Abstract
Animal models and behavioral paradigms are critical for elucidating the neural mechanism involved in complex behaviors, including social cognition. Both genotype and phenotype based models have implicated the neuropeptide oxytocin (OT) in the regulation of social behavior. Based on the findings in animal models, alteration of the OT system has been hypothesized to play a role in the social deficits associated with autism and other neuropsychiatric disorders. While the evidence linking the peptide to the etiology of the disorder is not yet conclusive, evidence from multiple animal models suggest modulation of the OT system may be a viable strategy for the pharmacological treatment of social deficits. In this review, we will discuss how animal models have been utilized to understand the role of OT in social cognition and how those findings can be applied to the conceptualization and treatment of the social impairments in ASD. Animal models with genetic alterations of the OT system, like the OT, OT receptor and CD38 knock-out mice, and those with phenotypic variation in social behavior, like BTBR inbred mice and prairie voles, coupled with behavioral paradigms with face and construct validity may prove to have predictive validity for identifying the most efficacious methods of stimulating the OT system to enhance social cognition in humans. The widespread use of strong animal models of social cognition has the potential yield pharmacological, interventions for the treatment social impairments psychiatric disorders. This article is part of a Special Issue entitled Oxytocin, Vasopressin, and Social Behavior.
Keywords: Social cognition, Oxytocin, Autism, Therapeutics, Buspirone, Serotonin, Prairie voles, Animal models
Introduction
Despite the increasing diagnosis of autism spectrum disorders (ASD) over the last twenty years, there has been little progress in the development of pharmacological treatments for the social impairments that are at the core of this disorder. Animal models and behavioral paradigms with face, construct, and predictive validity are essential for the development of novel, biologically-based drugs to enhance social function. Consequently, increased attention has been given to the development of animal models that enable the study of social cognition and social dysfunction. Many of the most promising animal models do not model ASD per se, but were developed to study the basic neurobiology of social cognition. The neural systems identified in these models that regulate social cognition provide an entry point to look at systems that may be disrupted in ASD and other psychiatric disorders with social impairments. The oxytocin (OT) system has been identified in several models to be a potent regulator of prosocial behavior. As a result of these findings, investigation in ASD subjects has revealed some associated alterations in the OT system. More importantly, preliminary findings suggest that the OT system may be a viable pharmacotherapeutic target for the treatment of the social impairments of the disorder regardless of specific etiology. In this review, we will discuss how animal models have been utilized to understand the role of OT in social cognition and how those findings can be applied to the conceptualization and treatment of the social impairments in ASD.
Using animal models to understand ASD
Animal models are used to characterize the biological mechanisms of disease or to understand the normative processes disrupted in disease. The ultimate goal of using animal models is to identify pharmacological targets for interventions. As ASD, like most psychiatric diseases, is a genetically and phenotypically complex disorder, no one animal model can capture all of the core features of the disorder, see Table 1. Animal models relevant to the social impairments of ASD can be divided into three categories: 1) genotype based, in which genes that regulate social cognition are altered in transgenic rodents, 2) phenotype based, in which normative or dysfunctional social behaviors are modeled, and 3) environmental based, in which environmental insults linked with social impairments are recapitulated. Animal models are used in conjunction with behavioral paradigms that enable the quantification of specific behaviors reflecting the animal’s cognitive or emotional state. Strong models have a combination of three features: 1) face validity, the model shares behavioral phenotypes with the disorder, 2) construct validity, the model shares a common biological mechanism with the disorder, and 3) predictive validity, drugs that have a specific effect in the model have a parallel effect in humans. Behavioral paradigms relevant to the social impairment in ASD should enable the quantification of variability in social cognition (face validity) based on evolutionarily common neurobiological mechanisms (construct validity). Ideally these behavioral paradigms will also prove to have predictive ability for identifying drugs capable of enhancing social cognition in human subjects in ASD and other psychiatric conditions with impairments in the social domain. Identifying the strengths and limitations of the individual animal models and behavioral paradigms may be useful in identifying the most clinically efficacious pharmacological methods of enhancing social functioning through the modulation of the OT system, see Table 2.
Table 1.
Using of Animal Models. Basic terminology used to describe how animal models and behavioral paradigms are classified, evaluated and used to assess social cognition.
| Terminology | Type | Definition | Example |
|---|---|---|---|
| Animal model | Genotype based | Mice (in most cases) genetically engineered to contain genetic alterations either associated with ASD or causative of monogenic forms of ASD |
|
| Laboratory animals used to characterize a disease or the normative processes disrupted in a disease. | |||
| Environment based | Animals (typically rodents or primates) that have undergone an environmental manipulation linked with ASD |
|
|
| Phenotype based | Animals social behavior parallels either functional or disordered human phenotypes |
|
|
| Validity | Construct validity | Model replicates etiological or neurobiological bases of the human condition being represented | |
| Relationship of the animal model or behavioral paradigm to the disease or behavior it is intended to represent. | |||
| Predictive validity | The outcome of a manipulation in an animal model is indicative of the effect on the human condition |
|
|
| Face validity | The measured phenootypes in the model are phenomenologically similar to the human condition |
|
|
| Behavioral paradigm | Isolation induced vocalization | Frequency and intensity of ultrasonic vocalizations emitted by pups, when separated from their dams, is indicative of social anxiety | |
| An experimental test used to quantify a specific behavior in the laboratory, usually representative of an animal’s cognitive or emotional state. | |||
| Social recognition test | Ability of a rodent to recall a familiar individual, as measured by decreased time spent in olfactory investigation of a familiar as opposed to novel conspecifics, as a measure of social memory | ||
| Social preference test | Degree of preference expressed, as measure by time in proximity, to social vs. nonsocial and novel vs. familiar stimuli a proxy for social motivation | ||
| Partner preference test | Formation of a social bond, as measured by twice as much time spent huddling with familiar as opposed to novel animal. |
indicates species typical performance in the behavioral task.
indicates abnormal performance associated with social impairment.
Table 2.
Relevance of animal models for oxytocin-based drug discovery.
| Treatment strategy | Description | Potential predictive model
|
|||||
|---|---|---|---|---|---|---|---|
| Genotype based models
|
Phenotype based models
|
||||||
| OT-KO | OTR-KO | CD38-KO | Monogenic ASD models | Inbred mice | Microtine rodents | ||
| Intranasal OT | Peripheral route of administration with hypothesized preferential access across the BBB. | + | −* | + | +* | + | + |
| Non-peptide agonists | Small molecule agonists for the OT receptor that cross the BBB. | + | −* | + | +* | + | + |
| OT releasers | Agonists for receptors on OT neurons or intracellular signaling molecules that promote OT release. | − | −* | + | +* | + | + |
| P-LAP inhibitors | Inhibits the enzymatic activity of placental leucine aminopeptidase, to prevent the degradation of OT | − | −* | − | +* | + | + |
The potential efficacy of OT based therapeutics can be best evaluated using different animal models of social cognition (+ indicates validity of testing that form of OT-based therapy in that specific model, − indicates lack of validity of testing that form of OT-based therapy in that specific model). OTKO and CD38KO mice are best utilized to test the effects of OT and OT receptor agonists, as the effect of OT in the social recognition test in these models is well characterized. Phenotype based models can be used to test all classes of OT-based therapies, though the best characterized behavioral endpoint uses the partner preference in female prairie voles. The use of OTR-KO mice has the most construct validity for the small subpopulation of ASD patients altered OTR expression, though the mechanism of action of OT-based drugs would be less clear in this model (−* indicates the model couldn’t be used to identifying therapeutics that act at OT receptors, but the model could be useful in understanding signaling through alternative pathways). Mouse models of monogenic forms of autism also have the potential to test the efficacy of OT based therapies, however the effect of central OT on social phenotypes must first be better defined (+*).
ASD phenotypes in genotype based models
Pharmacological manipulation of the OT system in functional model systems has indicated that this neurohypophyseal peptide not only coordinates the physiology of reproduction, but also promotes several elements of prosocial behavior, including parental behavior and complex social interactions between adults. Studies in mice with a genetically altered OT system have confirmed the role for OT in the regulation of social behavior. The establishment and verification of behavioral paradigms that capture variation in social cognition enables the attribution of specific phenotypes to the neurochemical system. Three lines of mice with genetically altered OT systems have been evaluated in a battery of social behavioral paradigms that capture a number of features relevant to the ASD phenotype. Each mouse line models the perturbation of a different level of OT signaling, release of the peptide in CD38 knock-out mice (CD38KO; the CD38 enzyme regulates the Ca2+ dependent secretion of OT, see Higashida in this issue for a full review), the peptide itself in OT knock-out mice (OTKO) and the peptide receptor in OT receptor knock-out (OTRKO) mice, to produce a detailed picture of the contributions of the OT system to discrete components of functional social cognition.
Social information processing and recognition
Impairments in social cognition have been identified in all three models of altered OT signaling in multiple behavioral paradigms. The most robust social deficits can be seen in the social recognition paradigm. Social information in rodents is primarily conveyed through olfactory cues. Rodents can differentiate between individuals based on their olfactory signature and maintain that memory for up to two hours. Social recognition of a conspecific can be detected by a decrease in olfactory investigation of the familiar animal (habituation) compared to a novel animal (Winslow and Camacho, 1995). Injection of OT into the lateral septum (Popik et al., 1992), medial preoptic areas (Popik and van Ree, 1991) and olfactory bulb (Dluzen et al., 1998) of rats enhances social recognition. Global elimination of OT, the OT receptor or CD38 in genetically engineered knock-out mice prevents the recognition of familiar conspecifics (Ferguson et al., 2000; Higashida et al., 2011a; Jin et al., 2007; Takayanagi et al., 2005). Male knock-out mice showed complete social amnesia, with no decrease in olfactory investigation after repeated exposures to a single animal, see Fig. 1 (Ferguson et al., 2000; Jin et al., 2007; Takayanagi et al., 2005). Despite a profound social memory deficit, the knock-out mice have normal sensory and nonsocial learning and memory capabilities. These mice habituate normally to non-social odors, like lemon scent. Mice with altered OT systems appear to have a specific deficit such that they are unable to use perceived social cues to recognize familiar individuals. The selective social impairments of the knock-out mice provide strong face validity for the models within the social recognition paradigm for the disruption of social cognition in ASD. The contribution of OT to functional social recognition may even be more subtle than social vs. non-social discrimination. Forebrain-specific OT receptor knock-down indicates that OT-dependent social recognition deficits are specific to intra but not inter mouse strain comparisons. This suggests the OT receptor is primarily involved in “fine” social discriminations, like those between specific mice, as opposed to “gross” between strain judgments (Macbeth et al., 2009). The “fine tuning” function of OT corresponds to the often intact pedantic emotion recognition among individuals with social deficits despite impairments in the context of a complex social interaction (Bartz et al., 2011).
Fig. 1.
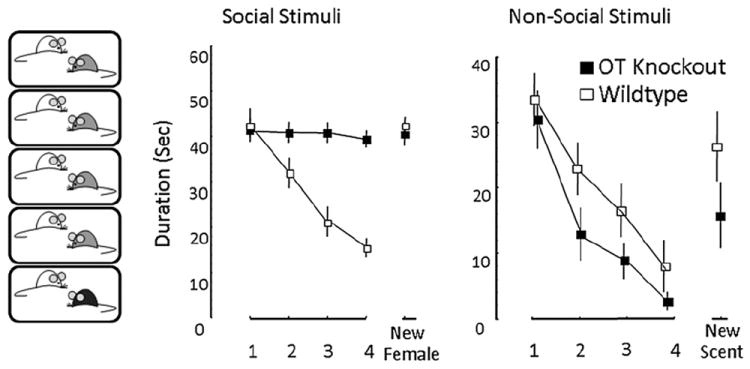
Social recognition behavioral paradigm. Recognition of a familiar odor (either social or non-social) is indicated by a decrease in duration of time in olfactory investigation of the scent. In the paradigm, the test animal is presented with either a single mouse (left graph) or cotton swab scented with a non-social odor (right graph) four times sequentially. The fifth presentation is a novel mouse or novel non-social odor. Recognition of the repeated stimulus should elicit a decrease in investigation duration. Presentation of the novel stimulus should result in a return to baseline levels of investigation. The OTKO mouse fails to show a reduction in the duration of olfactory investigation of social stimuli, but does show a habituation to the non-social stimuli. Similar patterns of olfactory investigation for social and non-social stimuli can be observed in comparisons of OTRKO and CD38KO mice to wild-type littermates.
The deficits in the social recognition paradigm can be rescued in all three knock-out lines by a single intracerebroventricular injections of OT given prior to, but not after the initial social exposure (Ferguson et al., 2001; Jin et al., 2007). This suggests that OT facilitates the encoding and not the consolidation or recall of social information. We hypothesize that this enhancement of encoding may be due to OT’s effect of enhancing the saliency of social stimuli. While this effect is consistent with the genetic alterations in CD38KO and OTKO, which have decreased levels of circulating OT, the efficacy of the OT in restoring social recognition with the OTRKO line is particularly intriguing. Central injections of either OT or vasopressin rescue the impaired social phenotypes in this model questioning the role of strict OT–OT receptor interactions in social deficits of this model. Co-administration of a vasopressin receptor 1a (V1aR) antagonist along with OT in this model prevents the rescue effects, implicating an alternative pathway (Sala et al., 2011). The overlap of the OT and vasopressin systems highlighted by the findings in the OTRKO mouse emphasizes the complicated pharmacology involved in developing OT-related pharmacotherapies for ASD and the potential alteration of both neuropeptide systems in the disease state (Ring, 2011). Evidence from the knock-out models suggests that while OT may be a viable therapeutic strategy for the treatment of social impairments, further exploration into the cross talk between that OT and vasopressin system that may occur in ASD is necessary for the determination of the ideal pharmacological intervention.
Social comfort
OT also plays a role in social phenotypes, like separation-induced vocalization and locomotion, which are measures of isolation-induced anxiety. Separation-induced vocalization is used as a measure of perceived distress and desire for reunification in response to social isolation. Mouse pups emit these types of ultrasonic calls when separated from their dam and littermates. In this behavioral paradigm, the subsequent vocalizations are quantified, with frequency of calls reflecting the distress associated with isolation. OTKO, OTRKO and CD38KO mouse pups emit fewer of these distress calls when separated from their mother than wild-type littermates, with the most profound reduction seen in the OTKO pups (Higashida et al., 2011b; Liu et al., 2008; Takayanagi et al., 2005; Winslow et al., 2000). In response to the same separation paradigm, OTKO pups make fewer attempts to reunite with their dam by crossing under a barrier (Ross and Young, 2009). The reduced reunification driven locomotion, is interestingly accompanied by an increase in general locomotor and exploratory behavior in OTRKO and CD38KO pups compared to wild-type mouse pups. Increased locomotion is thought to reflect a decrease in isolation-induced anxiety. Thus mouse pups with genetically altered OT systems appear to be less anxious than wild-type mice in response to social isolation. Abnormal responses to social stress are also seen in individuals with autism. Children with high-functioning autism have altered a neuroendocrine response to the Trier Social Stress Test, showing decreases in cortisol whereas the controls show increases in cortisol in response to the stressor, suggesting a diminished social stress response (Levine et al., 2012).
Anxiety
In contrast to decreased isolation induced anxiety, though, mice with genetically altered OT systems have increased general anxiety levels. OTKO mice spend less time in the open arms of the elevated plus maze (Mantella et al., 2003), a behavioral paradigm assessing anxiety, and show heightened corticosterone responses to environmental stressors compared to wild-type mice (Amico et al., 2008). The anxiety related behavior could be decreased with central injections of OT (Mantella et al., 2003). The anxiolytic effects of OT can be seen in both genetically modified and wild-type mice. Central and peripheral OT injections decrease anxiety behavior the elevated maze paradigm and in the four-plate paradigm in wild type mice, the effects of which can be blocked with a centrally acting OT receptor antagonist (Ring et al., 2006). Based on the anti-anxiety effects of OT, it has been speculated that the pro-social effects of OT may be mediated through a reduction in socially induced anxiety. Further work is needed to determine the extent to which OT promotes social behavior independently from its anxiolytic effects.
Other ASD relevant phenotypes
Interestingly, alteration of the OT system has recently been found to affect other clinical features associated with ASD. The OTRKO mouse displays non-social phenotypes that are consistent with ASD. OTRKO mice exhibit impaired cognitive flexibility. In an appetite-motivated T-maze test, the OTRKO animals are significantly slower to learn reversals of the baited arm than wildtype littermates, despite similar initial acquisition rates (Sala et al., 2011). This phenotype is consistent with the limited behavioral patterns inflexible to environmental demands frequently seen in individuals with ASD. Epileptic seizures are comorbid with ASD in 10–30% of cases (Gabis et al., 2005). OTRKO mice also have an increased vulnerability to pentylenetetrazole-induced seizures. Injection of the pro-epileptic drug induces spike train activity associated with clonic–tonic seizures in the knockout animals as opposed to the milder myoclonic seizure activity seen in wildtype littermates (Sala et al., 2011). These co-morbid findings in the OTRKO mouse suggest there may be overlap in the neurobiological systems that contribute to the complex phenotypes associated with ASD.
Evidence for construct validity
Based the profound social impairments associated with genetic modifications of the OT system in animal models, OT has been proffered as a candidate neurochemical system in the etiology ASD. Variation in the molecular, genetic and epigenetic regulation of the OT system has been associated with ASD. Single nucleotide polymorphisms in the OT receptor gene and haplotypes of these polymorphisms have been found within ethnically divergent populations to be associated with ASD, though no single haplotype has been consistently identified (Jacob et al., 2007; Lerer et al., 2008; Liu et al., 2010; Wermter et al., 2010; Wu et al., 2005). Several genome-wide linkage studies have been used to analyze autistic populations, the largest of which found a linkage peak over the 3p25 chromosomal, the location of the OTR gene (Barrett et al., 1999; Campbell et al., 2011; Lauritsen et al., 2006; McCauley et al., 2005; Philippe et al., 1999; Ylisaukko-oja et al., 2006). Interestingly, deletions or hypermethylation of the p25 region of chromosome 3 has also been found in a few autism cases (Gregory et al., 2009; Sebat et al., 2007). Hypermethylation of the OXTR promoter is associated with decreased OXTR mRNA in the temporal cortex. Peripheral levels of the peptide have also been reported to be altered in the ASD population compared, however the direction of this alteration is unclear (Jansen et al., 2006; Modahl et al., 1998). Despite the evidence linking alterations of the OT system with ASD, OT has neither emerged as a biomarker for the disorder nor a major genetic contributor. The critical role of OT in the regulation of social behavior in genetic mouse models, though, reinforces its contribution to the production of functional social behavior. The absence of dramatic alteration of the OT system in the ASD population, though, suggests the feasibility of enhancing the OT system to promote functional social cognition, irrespective of the cause of the social impairments in the disorder. Novel tools for investigating OT receptor density in the brains of patients, such as OTR PET ligands, would be potentially useful for identifying those who would likely be unresponsive to OT based therapies.
Oxytocin and monogenic forms of ASD
Of all rodent models of ASD, those based on monogenic forms of autism have the highest level of construct validity. Whereas alterations of the OT receptor gene are associated with ASD, alterations of genes including FMR1, UBE3A, DHCR7, and MeCP2 are considered causative of specific monogenic forms of autism (Kotulska and Jozwiak, 2011). Interestingly, mouse models recapitulating the genetic variation associated with monogenic autism disorders often show deficits in behavioral paradigms of social cognition (Ey et al., 2011). Despite some phenotypic similarities with OT deficient mouse models, most monogenic forms of autism are not associated with dysregulation of the OT system. Only Smith–Lemli–Opitz Syndrome (due to a mutation of the DHCR7 gene) may contain OT dysfunction in its etiology due to cholesterol’s modulatory role in the function of the OT receptor (Bukelis et al., 2007). Preliminary evidence, though, suggests that pharmacological manipulation of the OT system could enhance social function in monogenic forms of autism irrespective of the peptide’s etiological contribution. Intranasal OT increases eye gaze to social interactors and decreases cortisol response to social interactions in individuals with Fragile X Syndrome (Hall et al., 2011). The efficacy of OT in a population not characterized by OT dysregulation suggests that the peptide may be beneficial in a number of disorders with social impairments and that mouse models of monogenic forms of autism may be useful for assessing the efficacy of OT based drug therapies (including those discussed in the Therapeutic strategies for targeting the OT system section) in behavioral paradigms of social cognition.
ASD related phenotype based models
As ASD is a disorder currently diagnosed by distinct behavioral phenotypes rather than genetic or physiological biomarkers, an alternative approach in modeling the disorder is to study animal models with relevant phenotypes, in this case variation in social behavior. Social cognition in humans, like most behavioral traits, exists on a continuum, such that some individuals are extremely adept at navigating social encounters, while others have limited abilities. People with ASD fall at the low end of this continuum and are diagnosed, in part, by how they deviate from the mean in social functioning. The same variation in social behavior can be seen in animal models that contain behavioral diversity. Animal models that encompass a continuum of social behavior, with both highly social and asocial constituents, allow for the comparative investigation of the mechanisms that contribute to variation in social cognition. OT has emerged as a distinguishing feature between constituents that fall at the high and low end of social function. Two animal models, inbred mice and microtine rodents, have been used to phenotypically characterize variation in social behavior and comparatively identify the neurobiological structures that underlie this variation.
Inbred mouse models of social variability
Laboratory mice have been selectively bred to create hundreds of genetically distinct inbred lines. The genetic and phenotypic variation between these lines has been mined to identify inbred strains of mice with phenotypes relevant to autism based on high throughput behavioral screening assays. Several strains of inbred mice have been identified to have lower levels of sociability and the species typical preference for social novelty compared to other strains of mice, using tests of social recognition, reciprocal social interactions, social approach and social preference tasks (Brodkin, 2007; Silverman et al., 2010). Thus these inbred strains have face validity in the sense that their behavioral phenotype is relevant to the endophenotype in ASD, but it is unclear if they have construct or predictive validity for drug discovery. Of all the strains identified to have phenotypical similarities with ASDs, only the BTBR T+tf/J (BTBR) inbred strain, also shows alterations in the OT system, therefore we will limit our discussion to this strain.
BTBR mice have low levels of social play initiation as juveniles (Bolivar et al., 2007) and preference for social interaction, as measured in the social preference test, as adults (Moy et al., 2007). The language impairments and repetitive and stereotyped behaviors of ASD are also recapitulated in BTBR mice, which have atypical ultrasonic vocalizations during social interactions and high levels of repetitive grooming. Unlike the vocalization alterations seen in the OT related deficit models, BTBR pups emit louder and more frequent calls when isolated than other more social inbred stains of mice (Scattoni et al., 2008). As adults, though, the BTBR mice vocalize less and scent mark, a species-typical form of olfactory communication, less than more highly social strains in response to a social stimuli (Wohr et al., 2011). Interestingly, these behavioral similarities to ASD are accompanied by an increase in OT peptide levels in the paraventricular nucleus of the hypothalamus (Silverman et al., 2010). The contribution of the altered peptide levels in the low social BTBR mice has yet to be examined.
When using comparative variation models, it is important to keep in mind that the variation in the model arose through an independent selection history that does not necessarily match the evolution of the disorder. The altered systems in the model may not be altered in the disease to which it is phenotypically similar. In particular, inbred mice were all developed through human guided artificial selection and not through natural selection, which shapes non-domesticated species. Consequently, the selective pressures that led to a divergence of brain structure between high social and low social inbred mice are not the same as those that could have led to the same outcome in the natural environment. This may limit the construct and perhaps the predictive validity of inbred mouse strains for the development of pharmacological approaches to enhance social function.
Social variation in microtine rodents
In contrast to the comparative exploration of inbred mice strains, microtine rodents (voles) allow for the investigation of natural variation in social behavior. The Microtus genus exhibits a large amount of natural diversity in social behavior within its constituent species, from the highly affiliative, socially monogamous prairie voles (Microtus ochrogaster) to the asocial, non-monogamous meadow (M. pennsylvanicus) and montane voles (M. montanus). Additionally, as these are outbred species, there is also a large amount of intra-species social behavioral diversity. Both inter- and intra-species variation in social behavior have been linked to variation in the OTR system (Ross and Young, 2009). Highly social prairie voles have much higher levels of OT receptors in the nucleus accumbens, a reward center of the brain, than the asocial meadow voles, see Fig. 2 (Insel and Shapiro, 1992). In contrast, the distribution of the OT peptide is highly conserved among rodents (Ross et al., 2009a). Central administration of OT in prairie voles promotes the formation of social bonds between conspecifics (Williams et al., 1994). Correspondingly, administration of an OT receptor antagonist into the nucleus accumbens or the prefrontal cortex inhibits the formation of these bonds (Young et al., 2001). It is hypothesized that the presence of OT receptors in the nucleus accumbens allows for the pairing of neural encoding of social information, with reinforcement learning, mediated by dopamine signaling, thereby promoting a conditioned partner preference (Young and Wang, 2004). Variation in the density of OT receptors in the nucleus accumbens within prairie voles is also associated with intra-species variation in social motivation. Female prairie voles with high density of receptors in the nucleus accumbens are more likely to display alloparental behavior, in spontaneous parental care tests, than those with low receptor densities (Olazabal and Young, 2006a; Olazabal and Young, 2006b). Females with experimentally increased OTR expression in the nucleus accumbens form pair bonds more quickly, but interestingly do not show elevated alloparental behavior if the OTR manipulation is performed in adults (Ross et al., 2009b). However, if OTR levels are increased as juveniles, both partner preference and alloparental behavior is enhanced, suggesting a cumulative or “organizational” role of OTR in this regions for shaping some aspects of social behavior (Keebaugh and Young, 2011; Ross et al., 2009b).
Fig. 2.
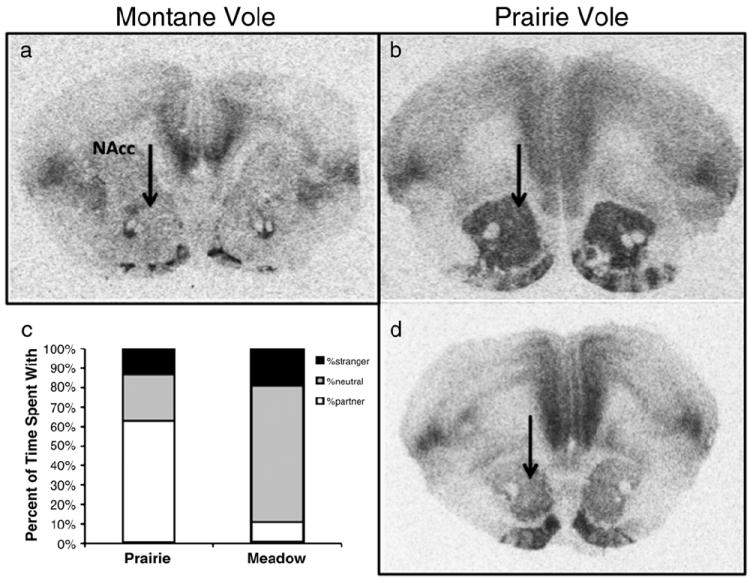
Variation in oxytocin receptor levels and partner preference. Asocial meadow voles (a) have lower levels of OT receptors in the nucleus accumbens (NAcc-indicated by black arrow) than highly social prairie voles (b), as seen in autoradiographic images of oxytocin receptor density. There is also individual variation within prairie voles in the levels of OT receptor in the NAcc (b, high d, low). The variation in receptor density, both across species and within prairie voles, corresponds to variation in partner preference behavior and alloparental behavior. Prairie voles spend more time in proximity of their familiar partner than either alone or with a novel stranger, while meadow voles spend more of their time in the neutral “nonsocial” chamber than with either conspecific while in the partner preference test (c).
While behavioral paradigms assessing prosocial behavior, like spontaneous parental care tests and the partner preference test, were originally developed to explore basic neurobiology in the prairie vole, they may have important face, construct and predictive validity for screening drugs that may enhance social motivation and social cognition more generally. For example, pair bonding is a type of social learning in which the social cues of the partner become associated with the reinforcing aspects of their interaction. This process requires that the social cues of the partner be highly salient and the encoding of those cues be linked to the reward system. Therefore, systems involved in social motivation, social information processing and learning and memory are all involved in the formation of a pair bond. The partner preference test utilizes the natural pair bonding behavior of the prairie vole to give a discrete experimental readout of social learning and expression. The conditions under which social bonds can be learned and expressed within the behavioral test are well established. These conditions can be augmented such that the learning phase of social bonding occurs under suboptimal conditions (e.g. decreased time and value of social stimuli). Experimental manipulations (genetic, behavioral or pharmacological) consequently can be made to try to promote the formation of social bonds under the suboptimal conditions, allowing for the identification of prosocial manipulations. This suboptimal pairing paradigm was used to first identify the critical role of OT in partner preference formation, as a central injection of OT was able to induce a partner preference under a brief cohabitation condition in which it would not otherwise form (Williams et al., 1994). More recently, this paradigm has been used specifically to screen for drugs that enhance social learning, which could be used in combination with social skills behavioral therapy to treat the social impairments of ASD (Modi and Young, 2011). We propose that drugs given prior to the social learning phase of the partner preference paradigm (e.g. prior to cohabitation) that accelerate bond formation, perhaps by modulating social motivation, social information processing, or learning and memory, may also work in accelerating the beneficial effects of behavioral therapies in ASD based on social reinforcement. Thus the microtine rodent natural variation phenotype model functions both comparatively for the identification of neural substrates underlying different levels of sociability and may also have predictive validity for the identification of novel prosocial therapeutics.
Therapeutic strategies for targeting the OT system
Based on the work in animal models, we propose that OT plays a critical role in 1) increasing the saliency of social stimuli and 2) linking the encoding of those stimuli to social reward and reinforcement. Thus OT can rescue social recognition in the deficit OTKO mouse, and also accelerate partner preference formation in a prairie vole with a functional OT system. This is important because targeting the OT system to increase the saliency of social stimuli and potentially enhance the interaction of the social processing and reward systems should be effective irrespective of the role of the OT system in the pathophysiology of ASD. Consequently, targeting the central OT system has considerable therapeutic potential for ameliorating the social deficits of not only ASD, but a number of psychiatric diseases characterized by social impairments (Ring, 2011).
The most direct approach to enhancing the OT system is peripheral administration of the peptide or its analogs. Due to its peripheral use in accelerating labor, biologically stable analogs, like pitocin, have been developed and approved for clinical use (Page, 1954). The initial studies of the therapeutic use of OT in the treatment of ASD made use of this compound. Intravenous administration of high doses of pitocin was found to both improve the retention of social information and reduce repetitive and stereotypic behaviors in individuals with high functioning autism and Asperger’s (Hollander et al., 2003; Hollander et al., 2007). However, while these studies provided proof of concept that the OT system can be targeted to treat ASD, there are still several limitations to its widespread clinical use based on the physiochemical properties of the peptide. OT, due to the actions of aminopeptiases, is metabolically unstable and has short half-life both in central (20 min) and peripheral circulation (about 5 min) (Mens et al., 1983). In addition, as a large, charged peptide, OT has poor penetration of the blood brain barrier (Ermisch et al., 1985; Landgraf and Neumann, 2004). Both properties of OT limit the potential for peripheral administration of the peptide to promote central neurotherapeutic effects (Ring, 2010). Thus, alternative strategies are needed to increase the central activation of the OT system for the treatment of the social impairments associated with ASD.
Intranasal OT
To circumvent the limitations posed by OT’s poor penetration of the blood brain barrier, several groups have administered the peptide intranasally. It is hypothesized that intranasal administration of peptides allows for passage through clefts in the nasal epithelium into the cerebral spinal fluid (Illum, 2000). Intranasal administration of other peptides, including vasopressin, results in a sustained increase in central levels for over an hour (Born et al., 2002), but there is not yet direct molecular evidence that OT accumulates in the brain in the same fashion. Recently, a large number of studies have looked at the behavioral effects of intranasal OT in humans and found that the peptide enhances a broad range of social and perceptual abilities. Intranasal OT increases the perception of socially important information, like emotion recognition (Domes et al., 2010), empathic perception (Bartz et al., 2010), facial identity (Rimmele et al., 2009) and the response to biological motion (Keri and Benedek, 2009), see (Bos et al., 2011) for a detailed review. It also promotes interpersonal relationships though the enhancement of socially reinforced learning (Hurlemann et al., 2010), trust (Kosfeld et al., 2005) and the experience of attachment (Buchheim et al., 2009). These effects may be, in part, mediated by increased reward associated with social encounters, due the interaction of the striatal OT and dopamine systems after intranasal administration (Rilling et al., 2011). Three studies have also looked at the prosocial effects of OT in ASD subjects. Guastella et al. (2010) found that OT improves the recognition of emotions from faces in autistic populations. Similarly, Hollander et al. (2007) found that the peptide increases the retention of social content in speech. Most recently, Andari et al. (2010) found that OT increases social interaction, trust and attention to socially informative stimuli. However, whether the effects of intranasal OT are mediated by activation of central OT receptors, or OT receptors in peripheral tissues, which then impact brain activity, remains to be determined. This distinction is currently one of the most important clinical questions in guiding therapeutic approaches for manipulating the system. Non-human primate models will likely be useful in making this critical determination. A more thorough understanding of the mechanism of action will be essential to inform the further development of drug strategies targeting the OT system.
Non-peptide agonists
Alternatives to synthetic OT are non-peptide, small molecule agonists, partial agonists or positive allosteric modulators, which could target and activate the OT receptor without the physical limitations of peptide agonists. The benefit of non-peptide agonists lies in the potential for increased stability, central penetration and oral bioavailability, making the agonist more amenable to classical routes of pharmacotherapeutic administration (Ashworth et al., 2006; Pitt et al., 2004). In addition, the increased epitope specificity possible with small, non-peptide agonists potentially allows for increased selectivity for the OTR over the structurally similar vasopressin receptors (Hawtin et al., 2001). Two groups have identified non-peptide molecules with high affinity for the OTR and have potent agonistic properties. WAY-267464 is a first generation agonist developed by Wyeth Pharmaceuticals with 87% of the intrinsic efficacy of OT and is 100 times more selective for the OTR than any other members of the OT/vasopressin receptor family (Ring et al., 2010). The compound produces anxiolytic effects in rodent behavioral paradigms similar to those seen with OT, which can be blocked by application of the OTR antagonist (Ring et al., 2010). However, the effects of WAY-267464 have not yet been reported in paradigms assessing social cognition. Ferring Pharmaceuticals also developed a potent nonpeptide agonist, which maximally stimulates the OTR to the same degree as OT and is 25 times for selective for the OTR than any other receptors in the family (Pitt et al., 2004). Behavioral efficacy of the Ferring agonist, though, has yet to be demonstrated. Behavioral effects of positive allosteric modulators of the OTR have not been reported, but these would have the advantage that they would only stimulate the OTR in the context of endogenous OTR activation, making that activation more potent.
Enhancing endogenous OT release
As an alternative to exogenous administration of OT or OT agonists, the endogenous OT system can be manipulated to increase central levels. One method of increasing central OT levels is by promoting the release of the peptide from OT producing neurons. Stimulation of receptors that regulate oxytocinergic neurons, through pharmacological manipulation, has been used to promote both central and peripheral OT release (Bagdy and Kalogeras, 1993; Sabatier et al., 2003). Receptors identified as being expressed on oxytocinergic neurons or on cells that interact with oxytocinergic neurons can be assayed to determine their ability to stimulate OT release and their subsequent therapeutic potential. Receptor systems are targeted based on their interactions with the OT system and not based on their other endogenous functions, though those functions should be considered as potential side effects at the clinical level. For example, stimulation of melanocortin receptors on the oxytocinergic neurons of the supraoptic neurons (SON) induces central, but not peripheral release of OT in rats, and a melanocortin 4 receptor (MC4R) antagonist blocks this effect (Sabatier, 2006). Thus MC4R agonists would presumably increase central OT release, circumventing the limitations of peripheral peptide administration. Furthermore, if this mechanism promotes both somatodendritic release and release from axon collateral dense core vesicles, OT would be elevated in brain regions most responsive to the peptide. Preliminary studies suggest that administration of a melanocortin receptor agonist promotes partner preference development in prairie vole (Modi and Young, unpublished data). However, the MC4R also has been targeted in drug development to reduce appetite and to increase sexual arousal, which may be confounding side effects in the treatment of disorders such as autism. These other activities of MC4R agonists must be taken into consideration in a drug discovery context.
The serotonin system is also involved in the regulation of OT secretion. Both serotoninergic fibers and receptors are located in the oxytocinergic SON and paraventricular nuclei of the hypothalamus, where they regulate the release of neurohypophyseal hormones (Jorgensen et al., 2003). Specifically, agonists of the 5-HT1a receptor, including 8-OHDPAT, DOI and buspirone, cause substantial increases in plasma OT levels, see Fig. 3a (Bagdy and Kalogeras, 1993; Uvnas-Moberg et al., 1996). 5-HT1a receptor-induced release of OT is even thought to underlie the prosocial effects of the hyperserotonergic drug of abuse, MDMA (Thompson et al., 2007). Buspirone, a clinically available partial 5-HT1a agonist, in addition to increasing peripheral levels of OT, promotes OT-dependent prosocial behaviors. As a proof of principle for the idea that stimulating OT release may be a viable means of enhancing social cognition, we performed a study to determine whether buspirone would accelerate partner preference formation in a suboptimal paradigm in female prairie voles.
Fig. 3.
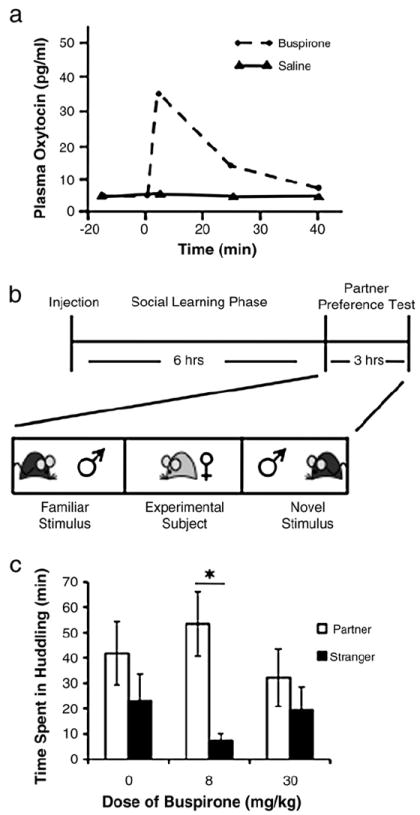
The effects of buspirone on OT release and partner preference formation in prairie voles. (a) Peripheral administration of buspirone (0.7 mg/kg intravenous), a 5-HT1a partial agonist, induces an increase in plasma OT levels (graph modified from Bagdy and Kalogeras, 1993). (b) The partner preference paradigm was used to assess the effect of buspirone on the formation of OT-dependent pair bonds. Buspirone (0, 8, 30mg/kg) was administered to non-sexually receptive, adult female prairie voles via intraperitoneal injection immediately prior to a six-hour cohabitation with an adult, sexually experienced male prairie vole. After the social learning phase, the formation of a partner preference by the experimental subject was tested in the partner preference test. During the test, the male partner was tethered in one arena of a three-chambered linear test box and a novel male was tethered in the opposite chamber. The experimental female was allowed to freely wander the test box for three hours and the amount of time spent huddling with either the partner or stranger was recorded using TopScan behavioral analysis software (CleverSys Inc. Reston, VA). (c) Females receiving an 8 mg/kg dose of buspirone but not a control injection or 30mg/kg dose formed a robust pair bond under suboptimal social learning conditions, as indicated by spending greater than twice the amount of time with the partner than the stranger (* indicates a significant partner preference). A comparison using a 2-way ANOVA revealed a significant main effect of stimulus animal (F(1,27)=9.262, p=0.004) no other significant main effects or interactions were found. To determine which treatments resulted in significantly more time spent with the partner than the stranger, post-hoc Student’s t-tests were performed with Bonferroni corrections of the p-value. Peripheral administration of 8 mg/kg of buspirone resulted in significantly more time spent with the partner than the stranger (p<0.003; Student’s t-test, Bonferroni level set at p<0.01), no other treatments resulted in a preference.
Twenty-nine female prairie voles were ovariectomized and given two days to recover. Females were then injected intraperitoneally with either vehicle (saline), 8 mg/kg or 30 mg/kg buspirone hydrochloride (Sigma-Aldrich, St. Louis, MO) and immediately cohabitated with an adult sexually experienced male prairie vole. After six hours of cohabitation, the experimental females were tested for partner preference, as previously described using an automated behavioral analysis system, see Fig. 3b (Ahern et al., 2009;Modi and Young, 2011). Animals receiving saline or the high dose of buspirone failed to display a partner preference. However, those animals receiving 8 mg/kg displayed a significant partner preference, see Fig. 3c. Thus buspirone, which acts on the 5-HT1a receptors to stimulate OT release, has the same behavioral effect on social bonding in female prairie voles as does a central injection of OT. We hypothesize that the accelerating effects of buspirone on partner preference are mediated through the OT system, but confirmation of this through co-infusion with OT antagonist is needed. The serotonin system is also intimately involved in the regulation of social interactions and anxiety thus it is possible that this effect is mediated by an OT independent mechanism. It is possible based on buspirone current clinical use as an anxiolytic that the effect on partner preference could be due to a reduction in anxiety rather than the promotion of social cognition. However, buspirone is just one example of indirectly stimulating the OT system to achieve the potential social behavioral benefits of the peptide. There are potentially numerous drugable receptor targets on oxytocinergic neurons that could be used to enhance OT release. This potential highlights the need for a systematic characterization of the receptors on OT neurons, so that the pathway with the greatest efficacy and least off target effects can be identified for pharmacotherapeutic use.
The OT system can also be pharmacologically enhanced by activating intracellular mechanisms, like the CD38 enzyme. Retinoids are a class of compounds related to vitamin A (retinol) including all-trans-retinoic-acid (ATRA), which has been proposed as a novel therapeutic strategy targeting the OT in the treatment of ASD. ATRA, a high-affinity ligand for retinoic acid receptors, is a potent inducer of CD38 and thus presumably OT release (Kishimoto et al., 1998). Cell lines derived from the lymphoblastoid cells of individuals with ASD have lower expression of CD38 mRNA than those derived from a control population (Lerer et al., 2010). Treatment of the ASD derived cells lines with ATRA results in increased CD38 mRNA expression, the same occurs in the control derived lines to a lesser extent (Ebstein et al., 2011). This suggests that ATRA could potentially be used to increase CD38 levels in individuals with autism, and in turn increase OT release through the endogenous inducer function of the protein.
Oxytocinase inhibitors
In addition to regulation of extracellular concentrations of OT through regulation of release, OT levels are also regulated through enzymatic degradation. Extracellular OT is limited by the enzymatic actions of aminopeptidases, which degrade the peptide to limit its functional activity. Placental leucine aminopeptidase (P-LAP, also known as insulin regulated amino peptidase) preferentially degrades OT by cleaving the peptide bond between theN-terminal cysteine and the adjacent tyrosine residue to inactivate the hormone (Tsujimoto and Hattori, 2005). Peripherally, P-LAP is released from the placenta and increases in maternal serum during pregnancy to maintain appropriate OT levels throughout pregnancy. P-LAP is also expressed in the brain, selectively in neurons (Matsumoto et al., 2001). High levels of P-LAP are expressed in selected olfactory regions, throughout the hippocampus and co-localized with OT and vasopressin neurons in the hypothalamus (Fernando et al., 2005). The overlapping distribution of the enzyme with OTR rich brain regions suggests that the enzyme plays a role in maintaining brain OT levels. Recently, competitive peptide inhibitors of the enzyme have been identified, including amastatin, angiotensin IV and LVV-hemorphin 7, which bind specifically with high affinity to the catalytic site to prevent aminopeptidase activity (Lew et al., 2003; Mizutani et al., 1992). The inhibitors have been used to facilitate memory in a number of paradigms and thus may be a viable strategy for promoting behavioral effects by inhibiting brain peptide degradation (Albiston et al., 2011; Chai et al., 2008). To further investigate therapeutic potential, the effect of P-LAP inhibitors should be compared to those of central OT in behavioral paradigms of social cognition, like social recognition in mice or partner preference in the prairie vole. However, it must be considered that aminopeptidases are not specific for OT and therefore, like many potential pharmacological approaches, the effects on other systems, and the consequential side effects, must be scrutinized.
Oxytocin manipulations as adjuncts to behavioral therapies
OT based therapies of any class may be most beneficial for the treatment of social impairments as a pharmacological adjunct to behavioral therapies. It is hypothesized that OT acts to enhance the saliency of social information and to assign social stimuli a positive valence. In that capacity, many of the initial studies of OT on functional human social cognition have demonstrated short-term prosocial effects evident in discrete experimental measures, including socially reinforced learning (Hurlemann et al., 2010). However, it is unclear if OT treatment alone can induce long-term improvement in measures of holistic social cognition. We propose that the saliency and valence effects of OT could enhance the acquisition of social skills taught as a part of structured behavior therapy program, like applied behavioral analysis. Many of the sub-skills already taught as a part of social skills training paradigms, like maintaining eye contact and understanding facial expressions, are modulated by OT(Guastella et al., 2008; Van Ijzendoorn and Bakermans- Kranenburg, 2011). Administration of a drug that enhances the function of the OT system may enable the patient to better attend to and from positive associations with the relevant social information presented. This treatment model is reflected in the design of one of the behavioral paradigms discussed, the partner preference test, in that potential prosocial drugs are administered prior to the social learning phase, so that the animals are receiving both pharmacological and behavioral stimulation simultaneously. The partner preference test could be used predictively to identify OT based therapies that would be most efficacious in a combined pharmacological/behavioral treatment strategy (Modi and Young, 2011).
Conclusion
Irrespective of the contribution of the OT system to the pathophysiology of the disorder, the OT system has a strong therapeutic potential for the treatment of social impairments in ASD and a number of other psychiatric disorders, based on its critical role in the modulation of social cognition in animal models. The potential benefits of OT, though, are limited by the biophysical properties of the peptide. This necessitates the development and validation of animal models and behavioral paradigms of social cognition to identify the most efficacious methods of upregulating the OT system. The potential use of genetic based and phenotype based animal models in the context of the various therapeutic approaches for targeting the OT system, can be seen in Table 2. Thus far, the potential benefits of pharmacologically enhancing the OT system have been limited by the scale of the available methods to manipulate the system. The development of more potent methods stimulating the OT system could have profound effects on social cognitive processes, which could be harnessed clinically. Thus, while OT may play a negligible role in the induction of social impairments of ASD it may still have a meaningful effect on the manifestation of the disorder. It is imperative that the field work to fill in the knowledge gaps in the relationship between the OT system and human social cognition, including characterization of the human functional and disorder OT system, the mechanism of action of intranasal OT, and the long-term efficacy of OT administration to realize the clinical potential of OT in the treatment of social impairments.
Acknowledgments
We would like to acknowledge funding support fromNIH MH064692 (LJY) and RR00165 to YNPRC.
References
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiston AL, Diwakarla S, Fernando RN, Mountford SJ, Yeatman HR, Morgan B, Pham V, Holien JK, Parker MW, Thompson PE, Chai SY. Identification and development of specific inhibitors for insulin-regulated aminopeptidase as a new class of cognitive enhancers. Br J Pharmacol. 2011;164:37–47. doi: 10.1111/j.1476-5381.2011.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico JA, Cai HM, Vollmer RR. Corticosterone release in oxytocin gene deletion mice following exposure to psychogenic versus non-psychogenic stress. Neurosci Lett. 2008;442:262–266. doi: 10.1016/j.neulet.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel J, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth DM, Batt AR, Baxter AJ, Broqua P, Haigh RM, Hudson P, Heeney CM, Laporte R, Penson AM, Pitt GR, Robson PA, Rooker DP, Tartar AL, Yea CM, Roe MB. Nonpeptide oxytocin agonists. Drugs Future. 2006;31:345. [Google Scholar]
- Bagdy G, Kalogeras KT. Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain Res. 1993;611:330–332. doi: 10.1016/0006-8993(93)90521-n. [DOI] [PubMed] [Google Scholar]
- Barrett S, Beck JC, Bernier R, Bisson E, Braun TA, Casavant TL, Childress D, Folstein SE, Garcia M, Gardiner MB, Gilman S, Haines JL, Hopkins K, Landa R, Meyer NH, Mullane JA, Nishimura DY, Palmer P, Piven J, Purdy J, Santangelo SL, Searby C, Sheffield V, Singleton J, Slager S. An autosomal genomic screen for autism. Collaborative linkage study of autism. Am J Med Genet. 1999;88:609–615. doi: 10.1002/(sici)1096-8628(19991215)88:6<609::aid-ajmg7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Ochsner KN. Oxytocin selectively improves empathic accuracy. Psychol Sci. 2010;21:1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interctions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, Honk JV. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2011.01.002. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, O’Connor MF, Gundel H. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 2009;34:1417–1422. doi: 10.1016/j.psyneuen.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukelis I, Porter FD, Zimmerman AW, Tierney E. Smith–Lemli–Opitz syndrome and autism spectrum disorder. Am J Psychiatry. 2007;164:1655–1661. doi: 10.1176/appi.ajp.2007.07020315. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EA, Levitt P. Association of oxytocin receptor (OXTR) gene variants with multiple phenotypes domains of autism spectrum disorder. J Neurodev Disord. 2011;3:101–112. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SY, Yeatman HR, Parker MW, Ascher DB, Thompson PE, Mulvey HT, Albiston AL. Development of cognitive enhancers based on inhibition of insulin-regulated aminopeptidase. BMC Neurosci. 2008;9(Suppl 2):S14. doi: 10.1186/1471-2202-9-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Mankuta D, Yirmiya N, Malavasi F. Are retinoids potential therapeutic agents in disorders of social cognition including autism? FEBS Lett. 2011;585:1529–1536. doi: 10.1016/j.febslet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Ruhle HJ, Skopkova J, Hrbas P, Landgraf R. On the blood–brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- Ey E, Leblond CS, Bourgeron T. Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 2011;4:5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RN, Larm J, Albiston AL, Chai SY. Distribution and cellular localization of insulin-regulated aminopeptidase in the rat central nervous system. J Comp Neurol. 2005;487:372–390. doi: 10.1002/cne.20585. [DOI] [PubMed] [Google Scholar]
- Gabis L, Pomeroy J, Andriola MR. Autism and epilepsy: cause, consequence, comorbidity or coincidence? Epilepsy Behav. 2005;7:652–656. doi: 10.1016/j.yebeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7 doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, McCarthy BE, Parker KJ, Reiss AL. Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.07.020. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtin SR, Howard HC, Wheatley M. Identification of an extracellular segment of the oxytocin receptor providing agonist-specific binding epitopes. Biochemistry. 2001;354:465–472. doi: 10.1042/0264-6021:3540465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Kikuchi M. CD38 and its role in oxytocin secretion and social behavior. Horm Behav. 2011a doi: 10.1016/j.yhbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Munesue T, Kikuchi M, Minabe Y, Lopatina O. Cd38 gene knock-out juvenile mice: a model of oxytocin signal defects in autism. Biol Pharmacol Bull. 2011b;34:1369–1372. doi: 10.1248/bpb.34.1369. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Wiegant VM, Westenberg HG, Lahuis BE, van Engeland H. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord. 2006;36:891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Riis M, Knigge U, Kjaer A, Warberg J. Serotonin receptors involved in vasopressin and oxytocin secretion. J Neuroendocrinol. 2003;15:242–249. doi: 10.1046/j.1365-2826.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Benedek G. Oxytocin enhances the perception of biological motion in humans. Cogn Affect Behav Neurosci. 2009;9:237–241. doi: 10.3758/CABN.9.3.237. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Hoshino S, Ohori M, Kontani K, Nishina H, Suzawa M, Kato S, Katada T. Molecular mechanism of human CD38 gene expression by retinoic acid. Identification of retinoic acid response element in the first intron. J Biol Chem. 1998;273:465–483. doi: 10.1074/jbc.273.25.15429. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kotulska K, Jozwiak S. Autism in monogenic disorders. Eur J Paediatr Neurol. 2011;15:177–180. doi: 10.1016/j.ejpn.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB, Als TD, Dahl HA, Flint TJ, Wang AG, Vang M, Kruse TA, Ewald H, Mors O. A genome-wide search for alleles and haplotypes associated with autism and related pervasive developmental disorders on the Faroe Islands. Mol Psychiatry. 2006;11:37–46. doi: 10.1038/sj.mp.4001754. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland adaptive behavior scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Israel S, Yaari M, Nemanov L, Mankuta D, Nurit Y, Ebstein RP. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Res. 2010;3:293–302. doi: 10.1002/aur.156. [DOI] [PubMed] [Google Scholar]
- Levine TP, Sheinkopf SJ, Pescosolido M, Rodino A, Elia G, Lester B. Physiologic arousal to social stress in children with autism spectrum disorders: a pilot study. Res Autism Spectr Disord. 2012;6:177–183. doi: 10.1016/j.rasd.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RA, Mustafa T, Ye S, McDowall SG, Chai SY, Albiston AL. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP) J Neurochem. 2003;86:344–350. doi: 10.1046/j.1471-4159.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- Liu HX, Lopatina O, Higashida C, Tsuji T, Kato I, Takasawa S, Okamoto H, Yokoyama S, Higashida H. Locomotor activity, ultrasonic vocalization and oxytocin levels in infant CD38 knockout mice. Neurosci Lett. 2008;448:67–70. doi: 10.1016/j.neulet.2008.09.084. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, Nishida H, Hashimoto O, Nakagami R, Tochigi M, Umekage T, Kano Y, Miyagawa T, Kato N, Tokunaga K, Sasaki T. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intra-strain but not inter-strain, social recognition. Genes Brain Behav. 2009;8:558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Nagasaka T, Hattori A, Rogi T, Tsuruoka N, Mizutani S, Tsujimoto M. Expression of placental leucine aminopeptidase/oxytocinase in neuronal cells and its action on neuronal peptides. Eur J Biochem. 2001;268:3259–3266. doi: 10.1046/j.1432-1327.2001.02221.x. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Li C, Jiang L, Olson LM, Crockett G, Gainer K, Folstein SE, Haines JL, Sutcliffe JS. Genome-wide and ordered-subset linkage analyses provide support for autism loci on 17q and 19p with evidence of phenotypic and interlocus genetic correlates. BMC Med Genet. 2005;6 doi: 10.1186/1471-2350-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wilmersma Greidanus TB. Penetration of neurophyophyseal hormones from plasma into cerebrospinal fluid: half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Mizutani S, Yokosawa H, Tomoda Y. Degradation of oxytocin by the human placenta: effect of selective inhibitors. Acta Endocrinol (Copenh) 1992;127:76–80. doi: 10.1530/acta.0.1270076. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. D-cycloserine facilitates socially reinforced learning in an animal model relevant to autism spectrum disorders. Biol Psychiatry. 2011;70:298–304. doi: 10.1016/j.biopsych.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Page EW. The usefulness of intravenous pitocin infusions in obstetrics. West J Surg Obstet Gynecol. 1954;62:125–135. [PubMed] [Google Scholar]
- Philippe A, Martinez M, Guilloud-Bataille M, Gilberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, Van Maldergem L, Penet C, Feingold J, Brice A, Leboyer M. Genome-wide scan for autism susceptibility genes. Paris Autism Research International Sibpair Study. Hum Mol Genet. 1999;8:805–812. doi: 10.1093/hmg/8.5.805. [DOI] [PubMed] [Google Scholar]
- Pitt GR, Batt AR, Haigh RM, Penson AM, Robson PA, Rooker DP, Tartar AL, Trim JE, Yea CM, Roe MB. Non-peptide agonists. Bioorg Med Chem Lett. 2004;14:4585–4589. doi: 10.1016/j.bmcl.2004.04.107. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur J Pharmacol. 1991;1:555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Popik P, Vos PE, van Ree JM. Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav Pharmacol. 1992;3:351–358. [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.07.013. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH. A complicated picture of oxytocin action in the central nervous system revealed. Biol Psychiatry. 2011;69:818–819. doi: 10.1016/j.biopsych.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, Sukoff Rizzo SJ, Luo B, Beyer CE, Logue SF, Marquis KL, Hughes ZA, Rosenzweig-Lipson S. Receptor and behavioral pharmacology ofWAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N. alpha-Melanocyte-stimulating hormone and oxytocin: a peptide signaling cascade in the hypothalamus. J Neuroendocrinol. 2006;18:703–710. doi: 10.1111/j.1365-2826.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social decifics, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M, Hattori A. The oxytocinase subfamily of M1 aminopeptidases. Biochim Biophys Acta. 2005;1751:9–18. doi: 10.1016/j.bbapap.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Hillegaart V, Alster P, Ahlenius S. Effects of 5-HT agonists, selective for different receptor subtypes, on oxytocin, CCK, gastrin and somatostatin plasma levels in the rat. Neuropharmacology. 1996;35:1635–1640. doi: 10.1016/s0028-3908(96)00078-0. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.07.008. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- Wermter AK, Kamp-Becker I, Hesse P, Schulte-Korne G, Strauch K, Remschmidt H. Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:629–639. doi: 10.1002/ajmg.b.31032. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology. 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Alarcón M, Cantor RM, Auranen M, Vanhala R, Kempas E, von Wendt L, Järvelä I, Geschwind DH, Peltonen L. Search for autism loci by combines analysis of autism genetic resource exchange and Finish families. Ann Neurol. 2006;59:145–155. doi: 10.1002/ana.20722. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]


