Abstract
Endothelial progenitor cells (EPCs) play a significant role in multiple biological processes such as vascular homeostasis, regeneration, and tumor angiogenesis. This makes them a promising cell of choice for studying a variety of biological processes, toxicity assays, biomaterial–cell interaction studies, as well as in tissue-engineering applications. In this study, we report the generation of two clones of SV40-immortalized EPCs from umbilical cord blood. These cells retained most of the functional features of mature endothelial cells and showed no indication of senescence after repeated culture for more than 240 days. Extensive functional characterization of the immortalized cells by western blot, flow cytometry, and immunofluorescence studies substantiated that these cells retained their ability to synthesize nitric oxide, von Willebrand factor, P-Selectin etc. These cells achieved unlimited proliferation potential subsequent to inactivation of the cyclin-dependent kinase inhibitor p21, but failed to form colonies on soft agar. We also show their enhanced growth and survival on vascular biomaterials compared to parental cultures in late population doubling. These immortalized EPCs can be used as a cellular model system for studying the biology of these cells, gene manipulation experiments, cell–biomaterial interactions, as well as a variety of tissue-engineering applications.
Introduction
Endothelial progenitor cells (EPCs), the circulating pool of progenitor cells with potential to differentiate into functional endothelial cells (ECs), have been proposed as a vital cell source for therapeutic neovascularization, vascular repair, studies on tumor angiogenesis, tissue-engineering efforts, and as a biomarker for a variety of disease states.1–5 Adult peripheral blood as well as umbilical cord blood (UCB) have been used as a good source for various kinds of stem cells, including hematopoietic stem cells, mesenchymal stem cells, and EPCs. The identification and characterization of adult circulating EPCs by Asahara et al. led to the expansion in the field of circulating EC biology.6 Later studies substantiate the existence of these cells, their bone marrow origin, and their contribution in the regeneration of new blood vessels in adults.7,8 They are characterized by their unique surface feature of CD34+, AC133+, and VEGFR2+expression and the loss of AC133 upon endothelial maturation in culture.9,10 The physiological role of EPCs is not yet clearly established. These cells play a key role in promoting neovascularization of ischemic tissues in nude mice and in developing into neovessels in vivo when seeded in decellularized grafts.11 They also express a variety of potent vasoactive regulators of functional ECs such as nitric oxide (NO), VE Cadherin, von Willebrand factor (vWF), and are involved in the protection against tissue degeneration due to vascular damage or dysfunction.12 The number of EPCs is inversely correlated with the risk for coronary artery diseases, indicating their potential use as a prognostic tool for cardiovascular diseases.13 Several studies substantiated the importance of circulating EPCs in tumor vascularization.14–18 All these properties make this unique cell population an ideal choice for various tissue-engineering applications as well as to study variety of vascular diseases in in-vitro conditions and in tumor vascularization models.19–21 Currently, there are two subsets of EPCs that have been identified from peripheral blood and UCB. The early EPCs are more like mature ECs with enhanced angiogenic ability and less multiplication potential and assumed to be of myeloid origin than the late EPCs with enhanced proliferation potential.22 Even though late EPCs possess enhanced proliferation potential in defined culture conditions than the early progenitors, it is difficult to maintain these cells in culture beyond 100 days, necessitating repeated isolation of EPCs.23
A clear definition of their identity, functional characterization, and other tissue-engineering applications involving this unique cell type requires a defined homogenous functional cell line with unlimited proliferation potential. Overexpression of human telomerase and viral oncoproteins such as E6, E7, and SV40 has been evaluated for immortalization of a variety of diploid cells, including ECs.24–26 Even though the introduction of telomerase is considered as a promising method to immortalize normal cells compared to other approaches, several studies indicate that telomerase can only extend lifespan of cells.27–29 In this work, we have generated two EC lines by stably introducing SV40T large antigen using retroviral infection. Both the cell lines retained the functional property of mature ECs and proliferated continually in culture without any manifestation of senescence-like phenotype for more than 150 population doubling (PD) compared to the vector control cells that failed to grow beyond 40–50PD in culture. We also show that the cells attached and proliferated well in vascular graft materials even after a PD of 80. These cell lines form a better choice to study a multitude of biological signaling centered on such cell type, their multilineage differentiation potential and also applications involving, vascular material compatibility studies, tumor angiogenesis, in vitro drug testing, and gene manipulation studies.
Materials and Methods
Cell isolation and purification
UCB was collected from the Sree Avittom Thirunal Hospital after approval by the Human Ethics Committee. Thirty to 40 mL of fresh human cord blood was collected from healthy full-term new borns, diluted 1:1 with phosphate-buffered saline (PBS), and overlaid on Ficoll-Hypaque. Mononuclear cells (MNCs) were obtained by density-gradient centrifugation and washed three times with PBS. The MNCs were resuspended in endothelial cell growth medium-2 (EGM-2) supplemented with the EGM-2 MV-Single Quots (Lonza) containing vascular EC growth factor, basic fibroblast growth factor, insulin-like growth factor-1, epidermal growth factor, ascorbic acid, hydrocortisone, and 10% fetal bovine serum (FBS). The cells were seeded on a collagen-coated surface. After initial 3-day culture, nonadherent cells were removed by gentle aspiration, and a new complete medium was added. CD34+ cells were purified from UCB using magnetic cell separation with CD34+ microbead columns until purity was >90%. These cells were also seeded on collagen-coated dishes as earlier. Human umbilical cord vein ECs (HUVECs) were isolated by collagenase enzyme digestion as per our earlier published method.30
Antibodies and reagents
Antibodies for endothelial NO synthase (eNOS) and vWF were purchased from Sigma-Aldrich. Antibodies against p21, P-Selectin, human telomerase reverse transcriptase (hTERT), HSC 70, and beta-actin were purchased from Santa Cruz Biotechnology. Antibodies for GPX1, Rb, and p53 were purchased from Cell Signaling Technology. The fluorescent dye calcium AM and Matrigel were procured form BD Biosciences. Ac-Dil-LDL, Hoechstm and Alexa-546-labeled secondary antibodies were purchased from Molecular Probes. CD45 PE, VEGFR-2 PE, respective isotope IgG controls, and CD34 magnetic beads were procured from Miltenyi Biotec. The NO sensor probe 4,5-diaminofluorescein diacetate (DAF-2 DA) was procured from Molecular Probes.
Retroviral infection
Amphotropic retrovirus was produced by transfection of the packaging cell line Amphophoenix with the retroviral expression vector pBABE neo-LT antigen (addgene plasmid 178031), using lipofectamine as per the manufacturer's instruction. The culture supernatant was collected after 48 h of transfection and filtered using 0.45-μm cellulose acetate filter to remove cell debris. The EPCs in the earlier passages were infected with the viral supernatant along with 8 μg/mL of polybrene. The infection was repeated two cycles at interval of 3 days. The infected cells were maintained in 100 μg per mL G418 for 1 month. For vector control experiments, the supernatant collected after transfection of Amphophoenix cells with a pBABE neoexpression vector was used to infect the parallel cultures.
Cell proliferation assay
The proliferation potential of cells was analyzed from PD time measurements. Briefly, at subconfluence, the cells were trypsinized and counted. The PD time was estimated using the equation, d=t/log2N, where d is the PD time; t is the time of cells in culture; N is the total number of cell counted during trypsinization.
Flow cytometry analysis
Cells at indicated passages were trypsinized and washed with a serum-containing medium, followed by fixing with paraformaldehyde for 10 min. The cells were incubated in presence of primary antibodies CD45 PE and VEGFR2 PE, and were analyzed by FACSAria using respective channels. For staining the cells with P-Selectin and VE-Cadherin, the cells after permeabilization were incubated with the respective primary antibody, followed by a secondary antibody conjugated with Alexa 546, and analyzed by FACSAria (Becton Dickinson). All flow cytometry experiments were carried out in triplicate.
Immunofluorescence staining
The cells were grown on a 12-mm coverglass in 24-well plates for 48 h. The cells were then washed with PBS and fixed with 4% paraformaldehyde. After blocking the monolayer with 3% bovine serum albumin in PBS, the cells were permeabilized with 0.2% Triton X-100 in PBS. The cells were subsequently incubated with the respective primary antibodies at a concentration of 5 μg/mL, for 4 h at 37°C. This was followed by incubation with Alexa-546-conjugated secondary antibody for 45 min. The monolayer was extensively washed and mounted with glycerol and imaged under a fluorescent microscope using FITC/Rhodamine/Hoechst filter sets (Nikon TE2000E). Images were captured with a Retiga Exi camera (Photometrics) and analyzed with NIS element software (Nikon).
Senescence associated-β gal activity
The cells were fixed and stained with senescence associated-β (SA-β) gal using the SA-β gal staining kit from Cell Signaling Technology as per the manufacturer's instructions. Cells were counted under the microscope, and a minimum of 500 cells were taken for each group. The percentage of β-gal-positive cells from the total number of cells was calculated (n=4).
Endothelial tube formation assay in Matrigel
The potential of ECs to form capillary-like tubular structures was analyzed by growing cells on Matrigel-coated 96-well plates (BD Biosciences) according to the manufacturer's suggestions. After 16 h of growth on the Matrigel surface, tube formation was analyzed under a phase-contrast microscope using 4× objective.
Uptake of Ac-Dil-LDL
The cells grown on 96-well imaging plates were incubated with 5 μg/mL of acetylated low-density lipoprotein (LDL), labeled with 1,1′-dioctadecyl-3,3,3′,3′ tetramethyl-indocarbocyanine perchlorate, Ac-Dil-LDL, for 4 h at 37°C and 5% CO2. The cells were imaged with rhodamine filter sets of a fluorescent microscope using 40× objective. Images were captured with a Retiga Exi camera (Photometrics) and analyzed with NIS element software (Nikon)
Measurement of NO production
Intracellular NO was determined using the NO-sensitive probe DAF-2 DA in live cells by microscopy as per the standard protocol. Briefly, cells were incubated with of DAF-2 DA at a concentration of 2 μM for 10 min. The cells were further incubated in a fresh medium for 30 min at 37°C to allow complete de-esterification of the intracellular diacetate, followed by nuclear staining with Hoechst for 5 min. The cells were visualized under FITC and DAPI filter sets using 10× objective and photographed with a Retiga Exi camera.
Cell seeding onto vascular materials
Both expanded polytetrafluoroethylene (ePTFE) and Dacron vascular graft materials were used for cell seeding. The EPCs at PD30, PD50, and SV40T at PD50 and PD100 were used for cell seeding. For calculating cell attachment onto these materials, the graft materials were fixed on 12-well plates using a glass ring. After extensive washing of the surface with a serum-free medium, a fixed number of cells (20×103 cells per cm2 area) was seeded onto these surfaces with 5% FBS. After 4 h, the unattached cells were collected by gentle washing and counted to calculate the percentage of cells attached to the surface. For visualizing the uptake of LDL by SV40T cells on ePTFE, cells seeded on to the ePTFE graft materials were fixed on 24-well plates. The cells were incubated with Ac-Dil-LDL, 5 μg/mL for 4 h. Later, the graft materials were fixed onto a glass coverslip over an O-ring with the cells surface inverted. Then, the cells on the material surface was imaged with an Epi fluorescent microscope (Nikon TE 2000E) using Rhodamine filter and photographed. For analyzing the cell growth on different materials, ePTFE or Dacron pieces were fixed on 6-well plates and seeded with 10×104 cells per well. At the end of 24 and 48 h, the cells were harvested by trypsinization and counted using a counting chamber.
Western blot
To prepare whole-cell extracts for western blot, cells grown on 100-mm dishes were harvested, washed thrice in PBS, and lysed in a lysis buffer (50 mM Tris–HCl [pH 7.4], 1% NP-40, 40 mM NaF, 10 mM NaCl, 10 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 10 mM dithiothreitol, and 1 mg/mL each of leupeptin and aprotinin). The cell lysate was resolved by SDS-PAGE, and the separated proteins were transferred to a polyvinylidene difluoride membrane by a wet transfer method using electrotransfer apparatus (Bio-Rad). After blocking with 10% nonfat milk powder in Tris-buffered saline containing 0.2% Tween-20, the membrane was incubated with the primary antibody, followed by HRP-conjugated secondary antibody. Proteins were visualized by an ECL kit (GE Healthcare; RPN 2132).
Soft-agar colony assay
For determining anchorage-independent growth potential of the cells, soft-agar colony assay was performed as per standard protocol. Briefly, 5000 cells per well were prepared in a 0.3% agarose and layered on the bottom layer of 1% agar in a 24-well plate and allowed to grow for 21 days. The colonies were stained with 0.05% crystal violet and were photographed using 4× objectives.
Cell cycle analysis
Cells were trypsinized, washed, and fixed with 70% ethanol for 20 min, followed by RNase treatment for 20 min at room temperature. The cells were stained with propidium iodide and were analyzed using FACSAria.
Cell death analysis by tetramethyl rhodamine methyl ester
The cells growing on ePTFE were stained with 50 nm of tetramethyl rhodamine methyl ester (Invitrogen) in a serum-containing medium for 15 min. The cells on the material were placed on a glass coverslip over an O-ring as described earlier. These cells were also stained with Calcium AM (BD Biosciences) before imaging, according to the manufacturer's protocol.
Karyotyping
Chromosome preparations were made using exponentially growing cells after treatment with colchicine 5 μg/mL for 6–8 h. The cells after trypsinization were treated with 0.5% KCl for 15 min and fixed with 3:1 methanol–glacial acetic acid. The suspension of cells after washing were dropped on cold wet slides and stained with 4% Giemsa solution as per standard protocol. Twenty metaphase spreads were analyzed for each group (n=4).
Telomerase activity assay
Telomerase activity was assayed using a TeloTAGGGTelomerase PCR ELISA PLUS detection kit (Roche), according to manufacturer's instructions. Briefly, the cell extract was separately prepared from 2×105cells, and 5 μg of protein was used for polymerase chain reaction (PCR) amplification using the biotin-labeled primers provided in kit. PCR products were denatured and hybridized to a digoxigenin-labeled detection probe specific for telomeric repeats. For detection, an antibody against digoxigenin conjugated to horse peroxidase and its substrate TMB was employed. Absorbance (A) was determined at 450 nm using a reference wavelength of ∼690 nm. Three independent experiments were done.
Results
Isolation of EPCs from UCB
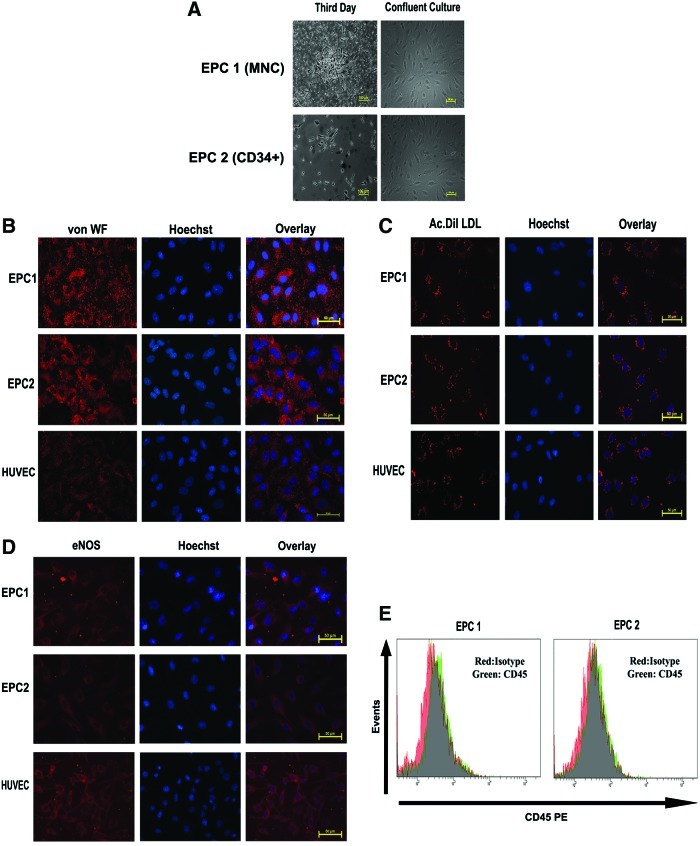
Two methods were employed to isolate EPCs from UCB. In the first method, MNCs isolated from UCB were seeded on collagen-coated dishes in EGM-2 as described by Asahara et al.6 In the second approach, CD34-positive cells were enriched from MNC preparation by MACS sorting and seeded on collagen-coated dishes. In both the plates, unattached cells were removed after 48 h while attached cells were maintained continuously in the EGM-2 supplemented with 10% FBS and growth factors until growing colonies were visible. Both the approaches reproducibly generated EC-like colonies as shown in Figure 1A (n=6). As shown in the figure, both methods yielded morphologically similar cultures with cobblestone morphology upon confluence. The cells were characterized for endothelial phenotype by a battery of markers. Cells isolated from both MNCs as well as CD34+ cells showed intense staining for vWF, uptake of Ac-Dil-LDL, and eNOS (Fig. 1B–D). Flow cytometry analysis confirmed that cells were negative for the Pan hematopoietic marker CD45 (Fig. 1E).
FIG. 1.
(A) Mononuclear cells (MNCs) isolated from umbilical cord blood (upper panel) or CD34+ MACS-sorted cells were plated on endothelial cell growth medium-2 (EGM-2) on collagen-coated dishes. A representative image on 3rd day and confluent culture is shown (Mag. 10×). (B) The expanded endothelial progenitor cells (EPCs) emerged from MNC (EPC1) and CD34+ MACS-sorted cells (EPC2) grown on glass coverslips were fixed and stained with von Willebrand factor (von WF) primary antibody followed by Alexa-546-conjugated secondary antibody. The cells were also stained with Hoechst to visualize nuclei. Human umbilical cord vein endothelial cells (HUVECs) were employed as positive control. As seen, the cells from both cultures showed intense granular staining similar to HUVECs. (C) The indicated cells were incubated with Ac-Dil-LDL for 4 h and imaged with a fluorescent microscope after staining with Hoechst. Both EPC1 and EPC2 showed internalization of Ac-Dil-LDL to the same extent as HUVECs. (D) The indicated cells were stained with endothelial nitric oxide synthase antibody followed by Alexa 546 secondary antibody and imaged as described above. (E) Both EPC1 and EPC2 were incubated with CD45 PE antibody or isotope IgG, followed by FACS analysis. Both the cells showed negative expression for CD45. Color images available online at www.liebertpub.com/tec
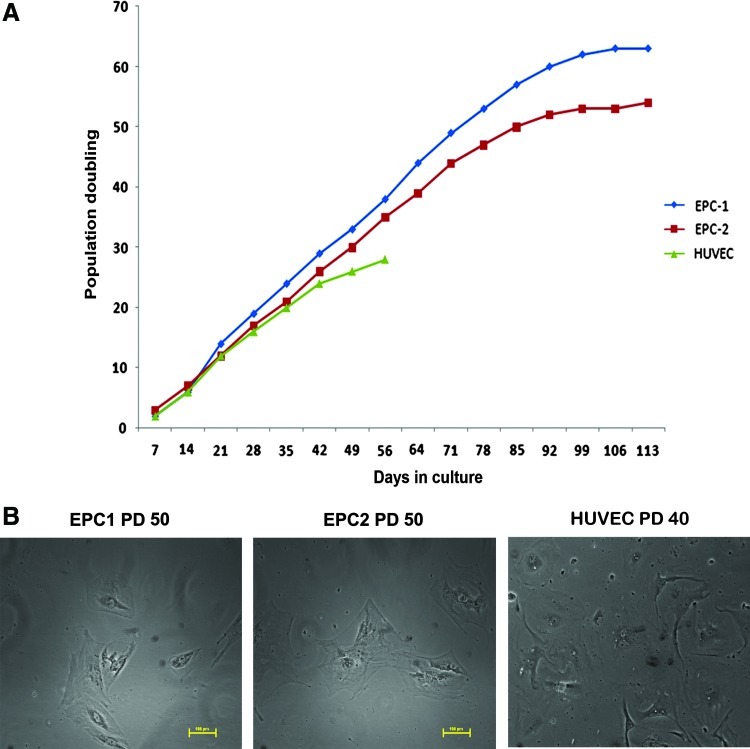
Proliferation state and senescence
The growth potential of both cell lines was analyzed for PD in a continuous culture. As shown in Figure 2A, the cells showed enhanced PD compared to HUVECs. HUVECs showed a decline in cell growth doubling after 30PD; however, EPCs showed enhanced proliferation up to 85–95 days. After this period, the proliferation rate of the cells was reduced. Previously, several studies also suggested that late outgrowth of cells can be maintained in culture for a long time owing to reduction in senescence.32–34 These results suggest that endothelial outgrowth colonies are more similar to late-outgrowth colonies described earlier. After 50PD in culture, cells showed flattened morphology, indicating their entry into senescence (Fig. 2B). Overall, as evidenced from the results, despite an enhanced proliferation rate, EPCs are also prone to senescence in a delayed manner. As shown in the figure, most cultures failed to grow beyond 100 days.
FIG. 2.
(A) The growth kinetics of EPC1, EPC2, and HUVECs are shown using the population doubling (PD) calculated at each passage. (B) A representative image showing flattened senescent cells at PD60 in both EPC1 and EPC2, as well as HUVECs at PD40. Color images available online at www.liebertpub.com/tec
Immortalization of progenitor cells
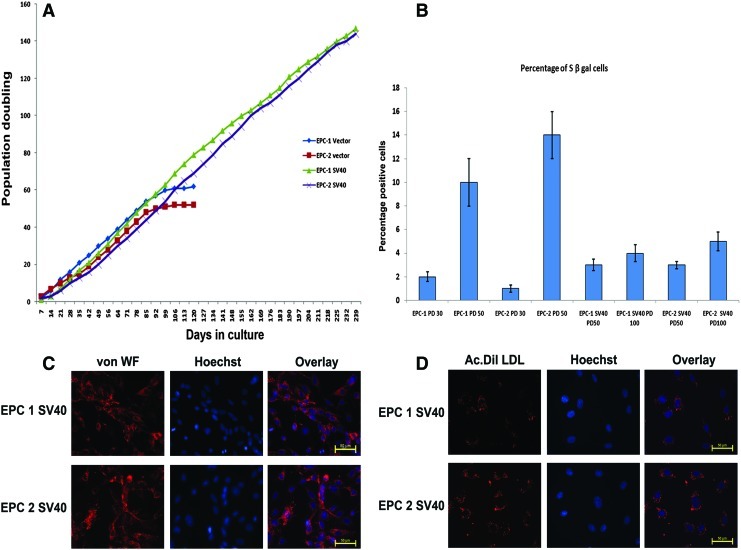
Since the above-described results showed that EPCs were also prone to senescence and senescence-associated suppression of proliferation, early passage cells were infected with SV40 T large antigen by retroviral infection. Three of the expanded cultures from individual donors were used for infection. Briefly, cells in early passage were exposed to two cycles of infection. Vector-transfected and SV40LT antigen-transfected cells were maintained in a G418-containing medium for 4 weeks. After PD of 50, the vector-transfected cells showed decline in cell proliferation (Fig. 3A). However, the SV40-immortalized clones grew beyond 240 days with PD of 110. Similarly, until PD of 100, the SV40-immortalized cells showed a less number of S β gal-positive cells compared to vector control at PD50 (Fig. 3B). To characterize these cells, SV40-infected clones from MNC as well as CD34-positive cultures were stained for vWF and Ac-Dil-LDL at PD100. The cells retained the ability to incorporate the LDL as well as vWF expression similar to the early passage EPCs (Fig. 3C, D). Overall, these results indicate that the two clones of SV40-infected cells possess unlimited replication potential and also retain the functional characteristics of mature ECs.
FIG. 3.
(A) The growth kinetics of the immortalized EPCs (EPC1 SV40 and EPC2 SV40) and respective vector-transfected cells are shown. (B) The percentage of senescent cells from the respective PD for different cells are shown after staining the cells for the S β gal stain as described (n=4). (C) The immortalized cells, EPC1 SV40 and EPC2 SV40, were grown on glass coverslips and stained for von WF as described. (D) The indicated cells were incubated with Ac-Dil-LDL and representative fluorescent images are shown. Color images available online at www.liebertpub.com/tec
Advanced functional features in SV-infected cultures
Among multiple methods of immortalization, telomerase is widely preferred for diploid cells because of safety issues compared to SV40, E6, or E7 viral oncogene protein. Since small T-antigen confers a transformed phenotype in cooperation with other oncogenes, in this study, we employed SV40T large antigen only to preserve functional features and to suppress transformation-induced changes. Since endothelial function is highly dependent on the expression of tightly regulated multitude of EC-specific signaling molecules, the key endothelial-specific proteins were analyzed in immortalized cells along with the surface markers.
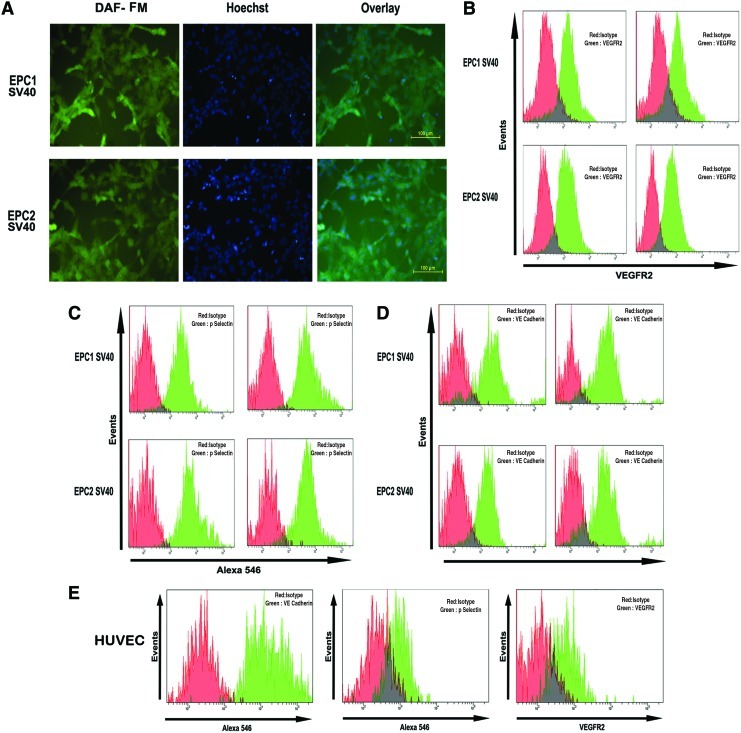
The most important vasoactive molecule synthesized by ECs that contributes to the maintenance of vascular tone is NO. The cell's ability to release NO was determined by fluorescent microscopy after staining cells with 4-amino-5-methylamino-2′,7′-difluorescein (DAF-FM), indicating that the SV40T cells retained expression of NO-associated fluorescence (Fig. 4A). Similarly, flow cytometry results substantiated that SV40 clones retained the expression of surface markers such as VEGFR2, P-Selectin, and VE- Cadherin (Fig. 4B–E).
FIG. 4.
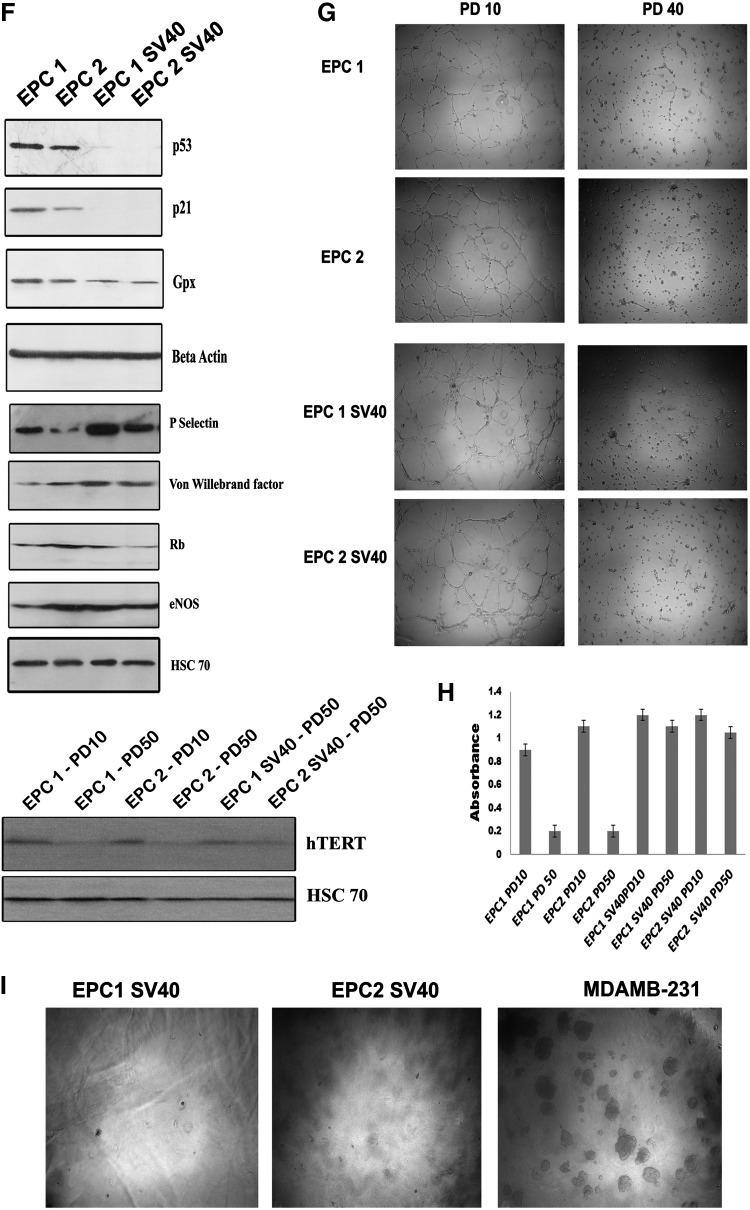
(A) EPC1 SV40 and EPC2 SV40 cells were incubated with the intracellular nitric oxide sensor 4-amino-5-methylamino-2′,7′-difluorescein (DAF-FM). The DAF fluorescence was detected using an FITC filter. The cells were also stained with Hoechst to visualize the nuclei. (B) EPC1 SV40 and EPC2 SV40 cells were incubated with VEGFR-2 PE and analyzed by FACS. The red histogram indicates the respective isotype IgG. (C) The above panel of cells was fixed and incubated with a primary antibody against P-Selectin followed by Alexa 546 secondary antibody. The respective isotype IgG control is represented as red histogram. (D) The above panel of cell lines was processed for VE cadherin immunostaining as described above. (E) HUVECs stained with above panel of antibodies served as control. (F) EPC1, EPC2, and the respective immortalized clones were grown on 100-mm dishes. The protein was extracted from subconfluent cultures as described. About 80 μg of protein was resolved on SDS–PAGE, followed by western blotting using the antibody indicated. Beta-actin and HSC-70 served as loading control. (G) EPC1, EPC2, and the respective SV40-immortalized cells were grown on Matrigel-coated dishes. After 16 h, the tubules formed were visualized using 4× objective. (H) Telomerase activity was assayed with a TeloTAGGGTelomerase PCR ELISAPLUS detection kit as described. The absorbance values determined at 450 nm using a reference wavelength of ∼690 nm used for plotting the graph (n=3). (I) The immortalized EPC1, EPC2, and the breast cancer cell line MDAMB-231 were grown on soft-agar colony assay. The representative images are shown. Color images available online at www.liebertpub.com/tec
SV40-immortalized cells were characterized by western blot for the expression of eNOS, vWF, P-Selectin, glutathione peroxidase, p53, p21, and retinoblastoma in vector-transfected cells at PD30- and SV40-infected cells at PD100. SV40-infected cells showed a marked decline in the p53 level compared to the parental cells, substantiating integration of SV40 large T antigen (Fig. 4F). Consistently, the CDK inhibitor p21 expression was significantly reduced in the immortalized cells. The results suggests that p53 mediated p21 cell cycle regulation is impaired, which allows enhanced proliferation of the cells and helped them to escape senescence. Interestingly, the cells showed enhanced expression of eNOS, vWF even upto PD100. When, compared to uninfected cultures, both the SV40 cells expressed detectable level of telomerase expression (Fig. 4F). Telomerase activity analysis using Telomerase PCR ELISA also showed maintenance of telomerase activity in SV40-immortalized cells even at PD50. However, the uninfected cultures showed decreased telomerase activity at PD50 (Fig. 4H).
In vitro tube formation on Matrigel has been used as a strategy to characterize the angiogenic potential of ECs. Both the EPCs at early passage formed extensive tubules on Matrigel (Fig. 4G). However, SV40 T cells after PD of 40 failed to show complete tube formation despite of their enhanced proliferation rate and retention of most functional attributes. Interestingly, after infection, the early passage cells retained the tube formation capacity. Consistent with this finding, the late-passage uninfected cells also showed reduction in tubule formation potential (Fig. 4G).
Further, we analyzed the anchorage-independent cell growth potential of SV40 LT-expressing cells using soft agar colony assay as described earlier. Interestingly, both the clones failed to form visible colonies on soft agar similar to the untransfected EPCs (Fig. 4I). However, the tumorigenic breast cancer cell line MDAMB-231 showed significant colony formation in soft agar. To address genomic stability of the immortalized cultures over the normal EPCs, we have used normal EPCs at PD30 and immortalized cultures at PD50 and PD100 for karyotyping. Metaphase spreads from immortalized cultures at PD50 and parental culture at PD30 were diploid in nature; however, immortalized cultures at PD100 showed significant chromosomal instability with more than 40% metaphase spread in hyperdiploid condition (Supplementary Fig. S1A–C; Supplementary Data are available online at www.liebertpub.com/tec). These results suggest that the immortalized cell, even though not transformed, repeated culture triggers chromosomal abnormality.
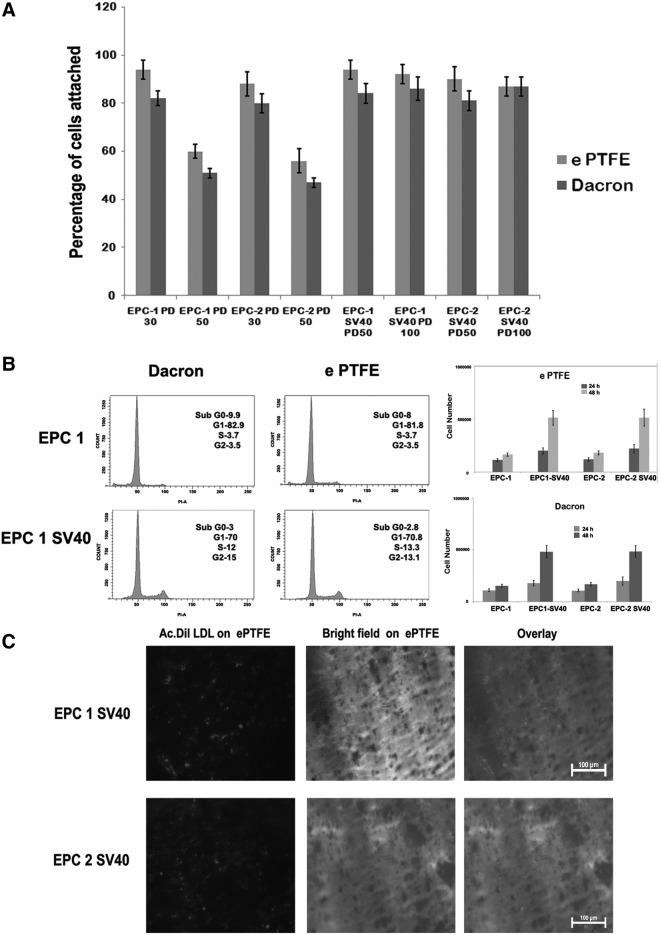
Cell attachment and cell cycle analysis on vascular graft surfaces
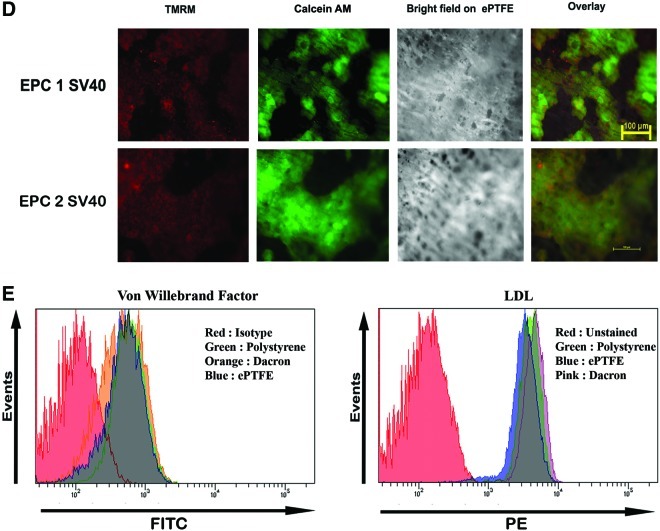
The attachment of cells to synthetic vascular graft is a prerequisite for its proliferation in these surfaces. We have analyzed cell attachment using both uninfected and infected cell population from different PDs. As shown in the Figure 5A, ability of cells to attach to this synthetic surface was significantly reduced in cells after PD of 50. However, SV40LT cells even upto 100PD retained enhanced cell attachment on to both the surfaces. Similarly, as shown in the cell cycle profile in Figure 5B, more immortalized cells are in the S and G2 phase on both materials compared to the vector-transfected cells at PD40. Consistent with these data, cell proliferation studies also showed improved cell growth on both Dacron and ePTFE compared to uninfected culture (Fig. 5B). Overall, our studies substantiate that the two clones of EPCs isolated from umbilical cord retained most functional features with strong cell growth potential on vascular biomaterials and form the best choice for tissue engineering as well as biomaterial–cell interaction studies. Similarly, SV40LT-immortalized cells also showed the uptake of Ac-Dil-LDL after transplantation on to ePTFE graft pieces (Fig. 5C). The SV40LT cells grown on ePTFE retained the transmembrane potential of mitochondria, one of the sensitive markers of apoptosis, indicating that they are also capable of withstanding material surface-induced damage (Fig. 5D). Additionally, we have analyzed the vWF expression and uptake of Ac-Dil-LDL on cells maintained in e PTFE, Dacron, and tissue culture polystyrene by FACS. The cells retained same level of vWF expression and LDL receptor internalization irrespective of the material surface (Fig. 5E).
FIG. 5.
(A) The EPC1 and EPC2 and the respective SV40-immortalized cells at the indicated PD were allowed to attach on to different surfaces. The percentage of cell attachment is shown (n=4). (B) The EPC1 vector-transfected and EPC1 SV40 cells at PD40 were allowed to grow for 24 h on Dacron and expanded polytetrafluoroethylene (ePTFE) fixed on 12-well plates. Then, the cells were trypsinized and analyzed for cell cycle status as described. The percentage of cells at each phase is also indicated. The cell proliferation analysis data for the indicated cells on Dacron and ePTFE are also shown (right). (C) The SV40-immortalized cells were grown on ePTFE for 24 h. Then, the cells were stained with Ac- Dil-LDL for 4 h. The epifluorescence image of internalized low-density lipoprotein (LDL) is shown. (D) The EPC1 SV40 cells and EPC2 SV40 cells were allowed to grow on ePTFE for 24 h. Then, the cells were stained with mitochondrial transmembrane potential-sensitive dye tetramethyl rhodamine methyl ester (TMRM) and calcium AM as described. Both TMRM and Calcium AM channels are shown. As seen, all the calcium-AM-positive cells retained granular red fluorescence, indicating maintenance of mitochondrial membrane potential. (E) The EPC1 SV40 cells were allowed to grow on Dacron, e PTFE, or tissue culture polystyrene for 24 h. Then, the cells were stained with von WF and analyzed by FACS (left). The same cells grown on same group of surfaces were also incubated with Ac-Dil-LDL and uptake was analyzed by FACS (right). Color images available online at www.liebertpub.com/tec
Discussion
EPCs constitute a small fraction of circulating progenitor cell types that can differentiate into functional ECs, and so remain as an ideal cell choice for various biological applications, including tissue engineering, gene delivery, and therapeutic angiogenesis, as well as for testing of investigational new drugs.35–38 Their presence in circulation is also used as a surrogate marker for a variety of pathologies, including heart disease and cancer.39–41 However, their widespread use for diverse applications and experimental investigations is often limited by the low abundance of this population. Ex vivo expansion of EPCs led to the identification of two distinct fractions of EPCs based on their proliferation state. In optimized conditions, late EPCs can be maintained up to 120 days when compared to early EPCs that fail to grow beyond 80 days.32 Even though late EPCs possess enhanced growth potential in culture, they are prone to senescence-related replicative arrest as shown here. Primary diploid cells have limited lifespan before reaching permanent growth arrest called replicative senescence.42 We here show that onset of replicative senescence in EPCs is delayed compared to HUVECs. However, after 60–80 days of continuous culture, they are also prone to loss of proliferation rate. The SA-β gal activity assay, an indication of senescence, shows a gradual increase after 80 days of continuous growth in culture, indicating that they are not senescence resistant despite being progenital type.
Although circulating EPCs were identified and characterized four decades ago, their function and significance of their enhanced presence in a subset of pathologies still remain obscure.43,44 Further research on the biology of these cells and to study its role in neovascularization, cell–material interaction or drug testing requires a characterized cell line with extended life span. Earlier Qiu et al. reported postnatal neovascularization potential of SV40-immortalized CD34+ cord blood cells.45 In this study, we demonstrate that EPCs are prone to senescence in culture, and this can be prevented by expression of SV40T antigen that bypass senescence through inactivation p53 and stabilization of telomerase. Previously, SV40 whole genome as well as early region of SV40 have been used to immortalize a variety of cells.46,47 These viral proteins bind to pRB and p53 tumor suppressor proteins that render cells to acquire enhanced proliferation by bypassing replicative senescence.31 The two clones failed to form colonies on soft agar, suggesting that they can be used as a functional cell source for variety of tissue-engineering applications without concern for transformation-induced functional loss. This could be because the expression vector harbors only SV40 LT antigen not the small T antigen. Studies indicate that SV40 small antigen is necessary to transform the cells in cooperation with other oncoproteins.31 Successful immortalization of subset of fibroblasts, epithelial cells, and ECs was reported with reintroduction of hTERT. However, several studies failed to immortalize ECs only with hTERT, emphasizing the need for additional oncoproteins such as E6 or E7.28,29
Even though the cells retained most functional features after SV40 immortalization, cells also showed lower expression of p53, p21, Gpx, and karyotypic abnormality in long-term culture. Changes in several key cell cycle regulatory proteins are inevitable for immortalization either by hTERT or by SV40.48 Such changes often may lead to abnormal cell physiology compared to the normal mortal cells that may render the cells more resistant to cell death. Further studies are needed to address these concerns. The results indicate that even though immortalized cells form ideal cell tool for studying cell material or in vitro drug-testing applications, enhanced proliferation potential is achieved with changes in certain key cell cycle regulators. Despite this, immortalized cells retained most of the differentiated properties of ECs, emphasizing their potential value as an EC model.
In the current study, we employed the immortalized cells as the possible cell of choice in transplantation studies on vascular biomaterials, since it retained most functions of mature ECs. The early passage cells showed the ability to attach to both ePTFE and Dacron surfaces; however, significantly a less number of cells were attached when the cells are from PD60 or above. Interestingly, the immortalized cells retained its ability to attach and proliferate on to the surface up to 240 days. The SV40T-immortalized cells retained intact mitochondrial membrane potential both in Dacron and ePTFE. The results emphasize that SV40 immortalization retained most of the functional features of the cells with unlimited proliferation potential. Hence, the cells developed can be a valuable cell source for a variety of experimental signaling studies as well as toxicological, biomaterial–cell interaction studies, and drug-testing applications.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Department of Biotechnology (BT/PR7560/MED/14/1031), Government of India, and Life Science Research Board (DLS/81/4822). The authors would like to thank Dr. Bob Weinberg, for the expression vector SV40 TL, and Dr. Garry P. Nolan, for the Amphophoenix packaging cell line. The authors also thank the flow cytometry facility of RGCB for their technical help in FACS experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ahn J.B. Rha S.Y. Shin S.J. Jeung H.C. Kim T.S. Zhang X., et al. Circulating endothelial progenitor cells (EPC) for tumor vasculogenesis in gastric cancer patients. Cancer Lett. 2010;288:124. doi: 10.1016/j.canlet.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Andreou I. Tousoulis D. Tentolouris C. Antoniades C. Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: clinical implications and perspectives. Atherosclerosis. 2006;189:247. doi: 10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Arap W. Pasqualini R. Engineered embryonic endothelial progenitor cells as therapeutic Trojan horses. Cancer Cell. 2004;5:406. doi: 10.1016/s1535-6108(04)00121-7. [DOI] [PubMed] [Google Scholar]
- 4.Au P. Daheron L.M. Duda D.G. Cohen K.S. Tyrrell J.A. Lanning R.M., et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J.H. Hur J. Yoon C.H. Kim J.H. Lee C.S. Youn S.W., et al. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. J Biol Chem. 2004;279:49430. doi: 10.1074/jbc.M402088200. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Asahara T. Masuda H. Takahashi T. Kalka C. Pastore C. Silver M., et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 8.Boos C.J. Goon P.K. Lip G.Y. Circulating endothelial progenitor cells. N Engl J Med. 2005;353:2613. author reply 2616. [PubMed] [Google Scholar]
- 9.Gehling U.M. Ergun S. Schumacher U. Wagener C. Pantel K. Otte M., et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106. [PubMed] [Google Scholar]
- 10.Masouleh B.K. Baraniskin A. Schmiegel W. Schroers R. Quantification of circulating endothelial progenitor cells in human peripheral blood: establishing a reliable flow cytometry protocol. J Immunol Methods. 2010;357:38. doi: 10.1016/j.jim.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Singh S. Wu B.M. Dunn J.C. Accelerating vascularization in polycaprolactone scaffolds by endothelial progenitor cells. Tissue Eng Part A. 2011;17:1819. doi: 10.1089/ten.tea.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasu T. Differentiation of endothelial progenitor cells: a useful biomarker? Circ J. 2009;73:1199. doi: 10.1253/circj.cj-09-0363. [DOI] [PubMed] [Google Scholar]
- 13.Massa M. Rosti V. Ferrario M. Campanelli R. Ramajoli I. Rosso R., et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 14.Gao D. Nolan D.J. Mellick A.S. Bambino K. McDonnell K. Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 15.Shaked Y. Ciarrocchi A. Franco M. Lee C.R. Man S. Cheung A.M., et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 16.Melero-Martin J.M. Dudley A.C. Concise review: vascular stem cells and tumor angiogenesis. Stem Cells. 2011;29:163. doi: 10.1002/stem.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mund J.A. Case J. The role of circulating endothelial progenitor cells in tumor angiogenesis. Curr Stem Cell Res Ther. 2011;6:115. doi: 10.2174/157488811795495468. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Padura I. Bertolini F. Circulating endothelial cells as biomarkers for angiogenesis in tumor progression. Front Biosci (Schol Ed) 2009;1:304. doi: 10.2741/s28. [DOI] [PubMed] [Google Scholar]
- 19.Zhu C. Ying D. Mi J. Li L. Zeng W. Hou C., et al. Development of anti-atherosclerotic tissue-engineered blood vessel by A20-regulated endothelial progenitor cells seeding decellularized vascular matrix. Biomaterials. 2008;29:2628. doi: 10.1016/j.biomaterials.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Achneck H.E. Jamiolkowski R.M. Jantzen A.E. Haseltine J.M. Lane W.O. Huang J.K., et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti implants. Biomaterials. 2011;32:10. doi: 10.1016/j.biomaterials.2010.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sales V.L. Mettler B.A. Engelmayr G.C., Jr. Aikawa E. Bischoff J. Martin D.P., et al. Endothelial progenitor cells as a sole source for ex vivo seeding of tissue-engineered heart valves. Tissue Eng Part A. 2010;16:257. doi: 10.1089/ten.tea.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchentouf M. Forner K. Cuerquis J. Boulassel M.R. Routy J.P. Waller E.K., et al. A novel and simplified method of culture of human blood derived early endothelial progenitor cells for the treatment of ischemic vascular disease. Cell Transplant. 2011;20:1431. doi: 10.3727/096368910X557164. [DOI] [PubMed] [Google Scholar]
- 23.Blann A.D. Pretorius A. Circulating endothelial cells and endothelial progenitor cells: two sides of the same coin, or two different coins? Atherosclerosis. 2006;188:12. doi: 10.1016/j.atherosclerosis.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Chapman E.J. Hurst C.D. Pitt E. Chambers P. Aveyard J.S. Knowles M.A. Expression of hTERT immortalises normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene. 2006;25:5037. doi: 10.1038/sj.onc.1209513. [DOI] [PubMed] [Google Scholar]
- 25.Davies B.R. Steele I.A. Edmondson R.J. Zwolinski S.A. Saretzki G. von Zglinicki T., et al. Immortalisation of human ovarian surface epithelium with telomerase and temperature-sensitive SV40 large T antigen. Exp Cell Res. 2003;288:390. doi: 10.1016/s0014-4827(03)00218-0. [DOI] [PubMed] [Google Scholar]
- 26.Rood P.M. Calafat J. von dem Borne A.E. Gerritsen W.R. van der Schoot C.E. Immortalisation of human bone marrow endothelial cells: characterisation of new cell lines. Eur J Clin Invest. 2000;30:618. doi: 10.1046/j.1365-2362.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- 27.Bodnar A.G. Ouellette M. Frolkis M. Holt S.E. Chiu C.P. Morin G.B., et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 28.Elenbaas B. Spirio L. Koerner F. Fleming M.D. Zimonjic D.B. Donaher J.L., et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn W.C. Counter C.M. Lundberg A.S. Beijersbergen R.L. Brooks M.W. Weinberg R.A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 30.Santhosh Kumar T.R. Krishnan L.K. Endothelial cell growth factor (ECGF) enmeshed with fibrin matrix enhances proliferation of EC in vitro. Biomaterials. 2001;22:2769. doi: 10.1016/s0142-9612(01)00020-5. [DOI] [PubMed] [Google Scholar]
- 31.Hahn W.C. Dessain S.K. Brooks M.W. King J.E. Elenbaas B. Sabatini D.M., et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmermans F. Van Hauwermeiren F. De Smedt M. Raedt R. Plasschaert F. De Buyzere M.L., et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 33.Imanishi T. Tsujioka H. Akasaka T. Endothelial progenitor cell senescence—is there a role for estrogen? Ther Adv Cardiovasc Dis. 2010;4:55. doi: 10.1177/1753944709353173. [DOI] [PubMed] [Google Scholar]
- 34.Erusalimsky J.D. Kurz D.J. Endothelial cell senescence. Handb Exp Pharmacol. 2006;176(Pt2):213. doi: 10.1007/3-540-36028-x_7. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs S. Motta A. Migliaresi C. Kirkpatrick C.J. Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells as a potential source of autologous cells for endothelialization of silk fibroin biomaterials. Biomaterials. 2006;27:5399. doi: 10.1016/j.biomaterials.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Kim K.L. Han D.K. Park K. Song S.H. Kim J.Y. Kim J.M., et al. Enhanced dermal wound neovascularization by targeted delivery of endothelial progenitor cells using an RGD-g-PLLA scaffold. Biomaterials. 2009;30:3742. doi: 10.1016/j.biomaterials.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 37.Suh W. Kim K.L. Kim J.M. Shin I.S. Lee Y.S. Lee J.Y., et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z. Ito W.D. Hopfner U. Bohmert B. Kremer M. Reckhenrich A.K., et al. The role of single cell derived vascular resident endothelial progenitor cells in the enhancement of vascularization in scaffold-based skin regeneration. Biomaterials. 2011;32:4109. doi: 10.1016/j.biomaterials.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 39.Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 40.Sun B. Zhang S. Ni C. Zhang D. Liu Y. Zhang W., et al. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005;14:292. doi: 10.1089/scd.2005.14.292. [DOI] [PubMed] [Google Scholar]
- 41.Suriano R. Chaudhuri D. Johnson R.S. Lambers E. Ashok B.T. Kishore R., et al. 17Beta-estradiol mobilizes bone marrow-derived endothelial progenitor cells to tumors. Cancer Res. 2008;68:6038. doi: 10.1158/0008-5472.CAN-08-1009. [DOI] [PubMed] [Google Scholar]
- 42.Yang D.G. Liu L. Zheng X.Y. Cyclin-dependent kinase inhibitor p16(INK4a) and telomerase may co-modulate endothelial progenitor cells senescence. Ageing Res Rev. 2008;7:137. doi: 10.1016/j.arr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y. Weisdorf D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timmermans F. Plum J. Yoder M.C. Ingram D.A. Vandekerckhove B. Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu H.Y. Fujimori Y. Nishioka K. Yamaguchi N. Hashimoto-Tamaoki T. Sugihara A., et al. Postnatal neovascularization by endothelial progenitor cells immortalized with the simian virus 40T antigen gene. Int J Oncol. 2006;28:815. [PubMed] [Google Scholar]
- 46.Srinivasan A. Peden K.W. Pipas J.M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J Virol. 1989;63:5459. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J. Rice P.W. Gorsch L. Abate M. Cole C.N. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992;66:2780. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toouli C.D. Huschtscha L.I. Neumann A.A. Noble J.R. Colgin L.M. Hukku B., et al. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene. 2002;21:128. doi: 10.1038/sj.onc.1205014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.