Abstract
Background
This study was designed to evaluate toxicity and preliminary efficacy of 2 cycles of concomitant standard dose/schedule of 153Sm-lexidronam plus Q 3 weeks schedule escalating doses of docetaxel in metastatic castration-resistant prostate cancer (mCRPC).
Methods
mCRPC patients with progressive bone metastases were treated in 4 cohorts. Docetaxel doses were escalated from 50, 50, 0 mg/m2 (on days 1, 22, 43, per 12-week cycle) to 75, 75, 75 mg/m2. 153Sm-lexidronam was administered on days 2 (Q 12 weeks) at dose of 1 mCi/kg/cycle (maximum of 2 cycles).
Results
Thirteen patients received an average of 3.6 doses of docetaxel (range, 2–6 doses, median 4) and 1.5 doses of 153Sm-lexidronam (range, 1–2, median 2). Toxicity was primarily hematologic. There were total 35 episodes grade 3/4 neutropenia with a median 7 (range 7–14) days to recovery to ≤grade 1. One dose limiting grade 3 thrombocytopenia occurred on cohorts 3 and 4. Eight of 13 (62%) patients had PSA > 50% decrease as best response during the treatment. Median time to bone disease progression was 5.2 months (range 91 days–10 months+); 6/13 (46%) patients had stable/improved bone scans at 6 months and 6/6 (100%) symptomatic patients had improvement in pain.
Conclusions
Concurrent 6-month administration of 4 doses (75 mg/m2) of standard Q 3 weeks schedule of docetaxel with 2 Q 3 months infusions of 1 mCi/Kg 153Sm-lexidronam is feasible with reversible bone marrow suppression, and deserves further testing in mCRPC patients with extensive bone metastasis.
Keywords: 153Sm-lexidronam, Metastatic prostate cancer, Docetaxel, Phase I
1. Introduction
Bone metastases in prostate cancer cause considerable morbidity and dramatically reduce patients’ quality of life [1]. Bone targeted therapy holds great promise for improving outcomes in metastatic castration-resistant prostate cancer (mCRPC) (reviewed in [2–4]).
At the present time, the combination of docetaxel and prednisone represents the standard chemotherapy approved for patients with mCRPC [5]. Sm-153 lexidronam (Samarium-153 Lexidronam, 153Sm-lexidronam, 153Sm-ethylene diamine tetramethylene phosphonate, 153Sm-EDTMP, Sm-153) is a complex of radioactive samarium and a tetraphosphate chelator, approved by FDA for the palliation of pain for patients with mCRPC. This bone-seeking radiopharmaceutical has special affinity to skeletal metastasis [6,7]. Previous trials showed that 153Sm-lexidronam at the dose of 1.0 mCi/kg was safe and effective for the palliation of painful bone metastases [8,9]. 153Sm-lexidronam has a relatively short half life, thus allowing a high dose of radioactivity resulting in fast palliation and probably a strong biological effect. In addition, bone marrow recovery is relatively quick, making it possible to re-treat or to start other therapies early. Analysis of data from 55 patients receiving 2 or more doses of 153Sm-lexidronam showed that repeated dosing of 1.0 mCi/kg of Sm-153 was both safe and effective, and a reasonable treatment option in patients whose bone pain responds and then recurs after an initial dose, provided that adequate hematologic function is present at the time of drug administration [10]. Furthermore, the β particles of Sm-153 have a lower energy than those emitted from strontium 89, for example, and with less bone and bone marrow exposure. The major toxicity associated with 153Sm-lexidronam treatment is myelosuppression [11]. Other side effects are mild and less common, including a flare reaction which is an exacerbation of the pre-existing pain in the first hours to days from receiving the drug [12].
Ongoing clinical experience with the combination of bone-seeking radiopharmaceuticals and systemic chemotherapy indicate that this approach is feasible and potentially effective [13,14].
The combination regimen in this study was designed to explore the possible interactions between repeated doses of docetaxel and 153Sam-lexidronam. The radiosensitizing properties of docetaxel are well documented in preclinical and clinical experiments [15–17]. The most likely underlying mechanism is a G2/M block in the cell cycle induced by docetaxel and this will result in cycling cells at the most radiosensitive phase of the cell cycle [18,19]. Our preclinical in vitro data suggest that 24 hours following a dose of docetaxel, there was a maximum G2/M arrest, which significantly sensitizes cells to low dose rate radiation like that from Sam-153. (DeWeese et al., unpublished data). Other investigators have also documented synergistic effects between taxanes and radiation when the taxane is delivered to cancer cells prior to radiation, and have surmised the benefits are primarily a result of taxane-induced arrest of cells in the radiosensitive G2M phase [20]. Based on these observations, 153Sam-lexidronam was infused 24 hours after the administration of escalating doses of docetaxel, a sequencing schedule different from the studies mentioned above.
2. Patients and methods
This study was designed as a nonrandomized, open label, phase I trial consisting of 4 dose cohorts with 3 to 6 patients in each cohort depending on toxicity.
2.1. Patient eligibility
Patients aged 18 or older with mCRPC were eligible for study. Progression after conventional systemic treatments including docetaxel did not preclude eligibility. Other eligibility criteria included ECOG PS ≤ 2, life expectancy of 12 weeks or longer, adequate bone marrow (WBC ≥ 3500/mm3, ANC ≥ 1500/mm3, and platelet count ≥ 150,000/mm3 and hemoglobin ≥ 8.0 g/dl), hepatic, and renal function, and no evidence of CNS metastasis. Patients treated with chemotherapy or radiation therapy were required to be off treatment for ≥4 weeks prior to entry, with recovery from all toxicities (to grade 1 or grade 0). Patients without significant pain (pain score < 2) were allowed for the study. Peripheral neuropathy must be <grade 1. Patients were required to provide written informed consent prior to study enrollment. The study was approved by the Cancer Review Committee and the Institutional Review Board of The Sidney Kimmel Cancer Center at Johns Hopkins University.
2.2. Treatment and dose escalation
The 153Sm-lexidronam vial was stored frozen in a shielded container and thawed prior to use. 153Sm-lexidronam was administered intravenously over a 1-minute period. Standard radiation exposure precautions were applied to ensure minimal exposure to patients and health care workers. 153Sm-lexidronam was given in 12-week intervals. Each dose of docetaxel was infused IV over approximately 1 hour. Dexamethasone 8 mg PO was administered 12, 3, and 1 hour prior to each dose of docetaxel. The dose of docetaxel was escalated between patient cohorts, and doses of 153Sm-lexidronam were fixed (1 mCi/kg). The dose escalation scheme is listed in Table 1. Docetaxel was administered on days 1, 22 for cohort 1 to 3, and 1, 22, 43 for cohort 4; 153Sm-lexidronam was administered on day 2. A maximum of 2 cycles (12 weeks each) of treatment was given. Disease status was reassessed (with bone scan and PSA or CT scan) after every cycle of therapy (every 3 months).
Table 1.
Dose escalation scheme
| Cohort | Docetaxel dose mg/m2 (days 1, 22, 43) |
Samarium 153- EDTMP mCi/ kg IV (days 2) |
Patient number (n) |
|
|---|---|---|---|---|
| Cycle 1 | Cycle 2 (week 13) |
Cycles 1 and 2 | ||
| 1 | 50, 50, 0 | 50, 50, 0 | 1 | 3 |
| 2 | 75, 75, 0 | 50, 50, 0 | 1 | 3 |
| 3 | 75, 75, 0 | 75, 75, 0 | 1 | 4 |
| 4 | 75, 75, 75 | 75, 75, 75 | 1 | 3 |
2.3. Toxicity and response evaluation
CBC with differential was obtained within 48 hours prior to each dose of docetaxel; then weekly while on study. An ANC > 1500/mm3 and platelets ≥ 150,000/mm3 were required prior to docetaxel or 153Sm-lexidronam infusion. Follow-up study included weekly CBC with differential, toxicity notation, pain medication diary, and PSA Q 3 weeks. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0. After completion of protocol, patients were monitored every 3 months with PSA and bone scan or CT scan, and pain medication diary until progression of disease.
2.4. Definition of dose-limiting-toxicity (DLT)
DLT was defined as follows: (1) grade 4 febrile neutropenia with documented infection; (2) grade 4 neutropenia lasting >7 days; (3) grade 3 hematological toxicity not recovered to grade 1 in 4 weeks; (4) grade 4 thrombocytopenia (platelet counts < 20,000/µl) > 7 days; (5) grade 3 or 4 nonhematologic toxicity, except manageable nausea, vomiting, diarrhea, or fatigue. The maximal tolerated dose (MTD) was defined as the dose where <2/6 patients (initially enrolled + 3 additional patients) developed DLT.
2.5. Dose reductions for docetaxel toxicity
Patients developing grade 4 neutropenia lasting ≤7 days or grade 4 thrombocytopenia that recovered within 4 weeks, remained on study and had a 50% dose reduction of docetaxel. No growth factors were employed. Patients were removed from the study for any of the following reasons: (1) withdrawal of consent; (2) disease progression, which is defined by a confirmed PSA rise at least 1 week apart and/or evidence of disease progression on bone scan, CT scan or physical examination; (3) development of DLT; (4) grades 3 or 4 thrombocytopenia not recovered to ≤ grade 0 after 4 weeks; (5) treatment delay of more than 4 weeks due to toxicity; (6) grade 4 hypersensitivity reactions to docetaxel; and (7) violation of the study protocol or patient noncompliance.
2.6. Pain assessment
Pain medication was recorded daily for patients on the sheet provided. Pain score was calculated using the following point system: 1 point: standard tablet or capsule of non-narcotic analgesic; 2 points: standard tablet or capsule of narcotic analgesic (i.e., codeine 30 mg, morphine 5 mg, hydromorphone 2 mg, anileridine 25 mg, or oxycodone 15 mg). For parenteral or transdermal narcotics, doses of 1 mg/h of morphine, hydromorphone 1 mg/h, or fentanyl patch 25 µg/h receive a score of 2 per hour of narcotic administration. The daily score is the sum of the points for all medications taken by a patient in 1 day. The pain diary was completed (in patients who initially present with pain) daily while on study drugs and was checked by a research nurse on a regular basis.
2.7. Statistical analysis
No statistical analysis was performed. All the data are primarily descriptive.
3. Results
3.1. Patient characteristics
A total of 13 patients were enrolled from May 2005 to February 2008 (see Table 2). All patients had castration resistant adenocarcinoma of prostate cancer with progressive metastatic disease in the bone upon entry in the study. Seven patients (54%) had more than 3 hormonal manipulations. Three patients had prior docetaxel and another 3 patients had prior palliative bone radiation treatment.
Table 2.
Patient characteristics (n = 13)
| No. of patients | 13 |
| Age, years | |
| Median | 69 |
| Range | 58–76 |
| ECOG performance status | |
| 0 | 6 |
| 1 | 7 |
| Baseline PSA (ng/ml) | |
| Median | 57.1 |
| Range | 9–832.5 |
| Site of metastasis | |
| Bone | 13 |
| Soft tissue | 2 |
| Lung | 0 |
| Liver | 0 |
| Lymph nodes | 2 |
| Pelvic mass | 2 |
| Prior hormonal manipulations | 13 |
| 1 | 2 |
| 2 | 4 |
| ≥3 | 7 |
| Prior palliative radiation treatment | 3 |
| Prior immunotherapy (vaccine) | 3 |
| Prior docetaxel-based chemotherapy | 3 |
All patients received at least 1 cycle of treatment. Four (31%) patients completed 2 cycles of treatment as planned. All data from the patients enrolled were used for toxicity analysis.
Thirteen patients received total 20 cycles in 4 cohorts, with total 47 doses of docetaxel and 20 doses of 153Sm-lexidronam. An average of 3.6 doses of docetaxel per patient was administered (range, 2–6 doses, median 4) and 1.5 doses of 153Sm-lexidronam (range, 1–2, median 2).
3.2. Toxicity
Overall the toxicities were expected. No grade 3/4 toxicities occurred except bone marrow suppression. Two patients in cohort 4 required dose reduction for docetaxel to 50%. Three of 13 (23%) patients (in cohort 3 and cohort 4) received 4 full doses (75 mg/m2) docetaxel and 2 doses of 153Sm-lexidronam without DLT.
The most common nonhematologic grade 1 or 2 toxicities included fatigue, alopecia, altered taste, skin rash, and GI discomfort such as nausea, anorexia, and constipation. Toxicity was primarily hematologic (shown in Tables 3 and 4). Only 1 pt in cohort 4 came off study because of G3 thrombocytopenia (nadir 28,000) with duration of 9 weeks (DLT). This patient was taxane naïve and 70 years old with normal platelet count at the baseline. No serious unexpected adverse events were seen. There were total 35 (cycle 1) and 11 (cycle 2) episodes grade 3/4 neutropenia with a median 7 (range 7–14) days to recovery to ≤grade 1 in all cohorts (Table 4).
Table 3.
Grade 3/4 hematological toxicity by cycle 1, use of G-CSF, and dose reductions
| Cohort | n | Grade 3 neutropenia (%) | Grade 4 neutropenia (%) | Febrile neutropenia | Use of G-CSF | Dose reductions |
|---|---|---|---|---|---|---|
| 1 | 3 | 3 (100) | 1 (33) | 0 | No | 0 |
| 2 | 3 | 3 (100) | 2 (67) | 0 | No | 2 |
| 3 | 4 | 3 (75) | 2 (50) | 0 | No | 0 |
| 4 | 3 | 3 (100) | 3 (75) | 0 | No | 2 |
Table 4.
Events of grade 3/4 neutropenia and thrombocytopenia which were thought to be possibly or definitely related to treatment
| Cohorts (no.) |
Cycle 1 | Cycle 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutropenia | Thrombo cytopenia |
Neutropenia | Thrombo cytopenia |
|||||||
| Nadir* | Duration (weeks) | Events (duration of weeks) |
Nadir* | Duration (weeks) | Events (duration of weeks) |
|||||
| 1 | 2 | >2 | 1 | 2 | >2 | |||||
| 1 (3) | 0.36–0.93 | 5 | 1 | 0 | 0 | 0.28–0.55 | 2 | 0 | 0 | 0 |
| 2 (3) | 0.35–0.88 | 4 | 1 | 0 | 0 | 0.68 | 1 | 0 | 0 | 0 |
| 3 (4) | 0.12–0.95 | 2 | 4 | 0 | 0 | 0.16–0.59 | 0 | 4 | 0 | 0 |
| 4 (3) | 0.08–0.59 | 3 | 5 | 0 | 0 | 0.15–0.62 | 3 | 1 | 0 | 1 (4) |
| Events | 14 | 11 | 0 | 0 | 6 | 5 | 0 | 1 | ||
Cells × 103/µl.
Three patients (23%) received docetaxel prior to enrollment of the study. Two of 3 (67%) in cohort 1 and cohort 3 were able to tolerate all full doses per protocol. One patient in cohort 3 (full dose docetaxel) developed prolonged grade 1 thrombocytopenia (>9 weeks) after cycle 1. This prevented him from receiving second dose of 153Sm-lexidronam and he was taken off the study. No further cohort expansion was planned. However, we proceeded to add cohort 4 with additional full dose of docetaxel on day 43 of each cycle. One patient was added to cohort 3 prior to approval of the amended protocol proposing the addition of cohort 4. One patient in cohort 4 showed prolonged grade 3 thrombocytopenia (>4 weeks), which was defined as DLT as well. There were no DLT seen at cohort levels 1 and 2.
The nadirs and the duration of neutropenia are listed in Table 4. Patients were not allowed to receive growth factors. No blood or platelet transfusions were needed for any patients.
3.3. Antitumor activity
Thirteen patients were enrolled and all of them completed at least 1 cycle of therapy. All patients showed progressive bone metastases prior to the study entry. Seven of 13 (54%) patients demonstrated evidence of progression after the first cycle of treatment. Nine patients did not complete the 24 week study due to progressive disease or toxicity. Six of 13 (46%) patients had stable bone scans at 6 months. The median time to bone progression was 5.2 months (range 91 days–10 months+).
3.4. Pain response
Six patients had symptomatic bone pain with pain score ≥ 2 at the time of study entry, while 7 patients had pain score <2. Two patients had pain scores between 2 and 5, while 4 patients had pain score > 5. All these 6 patients (100%) had reduced pain score from baseline lasting for more than 2 weeks. The median duration of narcotic analgesics use in these patients was 9 weeks (range, 3–20).
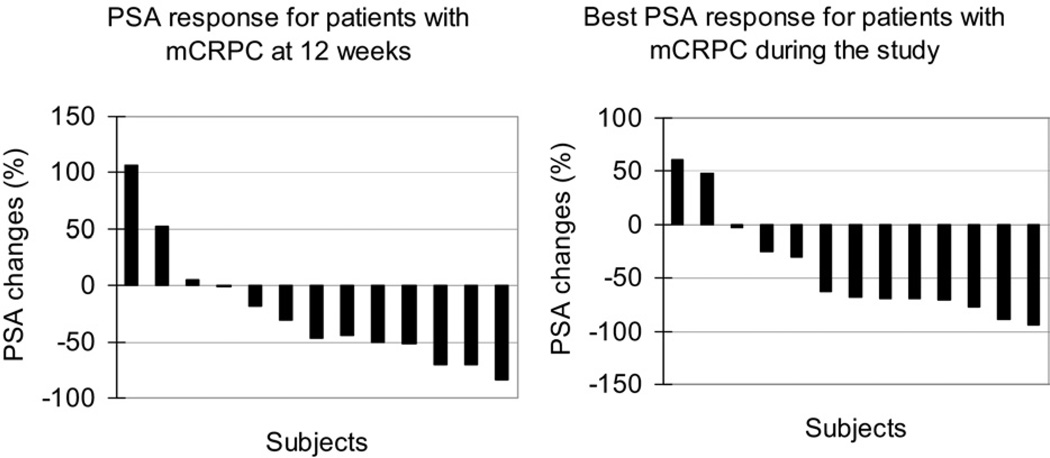
3.5. PSA changes
Figure 1 shows the waterfall plot analysis of the percent changes in PSA from baseline at 12 weeks after the initiation of treatment and the best response during the study. The median baseline PSA was 100.4 ng/ml (range 8.6 –1064). At the 12 weeks time point, 5/13 (38%) patients achieved 50% decrease. Eight of 13 (62%) patients achieved more than 50% decrease as best response during the study.
Fig. 1.
Waterfall plot of PSA declines per subject in this study, with each bar representing 1 patient’s lowest percent decline 12 weeks after initiation of treatment (left) and best response during the study (right) n = 13.
4. Discussion
The main purpose of this phase I study was to evaluate the safety of concurrent administration of standard dose schedule of docetaxel (75 mg/m2) with 2 full doses at every 3 months of 153Sm-lexidronam for the treatment of mCRPC.
Our experience confirms recent reports that the combination of 153Sm-lexidronam and docetaxel is safe and potentially effective [21]. Suttmann et al. reported their clinical experience with the combination in 12 mCRPC patients with diffuse bone metastases treated with a single standard dose of 1 mCi/kg 153Sm-lexidronam plus 6 weekly infusions of 35 mg/m2 docetaxel [14]. The overall toxicity was reported moderate, but 1 patient died due to neutropenic sepsis. Docetaxel was infused on days 1, 8, 15, 22, 29, and 36, and the only dose of 153Sm-lexidronam was given on day 7.
Tu et al. reported their experience in a heavily pretreated group of patients with mCRPC [22,23]. Patients were treated with 28 day cycles (docetaxel on days 1, 8, 15, and 153Sm-lexidronam was given on day 1 of each cycle of the combination) employing escalating dose of docetaxel from 25, 30, to 35 mg/m2 with a fixed dose (1 mCi/kg/cycle) of 153Sm-lexidronam. They concluded that weekly administration of docetaxel in combination with 2 cycles of 153Sm-lexidronam did not result in DLT.
Recently Morris et al. reported a phase I study with standard dosing schedule for docetaxel (Q 3 weeks between doses of 65 to 75 mg/m2) plus 153Sm-lexidronam (Q 6 weeks at escalating doses from 0.5 to 1 mCi/kg) [24]. 153Sm-lexidronam was given 6 hours before infusion of docetaxel at day 1. They concluded that docetaxel and 153Sm-lexidronam could be combined safely at full doses over repeated cycles. Incidence of neutropenia (68% for grade 3 and 18% for grade 4) and thrombocytopenia (0% for grade 3 and 4) was lower than what was observed in our study.
The design of this study is different from that of Tu et al. in that the docetaxel dose and schedule are different. We designed to use the accepted effective dosing and schedule of docetaxel for mCRPC [5]. Our design of this trial is very similar to that of Morris et al., although there are some differences in terms of scheduling. To maximize the radio-sensitivity of docetaxel, 153Sm-lexidronam was given on day 2 for a total of 2 cycles. We did see 1 patient with prolonged G3 thrombocytopenia in cohort 4 and also significant but transient bone marrow suppression in all cohorts. MTD was not determined in this study as well. In our study we determined that 3 to 4 full doses (75 mg/m2) of docetaxel at every 3 weeks cycle could be given concurrently with 2 doses of 153Sm-lexidronam.
The factors that may explain the difference in toxicity between our study and that of Morris et al. are (1) differences in patient populations; (2) the lower dose of 153Sm-lexidronam in 4 of 6 cohorts in their study; (3) prednisone 5 mg orally twice a day was given in their study vs. not given in ours; and (4) the different sequencing/timing between 153Sm-lexidronam and docetaxel (153Sm-lexidronam given 6 hours prior to docetaxel by Morris et al. vs. given 24 hours after docetaxel in ours). This is consistent with our hypothesis that docetaxel possesses radiosensitizing activity when given prior to the infusion of 153Samarium.
In summary, in this study we demonstrate that co-administration of docetaxel and Sm-153-lexidronam is feasible and results in reversible bone marrow suppression. Our preliminary data on efficacy confirms recent reports with this combination and the overall experience strongly suggests that this approach may be further explored as a first line treatment for patients with mCRPC.
Acknowledgments
The authors extend special thanks to Marianna Zahurak for assistance in statistical analysis of the data.
Footnotes
This work was supported by Sanofi-Aventis, Cytogen Inc., The prostate Cancer Foundation (PCF), and The James Kline research fund.
References
- 1.Costa L, Badia X, Chow E, et al. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 2.Bradley DA, Hussain M, Dipaola RS, et al. Bone directed therapies for prostate cancer. J Urol. 2007;178:S42–S48. doi: 10.1016/j.juro.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 3.Tu SM, Lin SH. Current trials using bone-targeting agents in prostate cancer. Cancer J. 2008;14:35–39. doi: 10.1097/PPO.0b013e318161d32d. [DOI] [PubMed] [Google Scholar]
- 4.Storey JA, Torti FM. Bone metastases in prostate cancer: A targeted approach. Curr Opin Oncol. 2007;19:254–258. doi: 10.1097/CCO.0b013e32805e8787. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Lamb HM, Faulds D. Samarium 153Sm lexidronam. Drugs Aging. 1997;11:413–418. doi: 10.2165/00002512-199711050-00007. Discussion 419. [DOI] [PubMed] [Google Scholar]
- 7.Liepe K, Kotzerke J. A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nucl Med Commun. 2007;28:623–630. doi: 10.1097/MNM.0b013e32825a6adc. [DOI] [PubMed] [Google Scholar]
- 8.Resche I, Chatal JF, Pecking A, et al. A dose-controlled study of 153Sm-ethylene diamine tetramethylene phosphonate (EDTMP) in the treatment of patients with painful bone metastases. Eur J Cancer. 1997;33:1583–1591. doi: 10.1016/s0959-8049(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 9.Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: A double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 10.Sartor O, Reid RH, Bushnell DL, et al. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109:637–643. doi: 10.1002/cncr.22431. [DOI] [PubMed] [Google Scholar]
- 11.Serafini AN. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases. Cancer. 2000;88:2934–2939. doi: 10.1002/1097-0142(20000615)88:12+<2934::aid-cncr9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Farhanghi M, Holmes RA, Volkert WA, et al. Samarium-153-EDTMP: Pharmacokinetic, toxicity, and pain response using an escalating dose schedule in treatment of metastatic bone cancer. J Nucl Med. 1992;33:1451–1458. [PubMed] [Google Scholar]
- 13.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: A randomized phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 14.Suttmann H, Grgic A, Lehmann J, et al. Combining 153Sm-lexidronam and docetaxel for the treatment of patients with hormone-refractory prostate cancer: First experience. Cancer Biother Radiopharm. 2008;23:609–618. doi: 10.1089/cbr.2008.0487. [DOI] [PubMed] [Google Scholar]
- 15.Karasawa K, Katsui K, Seki K, et al. Radiotherapy with concurrent docetaxel for advanced and recurrent breast cancer. Breast Cancer. 2003;10:268–274. doi: 10.1007/BF02966728. [DOI] [PubMed] [Google Scholar]
- 16.Mason KA, Hunter NR, Milas M, et al. Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res. 1997;3:2431–2438. [PubMed] [Google Scholar]
- 17.Geng CX, Zeng ZC, Wang JY, et al. Docetaxel shows radiosensitization in human hepatocellular carcinoma cells. World J Gastroenterol. 2005;11:2990–2993. doi: 10.3748/wjg.v11.i19.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradier O, Rave-Frank M, Lehmann J, et al. Effects of docetaxel in combination with radiation on human head and neck cancer cells (ZMK-1) and cervical squamous cell carcinoma cells (CaSki) Int J Cancer. 2001;91:840–845. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1142>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Distelmans W, Storme G. The effect of pretreatment with docetaxel (Taxotere; RP 56976) on irradiated subcutaneous MA 16/C murine tumors. J Exp Clin Cancer Res. 1999;18:167–172. [PubMed] [Google Scholar]
- 20.Kim JS, Amorino GP, Pyo H, et al. The novel taxane analogs, BMS-184476 and BMS-188797, potentiate the effects of radiation therapy in vitro and in vivo against human lung cancer cells. Int J Radiat Oncol Biol Phys. 2001;51:525–534. doi: 10.1016/s0360-3016(01)01658-3. [DOI] [PubMed] [Google Scholar]
- 21.Ricci S, Boni G, Pastina I, et al. Clinical benefit of bone-targeted radiometabolic therapy with 153Sm-EDTMP combined with chemotherapy in patients with metastatic hormone-refractory prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:1023–1030. doi: 10.1007/s00259-006-0343-8. [DOI] [PubMed] [Google Scholar]
- 22.S. Tu DJ, Mathew P, Logothetis CJ. Phase I study of concurrent weekly docetaxel (Tax) and repeated 153Sm-Lexidronam (Sam) in androgen-independent prostate cancer (AIPC). Proceedings of the 2008 ASCO Annual meeting; May 2008; Chicago. [Google Scholar]
- 23.Tu SM, Mathew P, Wong FC, et al. Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:3319–3324. doi: 10.1200/JCO.2008.20.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MJ, Pandit-Taskar N, Carrasquillo J, et al. Phase I Study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:2436–2442. doi: 10.1200/JCO.2008.20.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]