Abstract
The aim of this work was to study the effect of cryopreservation over the functionality of tissue-engineered constructs, analyzing the survival and viability of cells seeded, cultured, and cryopreserved onto 3D scaffolds. Further, it also evaluated the effect of cryopreservation over the properties of the scaffold material itself since these are critical for the engineering of most tissues and in particular, tissues such as bone. For this purpose, porous scaffolds, namely fiber meshes based on a starch and poly(caprolactone) blend were seeded with goat bone marrow stem cells (GBMSCs) and cryopreserved for 7 days. Discs of the same material seeded with GBMSCs were also used as controls. After this period, these samples were analyzed and compared to samples collected before the cryopreservation process. The obtained results demonstrate that it is possible to maintain cell viability and scaffolds properties upon cryopreservation of tissue-engineered constructs based on starch scaffolds and goat bone marrow mesenchymal cells using standard cryopreservation methods. In addition, the outcomes of this study suggest that the greater porosity and interconnectivity of scaffolds favor the retention of cellular content and cellular viability during cryopreservation processes, when compared with nonporous discs. These findings indicate that it might be possible to prepare off-the-shelf engineered tissue substitutes and preserve them to be immediately available upon request for patients' needs.
Introduction
Tissue engineering is showing an increasing advancement as time goes by while cell-scaffold constructs are expected to find a growing number of applications in the regeneration of human tissues. As the first engineered tissue substitutes are concurrently undergoing clinical trials, and foreseeing a growing demand for cultured cells and tissues, the tissue engineering community is becoming increasingly worried about the challenge of providing sufficient amounts of these products to the market.
One major such obstacle is related to preserving and storing “living” biomaterials. Maintaining large stocks of living tissues will become an important issue for manufacturers and/or distributors, for them to be able to ensure a steady supply of tissue substitutes. Hospitals will as well feel the need to create tissue banks due to unpredictable demand for specific tissues in clinical settings.1–2
Given that simple preservation techniques, such as refrigeration or tissue culture, have drawbacks including limited shelf-life, high cost, risk of contamination, or genetic drift, cryopreservation becomes a more viable option. This approach is based on the principle that chemical, biological, and physical processes are effectively “suspended” at cryogenic temperatures.2
The process of developing tissue substitutes can as well require long time spans, as long as several weeks. Starting from the isolation of autogenous cells to the in vitro expansion followed by the seeding and culture of those cells on a scaffold and finally to implantation,3–7 there is a resulting excessively long incapacitation of patients. To overcome this problem, an alternative approach, facilitating the large-scale clinical use of engineered tissues, would be the cryopreservation of constructs, that is, the cryopreservation of cells previously seeded and cultured onto scaffolds. This would be a way to generate a reliable source of readily available and ready to implant engineered constructs, greatly reducing the incapacitation time of patients.
Nevertheless, very few studies have focused on this problem.8–12 Thus, the present study aimed at analyzing the effect of cryopreservation over cells while seeded and contained into porous and nonporous constructs and the effect of cryopreservation over the morphological and mechanical properties of the scaffold itself that are critical for the engineering of various tissues. Porosity and mechanical properties are important features in tissue engineering scaffolding, particularly for hard/structural tissues such as bone. Scaffolds intended to replace tissues such as bone must preferably possess highly and fully interconnected open-pore geometry, not only to provide large surface area to volume ratios and to allowsufficient diffusion of nutrients and gases and removal of cellular metabolic waste for in vitro culture, but also to enable the tissue and vascularity ingrowth upon implantation. Apart from being able to maintain the spaces required for cell ingrowth and matrix production, scaffolds for bone tissue engineering should also a priori possess enough mechanical properties to provide structural support for neo-tissue formation upon implantation of the construct.13
Taking all of this into consideration, we submitted cell-seeded and nonseeded constructs to a standard cryopreservation procedure for a period of 7 days and then, after a short recovery period, compared their response, in terms of cell viability and proliferation and concerning the surface and mechanical material properties, to constructs collected immediately before cryopreservation.
Materials and Methods
Preparation of porous scaffolds and nonporous discs
Porous scaffolds and nonporous discs were produced using a polymeric mixture of corn starch and polycaprolactone (30/70 wt.%) designated by SPCL (Novamont). The nonporous discs were produced by injection moulding in a Klockner Ferromatic FM-20 machine using a mould that allowed obtaining discs with 8 mm diameter and 3 mm thickness. The porous scaffolds were produced through a pre-established fiber bonding methodology,14–15 briefly consisting of bonding fibers previously obtained by melt spinning by heat and pressure, and then cut into 8 mm diameter and 3.5–4 mm thick samples. All samples were sterilized with ethylene oxide before cell culture studies.
Cell seeding and culturing onto porous scaffolds and nonporous discs
Goat bone marrow stromal cells (GBMSC) were harvested from the iliac crest of adult goats and isolated as described elsewhere16 and then expanded in low-glucose Dulbecco's modified essential medium (DMEM; Sigma Chemical Co.) supplemented with 1% antibiotic/antimycotic (Sigma) and 10% fetal bovine serum (FBS; Sigma). When confluence was reached, cells were trypsinized and ressuspended (at passage 4). From the obtained cell suspension, and given the difference in surface area available for cell attachment, two different cellular concentrations were prepared: one cell suspension to seed 5×105 cells in 300 μL volumes, for the scaffolds, and the other to seed 1×105 cells in volumes of 200 μL onto the surface of starch and poly(caprolactone) (SPCL) discs. The rationale used for the cell seeding density used in this work was based on previously performed studies16 that showed that a ratio of 5:1 was appropriate for being able to seed a similar amount of these cells per surface area in both porous and nonporous scaffolds and allowing for the formation of extracellular matrix and osteoblastic differentiation. All the scaffolds/discs were placed in 24-well nonadherent plates to perform seeding.
After seeding, these samples were carefully transferred into the incubator and left there for 3 h, before adding 1,5 mL of DMEM basal medium. The samples were cultured for 7 days, and the medium changed every 2 or 3 days. Scaffolds and discs without cells were kept in the same conditions to be used as experimental controls.
Cryopreservation of cell-seeded porous scaffolds and nonporous discs
After 7 days of culture, half of the previously seeded constructs and unseeded scaffolds and discs, were collected for characterization assays, namely MTS, DNA quantification, scanning electron microscopy (SEM), microcomputerized tomography (micro-CT), and mechanical analysis while the other half of the constructs were cryopreserved, along with some more unseeded scaffolds and discs. For the cryopreservation step, a cryopreservative solution composed of dimethyl sulfoxide (DMSO) and FBS was used for suspending the seeded and unseeded scaffold and discs. The concentration of DMSO to use in the cryoprotective solution was determined by estimating the amount of cells in the scaffolds after 7 days of culture, using data obtained in previous studies.17 A period of 7 days of cryopreservation was chosen taking into consideration previous studies performed in the field, which state that the duration of the storage in liquid nitrogen (−196°C) has a negligible impact on constructs. Those studies used even shorter cryopreservation periods (less than 1 day).18 Thus, for each million of cells, a 10% concentration of DMSO was added to the cryopreservative solution. All seeded and unseeded scaffolds and discs were suspended in cryoprotective solution inside standard cryovials and placed inside a Statebourne Biosystem 24 cryogenic tank.
Thawing of cell-seeded porous scaffolds and nonporous discs after cryopreservation
After 7 days of cryopreservation, the constructs and unseeded scaffolds/discs were removed from the cryogenic tank and partially thawed in a 37°C water bath, removed from the cryovials, and placed in 24-well nonadherent plates. To each sample, a volume of cold DMEM basal medium with 20% FBS was added. The samples were further cultured for 9 days with the medium being changed every 2 or 3 days to allow cells to recover from the cryopreservation step. Cells and tissues that are submitted to cryopreservation always require a certain time to recover. Studies found in the literature have shown that, after thawing, cellular viability tends to decrease for a period of at least 7 days before stabilizing.19 This recovery culture period also allowed for a more prolonged and more efficient leaching of toxic DMSOs residues, in particular from the porous constructs. The recovery culture period was not prolonged for longer than 9 days since the strategy involved in a medically oriented usage of these constructs would consist of applying these constructs into tissue defects as quickly as possible after thawing, to reduce patient's immobilization time.
After this time, all the remaining samples were used for characterization assays, namely MTS, DNA quantification, SEM, micro-CT, and mechanical analysis.
Collection of cell-seeded porous scaffold and nonporous disc samples for biological analysis
After each culturing period, the collected cell-seeded cells/scaffolds samples were washed at least twice with 1 mL of sterile phosphate-buffered saline. In the case of the cells/scaffolds constructs to be used in DNA quantifications, each sample was transferred to a sterile tube with 1 mL of ultra-pure water. After this procedure, the tubes with samples were kept at a temperature of −80°C until further analysis.
MTS quantification
Cellular viability was quantitatively assessed by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (Promega) [33]. Culture medium without FBS and phenol red was mixed with MTS in a ratio of 5:1 and added to wells containing the constructs to be analyzed. The constructs were incubated in this solution for 3 h at 37°C in a 5% CO2 atmosphere. After the incubation period, the optical density was read in a microplate reader (Bio-Tek, Synergie HT) at 490 nm. A total of nine samples per study group/condition were analyzed and measurements were made in triplicate. The obtained results were normalized to the surface area of each type of sample used (discs or scaffolds).
DNA quantification
The previously frozen samples were defrosted at room temperature and submitted to an ultrasound bath for about 15 min, to ensure the removal of all cell content from the scaffolds. Double-stranded DNA (dsDNA) content was then measured using a fluorimetric PicoGreen dsDNA Quantification Kit (P7589; Invitrogen, Molecular Probes). Experimental samples and standards (0–2 μg/mL) were added to a white opaque 96-well plate. The procedure followed was based on the manufacturer's instructions. Fluorescence was quantified using a microplate reader (Bio-Tek, Synergie HT) at an excitation of A485/20 and at an emission of A528/20. A total of nine samples per study group/condition were analyzed and measurements were made in triplicate. The obtained results were normalized to the surface area of each type of sample used (discs or scaffolds).
Scanning electron microscopy
Constructs to be observed on the scanning electron microscope (Leica Cambridge S360, Leica Cambridge) were fixated in a 2.5% glutaraldehyde solution (Sigma-Aldrich) for 1 h at 4°C, dehydrated using an increasing concentration series of ethanol solutions (20%, 30%, 50%, 70%, 90%, and 100% (v/v)), left to dry overnight at room temperature, and finally sputter coated with gold in a Sputter Jeol JFC 1100 equipment. The controls (unseeded scaffolds/discs) were merely submitted to the sputter coating part of this procedure. A total of six samples per study group/condition were analyzed.
Microcomputerized tomography
Microcomputerized tomography was performed to quantify the porosity of the several scaffolds produced and their surface area. For this analysis, a SkyScan 1072 equipment was used. The X-ray source was set up to 40 KV, 248 μA with a magnification of 23.29×and an exposure time of 1.8 s that resulted in a resolution of about 11.32 μm/pixel. The measurements were performed on a total of nine samples per study group/condition. This analysis was not performed on discs since their porosity is negligible. The surface area of discs was calculated taking into consideration their height and diameter since these were composed of flat surfaces.
Atomic force microscopy
The roughness of the samples' (SPCL discs) surfaces was measured by atomic force microscopy (AFM). The analysis was performed on three samples per study group/condition on at least three spots per sample using tapping mode in a multimode scanning probe microscope (Veeco) connected to a NanoScope III (Veeco) with noncontacting silicon nanoprobes (ca. 300 kHz, set point 2–3 V; Nanosensors). All images were fitted to a plane using the first-degree flatten procedure included in the NanoScope software version 4.43r8.
Mechanical analysis
Scaffolds and discs were submitted to compression tests before and after cryopreservation for determining their compressive modulus, using a Universal tensile testing machine (Instron 4505 Universal Machine). The tests were performed under compression loading using a crosshead speed of 2 mm/min until 60% strain was reached. The compressive modulus was determined in the most linear region of the stress–strain curve using the secant method. A total of 10 samples per study group/condition were used for this analysis.
Statistics
DNA and MTS assays results are presented as mean±standard deviation. Statistical evaluation was performed using two-tailed paired Student t-tests, to assess the statistical differences between groups at different time points. Statistical significance was defined as p<0.05 for a 95% confidence interval.
Results
Viability, cellular content, and morphology of GBMSCs on porous scaffolds and nonporous discs before and after cryopreservation
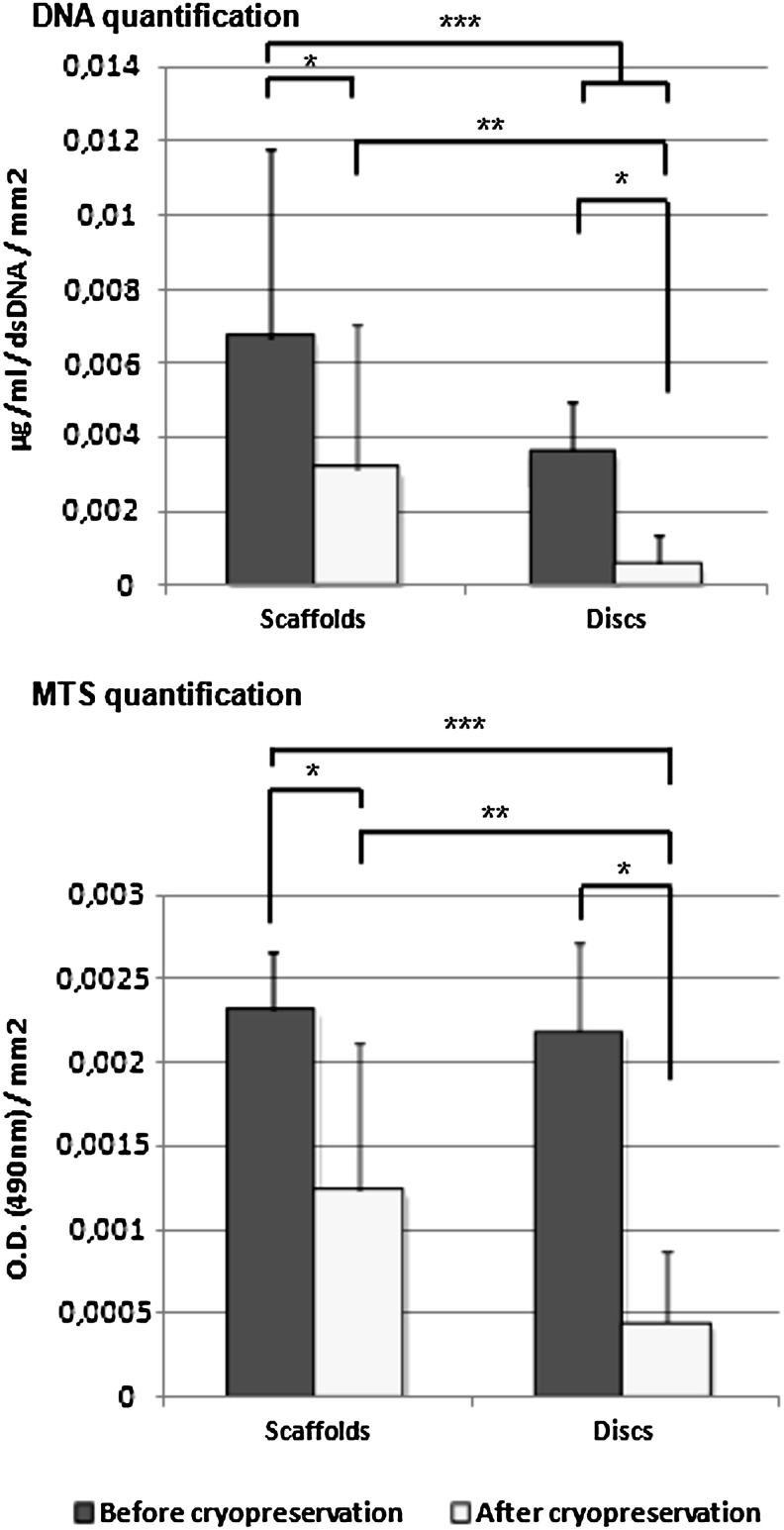
GBMSCs were successfully seeded and cultured under basal culture conditions on the porous scaffolds and nonporous discs for 7 days. Constructs were cryopreserved under standard conditions for 7 days, thawed, and then cultured for a further period of 9 days to allow for full cellular recovery and efficient leaching of DMSO residues. By examining the results obtained from the MTS and DNA analysis (Fig. 1) performed on seeded scaffolds and discs before and after cryopreservation and by normalizing these values with the surface area of each of both systems it was possible to determine that, despite an expected decrease observed upon cryopreservation, constructs were able to maintain cellular content and cellular viability. It was also possible to notice that cellular viability and DNA content were more efficiently maintained in porous scaffolds than in nonporous discs. The percentage of DNA/cellular content maintained in the constructs after cryopreservation was 46% for porous scaffolds and 15% for nonporous discs while the percentage of cellular viability was 54% for porous scaffolds and 20% for nonporous discs, when compared with values obtained before cryopreservation. This distinction between the behavior of cells in porous scaffolds and discs was particularly visible in the MTS data, which showed an evolution from statistically similar cellular viability between both groups before cryopreservation to a statistically and significantly higher cellular viability of cells in porous scaffolds after cryopreservation.
FIG. 1.
Quantification of cellular content by DNA quantification and cellular viability by MTS quantification normalized to surface area. *, **, and *** indicate statistical significance (p<0.05).
These results corroborated our expectations that cells seeded in porous scaffolds would be better preserved given that the scaffold's pores would retain the cryoprotectant more efficiently. As well, and also importantly, these results showed that, along with a prolonged recovery time for cellular viability stabilization, there was an effective removal of the cryoprotectant from the porous scaffold after cryopreservation. Cryoprotectants, in particular the one we used (DMSO), are known to be highly toxic for cells and, for this reason, it would be crucial not only to maintain the cryoprotectant inside the scaffold during cryopreservation but also to make sure it would be efficiently removed after cryopreservation. The efficiency of the cryoprotectant's removal contributed to the scaffold's specific porosity and architecture.
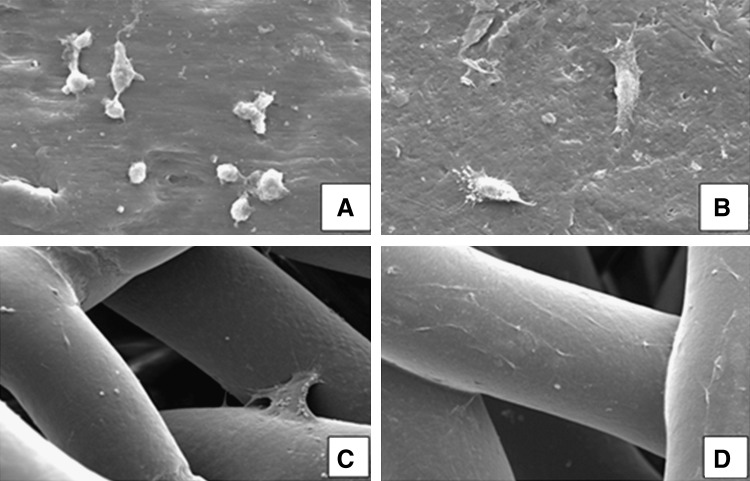
Through the SEM analysis (Fig. 2) it was possible to observe that the cellular morphology was not affected by the cryopreservation process and that cells remained well attached to the material's surfaces after thawing.
FIG. 2.
Scanning electron microscopy micrographs of starch and poly(caprolactone) (SPCL) discs and scaffolds before and after cryopreservation at magnification 1000×. GBMC-seeded SPCL discs before (A) and after (B) cryopreservation (magnification 1000×); GBMC-seeded SPCL scaffolds before (C) and after (D) cryopreservation (magnification 200×)
Morphology, surface topography, and architecture of porous scaffolds and nonporous discs before and after cryopreservation
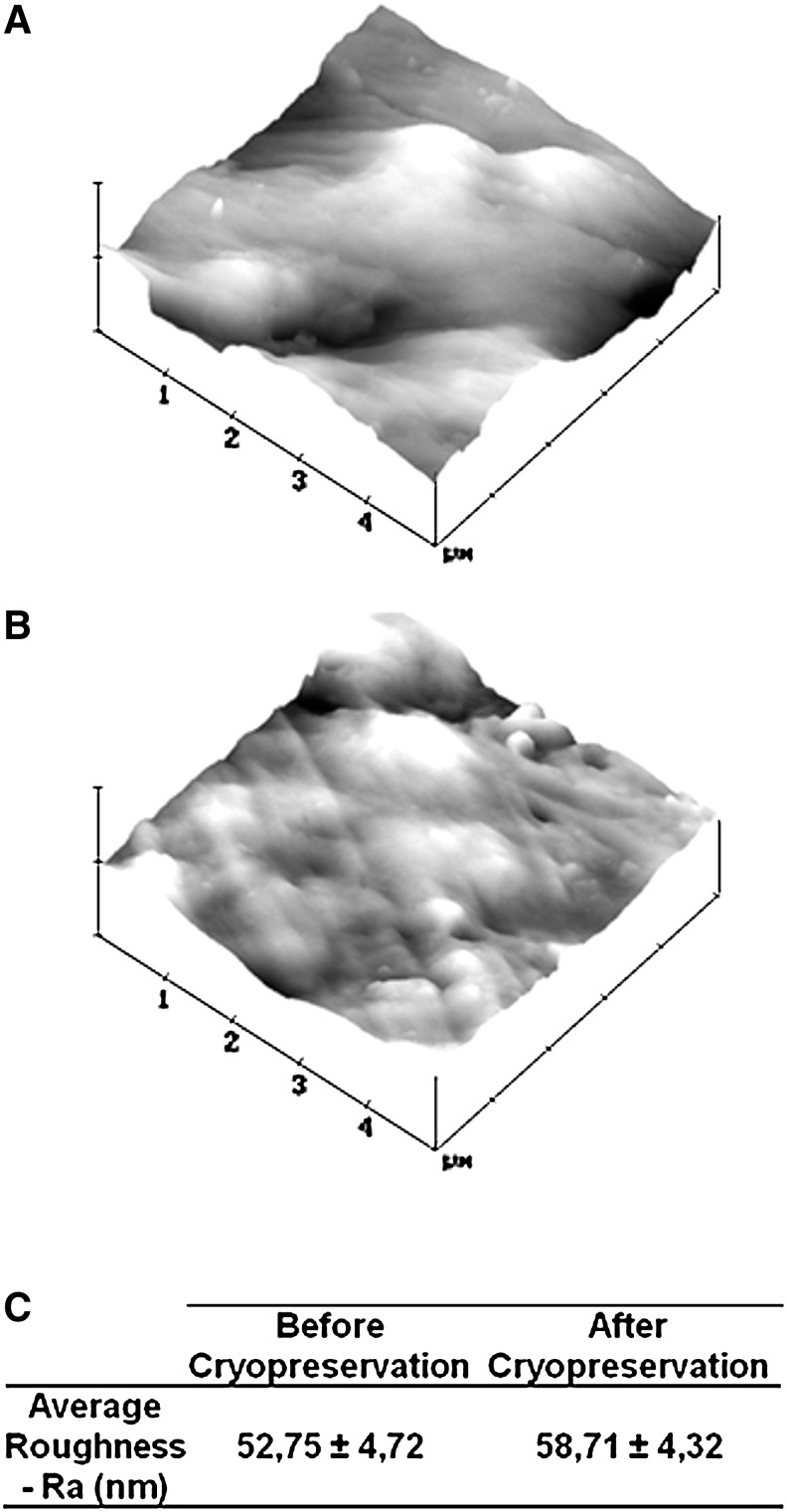
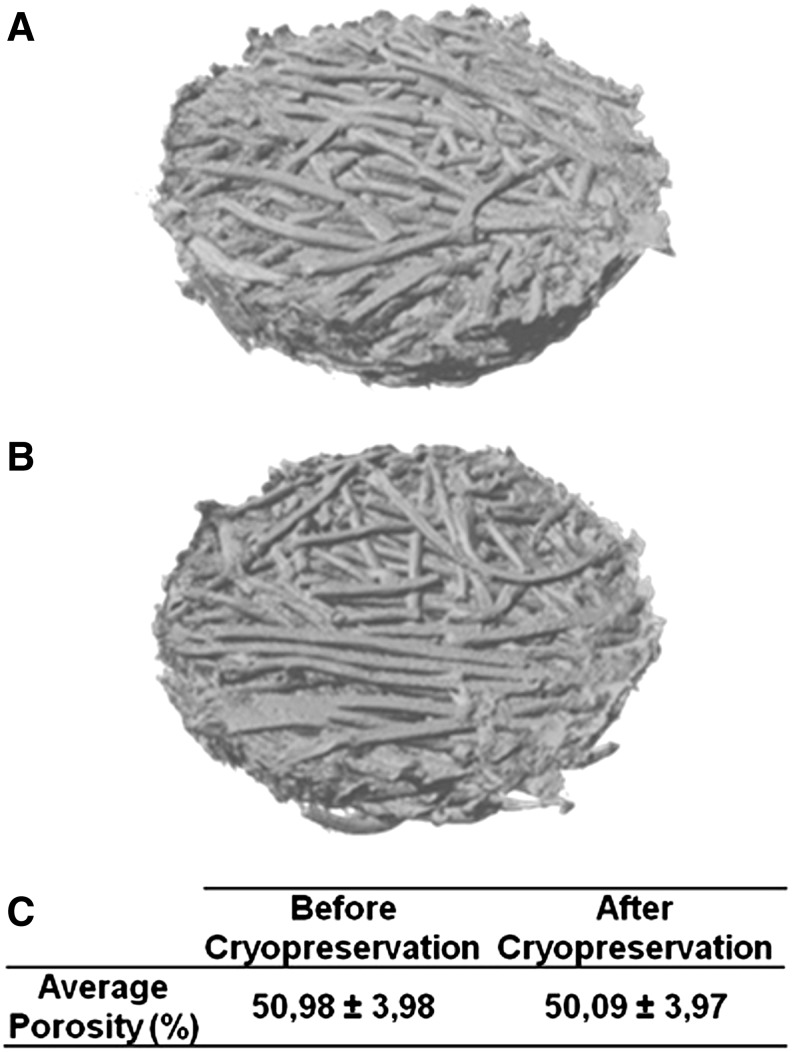
By comparing the SEM micrographs (Fig. 2) before and after cryopreservation it was possible to observe that the cryopreservation process did not change the material's surface morphology. After cryopreservation, the surface of the fibers of porous scaffolds and the surface of the nonporous discs showed the same smooth topography as before cryopreservation. This was more accurately confirmed by performing an AFM analysis (Fig. 3) on the surface of nonporous discs, which revealed no significant difference between the sample's roughness before and after cryopreservation. It was not possible to perform the same AFM analysis over the porous scaffolds given the very irregular fibrous architecture. In the same way, a micro-CT analysis and reconstruction was performed on the porous scaffolds showing that the cryopreservation process did not alter the general architecture of scaffolds and its porosity as shown in the performed micro-CT 3D reconstructions (Fig. 4A, B) and porosity quantitative analysis results (Fig. 4C).
FIG. 3.
Atomic force microscopy images of SPCL discs before (A) and after (B) cryopreservation and their average roughness (C). Values of average roughness before and after cryopreservation were not significantly different (p>0.05).
FIG. 4.
Microcomputerized tomography analysis of fiber-bonded scaffolds before (A) and after (B) cryopreservation. No significant difference was found in terms of scaffold morphology. A quantitative analysis was also performed to compare the total porosity of scaffolds before and after cryopreservation (C). The values of average porosity before and after cryopreservation were not significantly different (p>0.05).
Mechanical properties of porous scaffolds and nonporous discs before and after cryopreservation
Compression mechanical tests were performed on cryopreserved and noncryopreserved porous scaffolds and nonporous discs. The results showed that the cryopreservation process did not alter the mechanical compressive properties of scaffolds and discs since there was no significant difference found when comparing the values for Young's modulus before and after cryopreservation (Table 1).
Table 1.
Comparative Compression Mechanical Analysis on Scaffolds and Discs Before and After Cryopreservation
| |
Average young's modulus (Mpa) |
|
|---|---|---|
| Sample | Before cryopreservation | After cryopreservation |
| Scaffolds | 30.46±4.40 | 29.07±3.62 |
| Discs | 83.04±12.19 | 77.48±3.43 |
Values of average Young's modulus before and after cryopreservation were not significantly different (p>0.05).
Discussion
In this work, we were able to show that it is possible to maintain cellular content and cellular viability into cell-seeded constructs in standard cryopreservation conditions as shown by the MTS, DNA, and SEM results. Further, we were able to show that the porosity and pore interconnectivity found in porous scaffolds may in fact beneficially influence the viability and retention of cells in constructs exposed to cryopreservation processes. This observation may be explained by a possible retaining and protective effect exerted by the scaffold over the cells contained inside the scaffold's pores against the intrinsic damaging effects of cryopreservation.
As for the morphology of scaffolds and discs, we were able to assess that it was not damaged or altered by the cryopreservation process both at a macroarchitectural level and also in terms of superficial topography. This is an important observation since the scaffold's architecture and surface morphology are known to greatly influence the efficiency of cell adhesion and subsequent proliferation over material's surfaces.
Starch–polycaprolactone fiber mesh scaffolds have been extensively studied, demonstrating very promising results, both in vitro and in vivo, for applications in the tissue engineering field.16–17,20
We were also able to assess that the cryopreservation process did not alter the mechanical compressive properties of SPCL-based scaffolds and discs since there was no significant difference found when comparing the values for Young's modulus before and after cryopreservation.
This means that it would be possible to accurately define the construct's mechanical properties prior to cryopreservation aiming at specific tissue applications (in this case, given the mechanical properties of the studied scaffolds, mostly bone and cartilage) without having any detrimental effect caused by the cryopreservation process. The same principle applies to the architecture and surface topography of scaffolds that, as shown by the performed SEM, AFM, and micro-CT analysis, are also not affected by the cryopreservation process.
Overall, the obtained results show that it is possible to maintain viable cells and scaffolds properties upon cryopreservation of tissue-engineered constructs based on SPCL scaffolds and goat bone marrow mesenchymal cells using standard cryopreservation methods. Also, this study suggests that the architecture found in porous scaffolds favors the retention and viability of the construct's cellular content during cryopreservation processes, when compared to nonporous structures. Finally, these findings indicate that it may be possible to prepare off-the-shelf engineered tissue substitutes and preserve them to be immediately available upon request for patients.
Acknowledgments
This work was partially supported by the European Network of Excellence EXPERTISSUES (NMP3-CT-2004-500283). We also acknowledge the Portuguese Foundation for Science and Technology for the Ph.D. grant to Pedro Costa (SFRH/BD/62452/2009) and the research grant to Ana Dias in the scope of the research project VivoTissue (PTDC/CVT/67677/2006).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hirose M. Kotobuki N. Machida H. Kitamura S. Ohgushi H. Tateishi T. Osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells after thawing in culture. Mater Sci Eng C. 2004;24:355. [Google Scholar]
- 2.Karlsson J.O. Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17:243. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- 3.Fleming J.E., Jr. Cornell C.N. Muschler G.F. Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am. 2000;31:357. doi: 10.1016/s0030-5898(05)70156-5. [DOI] [PubMed] [Google Scholar]
- 4.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim B.S. Mooney D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 6.Perka C. Sittinger M. Schultz O. Spitzer R.S. Schlenzka D. Burmester G.R. Tissue engineered cartilage repair using cryopreserved and noncryopreserved chondrocytes. Clin Orthop Relat Res. 2000;378:245. doi: 10.1097/00003086-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 7.Vacanti C.A. Bonassar L.J. An overview of tissue engineered bone. Clin Orthop Relat Res. 1999;367(Suppl):S375. doi: 10.1097/00003086-199910001-00036. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi H. Ehashi T. Ohshima N. Jagawa A. Cryopreservation of fibroblasts immobilized within a porous scaffold: effects of preculture and collagen coating of scaffold on performance of three-dimensional cryopreservation. Artificial Organs. 2010;34:609. doi: 10.1111/j.1525-1594.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 9.Yin H. Cui L. Liu G. Cen L. Cao Y. Vitreous cryopreservation of tissue engineered bone composed of bone marrow mesenchymal stem cells and partially demineralized bone matrix. Cryobiology. 2009;59:180. doi: 10.1016/j.cryobiol.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Rupf T. Ebert S. Lorenz K. Salvetter J. Bader A. Cryopreservation of organotypical cultures based on 3D scaffolds. Cryoletters. 2010;31:157. [PubMed] [Google Scholar]
- 11.Bernemann I. Kuberka M. Glasmacher B. 71. Adapted freezing and thawing procedures to improve the cryopreservation of cell seeded scaffolds. Cryobiology. 2006;53:397. [Google Scholar]
- 12.Kofron M.D. Opsitnick N.C. Attawia M.A. Laurencin C.T. Cryopreservation of tissue engineered constructs for bone. J Orthopaedic Res. 2003;21:1005. doi: 10.1016/S0736-0266(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 13.Salgado A.J. Coutinho O.P. Reis R.L. Bone tissue engineering: state of the art and future trends. Macromolecular Biosci. 2004;4:743. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 14.Mendes S.C. Bezemer J. Claase M.B. Grijpma D.W. Bellia G. Degli-Innocenti F. Reis R.L. de Groot K. van Blitterswijk C.A. de Bruijn J.D. Evaluation of two biodegradable polymeric systems as substrates for bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S91. doi: 10.1089/10763270360697003. [DOI] [PubMed] [Google Scholar]
- 15.Gomes M.E. Holtorf H.L. Reis R.L. Mikos A.G. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:801. doi: 10.1089/ten.2006.12.801. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues M.T. Gomes M.E. Viegas C.A. Azevedo J.T. Dias I.R. Guzón F.M. Reis R.L. Tissue-engineered constructs based on SPCL scaffolds cultured with goat marrow cells: functionality in femoral defects. J Tissue Eng Regenerative Med. 2011;5:41. doi: 10.1002/term.287. [DOI] [PubMed] [Google Scholar]
- 17.Gomes M.E. Sikavitsas V.I. Behravesh E. Reis R.L. Mikos A.G. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J Biomed Mater Res A. 2003;67:87. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 18.Wen F. Magalhães R. Gouk S.S. Gajadhar B. Lee K.H. Hutmacher D.W. Kuleshova L.L. Vitreous cryopreservation of nanofibrous tissue-engineered constructs generated using mesenchymal stromal cells. Tissue Eng - Part C: Methods. 2009;15:105. doi: 10.1089/ten.tec.2008.0237. [DOI] [PubMed] [Google Scholar]
- 19.Lübke C. Sittinger M. Burmester G.R. Paulitschke M. Cryopreservation of artificial cartilage: viability and functional examination after thawing. Cells Tissues Organs. 2001;169:368. doi: 10.1159/000047904. [DOI] [PubMed] [Google Scholar]
- 20.Tuzlakoglu K. Bolgen N. Salgado A. Gomes M. Piskin E. Reis R. Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci: Mater Med. 2005;16:1099. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]