Abstract
The aim of the study was to determine whether reactive hypoglycaemia in pancreas transplant recipients that followed administration of glucagon-like peptide-1 (GLP-1) was associated with excessive insulin, insufficient glucagon, or both. Methodology involved six portally drained pancreas recipients who received GLP-1 (1.5 pmol/kg/min) or placebo infusion on randomized occasions during glucose-potentiated arginine testing. The second subject developed symptomatic hypoglycaemia [plasma glucose (PG) 42 mg/dl] 1 h after GLP-1 administration; subsequent subjects received intravenous glucose following GLP-1, but not placebo, infusion for PG levels <65 mg/dl. Following GLP-1 vs. placebo infusion, PG was lower (58 ± 4 vs. 76 ± 5 mg/dl; p < 0.05) despite administration of intravenous glucose. During hypoglycaemia, insulin levels and the insulin-to-glucagon ratio were greater after GLP-1 vs. placebo infusion (p < 0.05), while glucagon did not vary. It can be concluded from the study that GLP-1 can induce reactive hypoglycaemia in pancreas transplant recipients through excessive insulin secretion associated with an increased insulin-to-glucagon ratio.
Keywords: glucagon, glucagon-like peptide-1, insulin
Introduction
The incretin hormone glucagon-like peptide-1 (GLP-1) is normally secreted by intestinal L-cells in response to nutrient ingestion and stimulates postprandial insulin secretion in a glucose-dependent fashion. Thus, GLP-1 mimetic therapy for hyperglycaemia is expected to augment insulin secretion and consequently lower plasma glucose (PG) without causing hypoglycaemia. While investigating the effect of GLP-1 on insulin secretion during glucose-potentiated arginine testing in pancreas transplant recipients [1], we were surprised when the second subject developed symptomatic hypoglycaemia 1 h after GLP-1 infusion with clammy perspired skin and a PG of 42 mg/dl corrected by intravenous glucose administration. The next four subjects received intravenous glucose for PG levels <65 mg/dl from 30 to 60 min after the GLP-1 infusion ended. As GLP-1 increased insulin levels ~2.5-fold when compared with placebo by the end of the glucose-potentiated arginine test [1], we hypothesized that the greater insulin secretion associated with GLP-1 might suppress an appropriate increase in glucagon to hypoglycaemia, as evidenced by an increased molar ratio of insulin-to-glucagon during the period of reactive hypoglycaemia.
Subjects and Methods
Six pancreas transplant recipients with portal venous drainage participated [1] in the study. They were three men and three women aged 40 ± 2 years, BMI 23.3 ± 0.8 kg/m2, with type 1 diabetes for 32 ± 2 years who had undergone pancreas–kidney transplantation between 3 and 48 months before study, and had excellent pancreas (HbA1c 5.0 ± 0.2%) and kidney (serum creatinine 1.2 ± 0.2 mg/dl) graft function while taking prednisone (5 mg daily), tacrolimus and mycophenolate mofetil for immunosuppression. GLP-17–36 amide or matching placebo was infused on randomized occasions at a rate of 1.5 pmol/kg/min, with a double infusion rate for the first 10 min, from t = −30 to 60 min, with 5 g arginine injected under basal (t = 0 min) and ~230 mg/dl hyperglycaemic clamp (t = 55 min) conditions [1]. At t = 60 min both the GLP-1 or placebo, and glucose infusions were stopped. After the second subject experienced symptomatic hypoglycaemia 1 h after termination of the infusions, the remaining subjects had arterialized PG levels monitored every 10 min during the postinfusion observation period. Plasma samples collected from all the subjects up to 150 min to document levels of GLP-17–36 amide were subsequently assayed for glucose, insulin and glucagon as previously described [1] in order to determine the insulin-to-glucagon ratios during the period of hypoglycaemia.
Data are means ± standard error. Comparisons between GLP-1 and placebo infusions were performed by repeated measures analysis of variance (ANOVA) followed by the Wilcoxon matched pairs test with significance considered at p < 0.05 (two-tailed).
Results
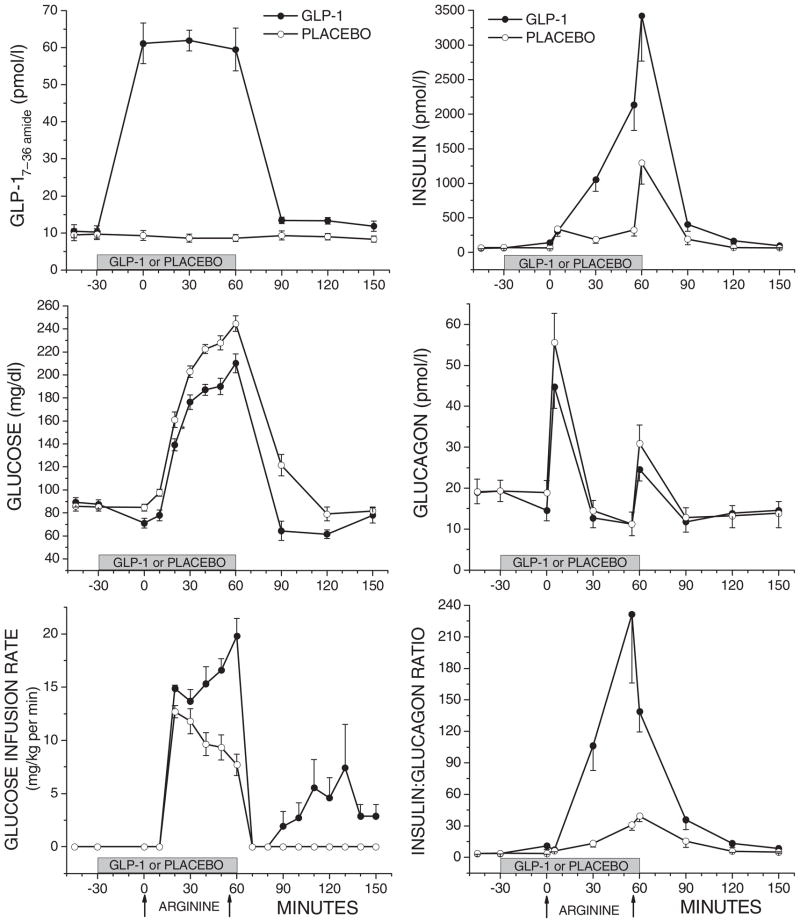
During the glucose-potentiated arginine test, levels of active GLP-1 were elevated approximately sixfold during GLP-1 infusion, then dropped back to preinfusion levels that remained slightly higher than those observed on the day of placebo infusion (p < 0.05; Figure 1). After the glucose-potentiated arginine test, PG was significantly lower following discontinuation of GLP–1 vs. placebo infusion (nadir glucose58 ± 4 mg/dl a median 30 min after GLP-1 vs. 76 ± 5 mg/dl a median 60 min after placebo; p < 0.05; Figure 1). The PG remained lower following GLP-1 vs. placebo infusion for 90 min even with the resumption of glucose administration after 30 min for PG levels <65 mg/dl in all but the first subject following GLP-1 infusion, and not in any subject following placebo infusion (p < 0.05; Figure 1). Insulin levels remained significantly greater after GLP-1 vs. placebo infusion (p < 0.05; Figure 1), while glucagon levels did not vary, resulting in a significantly increased molar ratio of insulin-to-glucagon during the period of hypoglycaemia (p < 0.05; Figure 1).
Figure 1.
Results during and after the glucose-potentiated arginine test under GLP-1 (-●-) or placebo (-○-) infusion. On the left are plasma levels of GLP-1, glucose and the glucose infusion rate during (t = 0–60 min) and after (t = 60–150 min) testing. On the right are corresponding plasma levels of insulin, glucagon and the insulin:glucagon ratio. To express glucose in mmol/l, multiply it by 0.0551.
Discussion
These results show reactive hypoglycaemia from 30 to 90 min after the administration of GLP-1. Reactive hypoglycaemia can occur when insulin levels are inappropriately elevated for the PG level and counter-regulatory glucagon secretion fails to activate. A PG level of 60 mg/dl normally activates glucagon secretion in pancreas recipients [2]. In this study glucagon levels were suppressed during the period of hypoglycaemia, including the symptomatic subject when the PG was 42 mg/dl. GLP-1 has been reported not to affect glucagon activation during hypoglycaemia induced by exogenous insulin [3], implying that hypoglycaemia should not occur with GLP-1-based therapies. However, here GLP-1 augmentation of endogenous β-cell insulin secretion likely inhibited glucagon secretion through a paracrine effect on α-cell function [4]. The resulting increased molar ratio of insulin-to-glucagon would prevent the increase in hepatic glucose production necessary to circumvent the development of hypoglycaemia [5].
While the experimental conditions were not physiologic, the plasma levels of glucose, GLP-1 and insulin achieved during the glucose-potentiated arginine test were in the range reported with alimentary forms of reactive hypoglycaemia that can occur 1–2 h after meal ingestion in some patients following upper gastrointestinal surgery [6,7]. In alimentary reactive hypoglycaemia, rapid nutrient delivery and absorption result in exaggerated postprandial levels of glucose, endogenous GLP-1, and consequently insulin. GLP-1 effects have also been associated with reactive hypoglycaemia in the absence of altered gastrointestinal anatomy, as evidenced by a patient with postprandial hypoglycaemia that resolved after removal of a GLP-1 secreting neuroendocrine tumour [8], and by some islet transplant recipients who experienced postprandial hypoglycaemia on GLP-1 mimetic (exenatide) therapy [9]. Indeed, administration of GLP-1 together with glucose in normal subjects induced reactive hypoglycaemia 30 min later [10], an identical time frame as reported here. Thus, in the presence of a normal islet β-cell mass, GLP-1 mimetics could induce reactive hypoglycaemia through excessive insulin secretion occurring together with suppressed glucagon and an increased molar ratio of insulin-to-glucagon as shown here in pancreas transplant recipients.
Acknowledgments
This work was supported by Public Health Services Research Grants P30-DK-19525 (Penn Diabetes Endocrinology Research Center Pilot Award to M.R.R.) and UL1-RR-024134 (Penn Clinical & Translational Research Center) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are indebted to Rebecca Mueller for her expert coordination of the study protocol and review of the manuscript, to Dr. Heather Collins of the Penn Diabetes Endocrinology Research Center for performance of the radioimmunoassay, to the nursing staff of the Penn Clinical & Translational Research Center for their subject care and technical assistance, and to Huong-Lan Nguyen for laboratory assistance.
References
- 1.Rickels MR, Mueller R, Markman JF, Naji A. Effect of GLP-1 on beta-and alpha-cell function in isolated islet and whole pancreas transplant recipients. J Clin Endocrinol Metab. 2009;94:181–189. doi: 10.1210/jc.2008-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paty BW, Lanz K, Kendall DM, Sutherland DER, Robertson RP. Restored hypoglycemic counterregulation is stable in successful pancreas transplant recipients for up to 19 years after transplantation. Transplantation. 2001;72:1103–1107. doi: 10.1097/00007890-200109270-00021. [DOI] [PubMed] [Google Scholar]
- 3.Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74:2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unger RH. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971;20:834–838. doi: 10.2337/diab.20.12.834. [DOI] [PubMed] [Google Scholar]
- 6.Gebhard B, Holst JJ, Biegelmayer C, Miholic J. Postprandial GLP-1, nore-pinephrine, and reactive hypoglycemia in dumping syndrome. Dig Dis Sci. 2001;46:1915–1923. doi: 10.1023/a:1010635131228. [DOI] [PubMed] [Google Scholar]
- 7.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 8.Todd JF, Stanley SA, Roufosse CA, et al. A tumour that secretes glucagon-like peptide-1 and somatostatin in a patient with reactive hypoglycaemia and diabetes. Lancet. 2003;361:228–230. doi: 10.1016/s0140-6736(03)12256-8. [DOI] [PubMed] [Google Scholar]
- 9.Froud T, Faradji RN, Pileggi A, et al. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy, and metabolic effects. Transplantation. 2008;86:36–45. doi: 10.1097/TP.0b013e31817c4ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toft-Nielsen M, Madsbad S, Holst JJ. Exaggerated secretion of glucagon-like peptide-1 (GLP-1) could cause reactive hypoglycaemia. Diabetologia. 1998;41:1180–1186. doi: 10.1007/s001250051049. [DOI] [PubMed] [Google Scholar]