]. Additionally, Kigerl et al. demonstrated that M2 macrophages can even overcome axonal growth inhibition caused by CSPG and myelin [18]. Indirect modulation of macrophages toward the M2 phenotype by using fibroblast growth factor (aFGF) can also result in the secretion of growth factors that can potentially help axonal regeneration after spinal cord injury in rats [50]. It has also been shown that certain cytokines that promote M2 macrophage formation, such as IL-10, moderately enhance neuroprotection [51]. The Schwartz laboratory has demonstrated that appropriate activation of microglia by IL-4 or IFN-γ differentially induces neurogenesis as well as oligodendrogenesis from adult stem cells [52]. However, to the best of our knowledge, exploiting macrophage phenotype modulation to promote regeneration in the PNS has not yet been reported.
Moreover, we showed that M1 and M2 macrophages can also affect SC proliferation and migration. Although it has been documented that CM from classically activated (M1) macrophages increases SC proliferation [40, 53], our study extends the known mitogenic effect of activated macrophages on SC to the M2 phenotype. Moreover, since SC infiltration is an integral component of axonal regeneration after sciatic nerve injury, we studied the effect of modulating macrophage activation states on SC migration too. Both M1 and M2 macrophages were shown to promote migration of SC. This result is in agreement with an in vitro study showing similar trend of M1 and M2 macrophages in recruitment of vessel associated progenitor cells [54]. The study showed that both M1 and M2 macrophages can induce chemotaxis of progenitor-like cells but with different capability and through different pathways. However, here we also showed that M2 macrophages increased the migration rate of SC 2-fold more than CM from M1 macrophages. These results also parallel another study which investigated the effect of CM from M1 and M2 macrophages on the migration of mouse myoblast-like cell [31]. Although it is clear that factors in the CM from M2 macrophages boost the migration rate, the precise mechanisms by which M2 macrophages trigger such a response are still unknown. However, preliminary gene expression analysis indicates that there are several genes in SC that are affected by macrophage CM migration (supplementary Fig. S3); in particular, stromal cell-derived factor-1 (SDF-1) demonstrates a trend similar to that observed with SC. Recently, it was demonstrated that the SDF-1 receptor, CXCR7, is an active component of SDF-1 signaling in SC [55], suggesting a possible autocrine mechanism. However, further investigation is necessary to identify the precise signaling pathway.
Further, to evaluate our regenerative bias/axonal growth model in other nerve regeneration approaches, PAN-MA nano-fiber film scaffold and autograft were also studied based on this model. Our data demonstrated that these two completely different regenerating conditions also fit the regenerative bias model which demonstrates that this model, independent of the approach, can be universally applied across other nerve regeneration approaches. However, the noticeable deviation from this linear correlation in high-regenerative-capacity conditions, such as autograft, clearly indicates the involvement of other critical factors in the process of rapid regeneration which this regenerative bias model cannot fully predict.
Although the axonal growth in the IL-4 containing scaffold in this study (as determined by the number of axons at the distal end) is still lower than that for autograft, from our experience with alternate strategies to enhance peripheral nerve repair such as presenting topographical cues or delivering trophic factors, regulating macrophage phenotype using immunomodulatory cytokines resulted in a considerably faster rate of axonal regeneration in this critically-sized nerve gap [4, 45]. In fact, the axonal growth rate, as defined by the length of axons in the nerve gap as a function of time, is faster by a factor of 2 compared to the best rate achieved in previous studies [4, 32] (Fig. 5 and Table S3). This data is significant because several studies have already clearly demonstrated that faster axonal growth substantially contributes to higher functional recovery [45, 56-58].
This approach, by minimizing the need for sophisticated delivery vehicle(s), demonstrates that short and early stimulation of macrophages can significantly influence the longer-term regenerative outcomes. The effectiveness of this approach possibly originates from the fact that besides the direct effects of different macrophage phenotypes on SC infiltration and axonal growth, macrophages play a key role in other aspects of the regenerative process. They actively participate in cell replacement via stem cell recruitment and homing as well as in the resident progenitor cell differentiation and proliferation [33, 54]. Their secretory products also influence the different phases of angiogenesis both in vivo and in vitro [59, 60].
Finally, in this study only three surface markers (CD68, CCR7, and CD206) were employed to characterize macrophages and their phenotypes participating in nerve regeneration. It is becoming increasingly evident that other ways of examining macrophage phenotype(s) including protein (cytokine and chemokine) analysis, and gene profiling can help to characterize a specific phenotype better and understand the extent to which each phenotype perform. Furthermore, as with most models, the M1/M2 classification scheme certainly oversimplifies the complexity of the PNS macrophage response. In fact, the present study identified macrophages that co-expressed markers for both or none of the M1 and M2 macrophage. Although the effects of these intermediate macrophages are unknown in the context of PNS regeneration, this model provides a useful conceptual framework. Future studies should examine additional macrophage-activation markers and cytokine expression to more extensively characterize the M1/M2 profile and the role these macrophages play in the peripheral nerve regeneration process. Moreover, this manuscript introduces the regenerative bias concept and further studies on the other types of nerve scaffolds need to be done to evaluate the model more comprehensively.
5. Conclusions
Our data demonstrates that modulation of macrophage phenotype at the site of peripheral nerve injury can favorably bias the endogenous regenerative potential after injury that obviates the need for the downstream modulation of multiple factors and has significant implications for the treatment of long peripheral nerve gaps. Moreover, the present study strongly suggests that more than the extent of macrophage presence, their specific phenotype at the site of injury influence the regenerative outcomes. These significant results are complementary to a growing body of evidence showing the direct correlation between macrophage phenotype and the regeneration outcome in other tissues.
Supplementary Material
Acknowledgments
We would like to thank Dr. Balakrishna Pai, Dr. Tarun Saxena and Johnathan Lyon in the Bellamkonda Laboratory for helpful scientific and editorial discussions. We would also like to thank Dr. Balakrishna Pai for generating the GFP-expressing RSC-96 Schwann cells used in the study. This work was supported by grants from the National Institutes of Health (NS44409, NS65109, 1R41NS06777-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfister B, Gordon T, Loverde J, Kochar A, MacKinnon S, Kacy Cullen D. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 2.Bellamkonda R. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–8. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Mukhatyar V, Karumbaiah L, Yeh J, Bellamkonda R. Tissue engineering strategies designed to realize the endogenous regenerative potential of peripheral nerves. Adv Mater. 2009;21:4670–9. [Google Scholar]
- 4.Kim Y, Haftel V, Kumar S, Bellamkonda R. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117–27. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madaghiele M, Sannino A, Yannas IV, Spector M. Collagen-based matrices with axially oriented pores. J Biomed Mater Res A. 2008;85:757–67. doi: 10.1002/jbm.a.31517. [DOI] [PubMed] [Google Scholar]
- 6.Fine EG, Decosterd I, Papaloïzos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee AC, Yu VM, Lowe JB, Brenner MJ, Hunter DA, Mackinnon SE, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez FJ, Verdú E, Ceballos D, Navarro X. Nerve guides seeded with autologous Schwann cells improve nerve regeneration. Exp Neurol. 2000;161:571–84. doi: 10.1006/exnr.1999.7315. [DOI] [PubMed] [Google Scholar]
- 9.Nie X, Zhang YJ, Tian WD, Jiang M, Dong R, Chen JW, et al. Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells. Int J Oral Maxillofac Surg. 2007;36:32–8. doi: 10.1016/j.ijom.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Spivey EC, Khaing ZZ, Shear JB, Schmidt CE. The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials. 2012;33:4264–86. doi: 10.1016/j.biomaterials.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–31. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokarram N, Bellamkonda RV. Overcoming endogenous constraints on neuronal regeneration. IEEE Trans Biomed Eng. 2011;58:1900–6. doi: 10.1109/TBME.2010.2103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110–22. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown M, Lunn E, Perry V. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. J Neurobiol. 1992;23:521–36. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- 15.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–40. [PubMed] [Google Scholar]
- 16.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, et al. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 17.Prewitt CMF, Niesman IR, Kane CJM, Houlé JD. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp Neurol. 1997;148:433–43. doi: 10.1006/exnr.1997.6694. [DOI] [PubMed] [Google Scholar]
- 18.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen ZL, Lassner F, Bader A, Becker M, Walter GF, Berger A. Cellular activity of resident macrophages during Wallerian degeneration. Microsurgery. 2000;20:255–61. doi: 10.1002/1098-2752(2000)20:5<255::aid-micr6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, et al. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83:175–85. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- 21.Griffin JW, George R, Ho T. Macrophage systems in peripheral nerves. A review. J Neuropathol Exp Neurol. 1993;52:553–60. doi: 10.1097/00005072-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Mosser D, Edwards J. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gratchev A, Kzhyshkowska J, Köthe K, Muller-Molinet I, Kannookadan S, Utikal J, et al. M [phi] 1 and M [phi] 2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473–86. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Edwards J, Zhang X, Frauwirth K, Mosser D. Biochemical and functional characterization of three activated macrophage populations. J Leukocyte Biol. 2006;80:1298–307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 27.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–99. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 28.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Pt A. 2008;14:1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 30.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2011;8:978–87. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements I, Kim Y, English A, Lu X, Chung A, Bellamkonda R. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30:3834–46. doi: 10.1016/j.biomaterials.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol. 2009;225:24–8. doi: 10.1016/j.expneurol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS medicine. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–65. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 36.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–51. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry V, Popovich PG, et al. Debate:“is increasing neuroinflammation beneficial for neural repair?”. J Neuroimmune Pharmacol. 2006;1:195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 38.Friedman SL. Mac the knife? Macrophages-the double-edged sword of hepatic fibrosis. J Clin Invest. 2005;115:29–32. doi: 10.1172/JCI23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morganti-Kossmann M, Rancan M, Stahel P, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-l regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Lett Nature. 1987;330:658–9. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz M, Butovsky O, Brück W, Hanisch U. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Stout R, Jiang C, Matta B, Tietzel I, Watkins S, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–9. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 43.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deonarine K, Panelli M, Stashower M, Jin P, Smith K, Slade H, et al. Gene expression profiling of cutaneous wound healing. J Trans Med. 2007;5:11–22. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodla M, Bellamkonda R. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balgude A, Yu X, Szymanski A, Bellamkonda R. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–84. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 47.Gölz G, Uhlmann L, Lüdecke D, Markgraf N, Nitsch R, Hendrix S. The cytokine/neurotrophin axis in peripheral axon outgrowth. Eur J Neurosci. 2006;24:2721–30. doi: 10.1111/j.1460-9568.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 48.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 49.Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29:3956–68. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo HS, Tsai MJ, Huang MC, Chiu CW, Tsai CY, Lee MJ, et al. Acid fibroblast growth factor and peripheral nerve grafts regulate th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J Neurosci. 2011;31:4137–47. doi: 10.1523/JNEUROSCI.2592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–63. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 52.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar A, Pluchino S, et al. Microglia activated by IL-4 or IFN-[gamma] differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Baichwal R, Bigbee J, DeVries G. Macrophage-mediated myelin-related mitogenic factor for cultured Schwann cells. Proc Natl Acad Sci. 1988;85:1701–5. doi: 10.1073/pnas.85.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1-and MMP-9-dependent pathways. J Leukocyte Biol. 2009;85:779–87. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 55.Ödemis V, Boosmann K, Heinen A, Küry P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123:1081–8. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- 56.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 57.English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol. 2007;97:1127–34. doi: 10.1152/jn.01035.2006. [DOI] [PubMed] [Google Scholar]
- 58.Krick K, Tammia M, Martin R, Höke A, Mao HQ. Signaling cue presentation and cell delivery to promote nerve regeneration. Curr Opin Biotechnol. 2011;22:741–6. doi: 10.1016/j.copbio.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukocyte Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 60.Nucera S, Biziato D, Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.