Abstract
Background
The optimal number of 3-wk docetaxel plus prednisone (DP) cycles for metastatic castration-resistant prostate cancer (mCRPC) is unclear.
Objective
A retrospective analysis of two clinical trials was performed to evaluate the association of the number of cycles with overall survival (OS).
Design, setting, and participants
An exploratory analysis compared outcomes of 332 men who received DP in the TAX-327 trial, which stipulated up to 10 cycles, and 220 men who received DP in CS-205, a randomized phase 2 trial comparing DP plus AT-101 (bcl-2 inhibitor) versus DP plus placebo, which allowed up to 17 cycles.
Measurements
Patients who completed 10 cycles of DP without progression in both trials were included. Men in both arms of CS-205 were combined for analysis, as no significant differences in outcomes were observed. OS was estimated from the date of cycle 10 docetaxel infusion.
Results and limitations
The number of men receiving 10 cycles was similar (p = 0.26) in the two trials (166 [50.0%] in TAX-327 vs 99 [45.0%] in CS-205; the latter group received a median of five additional cycles). Six- and 12-mo estimated survival after cycle 10 was 92.2% (95% confidence interval [CI], 86.9–95.4%) and 74.6% (CI, 67.2–80.5%) in TAX-327, compared with 92.8% (CI, 85.5–96.5) and 63.4% (CI, 51.8–72.9%) in CS-205. Subanalyses suggested that <10 cycles may have a negative impact and prostate-specific antigen (PSA) declines at cycle 10 may carry a favorable impact. The significance of continued PSA declines up to 17 cycles is unclear. Limitations of a retrospective analysis apply.
Conclusions
A survival benefit was not detected with >10 cycles of DP in men with mCRPC in this retrospective hypothesis-generating analysis.
Keywords: Docetaxel, Prednisone, Metastatic castration-resistant, prostate cancer
1. Introduction
Docetaxel plus prednisone (DP) every 3 wk is established as the frontline chemotherapy for metastatic castration-resistant prostate cancer (mCRPC) [1–3]. However, the optimal number of cycles remains unclear with the TAX-327 and Southwest Oncology Group (SWOG) 9916 trials allowing up to 10 or 12 cycles, respectively, which were arbitrarily chosen due to toxicity concerns. The Prostate Cancer Clinical Trials Working Group (PCWG)–2 criteria recommend at least four cycles before evaluating response [4]. Given the potential benefit of docetaxel beyond 10 cycles, the issue of duration of frontline docetaxel is of clinical relevance.
A large, randomized, phase 2 trial was conducted by Ascenta Therapeutics (CS-205) evaluating the combination of AT-101, a BCL-2 antagonist, in combination with DP every 3 wk as front-line therapy for mCRPC [5]. This trial allowed up to 17 cycles of DP to allow for 1 yr of therapy. We hypothesized that an exploratory analysis comparing the survival of patients who completed 10 cycles of DP in TAX-327 and CS-205 might yield insights into the value of DP beyond 10 cycles.
2. Materials and methods
2.1. Patient population
TAX-327 was a phase 3 trial of 1006 men with mCRPC comparing first-line DP every 3 wk, weekly DP, or mitoxantrone plus prednisone every 3 wk. Patients were recruited internationally after institutional internal review board (IRB) approval [1,2]. Treatment continued for up to 30 wk or until toxicity, progression (symptomatic, World Health Organization [WHO] criteria, or prostate-specific antigen [PSA] criteria), or death. Imaging was performed every 6–9 wk. Measurable lesions were evaluated by WHO criteria [6].
CS-205 was a phase 2 trial that recruited 221 men with mCRPC who were randomized to DP combined with either placebo or AT101 [5]. The trial was approved by national ethics committees or IRBs and conducted in the Russian Federation and the United States. A maximum of 17 cycles was permitted unless unacceptable toxicity, progression by PCWG-2 criteria (symptomatic, Response Evaluation Criteria in Solid Tumors [RECIST], but not PSA progression alone), or death occurred [4]. AT101 was not continued after discontinuation of DP. Imaging was obtained every three cycles (approximately 9 wk) or at symptomatic progression. Patients who received cycle 10 in CS-205 and among those who received DP (every 3 wk) in TAX-327 were eligible for our analysis. Men in both arms of the CS-205 trial were combined for analysis, as no significant differences in outcomes were observed [5].
2.2. Statistical analysis
The primary analysis was to compare overall survival (OS) from cycle 10, day 1 of DP in the two trials, and secondary analyses were to investigate differences in OS for those with ≥30% PSA declines within 90 d of starting DP and for those removed for reasons other than progression or death. Additionally, PSA declines following cycle 10 were examined. Descriptive statistics were used to summarize patient characteristics and PSA changes. The Fisher exact test, χ2 test, and Wilcoxon rank-sum test were used to investigate for differences in characteristics. Patients were categorized into risk groups based on visceral disease, bone-scan progression, baseline anemia, and pain (good: no or one risk factor; intermediate: 2 risk factors; poor: three to four risk factors), or based on clinical subtypes (node only, bone-scan progression with or without nodal involvement, or visceral disease) according to PCWG-2 recommendations [4,7]. OS estimates were calculated from cycle 10 using the Kaplan-Meier method. Comparison of survival estimates was performed using the log-rank test and Cox proportional hazards models were used to estimate the prognostic ability of additional cycles of treatment. Logistic regression analysis was used with a stepwise selection process (entry criteria: p ≥ 0.10) to identify factors prognostic for receiving cycle 10 in a multivariable model. All tests were two-sided and p ≥ 0.05 was considered significant.
3. Results
3.1. Patients
In the TAX-327 study, 332 patients were treated on the DP regimen, of whom 166 (50.0%) received cycle 10 (Fig. 1,Table 1). Patients who received cycle 10 were more likely to have a baseline Karnofsky performance status ≥80% (92% vs 83%; p = 0.031), no significant pain (61% vs 47%; p = 0.015), no visceral involvement (84% vs 70%; p = 0.004), no prior progression due to measurable lesions (77% vs 67%; p = 0.067), and lower median alkaline phosphatase (183 vs 246 U/l; p = 0.059). Of the 221 patients in the CS-205 trial, one never received DP and 99 patients (45.0% of 220 who received DP) received cycle 10. Patients who received cycle 10 were on average younger (mean age: 68.1 vs 70.2 yr; p = 0.079) and less likely to have prior progression due to measurable tumor (17% vs 29%; p = 0.055). The probability of cycle 10 treatment was not significantly different (p = 0.26) between the trials. Combining the trials, a multivariate logistic regression model was constructed of factors prognostic for receipt of cycle 10. Higher age (odds ratio [OR]: 0.98 per year; 95% confidence interval [CI], 0.96–1.00; p = 0.035), alkaline phosphatase (OR: 0.97 per 100; 95% CI, 0.94–1.00; p = 0.065), visceral disease (OR: 0.64; 95% CI, 0.42–0.96; p = 0.029), and anemia (OR: 0.72; 95% CI, 0.51–1.03; p = 0.070) decreased the probability of receiving cycle 10 (c-statistic = 0.61).
Fig. 1.
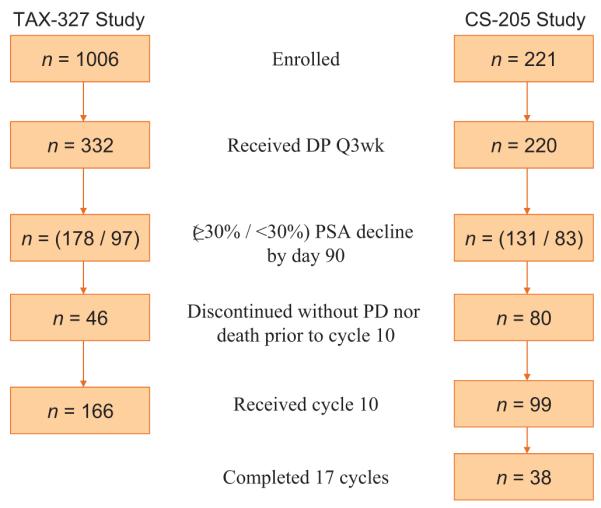
Study schema.
DP = docetaxel plus prednisone; Q3 wk = every 3 wk; PSA = prostate-specific antigen; PD = progressive disease.
Table 1.
Baseline characteristics of patients eligible for analysis1
| TAX-327 study (n = 166) | CS-205 study (n = 99) | p value | |
|---|---|---|---|
| Patients receiving cycle 10, no. (%)2 | 166 (50.0) | 99 (45.0%) | 0.26 |
| Baseline characteristics | |||
| Mean age, yr (± SD) | 67.1 (8.3) | 68.1 (7.6) | 0.39 |
| Gleason score, no. (%) | 0.121 | ||
| ≤7 | 71 (42.8) | 35 (35.4) | |
| 8–10 | 53 (31.9) | 44 (44.4) | |
| NA | 42 (25.3) | 20 (20.2) | |
| Good performance status3, no. (%) | 152 (91.6) | 95 (96.0) | 0.21 |
| Stage, no. (%) | 0.14 | ||
| 1–2 | 24 (15.7) | 23 (23.7) | |
| 3–4 | 129 (84.3) | 74 (76.3) | |
| NA | 13 | 2 | |
| Prior radiotherapy, no. (%) | 89 (53.6) | 52 (52.5) | 0.90 |
| Extent of disease, no. (%) | |||
| Bone metastases | 148 (89.2) | 88 (88.9) | 1.00 |
| Visceral disease | 26 (15.7) | 25 (25.3) | 0.076 |
| Measurable lesions | 61 (36.7) | 17 (17.2) | <0.001 |
| Evidence of progression, no. (%) | |||
| Bone scan | 116 (69.9) | 48 (48.5) | <0.001 |
| Increased PSA | 126 (75.9) | 84 (84.8) | 0.088 |
| Median PSA, ng/ml (range) | 98.1 (0.7–40740) | 71.4 (1.2–13056) | 0.39 |
| PSA <20 ng/ml, no. (%) | 24 (14.5%) | 16 (16.2%) | |
| Patients with significant pain, no. (%) | 65 (39.2) | 34/96 (35.4) | 0.60 |
| Median alkaline phosphatase level, U/ml (range) | 183 (40–4517) | 119 (44–1553) | <0.001 |
| Patients with anemia, no. (%) | 86 (51.8) | 52/98 (53.1) | 0.90 |
| Risk factors, no. (%) | 0.75 | ||
| 0–1 (good risk) | 69 (41.6) | 42 (44.2) | |
| 2 (intermediate risk) | 59 (35.5) | 35 (36.8) | |
| 3–4 (poor risk) | 38 (22.9) | 18 (18.9) | |
| NA | 0 | 4 | |
| PCWG-2 subtypes, no. (%) | 0.16 | ||
| Visceral disease | 26 (15.9) | 25 (25.3) | |
| Bone metastasis with/without nodal involvement | 127 (77.4) | 67 (67.7) | |
| Nodal disease only | 11 (6.7) | 7 (7.1) | |
| NA | 2 | 0 | |
| Cycle 10 | |||
| Median PSA at cycle 10 (range) | 13.6 (0.07–1658) | 26.1 (0.0–9180) | 0.006 |
| PSA <20 ng/ml, no. (%) | 96 (57.8) | 46 (46.5) | |
| Median PSA change from baseline, % | −84.5 | −62.3 | <0.001 |
| Completed cycle 10, no. (%)4 | 154 (92.8) | 87 (87.9) | 0.19 |
DQ3W = Every 3-wk docetaxel plus prednisone therapy; SD = standard deviation; PSA = prostate-specific antigen; NA = not available; PCWG-2 = Prostate Cancer Clinical Trials Working Group 2.
All p values and percentages are calculated excluding patients with missing data except for Gleason score p value, which is calculated including all patient data.
Percentage of patients receiving any DQ3W study treatment.
Defined as Karnofsky 80–100% (TAX-327 study) or ECOG 0-1 (CS-205 study).
Defined as receiving cycle 11 treatment (CS-205 study) or completed treatment per study protocol (TAX-327 study).
Characteristics of the 166 TAX-327 and 99 CS-205 patients who received cycle 10 demonstrated that pain, performance status, risk groups, and PCWG-2 clinical subtypes were similar (Table 1). While PSA was similar at baseline, men in the TAX-327 study had lower median PSA (13.6 ng/ml vs 26.1 ng/ml, p = 0.006) at cycle 10. Twelve (12.1%) CS-205 patients received cycle 10 but discontinued study therapy prior to cycle 11. Patients on CS-205 who received cycle 10 received a median of five additional cycles. The reason for discontinuation was completion of study medication for all but 12 (7.2%) of the TAX-327 men who received cycle 10 (10 men had disease progression and 2 had protocol violation). Up to and including cycle 10, 29.8% of TAX-327 and 34.1% of CS-205 patients had a dose modification (p = 0.30).
3.2. Comparison of survival in those completing 10 cycles
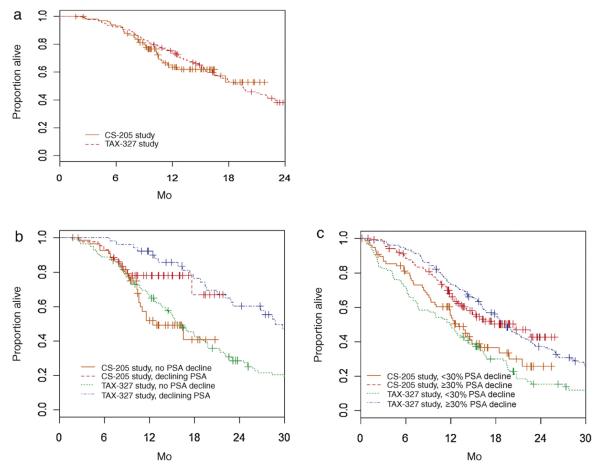
Due to longer follow-up period, survival information was more complete in TAX-327 (median follow-up of 23.3 mo vs 13.2 mo in men censored at analysis). Six-month and 12-mo estimated OS from cycle 10 was similar between the two cohorts (p = 0.72; Fig. 2a): 92.2% (95% CI, 86.9–95.4%) and 74.6% (95% CI, 67.2–80.5%) for TAX-327 patients compared with 92.8% (95% CI, 85.5–96.5) and 63.4% (95% CI, 51.8–72.9%) for those in CS-205. Results were also similar in subgroups including patients who had a declining PSA at cycle 10 compared to the previous PSA (Fig. 2b), or two consecutively measured declining PSAs at cycle 10 (data not shown), grouped by baseline risk groups or by PCWG-2 clinical subtypes (Table 2). Of the 99 CS-205 patients receiving cycle 10, 38 (38.4%) completed cycle 17, which represents 17.3% of all patients. At the time of analysis, six of these 38 patients died with 6-mo and 1-yr OS estimates of 84.7% (95% CI, 66.8%–93.4%) and 74.1% (95% CI, 44.2%–89.6%), respectively. These 38 patients’ baseline characteristics were not substantially different from the 61 who did not complete cycle 17.
Fig. 2.
Overall survival (a) from day 1 of cycle 10 and beyond day 90 for those with and without ≥30% prostate-specific antigen (PSA) decline by day 90, (b) for those with and without PSA decline from cycle 9 to cycle 10, and (c) for those with and without ≥30% PSA decline by day 90.
Table 2.
Overall survival from cycle 10 day 1 by study
| TAX-327 study (n = 166) | CS-205 study (n = 99) | p value | |
|---|---|---|---|
| Known death date, no. (%) | 132 (79.5) | 34 (34.3) | <0.001 |
| Poor risk (3–4 factors), no. | 38 | 18 | 0.44 |
| 6-mo, % (95% CI) | 84.2 (68.2–92.6) | 76.5 (48.8–90.5) | |
| 12-mo, % (95% CI) | 60.1 (42.7–73.7) | 39.3 (14.5–63.7) | |
| Intermediate risk (2 factors), no. | 59 | 35 | 0.80 |
| 6-mo, % (95% CI) | 89.8 (78.8–95.3) | 94.3 (79.0–98.5) | |
| 12-mo, % (95% CI) | 67.6 (54.0–77.9) | 53.4 (34.8–68.9) | |
| Good risk (0–1 factors), no. | 69 | 42 | 0.71 |
| 6-mo, % (95% CI) | 98.6 (90.2–99.8) | 97.6 (83.9–99.7) | |
| 12-mo, % (95% CI) | 88.4 (78.2–94.0) | 78.5 (59.4–89.4) | |
| PCWG-2 visceral disease subtype, no. | 26 | 25 | 0.26 |
| 6-mo, % (95% CI) | 100.0 (–) | 88.0 (67.3–96.0) | |
| 12-mo, % (95% CI) | 80.2 (58.8–91.3) | 54.2 (30.6–72.9) | |
| PCWG-2 bone metastases with/without nodal disease subtype, no. | 127 | 67 | 0.61 |
| 6-mo, % (95% CI) | 90.6 (84.0–94.5) | 93.9 (84.7–97.7) | |
| 12-mo, % (95% CI) | 73.2 (64.5–80.0) | 68.9 (54.9–79.3) | |
| PCWG-2 node-only disease subtype, no. | 11 | 7 | 0.058 |
| 6-mo, % (95% CI) | 100.0 (–) | 100.0 (–) | |
| 12-mo, % (95% CI) | 90.9 (50.8–98.7) | 31.3 (1.3–73.4) |
CI = confidence interval; PCWG = Prostate Cancer Clinical Trials Working Group.
3.3. Survival in those discontinuing early for reasons other than progression or death
One hundred and sixty-six patients came off study before cycle 10 in the TAX-327 study, four due to death, 116 due to progressive disease (PD), and 46 due to other reasons. Of the 46 non-PD or death patients, 19, 13, and 14 came off-study during cycles 1–3, 4–6, and 7–9, respectively, with better survival (hazard ratio [HR]: 0.81; 95% CI, 0.72–0.92 per cycle) observed from the date of last chemotherapy (1-yr OS: 21.1% vs 61.5% vs 78.6%, respectively) for those on treatment longer. Of 121 patients in the CS-205 study who came off-study before cycle 10, 41 were due to PD and 80 were due to other reasons. Survival time from last date of chemotherapy appeared improved (HR: 0.91; 95% CI, 0.79–1.03, per cycle) for patients remaining on-study longer (1-yr OS: 32.9% vs 46.9% vs 49.6%, respectively, among 24, 27, and 29 men off-study due to non-PD during cycles 1–3, 4–6, and 7–9, respectively). A landmark analysis, using landmarks of 90, 120, 150, and 180 d, comparing patients on or off therapy at these times, also displayed better OS for those on therapy (Table 3).
Table 3.
Survival time as landmark analyses for those on or off trial at these time points for reasons other than progression or death
| CS-205 study |
TAX-327 study |
|||||||
|---|---|---|---|---|---|---|---|---|
| Off study |
On study |
Off study |
On study |
|||||
| Landmark date, d | n | 1-yr OS (95% CI)* | n | 1-yr OS (95% CI) | n | 1-yr OS (95% CI) | n | 1-yr OS (95% CI) |
| 90 | 18 | 58.8 (32.5–77.8) | 175 | 64.4 (56.6–71.2) | 17 | 47.1 (23.0–68.0) | 244 | 73.7 (67.7–78.8) |
| 120 | 27 | 35.6 (18.0–53.8) | 159 | 65.0 (56.6–72.1) | 20 | 50.0 (27.1–69.2) | 222 | 72.9 (66.5–78.3) |
| 150 | 35 | 44.3 (27.2–60.1) | 137 | 66.0 (57.0–73.6) | 20 | 50.0 (27.1–69.2) | 205 | 73.6 (67.0–79.1) |
| 180 | 49 | 42.2 (27.1–56.5) | 118 | 62.0 (51.8–70.7) | 26 | 57.2 (36.1–73.6) | 182 | 74.1 (67.1–79.9) |
OS = overall survival; CI = confidence interval.
1-yr OS and CI are from the landmark date. To be included, the patient must be alive as of the landmark date and either off study due to reasons other than progression or death or still on study.
3.4. Survival in those with ≥30% prostate-specific antigen decline by day 90
Given that PSA declines ≥30% by day 90 is a moderate surrogate for OS and may reflect more favorable tumor biology, we hypothesized that a differential in OS by a longer course of DP may be identifiable in such patients. Of men with PSA declines ≥30% by day 90 after starting DP, 118 of 178 (66.3%) men in TAX-327 and 62 of 131 (47.3%) in CS-205 eventually received cycle 10. Mean 1-yr OS from day 90 was 73.5% (95% CI, 66.4–79.4%) among the TAX-327 men with PSA declines ≥30% and 67.0% (95% CI, 57.7–74.7%) among CS-205 men with PSA declines ≥30% (Fig. 2c). Conversely, 24 of 97 (24.7%) TAX-327 men and 37 of 83 (44.6%) CS-205 men received cycle 10 despite having PSA reductions <30% by day 90. Mean 1-yr OS from day 90 was 49.0% (95% CI, 38.7–58.6%) among the TAX-327 men with PSA declines <30% and 55.4% (95% CI, 43.0–66.1%) among CS-205 men with PSA declines <30% (Fig. 2c).
3.5. Timing of prostate-specific antigen declines
When examined from the beginning of both trials, patients were most likely to have declining PSA before cycle 5 (Fig. 3). On the first day of cycles 10, 13, and 17, respectively 44.9%, 50.0%, and 60.5% of CS-205 evaluable patients had declining PSA. Thus, >10% of all patients originally treated with DP had declining PSA at cycle 17 coupled with no objective PCWG-2-defined progression. One-year survival beyond cycle 10 was 78.0% (95% CI, 61.8%–87.9%) among 44 men with a PSA decline from cycle 9 to cycle 10 compared with 52.0% (95% CI, 36.2%–65.6%) among 55 men without PSA declines (HR: 0.49; 95% CI, 0.23–1.02; p = 0.056) (Fig. 2b). No significant difference in OS was observed with PSA declines in cycles 13 and 17, but this analysis was limited by few events (data not shown). One-year survival beyond cycle 10 was 92.2% (95% CI, 80.4%–97.0%) among 51 men with declining PSA at cycle 10 in TAX-327 compared with 66.8% (95% CI, 57.4%–74.6%) among 115 men without declining PSA (HR: 0.41; 95% CI, 0.28–0.62; p < 0.001 (Fig. 2b).
Fig. 3.
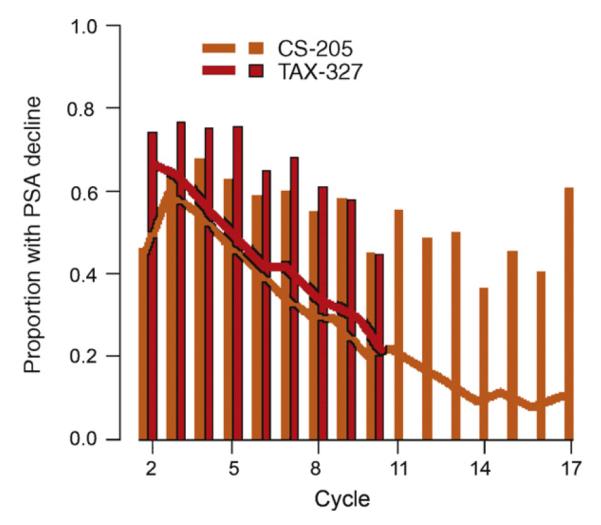
Proportion of patients with declining prostate-specific antigen (PSA) by cycle of treatment. Bars represent proportion of patients among those remaining on study treatment, and lines represent the proportion of patients (the denominator being the overall number of patients who received any docetaxel-prednisone therapy on the trial) who received further docetaxel and prednisone study treatment beyond a given cycle.
4. Discussion
This retrospective exploratory analysis comparing outcomes in men with mCRPC receiving 10 protocol-stipulated cycles of DP chemotherapy in the TAX-327 trial and those receiving ≥10 of the planned 17 cycles of DP in the CS-205 trial does not suggest an extension of OS with >10 cycles of therapy. The median progression-free survival on DP of 7.5 mo in a modern dataset also suggests that additional benefits from >10 cycles might be limited [8]. The limitations of an unplanned retrospective analysis are applicable, although all patients were treated on prospective trials with similar DP-based regimens. Our hypothesis that a PSA decline ≥30% in 90 d may be associated with a differential benefit with >10 cycles was not demonstrated[9,10]. Intriguingly, in the subset of men removed from both trials before cycle 10 for reasons other than progression or death, there was a trend toward longer survival with more cycles. The caveat is that this heterogeneous group of patients was removed from therapy for a variety of reasons, including toxicities, nonprotocol-defined progression, and personal decisions. Despite the caveats, these data suggest that stopping therapy earlier than 10 cycles might have a negative impact in those without PD.
The PSA declines beyond 10 cycles in CS-205 coupled with a survival benefit in those with PSA declines at cycle 10 are intriguing. Although this raises the possibility of continued benefit up to 17 cycles of DP in a subset of patients, the prognostic value of PSA declines beyond 90 d of initiating therapy is uncertain and there were no clear differences in OS between the trials for those with PSA declines at cycle 10. Conversely, the finding that PSA declines at cycle 10 in both trials demonstrated better survival might indicate that PSA decline at cycle 10 is a favorable prognostic factor and not necessarily predictive of benefit from additional cycles. The lower median PSA at cycle 10 in the TAX-327 group suggests a greater depth of response, which might potentially confound the identification of a benefit from additional cycles in the CS-205 trial. However, the clinical significance of this finding is unclear, since PSA declines are a suboptimal surrogate for antitumor activity. PSA response rates in the CS-205 trial were not statistically different in the arm receiving AT-101 in combination with DP [5].
Generally, systemic cytotoxic chemotherapy for most major advanced malignancies is administered up to six cycles. The concept of intermittent treatment was assessed in the Androgen-Independent Prostate Cancer Study of Calcitriol Enhancing Taxotere (ASCENT)-1 trial, where patients could suspend docetaxel if PSA declined ≥50% and attained a level ≥ 4 ng/ml, which occurred at a median of 22 wk [11]. On resumption of treatment for progression, 45.5% of patients exhibited a ≥50% PSA decline. However, the impact of intermittent therapy on survival is unclear in the absence of a prospective trial. It is unclear if a proportion of men in either trial was retreated with DP after discontinuing therapy or continued docetaxel after completion of the protocol. Indeed, cabazitaxel extended survival in a docetaxel-resistant population [12]. However, retreatment with docetaxel has not been proven to extend survival, and cabazitaxel, sipuleucel-T, and abiraterone acetate were not available at the time TAX-327 and CS-205 were conducted [13,14].
A prospective randomized trial comparing the continuation of DP (until progression or toxicity) versus stopping DP in those with stable or responding disease after 10 cycles may provide a definitive answer regarding the optimal number of cycles. Conversely, the strategy of switching to a different second-line agent as consolidation therapy in patients with stable or responding disease after a predefined number of cycles of first-line therapy has demonstrated benefits in non-small cell lung cancer [15–17]. Given scarce resources and the likely marginal or no benefit of continuing DP beyond 10 cycles, a similar strategy of 10 cycles of DP followed by second-line consolidation employing a biologic agent with nonoverlapping toxicities might yield greater benefits.
5. Conclusions
Our retrospective hypothesis-generating analysis suggests that >10 cycles of DP for mCRPC does not enhance survival.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Guru Sonpavde had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pond, Armstrong, Sonpavde.
Acquisition of data: Pond, Armstrong, Wood, Brookes, Leopold, Berry, de Wit, Eisenberger, Tannock, Sonpavde.
Analysis and interpretation of data: Pond, Armstrong, Sonpavde.
Drafting of the manuscript: Pond, Armstrong, Wood, Brookes, Leopold, Berry, de Wit, Eisenberger, Tannock, Sonpavde.
Critical revision of the manuscript for important intellectual content: Pond, Armstrong, Wood, Brookes, Leopold, Berry, de Wit, Eisenberger, Tannock, Sonpavde.
Statistical analysis: Pond, Armstrong.
Obtaining funding: None.
Administrative, technical, or material support: Pond, Armstrong, Sonpavde.
Supervision: Pond, Armstrong, Sonpavde.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: A.J. Armstrong has received research support and speaking honoraria from Sanofi-Aventis; B.A. Wood and L. Leopold are employees of and own stock in Ascenta Therapeutics; M. Brookes is an employee of Ascenta Therapeutics; W.R. Berry has received research support from Ascenta Therapeutics and research support and speaking honoraria from Sanofi-Aventis; R. de Wit and M.A. Eisenberger have received advisory board honoraria from Sanofi-Aventis; I.F. Tannock has received research support from Sanofi-Aventis; and G. Sonpavde has received research support from Ascenta Therapeutics and Sanofi-Aventis, and speaking honoraria from Sanofi-Aventis.
References
- [1].Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- [2].Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- [3].Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- [4].Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sonpavde G, Matveev V, Burke J, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. J Clin Oncol. 2011;29(Suppl 7) doi: 10.1093/annonc/mdr555. abstract 125. [DOI] [PubMed] [Google Scholar]
- [6].World Health Organization . WHO handbook for reporting results of cancer treatment. World Health Organization; Geneva, Switzerland: 1979. WHO criteria. (Offset publication no. 48.) [Google Scholar]
- [7].Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010;46:517–25. doi: 10.1016/j.ejca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [8].Kelly WK, Halabi S, Carducci MA, et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): Survival results of CALGB 90401. J Clin Oncol. 2010;28(Suppl) doi: 10.1200/JCO.2011.39.4767. abstract LBA4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Armstrong AJ, Garrett-Mayer E, Ou Yang YC, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965–70. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- [10].Petrylak DP, Ankerst DP, Jiang CS, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99–16. J Natl Cancer Inst. 2006;98:516–21. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- [11].Beer TM, Ryan CW, Venner PM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT investigators. J Clin Oncol. 2007;25:669–74. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- [12].De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- [13].Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- [14].de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- [16].Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- [17].Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]