Abstract
Pitch experiments aimed at evaluating temporal pitch mechanism(s) often utilize complex sounds with only unresolved harmonic components, and a low-pass noise masker to eliminate the potential contribution of audible distortion products to the pitch percept. Herein we examine how: (i) masker induced reduction of neural distortion products (difference tone: DT; and cubic difference tone: CDT) alters the representation of pitch relevant information in the brainstem; and (ii) the pitch salience is altered when distortion products are reduced and/or eliminated. Scalp recorded brainstem frequency following responses (FFR) were recorded in normal hearing individuals using a complex tone with only unresolved harmonics presented in quiet, and in the presence of a low-pass masker at SNRs of +15, +5, and −5 dB. Difference limen for F0 discrimination (F0 DL) was obtained in quiet and in the presence of low-pass noise. Magnitude of DT components (with the exception of components at F0 and 2F0), and the CDT components decreased with increasing masker level. Neural pitch strength decreased with increasing masker level for both the envelope-related (FFRENV) and spectral-related (FFRSPEC) phase-locked activity. Finally, F0 DLs increased with decreasing SNRs suggesting poorer F0 discrimination with reduction of the distortion products. Collectively, these findings support the notion that both DT and CDT, as reflected in the FFRENV and FFRSPEC, respectively, influence both the brainstem representation of pitch relevant information and the pitch salience of the complex sounds.
1. Introduction
Pitch experiments aimed at evaluating temporal pitch mechanisms often employ complex tone stimuli containing only higher peripherally unresolved harmonics along with a low-pass masker (Shackleton & Carlyon, 1994; Plomp, 1976; Kaernback & Bering, 2001; Pressnitzer et al., 2001; Moore & Gockel, 2011). This low-pass masker serves to eliminate the potential contribution of audible distortion products to the pitch salience (Plomp, 1967; Kaernback & Bering, 2001; Pressnitzer et al., 2001).
Distortion products at f2-f1 (the quadratic difference tone: DT), and the more extensively studied cubic difference tone at 2f1-f2 (CDT) have been characterized in: (i) pitch perception (Goldstein, 1967; Humes, 1980a; Smoorenburg, 1971; Wegel & Lane, 1924; Wilson, 1980; Zwicker, 1979, 1980); (ii) the discharges of the auditory nerve and anteroventral cochlear nucleus (Buunen & Rhode, 1978; Buunen et al., 1977; Goldstein & Kiang, 1968; Kim et al., 1980; Smoorenburg et al., 1976); (iii) the ear canal as an otoacoustic emission (Brown, 1987; Clark et al., 1984; Kemp & Brown, 1984; Kemp, 1979; Schmiedt, 1986; Lonsbury-Martin, Martin, Probst, & Coats, 1987; Martin, Lonsbury-Martin, Probst, Scheinin, & Coats, 1987; Gaskill & Brown, 1990; Martin, et al., 1990); and (iv) the scalp recorded brainstem FFRs (Chertoff & Hecox, 1990; 1992; Rickman et al., 1991; Krishnan, 1999; Pandya & Krishnan, 2004; Bhagat & Champlin, 2004; Elsisy & Krishnan, 2008). Both f2-f1 and 2f1-f2 distortion products are presumed to be generated by the active cochlear process associated with the nonlinearity of the outer hair cell motion (Rhode & Cooper, 1993; Robles, Ruggero, & Rich, 1997).
In addition to providing information on the functional integrity of the cochlear active process, these distortion products (f2-f1 and 2f-f2) are believed to influence perception of some pitch producing sounds (Smoorenburg 1970; Plomp 1965; Pressnitzer et al., 2001). The objective of the present study was to examine how, for complex tones containing only unresolved harmonics, neural versions of these distortion products, as reflected in the scalp-recorded human FFR, are altered by masking noise and how they influence the neural representation of pitch-relevant information. The FFR reflects sustained phase-locked neural activity in a population of neural elements within the rostral brainstem (Worden & Marsh, 1968; Marsh et al., 1974; Smith et al., 1975; Glaser et al., 1976), presumably the inferior colliculus (IC). FFRs have been shown to preserve information about formants of speech sounds (Krishnan, 1999, 2002; Krishnan & Parkinson, 2000; Aiken & Picton, 2008); pitch relevant information of both steady-state (Greenberg et al., 1987) and dynamic complex sounds including speech (Krishnan et al., 2004, 2005) and nonspeech (iterated rippled noise) stimuli (Swaminathan et al., 2008a, b; Krishnan & Gandour, 2009; Krishnan et al., 2009a, b); and neural correlates of distortion products at f2-f1 and 2f1-f2 (Chertoff et al., 1990, 1992; Rickman et al., 1991; Krishnan, 1999; Pandya & Krishnan, 2004; Bhagat & Champlain, 2004; Elsisy & Krishnan, 2008). It should be noted here that FFR does not preserve pitch-relevant information for all pitch eliciting stimuli (Gockel et al., 2011). Using a behavioral measure, the F0DL, we assess whether changes in the brainstem neural representation of pitch-relevant information, due to masking of the distortion products, are accompanied by changes in pitch salience.
2. Methods
2.1 Participants
Nine (3 male, 6 female) adult listeners (aged 18–29 years) participated in the FFR experiment, and six (3 male, 3 female) adult listeners (aged 21–26 years) participated in the behavioral experiment (three of whom also participated in the FFR experiment). Participants were native speakers of American English and none had more than 3 years of musical training (which occurred at least 4 years prior to the experiment). All participants had hearing thresholds of 15 dB HL or less at octave frequencies between 250 and 8000 Hz. All participants were Purdue University students who were paid for their participation. Each participant gave informed consent in compliance with a protocol approved by the Institutional Review Board of Purdue University.
2.2 Stimuli
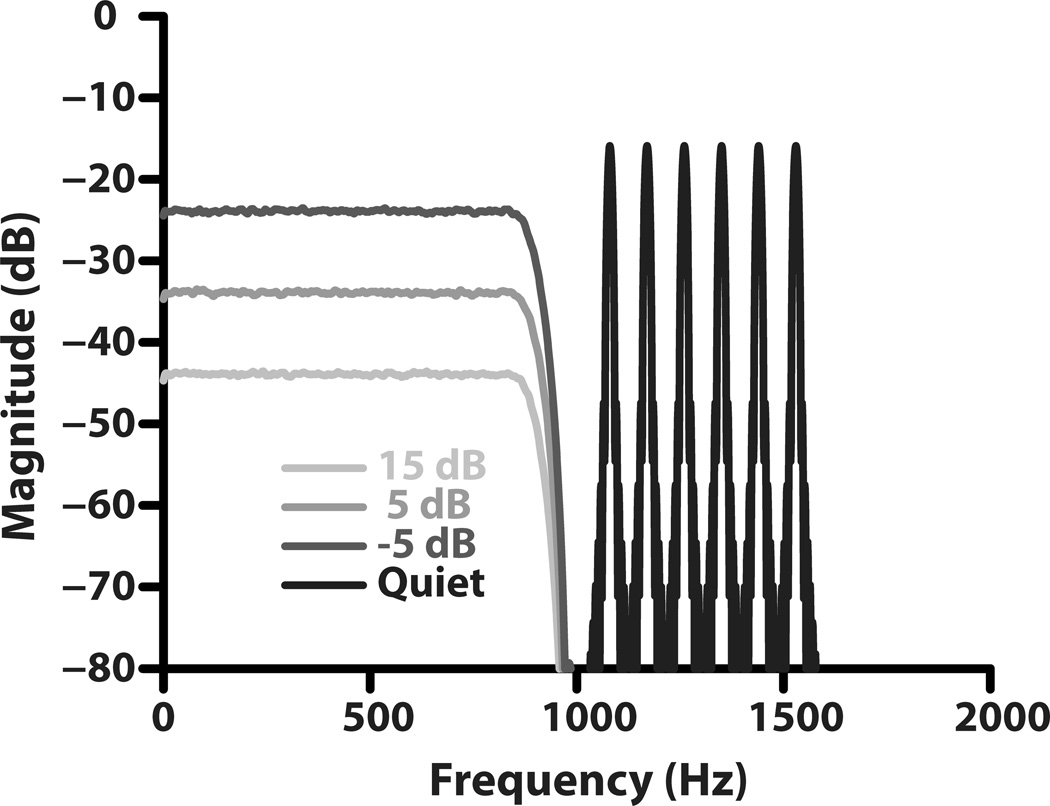
For both the FFR and behavioral experiments, the complex tone burst stimulus consisted of harmonics 12–17 of a 90 Hz fundamental, added in sine phase (Figure 1). A low-pass Gaussian noise (500 order FIR; Fpass=885 Hz, Fstop=965 Hz, Astop=60 dB) was gated with the complex tone burst to mask the distortion product components at SNRs of +15, +5, and −5 dB. The overall level of the tone complex was 75 dB SPL. The noise was frozen (same sample) and was the same within an experimental condition and across subjects, but different across all conditions. All stimuli had an overall duration of 150 ms including the 10 ms onset and offset ramps and were generated using a sampling rate of 20 kHz. Spectra of the averaged (1000 presentations) digital signal shown in Fig. 1 illustrate the experimental paradigm used for the study. For all stimuli sound-pressure level was measured using a 2 cc coupler (G.R.A.S, type RA0038) with a 1/2-inch microphone (Bruel and Kjaer type 4189) connected to a sound level meter (Bruel & Kjaer type 2230, linear weighting).
Figure 1.
Spectra of the tonal complex and low-pass noise masker to illustrate the experimental paradigm. Tonal complex contains six equal amplitude harmonics (12th–17th harmonic of a 90-Hz F0) falling in the peripherally unresolved region. Low-pass noise (500 order FIR; Fpass=885 Hz, Fstop=965 Hz, Astop=60 dB) spectra show the three levels used in the study. The spectrum of the low-pass noise shown represents an average of 1000 presentations of the noise stimuli.
2.3 FFR recording protocol
Participants reclined comfortably in an acoustically and electrically shielded booth. They were instructed to relax and refrain from extraneous body movement to minimize myogenic artifacts. Participants were allowed to sleep through the duration of the FFR experiment. FFRs were recorded from each participant in response to monaural presentation of the complex tone and low-pass noise stimuli to the right ear through a magnetically shielded insert earphone (Etymotic, ER-3A). Control recordings in a subset of participants, using the same procedures but with the eartip and the tubing of the insert earphones moved away from the ear, showed no response components above the noise floor. Stimuli were presented at a repetition rate of 3.93/sec. Responses were recorded to the complex tone in quiet, and in the presence of the three noise conditions. The presentation order of the experimental conditions was randomized both within and across participants. Alternating onset stimulus polarity was utilized with averaged responses to each onset polarity stored in two separate buffers. This enabled derivation of the phase-locked activity to the stimulus envelope only (FFRENV) by adding the responses to each polarity, and to the spectral components only (FFRSPEC) by subtracting the response to each polarity from each other (Greenberg et al., 1987; Krishnan, 1999; 2002; Aiken & Picton, 2008). Control of the experimental protocol was accomplished by a signal generation and data acquisition system (Intelligent Hearing Systems, SmartEP, Advanced Research Module).
FFRs were recorded differentially between a non-inverting (+) electrode placed on the midline of the forehead at the hairline (Fz) and an inverting (−) electrode placed on (i) the ipsilateral mastoid (A2); (ii) the contralateral mastoid (A1); and (iii) the 7th cervical vertebra (C7). Another electrode placed on the mid-forehead (Fpz) served as the common ground. All inter-electrode impedances were maintained at or below 1 kΩ. The raw EEG inputs were amplified by 200,000 and band-pass filtered from 30 to 3000 Hz (6 dB/octave roll-off, RC response characteristics). In addition, each averaged FFR was digitally band-pass filtered from 60 to 2500 Hz (500 order FIR; Fstop1=25 Hz, Fpass1=65 Hz, Fpass2=2490 Hz, Fstop2=2535 Hz, Astop=60dB) post acquisition. Sweeps containing activity exceeding +/− 30 µV were rejected as artifacts. Each FFR response waveform represents the average of 4000 artifact free trials (2000 sweeps for each stimulus polarity) over a 200 ms acquisition window using a sampling rate of 10 kHz.
2.4. FFR data analysis
FFR data from only the ipsilateral channel (high forehead-A2) is reported here since all response components were relatively more robust for this electrode configuration. Also, differences in response magnitude across recording configurations and/or absent distortion components in the contralateral recordings precluded combining data across recording configurations. Spectral analyses (Welch’s estimate of power spectral density using the default settings in Matlab) were performed on the FFRENV, and FFRSPEC data from individual subjects to estimate: (i) the magnitude of the DT distortion components at F0 (90 Hz) and at integral multiples 2F0 through 8F0 (720 Hz); (ii) the magnitude of the CDT distortion components at 810, 900 and 990 Hz; and (iii) the magnitude of response to the temporal fine structure in the stimulus waveform. FFRENV data were used to quantify the DT components while the FFRSPEC data were used to quantify both the CDT components and the phase-locked responses to the temporal fine structure. The magnitude of: (i) DT distortion component was expressed as the mean of the FFR response peaks at 270, 360, 450, 540, 630 and 720 Hz (response components at F0 and 2F0 were excluded because they may reflect neural phase-locking to stimulus envelope periodicity and not quadratic distortion products); (ii) CDT distortion component was expressed as the mean of FFR response peaks at 810, 900 and 990 Hz (it should be noted here that the presence of robust CDTs at only these three specific frequencies, and not at frequencies below 810 Hz or above 990 Hz is expected given the optimal f2/f1 ratio (1.13–1.25) for only the primaries producing these CDTs) and (iii) FFR response to the temporal fine structure in the stimulus waveform was expressed as the mean of the six spectral response peaks (harmonics 12–17).
To evaluate if reduction of the distortion products by the low-pass noise altered the pitch strength reflected in the FFRs, we quantified the neural periodicity strength for both the FFRENV and the FFRSPEC data by performing autocorrelation analyses (Cariani and Delgutte, 1996b; Krishnan et al., 2004; 2005; Krishnan and Plack, 2011). For each FFR, the normalized autocorrelation function (ACF) was computed in order to determine the dominant periodicities of the response. The first prominent peak in the ACF away from time-lag zero (that is the peak with a delay corresponding to the period of the 90-Hz F0) was taken as the magnitude of neural pitch strength (Krishnan et al., 2005; Krishnan & Plack, 2011). The magnitude of the ACF peak (the measure of neural pitch strength) has shown a high correspondence with the pitch salience of a stimulus in human behavioral experiments (Yost et al., 1996); in single-unit neurophysiologic studies of animals (Cariani and Delgutte, 1996a); and in human studies comparing behavioral and brainstem electrophysiological measures (Krishnan et al., 2010; Krishnan & Plack, 2011).
2.5. Statistical analysis of FFR data
In order to assess whether averaging over frequency within each of the FFRENV, FFRSPEC, and FFRSTIM (phase-locked response to the temporal fine structure of the stimulus waveform) spectra is justified, three separate three-way ANOVAs (with subjects as a random factor, and SNR and harmonic number as fixed factors) were performed on the individual spectral magnitudes at: (i) DTs (3F0 through 8F0); (ii) CDTs (810, 900, and 990 Hz); (iii) FFRSTIM components (1080 through 1530 Hz) The results revealed no significant interaction involving harmonic number for any of the three response types, suggesting that masking effects were similar across frequency. Subsequent to this verification, separate ANOVAs were performed on the following response components: F0, 2F0 DT; the mean of the 3F0-8F0 DT; and the mean of the FFRSPEC responses. For each FFR type, a two-way mixed model ANOVA with subjects as a random factor, and noise as a fixed factor was carried out to determine if the mean spectral magnitude of these components changed significantly with increase in the level of the low-pass masker. In addition, a separate two-way mixed model ANOVA was carried out to determine if changes in ACF magnitude (i.e. neural pitch strength) as a function of noise level were significant for FFRENV and FFRSPEC response waveforms.
2.6 Psychophysical estimate of F0 DL
To quantify changes in the salience of pitch with increase in level of the low-pass masker, we measured F0 DL (fundamental frequency difference limen). This measure has been used previously in pitch discrimination experiments to assess sensitivity to F0 differences, and as an indirect measure of pitch salience of complex sounds (Bernstein and Oxenham, 2003; Hall and Plack, 2009; Houtsma and Smurzynski, 1990; Shackleton and Carlyon, 1994). We reasoned that if distortion products influence pitch salience, then their systematic reduction by noise should alter the F0 DL estimate. That is, pitch discrimination ability should decrease as audible distortion products are reduced with increasing level of the low-pass masker. Testing consisted of four conditions (quiet plus the three masking conditions) using the same set of stimuli used for the FFR experiment. Six participants performed a two-interval forced-choice task (2IFC). For a given trial within a condition, they heard 2 sequentially presented sounds, one containing a reference stimulus, and one containing a comparison stimulus with a slightly higher pitch. Subjects were instructed to identify the interval in which the complex sound was perceived as higher in pitch. On each trial, the reference F0 was selected from a uniform random distribution ranging from 1 semitone below to 1 semitone above the original F0. This across trial frequency “roving” discourages listeners from comparing incoming tones against a fixed representation of the standard in long-term memory (Micheyl et al., 2010). Discrimination thresholds were measured using a two-down, one-up adaptive tracking procedure (Levitt, 1971). An initial starting F0 difference, fixed at +15%, was used to enable listeners to easily discriminate the two stimuli at all noise levels. Following two consecutive correct responses, the frequency difference between the reference and comparison stimulus was decreased for the subsequent trial, whereas it was increased following a single incorrect response. Frequency difference between reference and comparison intervals was varied using a geometric step size of 1.15 between response reversals. For each condition, 16 reversals were measured and the geometric mean of the last 12 was taken as the F0 DL. Two estimates were made per condition and the mean of the two runs used as the final F0 DL threshold. The behavioral experiment was conducted after the FFR experiment. A mixed-model ANOVA with subjects as a random factor, and SNR as a fixed factor was performed on the F0 DL data to evaluate the effects of noise level on F0 discrimination ability.
3. Results
3.1 FFR data
3.1.1. Effects of low-pass noise on the FFRENV components
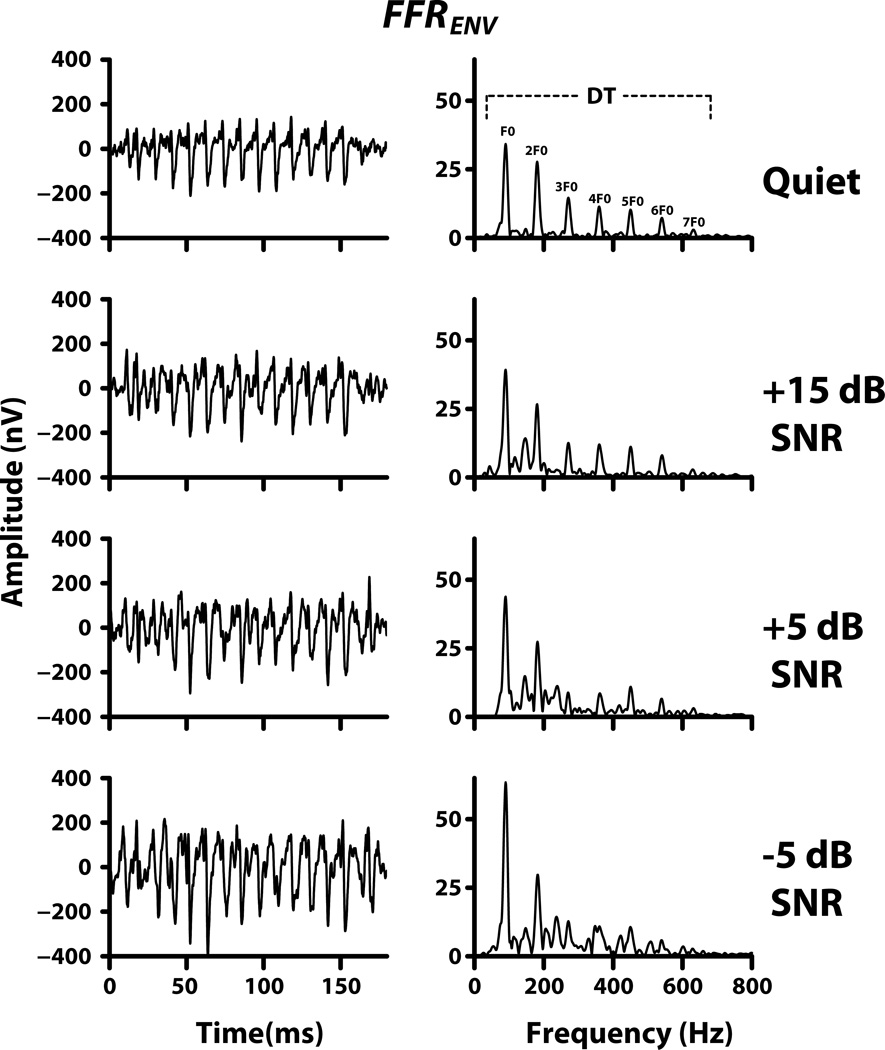
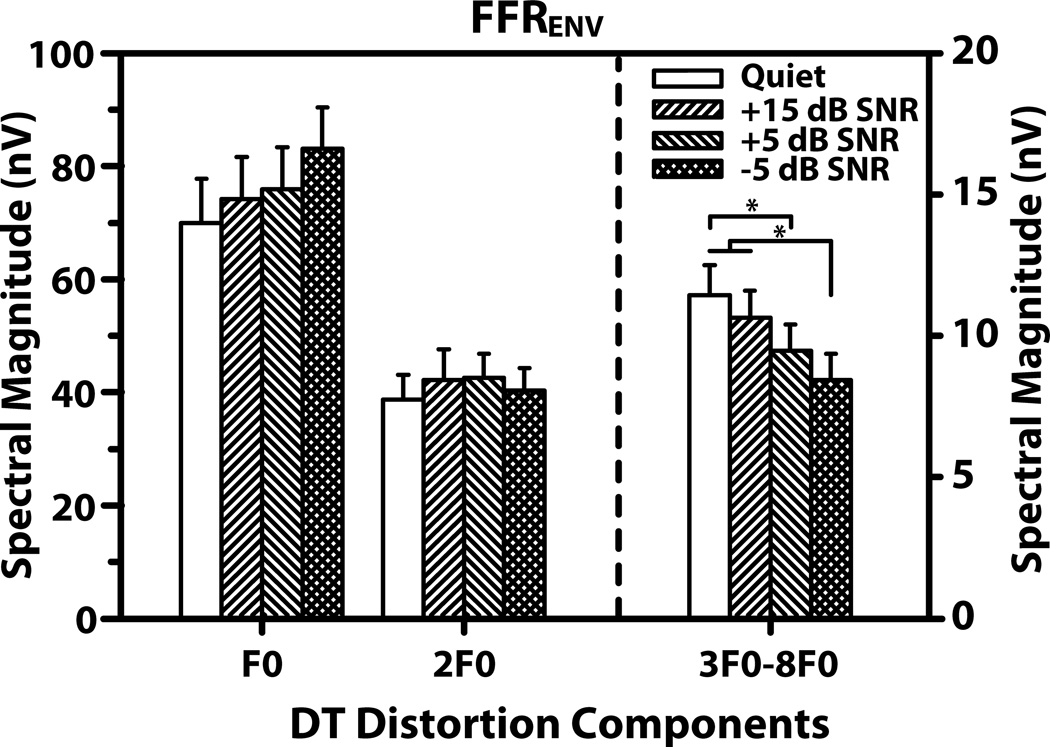
FFRENV waveforms and their spectra from a representative participant for the quiet and noise conditions are shown in Figure 2. The waveform data show robust phase locking to the fundamental periodicity of 90 Hz across all conditions with no discernible decrease in amplitude for the low-pass noise conditions. Consistent with this observation, the robust spectral peaks at F0 and 2F0 in the spectra show no reduction in magnitude across the experimental conditions. In contrast, the DT distortion components (3F0-8F0) show a decrease in magnitude as the low-pass noise is increased and are barely discernible at the highest noise level. The mean spectral magnitude of the DT distortion components at F0, 2F0, and the mean of the six DT distortion components (3F0-8F0) are plotted for the quiet and low-pass noise conditions in Figure 3. Only the 3F0-8F0-distortion component showed a reduction in magnitude with increase in noise level. No reduction in magnitude was observed for the DT distortion components at F0 and 2F0. Three separate two-way ANOVAs (with SNR as fixed factor and subjects as a random factor) were performed on the mean FFRENV DT components at F0, 2F0, and 3F0-8F0. No significant main effect of SNR was found for the components at F0 (F3,32 = 0.54, p = 0.660), and 2F0 (F3,32 = 0.15, p = 0.930). In contrast, the ANOVA for the mean of the 3F0-8F0 components gave a significant main effect (F3,24 = 10.13, p < 0.001) suggesting that the mean amplitude decreased with increasing noise level. Post hoc Tukey multiple comparisons (α=0.05) revealed that the mean magnitude of this DT for the −5 dB SNR condition was significantly smaller compared to that for the Quiet, and +15 SNR conditions (p <0.001). The comparison between the Quiet and +5 SNR condition was also statistically significant (p <0.010).
Figure 2.
FFRENV waveforms (left panels) and their spectra (right panels) from a representative participant are plotted for the quiet and three noise conditions. The multiple DT components are also identified. Note that these spectra were obtained from the electrical signals.
Figure 3.
Mean magnitude of F0, 2F0 (left axis), and 3F0-8F0 DT distortion components (right axis) for the quiet and three noise conditions. The asterisk indicates statistically significant differences in magnitude across experimental conditions. Note the difference in amplitude scale for each response type. Error bars correspond to 1 SEM.
3.1.2. Effects of low-pass noise on the FFRSPEC components
FFRSPEC waveforms and their spectra for the quiet and noise conditions from a single participant are shown in Figure 4. The FFRSPEC waveforms, unlike their FFRENV counterparts, do not show a simple robust periodic component, but instead a relatively smaller amplitude, complex waveform that appears to change little across the experimental conditions. While no measurable peak was present at F0=90 Hz, the spectral data clearly show robust peaks at frequencies of the unresolved harmonic components of the stimulus (FFRSTIM region identified by the vertical dashed lines). In addition, three spectral peaks (810, 900, and 990 Hz) at CDT distortion component frequencies are also clearly discernible. Note that the FFRSTIM components remain relatively unchanged with increasing noise level. In contrast, the CDT components decrease with increasing noise level and are barely discernible above the noise floor at −5 dB SNR.
Figure 4.
FFRSPEC waveforms (left panels) and their spectra (right panels) from a representative participant are plotted for the quiet and three noise conditions. Vertical dotted lines in the spectral plots demarcate the frequency regions containing the CDT distortion components and the FFR response to stimulus temporal fine structure (FFRSTIM). The low-pass noise region is also identified.
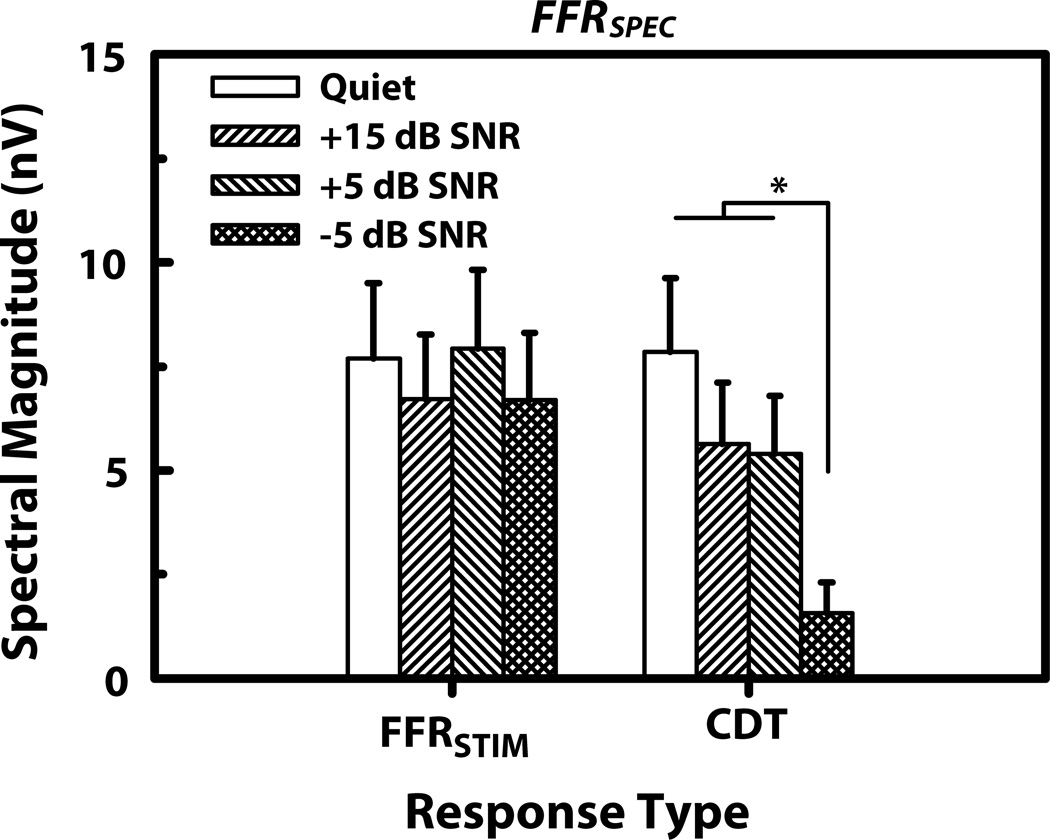
Mean spectral magnitude for these components shown in Figure 5 confirms this observation. A two-way ANOVA yielded a significant main effect of SNR on the mean magnitude of the CDT distortion components (F3,24 = 10.13, p < 0.001) but not for the mean of the six FFRSTIM components (F3,24 = 2.13, p = 0.120). Posthoc Tukey multiple comparisons (α=0.05) revealed that the magnitude of the mean CDT distortion components for the −5 dB SNR condition was significantly smaller compared to all other conditions (p <0.01).
Figure 5.
Mean magnitude of FFRSPEC response components for the stimulus temporal fine structure (FFRSTIM), and CDT distortion product components for the quiet and noise conditions. The asterisk indicates statistically significant differences in magnitude across experimental conditions. Error bars correspond to 1 SEM.
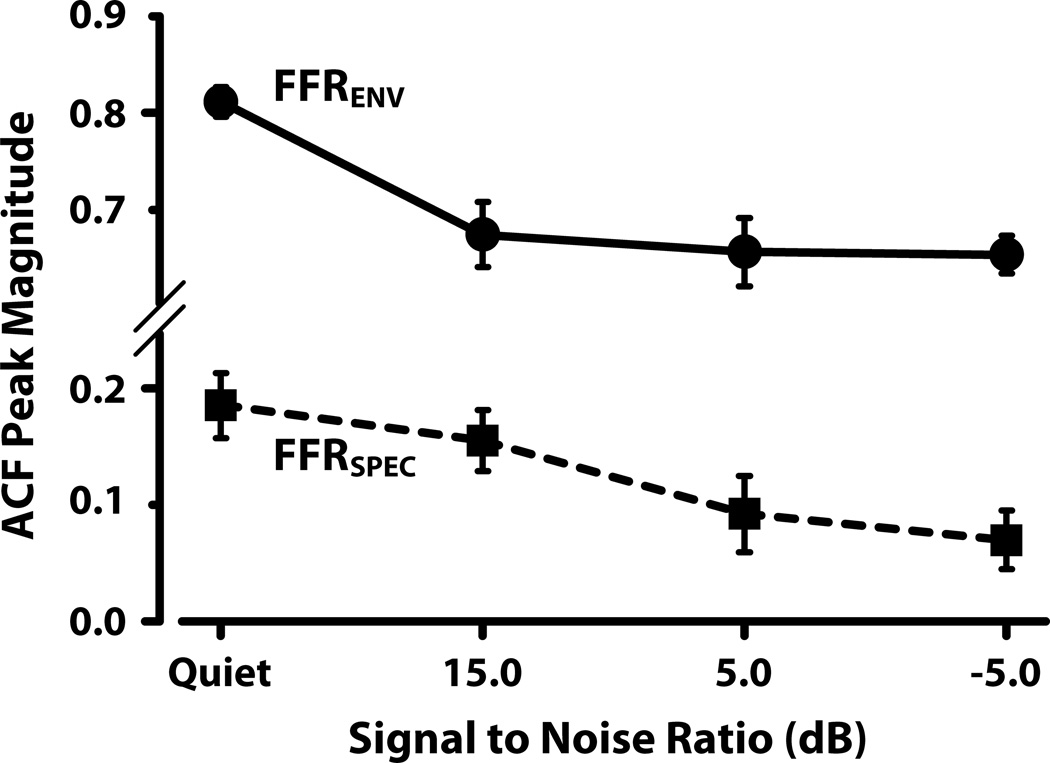
3.1.3. Effects of low-pass noise on neural periodicity strength
Mean normalized autocorrelation magnitude (first ACF peak after zero corresponding to the period of 90 Hz) for the FFRENV (solid circle), and FFRSPEC (solid square) waveforms are plotted as a function of noise level in Figure 6. For both, the ACF magnitude decreases as low-pass noise level is increased. ANOVA results indicated a significant main effect of SNR on strength of periodicity for both FFRENV (F3,24 = 27.66, p < 0.000), and FFRSPEC (F3,24 = 3.25, p = 0.039). Post hoc Tukey multiple comparisons (α = 0.05) revealed that ACF magnitude was statistically different only between the quiet and the −5 dB condition for the FFRSPEC data. For the FFRENV, ACF magnitude was significantly reduced for all noise conditions compared to the quiet condition, but there was no significant difference between the various noise levels.
Figure 6.
Mean autocorrelation (ACF peak amplitude) magnitude for FFRENV (circle) and FFRSPEC (square) data plotted for the quiet and noise conditions. The ACF peak magnitude, for both responses, corresponds to a delay equal to the period of the 90-Hz F0. Error bars correspond to +/− 1 SEM.
Overall, these results show that only the 3F0-8F0 DT distortion components, and the CDT distortion components are reduced in magnitude with increasing level of the low-pass masker with no reduction in the magnitude for F0, and 2F0 response components. Also, the periodicity strength of the FFRENV and FFRSPEC waveform sometimes decreases with increasing level of the masker. Taken together, these results suggest that neural representation of pitch-relevant information is degraded when distortion products are reduced or eliminated by the low-pass noise masker.
3.2. Effects of low-pass noise on F0 DL
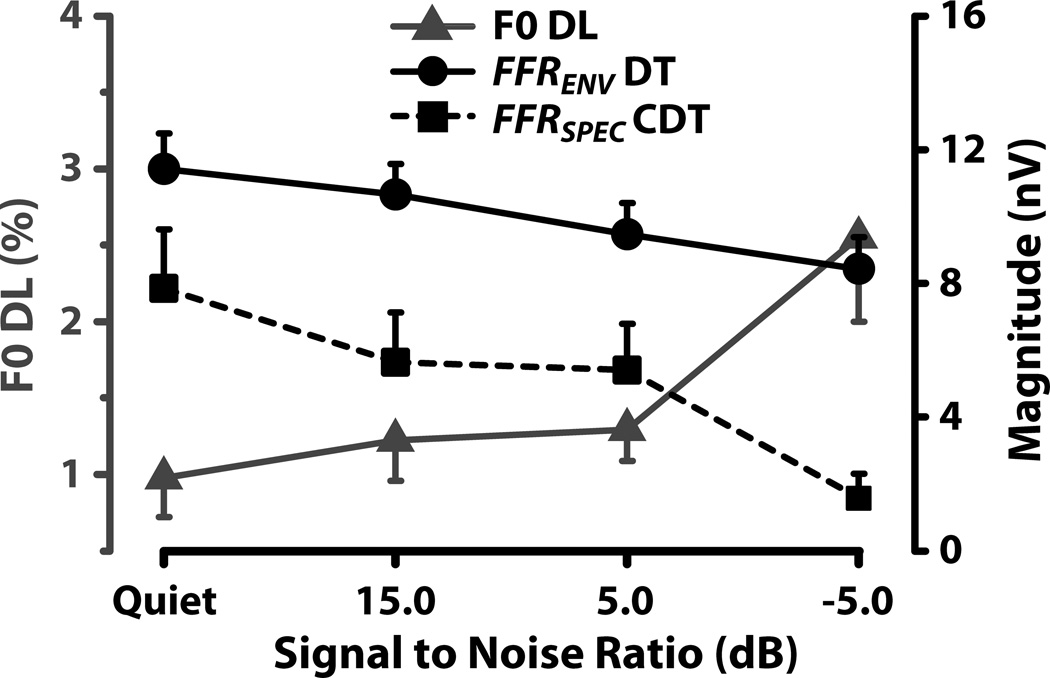
Mean F0 DLs (triangles), shown in Figure 7, increase with increase in the level of the low-pass noise, suggesting that F0 discrimination ability decreases with increasing level of the low-pass noise. A mixed model ANOVA with subjects as a random factor and SNR as a fixed factor revealed a significant main effect of SNR (F3,15 =9.71, p < 0.001) on behavioral F0 DLs. Post hoc Tukey adjusted multiple comparisons (α < 0.05) revealed a statistically significant difference between the quiet and all SNR conditions, and between the −5 and 5 dB SNR condition. In addition, a qualitative comparison of the F0 DL and the FFR distortion product magnitudes (Figure 7) reveals that increase in F0 DL thresholds with increase in noise level is associated with a decrease in the magnitude of both DT and CDT neural distortion components. Similarly, a qualitative comparison of the F0 DLs (Figure 7) with the ACFs of the FFR (Figure 6) reveals that increase in F0 DL thresholds with increase in noise level is associated with a decrease in the neural periodicity strength. This increase in F0 DL thresholds and the concomitant decrease in both the magnitude of neural distortion products and the neural periodicity strength with increase in noise level is consistent with the established view that distortion products may aid pitch discrimination.
Figure 7.
Mean F0 DL (triangle and left y-axis), mean magnitude of 3F0-8F0 DT distortion components (circle and right y-axis) and, mean magnitude of CDT distortion components (square and right y-axis) for the quiet and noise conditions. Error bars correspond to 1 SEM.
4. Discussion
The results of this study relate primarily to five main observations: (1) Magnitude of the mean 3F0-8F0 DT, and the mean CDT distortion component decreases with increasing level of a low-pass noise masker, but the magnitude of the DT components at F0 and 2F0 were not reduced; (2) FFR periodicity strength (derived from the ACF magnitude at peak delay corresponding to the 90 Hz F0) decreases as the level of the low-pass masker is increased suggesting that neural representation of pitch-relevant information in the brainstem is enhanced by the presence of distortion products; (3) Consistent with previous studies, F0 DL thresholds increase with increase in the level of the low-pass masker suggesting that the presence of distortion products may aid pitch perception; (4) Qualitative comparison of FFR neural (distortion products, and ACFs) data with the behavioral F0 DL measure suggests that increase in F0 DL thresholds with increase in the level of the low-pass masker is associated with a reduction in the magnitude of both the FFR distortion components and the periodicity strength of the FFR. These results taken together suggest that pitch-relevant information preserved in the phase locked neural activity generating the FFR may serve as an electrophysiological correlate of the behavioral pitch measure, although the FFR does not always reflect the perceived pitch (Gockel et al., 2011); and (5) FFR data show clear response peaks at the frequencies of the six unresolved harmonics (phase-locked responses to the fine structure in the stimulus waveform) which, as expected, are unaffected by the low-pass noise.
4.1. Neural and psychophysical correlates of distortion products
The presence of robust neural correlates of both DT and CDT distortion products in the scalp recorded FFR has been previously reported in both animals (Chertoff & Hecox, 1990; Rickman et al., 1991; Chertoff et al., 1992; Dolphin et al., 1994; Arnold & Burkard, 1998) and humans (Hall, 1979; Chambers et al., 1986; Greenberg et al., 1987; Krishnan, 1990; Pandya & Krishnan, 2004; Bhagat & Champlin, 2004; Elsisy & Krishnan, 2008; Gockel et al., 2011). Single unit physiologic experiments have reported the presence of these distortion products at several levels along the auditory pathway (auditory nerve: Goldstein & Kiang, 1968; Buunen & Rhode, 1978; Kim et al., 1980; cochlear nucleus: Faulstich & Kossl, 1999; Smoorenberg et al., 1976; inferior colliculus: Abel & Kossl, 2009; McAlpine, 2004; medial geniculate body: Horner et al., 1983; auditory cortex: Purcell et al., 2007). Psychophysical experiments have not only demonstrated the presence of audible distortion products (Bunnen et al., 1974; Wiegrebe & Patterson, 1999; Pressnitzer & Patterson, 2001; Oxenham et al., 2009) but have also used low-pass masking noise to eliminate or reduce their contribution in the evaluation of temporal pitch mechanisms (Moore & Sek 2000; Plack & White 2000; Carlyon et al. 2002; Grimault et al., 2002; Bernstein & Oxenham 2003; Oxenham et al., 2009). These distortion products are believed to influence perception of some pitch producing sounds (Smoorenburg 1970; Plomp 1965; Pressnitzer et al., 2001).
While the specific generator site(s) for FFR distortion products are yet to be determined, it is clear that a nonlinear process, presumably at or caudal to the FFR generators, must be present to generate these components. One possibility is that the FFR DT, and CDT components are generated by cochlear nonlinear mechanisms and subsequently transmitted to the auditory nerve (Kim et al., 1980; Goldstein & Kiang, 1968), and the FFR generators in the brainstem (Chertoff et al., 1990; Chertoff et al. 1991; Pandya & Krishnan, 2004; Krishnan & Elsisy, 2008). Thus, it is possible that both DT and CDT components in our FFR data may reflect far-field brainstem representations of more peripheral nonlinear processes. If this is true, then all of these distortion components should be significantly reduced in the presence of the low-pass noise masker. However, only the mean 3F0-8F0 DT and the mean CDT distortion component magnitude were reduced in the presence of the low-pass noise, with no change in the magnitude of the components at F0 and 2F0. While the reduction in magnitude for the mean 3F0-8F0 DT, and the mean CDT component by the low-pass noise may be interpreted to suggest a peripheral origin for these distortion products, the failure to see any magnitude reduction for F0 and 2F0 in the presence of the low-pass noise is not consistent with this view. Greenberg and colleagues (1987) also observed that the FFR component at F0 (in response to a complex tone containing mostly unresolved harmonics) was unaffected by a narrow-band noise centered at the F0 frequency. The authors interpreted this finding to suggest that the F0 component was generated by a mechanism other than intracochlear distortion.
It is possible that the FFRENV components, at least at F0, reflects neural phase-locking to stimulus envelope periodicity (the other mechanism) and not a quadratic distortion product and therefore would be less susceptible to masking (Hall, 1979; Smith et al., 1978; Greenberg et al., 1987; Krishnan & Plack, 2011; Gockel et al., 2011). If we assume that the low-pass noise used in this study masks frequency components within the noise passband, the F0 component must arise through phase-locking to the envelope created by interactions between the unmasked components located above the passband (i.e. the unresolved harmonics). While we would expect the F0 component to be the major envelope component, there could be a second (2F0) or even higher harmonics if the envelope waveform is not sinusoidal, and these should be equally resistant to noise as well. However, the observation of reduction in magnitude for DT components 3F0-8F0 is inconsistent with this explanation.
4.2. Contributions of FFR DT and CDT distortion products to pitch
Previous FFR studies largely focused on evaluating the characteristics of either the DT or the CDT distortion products (Chertoff & Hecox, 1990; Krishnan, 1990; Rickman et al., 1991; Chertoff et al., 1992; Dolphin et al., 1994; Arnold & Burkard, 1998; Pandya & Krishnan, 2004; Bhagat & Champlain, 2004; Elsisy & Krishnan, 2008). Unlike the current study, none of these previous studies evaluated the effects of a low-pass masker on either the magnitude of the neural distortion products (DT and CDT) or on the magnitude of the ACF of the FFR with an aim to assess the influence of distortion products on the neural representation of pitch-relevant information in the human brainstem.
The confirmation of the presence of DT and CDT distortion products by previous psychophysical and physiological experiments as well as our FFR data herein presents a compelling case to evaluate the contribution of these distortion products to central neural representations and the perceptual responses to complex sound stimuli. Equally important is the fact that the presence of these distortion components could serve as a potential confound in experiments designed to investigate auditory temporal processing. Although distortion products cannot fully account for the pitch percept of spectrally complex sounds (e.g., Schouten et al. 1962), there is strong evidence to suggest that audible cochlear distortion products can contribute to the pitch of complex sounds (Houtsma & Goldstein, 1972; Smoorenburg, 1970; Wiegrebe & Patterson, 1999; Pressnitzer & Patterson, 2001; Oxenham et al., 2009). These studies showed that F0 DLs for complex tones containing only unresolved harmonics increase when the distortion products are either masked or canceled, suggesting that cochlear distortion products (DT and CDT) may contribute to the pitch perception of complex sounds.
A qualitative comparison of FFR distortion products and FFR ACFs with behavioral F0 DL measure reveals that increase in F0 DL thresholds with increase in the level of the low-pass masker is associated with a reduction in the magnitude of both, the FFR distortion components and the neural periodicity strength. These results are compatible with the view that distortion products contribute to the neural representation of pitch-relevant information in the rostral brainstem and to the pitch salience. One possible explanation is that distortion-induced introduction of harmonic components in the resolved region enhances neural representation of pitch, and its salience. Once these distortion products are reduced or eliminated by low-pass noise, only the weaker envelope modulation cue from the unresolved region is available to mediate a relatively weaker pitch. This explanation is consistent with the reduction in both the neural pitch strength and the decreased ability to discriminate F0 as masker level is increased.
4.3. Neural phase-locking to temporal fine structure of the complex tone
The observation of robust peaks at frequencies of the unresolved harmonics in our FFRSTIM spectra is evidence of neural phase-locking to the temporal fine structure of the stimulus waveform. There is strong physiological evidence, at the auditory nerve level, supporting the presence of neural-phase-locking to the temporal fine structure of complex stimuli with only unresolved harmonics (Young & Sachs, 1979; Cariani & Delgutte, 1996a; 1996b; Meddis & O’Mard, 1997), as long as the unresolved components are within the frequency limit of phase-locking. In contrast, if all unresolved components are above the frequency limit of phase-locking, the only component visible in the neural response is at the envelope frequency. Thus it is clear that information about the temporal fine structure of the stimulus waveform, which may be relevant to pitch, is also available in the phase-locked neural activity in the rostral brainstem generating the FFR.
5.0 Conclusions
The FFR and behavioral results taken together suggest that distortion products (DT and CDT), evoked by a complex tone containing only peripherally unresolved harmonics may contribute to neural information relevant for pitch. Regarding pitch, the findings emphasize the importance of gaining a better understanding of how neural processing of this perceptual attribute at all stages along the auditory pathway may be influenced by distortion products. Moreover, the neural data from the auditory brainstem reinforce the importance of eliminating contributions of distortion products when evaluating purely temporal mechanisms of pitch. The scalp recorded FFR may be useful to evaluate the contribution of distortion products to neural encoding of complex sounds in humans.
Highlights.
Envelope and spectral FFRs preserve pitch relevant information.
Distortion product magnitude decreases with increasing masker level.
FFR pitch strength decreases when distortion products are masked.
Acknowledgments
Research supported by NIH R01DC008549 (A.K.). We also profusely thank the two reviewers for their patience and many thoughtful insights that have improved the quality of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel C, Kössl M. Sensitive response to low-frequency cochlear distortion products in the auditory midbrain. Journal of Neurophysiology. 2009;101(3):1560. doi: 10.1152/jn.90805.2008. [DOI] [PubMed] [Google Scholar]
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hearing Research. 2008;245(1–2):35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Arnold S, Burkard R. The auditory evoked potential difference tone and cubic difference tone measured from the inferior colliculus of the chinchilla. The Journal of the Acoustical Society of America. 1998;104:1565. doi: 10.1121/1.424368. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Oxenham AJ. Pitch discrimination of diotic and dichotic tone complexes: Harmonic resolvability or harmonic number? The Journal of the Acoustical Society of America. 2003;113(6):3323–3334. doi: 10.1121/1.1572146. [DOI] [PubMed] [Google Scholar]
- Bhagat S, Champlin C. Evaluation of distortion products produced by the human auditory system. Hearing Research. 2004;193(1–2):51–67. doi: 10.1016/j.heares.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Brown A. Acoustic distortion from rodent ears: a comparison of responses from rats, guinea pigs and gerbils. Hearing Research. 1987;31(1):25–37. doi: 10.1016/0378-5955(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Buunen TJ, Festen JM, Bilsen FA, van den Brink G. Phase effects in a three- component signal. The Journal of the Acoustical Society of America. 1974;55(2):297–303. doi: 10.1121/1.1914501. [DOI] [PubMed] [Google Scholar]
- Buunen TJ, Kate JH, Raatgever J, Bilsen FA. Combined psychophysical and electrophysiological study on the role of combination tones in the perception of phase changes. The Journal of the Acoustical Society of America. 1977;61(2):508–519. doi: 10.1121/1.381292. [DOI] [PubMed] [Google Scholar]
- Buunen TJ, Rhode WS. Responses of fibers in the cat's auditory nerve to the cubic difference tone. The Journal of the Acoustical Society of America. 1978;64(3):772–781. doi: 10.1121/1.382042. [DOI] [PubMed] [Google Scholar]
- Cariani PA, Delgutte B. Neural correlates of the pitch of complex tones. I. Pitch and pitch salience. Journal of Neurophysiology. 1996a;76(3):1698–1716. doi: 10.1152/jn.1996.76.3.1698. [DOI] [PubMed] [Google Scholar]
- Cariani PA, Delgutte B. Neural correlates of the pitch of complex tones. II. Pitch shift, pitch ambiguity, phase invariance, pitch circularity, rate pitch, and the dominance region for pitch. Journal of Neurophysiology. 1996b;76(3):1717–1734. doi: 10.1152/jn.1996.76.3.1717. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, van Wieringen A, Long CJ, Deeks JM, Wouters J. Temporal pitch mechanisms in acoustic and electric hearing. The Journal of the Acoustical Society of America. 2002;112(2):621–633. doi: 10.1121/1.1488660. [DOI] [PubMed] [Google Scholar]
- Chambers RD, Feth LL, Burns EM. The relationship between the human frequency-following responses at the low pitch of complex tones. The Journal of the Acoustical Society of America. 1986;80:1673–1680. doi: 10.1121/1.394279. [DOI] [PubMed] [Google Scholar]
- Chertoff ME, Hecox KE. Auditory nonlinearities measured with auditory-evoked potentials. The Journal of the Acoustical Society of America. 1990;87(3):1248–1254. doi: 10.1121/1.398800. [DOI] [PubMed] [Google Scholar]
- Chertoff ME, Hecox KE, Goldstein R. Auditory distortion products measured with averaged auditory evoked potentials. J Speech Hear Res. 1992;35(1):157–166. doi: 10.1044/jshr.3501.157. [DOI] [PubMed] [Google Scholar]
- Clark WW, Kim DO, Zurek PM, Bohne BA. Spontaneous otoacoustic emissions in chinchilla ear canals: correlation with histopathology and suppression by external tones. Hearing Research. 1984;16(3):299–314. doi: 10.1016/0378-5955(84)90119-9. [DOI] [PubMed] [Google Scholar]
- Dolphin WF, Chertoff ME, Burkard R. Comparison of the envelope following response in the Mongolian gerbil using two-tone and sinusoidally amplitude-modulated tones. The Journal of the Acoustical Society of America. 1994;96(4):2225–2234. doi: 10.1121/1.411382. [DOI] [PubMed] [Google Scholar]
- Elsisy H, Krishnan A. Comparison of the acoustic and neural distortion product at 2f1-f2 in normal-hearing adults. International Journal of Audiology. 2008;47(7):431–438. doi: 10.1080/14992020801987396. [DOI] [PubMed] [Google Scholar]
- Faulstich M, Kossl M. Neuronal response to cochlear distortion products in the anteroventral cochlear nucleus of the gerbil. The Journal of the Acoustical Society of America. 1999;105(1):491–502. doi: 10.1121/1.424586. [DOI] [PubMed] [Google Scholar]
- Gaskill SA, Brown AM. The behavior of the acoustic distortion product, 2f1-f2, from the human ear and its relation to auditory sensitivity. The Journal of the Acoustical Society of America. 1990;88(2):821–839. doi: 10.1121/1.399732. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Suter CM, Dasheiff R, Goldberg A. The human frequency-following response: its behavior during continuous tone and tone burst stimulation. Electroencephalography and Clinical Neurophysiology. 1976;40(1):25–32. doi: 10.1016/0013-4694(76)90176-0. [DOI] [PubMed] [Google Scholar]
- Gockel HE, Carlyon RP, Mehta A, Plack CJ. The frequency following response (FFR) may reflect pitch-bearing information but is not a direct representation of pitch. Journal of the Association for Research in Otolaryngology. 2011;12:767–782. doi: 10.1007/s10162-011-0284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. Combination Tones and Auditory Nonlinearity. The Journal of the Acoustical Society of America. 1967;41:1603. doi: 10.1121/1.1910396. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Kiang NYS. Neural correlates of the aural combination tone 2f1-f2. Proceedings of the IEEE. 1968;56(6):981–992. [Google Scholar]
- Greenberg S, Marsh JT, Brown WS, Smith JC. Neural temporal coding of low pitch. I. Human frequency-following responses to complex tones. Hearing Research. 1987;25(2–3):91–114. doi: 10.1016/0378-5955(87)90083-9. [DOI] [PubMed] [Google Scholar]
- Grimault N, Micheyl C, Carlyon RP, Collet L. Evidence for two pitch encoding mechanisms using a selective auditory training paradigm. Perception and Psychophysics. 2002;64(2):189–197. doi: 10.3758/bf03195785. [DOI] [PubMed] [Google Scholar]
- Hall JW., III Auditory brainstem frequency following responses to waveform envelope Periodicity. Science. 1979;205:1297–1299. doi: 10.1126/science.472748. [DOI] [PubMed] [Google Scholar]
- Hall DA, Plack CJ. Pitch processing sites in the human auditory brain. Cerebral Cortex. 2009;19(3):576–585. doi: 10.1093/cercor/bhn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner K, de Ribaupierre Y, de Ribaupierre F. Neural correlates of cubic difference tones in the medial geniculate body of the cat. Hearing Research. 1983;11(3):343–357. doi: 10.1016/0378-5955(83)90066-7. [DOI] [PubMed] [Google Scholar]
- Houtsma A, Goldstein JL. The central origin of the pitch of complex tones: Evidence from musical interval recognition. Journal of the Acoustical Society of America. 1972;51:520–529. [Google Scholar]
- Houtsma AJ, Smurzynski J. Pitch identification and discrimination for complex tones with many harmonics. The Journal of the Acoustical Society of America. 1990;87(1):304–310. [Google Scholar]
- Humes LE. Growth of L(f2--f1) and L(2f1--f2) with input level: influence of f2/f1. Hearing Research. 1980;2(2):115–122. doi: 10.1016/0378-5955(80)90033-7. [DOI] [PubMed] [Google Scholar]
- Kaernbach C, Bering C. Exploring the temporal mechanism involved in the pitch of unresolved harmonics. The Journal of the Acoustical Society of America. 2001;110:1039. doi: 10.1121/1.1381535. [DOI] [PubMed] [Google Scholar]
- Kemp D. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. European Archives of Oto-Rhino-Laryngology. 1979;224(1):37–45. doi: 10.1007/BF00455222. [DOI] [PubMed] [Google Scholar]
- Kemp D, Brown A. Ear canal acoustic and round window electrical correlates of 2f1-f2 distortion generated in the cochlea. Hearing Research. 1984;13(1):39–46. doi: 10.1016/0378-5955(84)90093-5. [DOI] [PubMed] [Google Scholar]
- Kim DO, Molnar CE, Matthews JW. Cochlear mechanics: nonlinear behavior in two-tone response as reflected in cochlear-nerve-fiber responses and in ear-canal sound pressure. The Journal of the Acoustical Society of America. 1980;67:1704–1721. doi: 10.1121/1.384297. [DOI] [PubMed] [Google Scholar]
- Krishnan A. Human frequency-following responses to two-tone approximations of steady- state vowels. Audiology and Neurotology. 1999;4(2):95–103. doi: 10.1159/000013826. [DOI] [PubMed] [Google Scholar]
- Krishnan A. Human frequency-following responses: representation of steady-state synthetic vowels. Hearing Research. 2002;166(1–2):192–201. doi: 10.1016/s0378-5955(02)00327-1. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT. The role of the auditory brainstem in processing linguistically-relevant pitch patterns. Brain and Language. 2009;110:135–148. doi: 10.1016/j.bandl.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Bidelman GM. Brainstem pitch representation in native speakers of Mandarin is less susceptible to degradation of stimulus temporal regularity. Brain Research. 2010;1313:124–133. doi: 10.1016/j.brainres.2009.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Bidelman GM, Swaminathan J. Experience-dependent neural representation of dynamic pitch in the brainstem. Neuroreport. 2009;20(4):408–413. doi: 10.1097/WNR.0b013e3283263000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Smalt CJ, Bidelman GM. Language-dependent pitch encoding advantage in the brainstem is not limited to acceleration rates that occur in natural speech. Brain Lang. 2010;114(3):193–198. doi: 10.1016/j.bandl.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Parkinson J. Human frequency-following response: representation of tonal sweeps. Audiology and Neurotology. 2000;5(6):312–321. doi: 10.1159/000013897. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Plack CJ. Neural encoding in the human brainstem relevant to the pitch of complex tones. Hearing Research. 2011 doi: 10.1016/j.heares.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Swaminathan J, Gandour JT. Experience-dependent Enhancement of Linguistic Pitch Representation in the Brainstem Is Not Specific to a Speech Context. Journal of Cognitive Neuroscience. 2009;21(6):1092–1105. doi: 10.1162/jocn.2009.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Xu Y, Gandour J, Cariani P. Encoding of pitch in the human brainstem is sensitive to language experience. Brain Research. Cognitive Brain Research. 2005;25(1):161–168. doi: 10.1016/j.cogbrainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Xu Y, Gandour JT, Cariani PA. Human frequency-following response: representation of pitch contours in Chinese tones. Hearing Research. 2004;189(1–2):1–12. doi: 10.1016/S0378-5955(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49(2):467–477. [PubMed] [Google Scholar]
- Lonsbury-Martin B, Martin G, Probst R, Coats A. Acoustic distortion products in rabbit ear canal. I. Basic features and physiological vulnerability. Hearing Research. 1987;28(2–3):173–189. doi: 10.1016/0378-5955(87)90048-7. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Harris F, Stagner B, Hawkins M, Martin G. Distortion product emissions in humans. I. Basic properties in normally hearing subjects. The Annals of otology, rhinology & laryngology. Supplement. 1990;147:3. [PubMed] [Google Scholar]
- Marsh JT, Brown WS, Smith JC. Differential brainstem pathways for the conduction of auditory frequency following responses. Electroencephalography and Clinical Neurophysiology. 1974;38:415–422. doi: 10.1016/0013-4694(74)90192-8. [DOI] [PubMed] [Google Scholar]
- Marsh JT, Brown WS, Smith JC. Far-field recorded frequency-following responses: Correlates of low pitch auditory perception in humans Responses d'entertainement enregistrees a distance: Correlates de la perception auditive des sons de faible hauteur chez l'homme. Electroencephalography and Clinical Neurophysiology. 1975;38(2):113–119. doi: 10.1016/0013-4694(75)90220-5. [DOI] [PubMed] [Google Scholar]
- Martin G, Lonsbury-Martin B, Probst R, Scheinin S, Coats A. Acoustic distortion products in rabbit ear canal. II. Sites of origin revealed by suppression contours and pure-tone exposures. Hearing Research. 1987;28(2–3):191–208. doi: 10.1016/0378-5955(87)90049-9. [DOI] [PubMed] [Google Scholar]
- McAlpine D. Neural Sensitivity to periodicity in the inferior colliculus: Evidence for the role of cochlear distortions. Journal of Neuroscience. 2004;92:1295–1311. doi: 10.1152/jn.00034.2004. [DOI] [PubMed] [Google Scholar]
- Meddis R, O’Mard L. A unitary model of pitch perception. The Journal of the Acoustical Society of America. 1997;102(3):1811–1820. doi: 10.1121/1.420088. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Davis K, Wrobleski DM, Oxenham AJ. Does fundamental-frequency discrimination measure virtual pitch discrimination? The Journal of the Acoustical Society of America. 2010;128:1930–1942. doi: 10.1121/1.3478786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Gockel HE. Resolvability of components in complex tones and implications for theories of pitch perception. Hearing Research. 2011;276(1–2):88–97. doi: 10.1016/j.heares.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Sek A. Effects of relative phase and frequency spacing on the detection of three-component amplitude modulation. The Journal of the Acoustical Society of America. 2000;108:2337. doi: 10.1121/1.1312362. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Micheyl C, Keebler MV. Can temporal fine structure represent the fundamental frequency of unresolved harmonics? The Journal of the Acoustical Society of America. 2009;125(4):2189–2199. doi: 10.1121/1.3089220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya PK, Krishnan A. Human frequency-following response correlates of the distortion product at 2F1-F2. J Am Acad Audiol. 2004;15(3):184–197. doi: 10.3766/jaaa.15.3.2. [DOI] [PubMed] [Google Scholar]
- Plack CJ, White LJ. Pitch matches between unresolved complex tones differing by a single interpulse interval. The Journal of the Acoustical Society of America. 2000;108:696. doi: 10.1121/1.429602. [DOI] [PubMed] [Google Scholar]
- Plomp R. Pitch of complex tones. Journal of the Acoustical Society of America. 1967;41(6):1526–1533. doi: 10.1121/1.1910515. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, Patterson RD, Krumbholz K. The lower limit of melodic pitch. The Journal of the Acoustical Society of America. 2001;109(5 Pt 1):2074–2084. doi: 10.1121/1.1359797. [DOI] [PubMed] [Google Scholar]
- Purcell DW, Picton TW, Pantev C. Cortical responses to the 2f-f combination tone measured indirectly using magnetoencephalography. The Journal of the Acoustical Society of America. 2007;122:992. doi: 10.1121/1.2751250. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Cooper NP. Two-tone suppression and distortion production on the basilar membrane in the hook region of cat and guinea pig cochleae. Hearing Research. 1993;66(1):31–45. doi: 10.1016/0378-5955(93)90257-2. [DOI] [PubMed] [Google Scholar]
- Rickman MD, Chertoff ME, Hecox KE. Electrophysiological evidence of nonlinear distortion products to two-tone stimuli. The Journal of the Acoustical Society of America. 1991;89(6):2818–2826. doi: 10.1121/1.400720. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA, Rich NC. Two-tone distortion on the basilar membrane of the chinchilla cochlea. Journal of Neurophysiology. 1997;77(5):2385–2399. doi: 10.1152/jn.1997.77.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA. Acoustic distortion in the ear canal. I. Cubic difference tones: effects of acute noise injury. The Journal of the Acoustical Society of America. 1986;79(5):1481–1490. doi: 10.1121/1.393675. [DOI] [PubMed] [Google Scholar]
- Schouten JF, Ritsma R, Cardozo BL. Pitch of the residue. Journal of the Acoustical Society of America. 1962 [Google Scholar]
- Shackleton TM, Carlyon RP. The role of resolved and unresolved harmonics in pitch perception and frequency modulation discrimination. Journal of the Acoustical Society of America. 1994;95(6):3529–3540. doi: 10.1121/1.409970. [DOI] [PubMed] [Google Scholar]
- Smith JC, Marsh JT, Brown WS. Far-field recorded frequency-following responses: evidence for the locus of brainstem sources. Electroencephalography and Clinical Neurophysiology. 1975;39(5):465–472. doi: 10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Smith JC, Marsh JT, Greenberg S, Brown WS. Human auditory frequency-following responses to missing fundamental. Science. 1978;201:639–641. doi: 10.1126/science.675250. [DOI] [PubMed] [Google Scholar]
- Smoorenburg GF. Pitch perception of two-frequency stimuli. The Journal of the Acoustical Society of America. 1970;48(4):924–942. doi: 10.1121/1.1912232. [DOI] [PubMed] [Google Scholar]
- Smoorenburg GF. Audibility region of combination tones. The Journal of the Acoustical Society of America. 1972a;52:603. [Google Scholar]
- Smoorenburg GF. Combination tones and their origin. The Journal of the Acoustical Society of America. 1972b;52:615. [Google Scholar]
- Smoorenburg GF, Gibson MM, Kitzes LM, Rose JE, Hind JE. Correlates of combination tones observed in the response of neurons in the anteroventral cochlear nucleus of the cat. The Journal of the Acoustical Society of America. 1976;59(4):945–962. doi: 10.1121/1.380954. [DOI] [PubMed] [Google Scholar]
- Swaminathan J, Krishnan A, Gandour JT. Pitch encoding in speech and nonspeech contexts in the human auditory brainstem. Neuroreport. 2008;19(11):1163–1167. doi: 10.1097/WNR.0b013e3283088d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan J, Krishnan A, Gandour JT, Xu Y. Applications of static and dynamic iterated rippled noise to evaluate pitch encoding in the human auditory brainstem. IEEE Transactions on Biomedical Engineering. 2008;55(1):281–287. doi: 10.1109/TBME.2007.896592. [DOI] [PubMed] [Google Scholar]
- Wegel RL, Lane C. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Physical Review. 1924;23(2):266. [Google Scholar]
- Wiegrebe L, Patterson RD. Quantifying the distortion products generated by amplitude-modulated noise. The Journal of the Acoustical Society of America. 1999;106:2709. doi: 10.1121/1.428099. [DOI] [PubMed] [Google Scholar]
- Worden FG, Marsh JT. Frequency-following (microphonic-like) neural responses evoked by sound. Electroencephalography and Clinical Neurophysiology. 1968;25(1):42–52. doi: 10.1016/0013-4694(68)90085-0. [DOI] [PubMed] [Google Scholar]
- Yost WA, Patterson R, Sheft S. A time domain description for the pitch strength of iterated rippled noise. The Journal of the Acoustical Society of America. 1996;99(2):1066–1078. doi: 10.1121/1.414593. [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. The Journal of the Acoustical Society of America. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]
- Zwicker E. Different behaviour of quadratic and cubic difference tones. Hearing Research. 1979;1(4):283–292. doi: 10.1016/0378-5955(79)90001-7. [DOI] [PubMed] [Google Scholar]
- Zwicker E. Nonmonotic behaviour of (2f1 - f2) explained by a saturation-feedback model. Hearing Research. 1980;2(3–4):513–518. doi: 10.1016/0378-5955(80)90088-x. [DOI] [PubMed] [Google Scholar]