Abstract
The effectivenes of cancer vaccines in inducing CD8+Tcell responses remains a challenge, resulting in a need for testing more potent adjuvants. Our objective was to determine the safety and immunogenicity of vaccination against melanoma-related antigens employing MART-1, gp100, and tysosinase paptides combined with the TLR-9 agonist PF-3512676 and local GM-CSF in-oil emulsion.
Using continuous monitoring of safety and a two-stage design for immunological efficacy, 20 immune-response evaluable patients were targetted. Vaccinations were given subcutaneously on days 1 and 15 per cycle (1 cycle=28 days) for up to 13 cycles. IFN-γ ELIspot was used as the primary assay measuring the frequency of peripheral antigen-specific CD8+T-cells at days 50 and 90 compared to baseline (target ≥9/20 immunologic responses). Clinical responses were measured by RECIST every 8 weeks.
Twenty two (including 20 immune-response evaluable) melanoma patients were enrolled. All had AJCC stage IV (5M1a, 6M1b, 11M1c) and most had previously received therapy. Eight had previously-treated brain metastases. An average of 3.5 cycles of vaccination per patient were administered. Clinical response data were available for 21 patients. There were 2PR and 8SD lasting 2–7 months. One patient with ongoing PR continued on treatment. At a median follow-up of 7.39 months (range 3.22–20.47), median PFS was 1.9 months (90%CI=1.84–3.68) and median OS was 13.4 months (90%CI=11.3-Inf). No regimen-related grade 3/4/5 toxicities were observed. There were 9/20 patients with positive ELISPOT at day 50 and/or day 90.
Our adjuvant regimen combining PF-3512676 and GM-CSF was safe and is worthy of further testing with these or alternative peptides, potentially in combination with antibodies that target immunoregulatory checkpoints.
Keywords: melanoma, vaccine, TLR9, PF-3512676, GM-CSF
INTRODUCTION
In advanced melanoma, the host immune response is compromised, with evidence of displaying strong melanoma antigen-specific Th2-type polarization1, and a tumor microenvironment that supports disease progression.2 Therefore, host immune tolerance of melanoma appears to be a hurdle to the therapy of advanced disease. Overcoming melanoma-induced immune suppression may be achieved through stimulation of dendritic cell (DC) receptors such as TLR-9 and CD40 leading to enhanced expression of costimulatory molecules on the surface of DC and enhanced antigen presentation.3, 4 Another strategy is to enhance T-cell activation by blocking negative signaling receptors such as programmed death ligand 1 (PD-1) or cytotoxic T-lymphocyte antigen-4 (CTLA4), or overcoming indoleamine deoxygenase (IDO) mediated suppressive effects or augmenting costimulatory functions (4-1BB/anti-CD137.5 Other key approaches to overcoming melanoma tolerance are cancer vaccines designed to increase immune recognition of tumor cells and to enhance and focus the antitumor effector immune response through tumor specific lymphocyte activation. The advantage of using cancer vaccination is the targeting of the host immune response and creating melanoma specific immunity. These include dendritic cell (DC)-based vaccination and other melanoma specific vaccines comprised of whole tumor cells, tumor-cell lysates or specific peptides. This is in addition to DNA vaccines, heat shock proteins (HSPs) and gene therapy. Vaccination with the multi-epitope peptide vaccine containing MART-1 (27–35), gp100 (209–217, 210M), and tyrosinase (368–276, 370D) peptides was tested in the Eastern Cooperative Oncology Group E1696 trial for metastatic melanoma with or without interferon (IFN)-α2b or GM-CSF (both given systemically) as immune adjuvants, in a 2×2 factorial design. A total of 120 subjects were enrolled, but only 75 had complete immunological data. CD8 T-cell responses to one or more of the 3 antigens targeted as measured by ELISPOT was invoked in 35% of subjects. ELISPOT assay responses were associated with prolongation in median survival. GM-CSF and IFNα2b, both given systemically, did not appear to influence the vaccine’s immunological and antitumor responses.6 Therefore, we attempted to evaluate more potent vaccine adjuvants by combining a TLR9 agonist (PF-3512676) and GM-CSF, in order to improve the outcome of vaccination against the 3 lineage antigens targeted by the triple peptide vaccine in E1696, as tested in this safety and immunogenicity study.
TLR9 agonists induce activation of DCs, resulting in increased surface expression of co-stimulatory molecules.4 Activation of DCs also initiates multiple effects, including natural killer cell activation, cytokine/chemokine production, and antigen presentation. This results in the induction of an antitumor adaptive immune response.4 PF-3512676 is a synthetic oligodeoxynucleotide (ODN) that activates TLR9 mimicking unmethylated CpG single-stranded DNA. It induces DC maturation and enhances antigen presentation.4 Tumor immunization strategies have been enhanced with the addition of CpG ODN as an adjuvant.7 DCs produce high amounts of IL-12 following stimulation with both CpG ODN (through TLR9) and CD40 Ligand.8 PF-3512676 has been used in a series of human phase I studies given in association with HBs antigen, and shown to exhibit a strong adjuvant effect.9, 10 In addition, PF-3512676 enhanced the number of antigen-specific T cells induced by vaccination with MART-1 peptide vaccination plus incomplete Freund’s adjuvant ~10-fold.11
Local administration of GM-CSF combined with tumor vaccines has been found to have beneficial effects on vaccine immune responses, believed to result from its effects on dendritic cells.12–15 GM-CSF incorporated with peptide vaccination in adjuvant was shown to significantly enhance cellular and humoral immunity to two HIV-1 MN vaccine constructs. GM-CSF synergized with IL-12 for CTL induction in BALB/c mice concomitant with suppression of Th2 cytokines IL-4 and IL-10.13 GM-CSF has also been tested in a series of human trials and noted to enhance T cell responses to multiple peptides when administered in an emulsion of GMCSF-in-adjuvant.14 On the other hand, Slingluff et al have reported that CD8+ and CD4+ T-cell responses to a multipeptide vaccine were lower when the peptides were administered with GM-CSF alone.16
The above considerations, as well as data from preclinical studies support the combination of CPG ODN and GM-CSF as immune adjuvants enhancing antigen-specific immune response compared to immunostimulatory strategies employing either agent alone.15, 17 We have therefore, conducted this safety and immunogenicity study of immunization with MART-1, gp100, and tyrosinase peptides, involving a potent immunological adjuvant approach combining PF-3512676 and GM-CSF, given with the peptides locally in oil-adjuvant.
PATIENTS AND METHODS
Patients
Patients at least 18 years of age were considered eligible if they had inoperable stage III or stage IV melanoma (cutaneous, ocular or mucosal), had measurable disease (RECIST), and typed serologically positive for HLA-A2. They were required to have adequate hematologic, renal, and liver function tests. Systemic therapy for melanoma was allowed but no prior vaccination with MART-1 (26–35, 27L), gp100 (209–217, 210M) and tyrosinase (368–376, 370D) peptides alone or in combination. Patients with a history of brain metastasis were eligible but must have had adequate surgical or radiologic treatment prior to enrollment. All patients provided a written informed consent.
Study Design and Treatment
This was a safety and immunogenicity single arm study of immunization with a multi-epitope peptide vaccine containing MART-1 (26–35, 27L), gp100 (209–217, 210M) and tyrosinase (368–376, 370D) peptides, given combined with the immunomodulators GM-CSF and PF3512676 in Montanide ISA oil adjuvant for HLA-A2+ patients. Using continuous monitoring of safety (Bayesian) along with a two-stage design for immunological efficacy, 20 immune-response evaluable patients were planned for enrollment.
Vaccinations were given on days 1 and 15 of each cycle (1 cycle = 28 days) for a maximum of 13 cycles (1 year). On each vaccination day, vaccination was administered at 3 locations subcutaneously, rotating truncal sites in the vicinity of the four nodal drainage groups of the four extremities. At each vaccination site, a combination of 100 mcg of one peptide, 80 mcg of GM-CSF and 0.6 mg of PF3512676 were given in an emulsion with Montanide ISA-51 VG. Therefore, a total daily dose of 240 mcg of GM-CSF and a total daily dose of 1.8 mg of PF3512676 was used.
We adopted peptide dosage used for the MAGE-A3 peptide by Marchand et. al.18, (i.e., 100 mcg of peptide). In a trial utilizing vaccination with PF3512676, MART-1 peptide (at 100 mcg), and IFA, Speiser at al. showed that the highest T-cell frequencies in the peripheral blood were reached 7–10 days after recall vaccination that declined shortly afterwards, similar to observations in mice. 11,19–21. A dosage of PF3512676 of 0.6 mg was used as suggested to be optimal by previous studies.9–11 We chose a total daily dose of 240 mcg of GM-CSF on days 1 and 15 of each cycle (1 cycle = 28 days), a dose was selected with which there was a safety margin in humans based on prior studies utilizing this approach.22,13, 14. This dose was divided into each of three vaccination sites, given in-adjuvant in an emulsion with each of the peptides and Montanide ISA-51 VG, so that the actual dose delivered at each vaccination site was 80 mcg.
For each vaccination site, a vaccine emulsion was prepared using a syringe method of vaccine preparation, utilizing a plastic, polypropylene barrel, non-rubber-tipped polyethylene plunger, luer-lock tip syringe, to generate the water-in-oil emulsion. The water-in-oil emulsion consisted of peptide (100 mcg/0.1 mL), GM-CSF (80 mcg/0.16 mL using lyophilized 500 mcg/vial reconstituted with 1 mL of sterile water), PF3512676 (0.6 mg/0.04 mL using 15mg/mL vial) and 0.20 mL of sterile saline. Peptide emulsions incorporating Montanide ISA-51 are most stable when the emulsifying reagent is added at a 1:1 ratio with the aqueous component. Thus, 0.5 mL of Montanide ISA-51 VG was added.
Toxicity and Response Assessments
The NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 descriptions and grading scales were utilized for AE grading and reporting. After enrollment of the first study patient, we spaced enrollment of the next 3 patients by intervals of 2 weeks. This allowed sufficient time for any acute, treatment-related AEs to emerge early in the trial and ensure that by the time the fourth patient was enrolled on study the previous three would have received 2–4 vaccinations and had 15–45 days of observation for possible toxicity. Vaccine safety throughout the clinical trial was monitored for evidence of regimen-limiting toxicity (RLT), defined as NCI CTCAE grade 2 or greater regimen-related (possibly, probably, or definitely related to vaccination) allergic reactions (including generalized urticaria), or any regimen-related grade 3 or greater adverse event. All patients receiving an initial vaccination were evaluable for the analysis of adverse events (AEs) and RLT.
The ELISPOT assay for quantitating peptide-reactive CD8+ T cells was utilized for this study for the detection of low frequency CD8+ T cell populations without ex vivo expansion.23, 24 Patient whole blood was collected in Na Heparin tubes and peripheral blood mononuclear cells were isolated within 4 hours by a standard Ficoll gradient centrifugation and cryopreserved until each patient time point was collected to eliminate inter-assay variability. We used IFN-γ ELISPOT for the assessment of the frequency of melanoma peptide-specific CD8+ T lymphocytes in the blood induced by this vaccination. The frequency of CD8+ T cells freshly isolated from blood was tested for immunoreactivity against the 3 HLA-A2-restricted peptides. The IFN-γ ELISPOT assay was performed as reported.6, 23, 24 Based on the data from Speiser at al.,11 we set the dates of PBMC collection for ELISPOT testing at baseline, day 50–53 (7–10 days after 4th vaccination) and day 91–94 (for those who went on to receive additional vaccinations). ELISPOT assays were performed using capture monoclonal antibodies (mAbs) antihuman interferon (IFN)-γ (Mabtech, USA) and detection biotinylated mAbs antihuman IFN-γ (Mabtech, USA). Patient CD8+ T-cell responders were positively isolated from PBMCs by immunomagnetic CD8 MicroBeads (Miltenyi Biotec, Germany). Wells were plated in triplicate and internal controls were used to test for spontaneous release of IFN-γ as well as for the ability to mount an immune response. For example, test wells were plated with CD8 responders + antigen-presenting cells (APC) T2 cell line and no peptide to evaluate for non-specific response, and similar wells were plated for each patient with the addition of the mitogen, phytohemagglutinin (PHA) to ensure all patients were capable of mounting an immune response. The CEF peptide pool (Axxora LLC) was also used as an additional positive control for detecting antigen specific CD8 cell response. CD8 cells were incubated in antibody-coated Elispot plates at 0.5×106cells/well with peptides at 10ug/L along with T2 APC for 20 hours to allow for immune recognition. After plate development, spot numbers were automatically determined by a computer-assisted video image analyzer (Cellular Technology Ltd, USA) with software ImmunoSpot 3. To calculate the number of T cells responding to a particular antigen, the mean numbers of spontaneous spots induced by APCs alone were subtracted from mean spot numbers induced by antigen-loaded APCs. By convention, a positive peripheral immune response by ELISPOT was defined as the number of reactive CD8+ T cells against any of the HLA-A2-restricted peptides MART-1, gp100, and tyrosinase which at least doubled as compared to baseline, and for which the increment was at least 10 spots.6, 25
For the purpose of tumor response assessment (RECIST criteria v.1), imaging staging studies were carried out every 8 weeks (2 cycles). Patients were classified as complete response (CR), partial response (PR), stable disease (SD) or disease progression (PD).
Statistical Methods
Using continuous monitoring of safety (Bayesian analysis) along with a two-stage design for immunological efficacy, up to 20 immune-response evaluable patients were planned for enrollment on this study. The primary study endpoint was safety of the investigational vaccine. Secondary endpoints included immunologic response, measured by IFN-γ ELISPOT assays for patient CD8+ T-cell reactivity against three HLA-A2-restricted peptides (MART-1, gp100, tyrosinase), objective tumor response (RECIST v.1), progression free survival (PFS) and overall survival (OS).
From study E1696, an estimated 30% of patients treated with vaccine alone were expected to show an immunologic response, i.e., one in which the number of reactive CD8+ T cells against any of the HLA-A2-restricted peptides MART-1, gp100, and tyrosinase (measured by ELISPOT assays) doubles (as compared to baseline) after 4 vaccinations, and for which the increment is at least 10 spots.6 Our immunologic objective was to increase this response rate to 60% or more by our investigational vaccine. We therefore planned to use a two-stage design for immunologic response: provided toxicity is acceptable, 10 patients that are evaluable for immunologic response were to be enrolled in stage 1. If 4 or more “responses” occurred, then an additional 10 patients evaluable for immunologic response were planned for stage 2 enrollment provided toxicity remains acceptable (N = 20 total patients evaluable for immunologic response). If 9 or more responses occurred by the end of stage 2, then we would consider our vaccination regimen to be potentially worthy of further study. (Design characteristics: α = 0.098 one-sided test; power = 91%; 65% chance of stopping by the end of stage 1 if the underlying immunologic response rate is only 30%). Progression-free survival and overall survival were estimated by the Kaplan-Meier method.
RESULTS
Patient Characteristics
Twenty two patients (11 male, 11 female), age 48–81 (median 66) were enrolled between 01/2009 and 12/2010. All had AJCC stage IV (5M1a, 6M1b, 11M1c) and most had previously received therapy (0–3 regimens). Eight patients had prior treated brain metastases. Table 1 summarizes the study population’s demographics and baseline patient characteristics.
Table 1.
Patient Demographics and Baseline Disease Characteristics (N=22 patients)
| Variable | No. of Patients (%) |
|---|---|
|
| |
| Age, years Median (Range) | 66 (48–81) |
|
| |
| Cutaneous | 17 (77) |
| Unknown primary | 4 (18) |
| Mucosal | 1 (5) |
|
| |
| Gender | |
| Female, | 11 (50) |
| Male | 11 (50) |
|
| |
| Performance Status | |
| 0 | 3 (14) |
| 1 | 19 (86) |
|
| |
| Prior Therapy | |
|
| |
| # Prior Regimens (range) | 0–3 |
|
| |
| Prior Brain metastases | 8 (36) |
|
| |
| AJCC stage | |
|
| |
| M1a | 5 (23) |
|
| |
| M1b | 6 (27) |
|
| |
| M1c | 11 (50) |
Treatment Details
Seventy eight cycles (156 vaccinations) had been administered as of 03/2011 (average 3.5 cycles per patient). Table 2 summarizes the treatment details and the reasons for discontinuation.
Table 2.
Treatment Details (N= 21* evaluable patients)
| Cycles completed | No. pts treated (%) | No. pts off study after treatment (%) | PD as Reason for D/C (%) | Toxicity as Reason for D/C (%) |
|---|---|---|---|---|
| 1 | 21/21 (100) | 0 | NA | NA |
| 2 | 21/21 (100) | 11/21 (52) | 11/11 (100) | 0 |
| 3 | 10/21 (48) | 1/21 (5) | 1/1 (100) | 0 |
| 4 | 9/21 (43) | 4/21 (19) | 4/4 (100) | 0 |
| ≥5 (5–12) | 5/21(24) | 4/21(19) | 4/4/(100) | 0 |
One additional patient considered non-evaluable received one vaccination
Efficacy
A total of 22 patients were enrolled on this study. One who received one vaccination and had a bleeding brain tumor at baseline despite adequate radiotherapeutic management was considered non-evaluable for efficacy. Another patient had no post-vaccination lymphocytes collected for ELISPOT. At the end of stage I enrollment of 10 immune response evaluable (have baseline and post-vaccination blood specimens for ELISPOT testing) patients, the study met the interim analysis criterion of at least 4 positive immune responses and, therefore, moved into stage II enrolment of 10 additional patients (Total N=21 evaluable for clinical efficacy and 20 evaluable for immunological efficacy).
Immunological Efficacy (Stages I and II)
Twenty patients were evaluable for immunological efficacy. Positive ELISPOT was defined as the number of reactive CD8+ T cells against any of the HLA A2-restricted peptides MART-1, gp100, and tyrosinase that doubled (as compared to baseline) after 4 vaccinations, and for which the increment was at least 10 spots. There were 8/20 patients with positive ELISPOT at day 50 and 5 (out of 10 patients with day 90 specimens) ELISPOT positive at day 90. One patient was negative at day 50 and positive at day 90. Therefore, there were a total of 9/20 patients with positive ELISPOT at day 50 and/or day 90. Among the ELISPOT positive patients, 6/9 had SD or PR as the best anti-tumor response and 3 had PD. Table 3 summarizes the immunologic response data. The cytotoxic T cell response rate to each peptide was similar at day 50 (N=20 patients), but was different at day 90 (N=10 patients). However, this analysis was limited by the small sample size.
Table 3.
Summary of tumor response data (RECIST): Best Response (N=22) and immunologic response (ELIspot) at day 50 and day 90 post-vaccination (N=20)
| Primary | Stage | Best Response (RECIST) | Duration of Resp. (Months) | History of brain metastases | Site of progression | ELIspot* | |
|---|---|---|---|---|---|---|---|
| Day 50 | Day 90 | ||||||
| 1. Unknown | M1a | PD | 0 | yes | brain, LN | n | NE |
| 2. Cutaneous | M1b | SD | 4 | 0 | LN, lung | n | n |
| 3. Cutaneous | M1c | PD | 0 | yes | subQ, LN | NE | NE |
| 4. Cutaneous | M1a | SD | 7 | 0 | LN | p | p |
| 5. Cutaneous | M1c | PR | 2 | 0 | liver | n | p |
| 6. Cutaneous | M1b | PD | 0 | 0 | lung | p | NE |
| 7. Cutaneous | M1b | SD | 2 | 0 | lung | n | n |
| 8. Cutaneous | M1b | PD | 0 | 0 | lung | p | NE |
| 9. Cutaneous | NE | NE | NE | yes | NE | NE | NE |
| 10. Unknown | M1c | PD | 0 | 0 | LN, subQ | n | NE |
| 11. Cutaneous | M1c | SD | 4 | yes | brain | p | n |
| 12. Unknown | M1c | SD | 2 | yes | brain, lung | n | n |
| 13. Unknown | M1c | SD | 6 | yes | brain | p | p |
| 14. Cutaneous | M1a | PD | 0 | 0 | subQ | n | NE |
| 15. Cutaneous | M1c | PD | 0 | yes | brain, bone, liver, lung | n | NE |
| 16. Cutaneous | M1c | PD | 0 | yes | brain | p | NE |
| 17. Cutaneous | M1c | PD | 0 | 0 | liver, lung, muscle, subQ | n | NE |
| 18. Cutaneous | M1a | PD | 0 | 0 | liver, LN, subQ | n | NE |
| 19. Cutaneous | M1a | PD | 0 | 0 | LN, subQ | n | NE |
| 20. Cutaneous | M1c | SD | 2 | 0 | LN | p | p |
| 21. Mucosal | M1b | SD | 2 | 0 | Lung | p | p |
| 22. Cutaneous | M1b | PR | 2+ | 0 | NA | n | n |
NE: non-evaluable; LN: lymph node; subQ: subcutaneous; NA: non-applicable; p=positive; n=negative
Twenty patients had day 50 samples for ELIspot testing and only 10 had day 90 samples
Tumor Response
Response data were available for 21 patients. Two patients (M1b, M1c) had PR and 8 (4M1c, 3M1b, 1M1a) had SD lasting 2–7 months. Among 7 evaluable patients with history of treated brain metastases, 6 had disease progression in the brain.
Survival
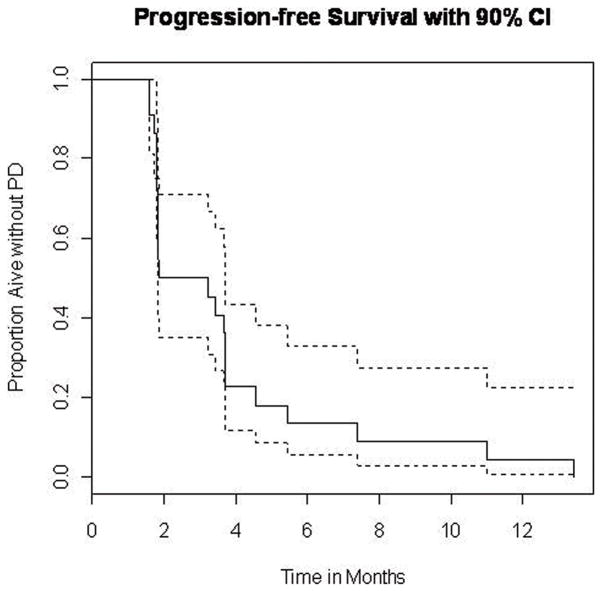
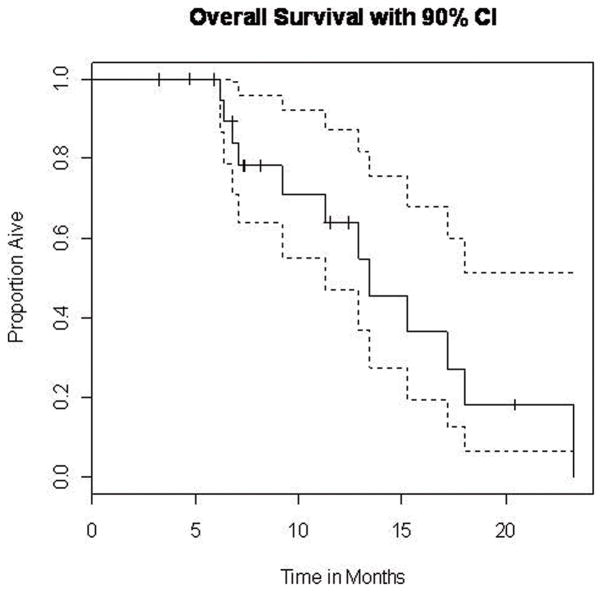
One patient with ongoing PR continued on treatment. All other patients had progressed and among these only 10 were still alive with a median follow up time of 7.39 months (range 3.22 to 20.47 months). No all patients have reached at least 1 year of follow up from first vaccination at the time of data cut-off. Among the first 11 patients enrolled on the study who had reached at least 1 year of follow up from first vaccination, 8 were alive at one year. Median PFS was 1.87 months (90% CI=1.84, 3.68). The Kaplan–Meier plot of the probability of PFS is shown in Figure 1. Median OS was 13.4 months (90% CI=11.3, Inf). The Kaplan–Meier plot of the probability of OS is shown in Figure 2.
Figure 1.
Kaplan – Meier plot of the probability of progression-free survival (N=21). The estimated median is 1.87 months (90% CI=1.84, 3.68).
Figure 2.
Kaplan – Meier plot of the probability of overall survival (N=21). The estimated median is 13.4 months (90% CI=11.3, Inf).
Safety
Table 4 summarizes AEs by severity that were considered possibly, probably or definitely related to the study regimen. No regimen-related grade 3/4/5 toxicities were observed.
Table 4.
Adverse events considered possibly, probably or definitely related to the study regimen presented by worst grade (CTCAE v.3) (N=22 patients)
| Type | All Grades | Grade 1 | Grade 2 | Grade 3/4 | ||||
|---|---|---|---|---|---|---|---|---|
| No. Patients | % | No. Pts. | % | No. Pts. | % | No. Pts. | % | |
| Constitutional | ||||||||
| Allergic rhinitis | 3 | 14 | 2 | 9 | 1 | 5 | 0 | 0 |
| Fatigue | 9 | 41 | 7 | 32 | 2 | 9 | 0 | 0 |
| Fever | 4 | 18 | 4 | 18 | 0 | 0 | 0 | 0 |
| Insomnia | 3 | 14 | 2 | 9 | 1 | 5 | 0 | 0 |
| Rigors/chills | 3 | 14 | 3 | 14 | 0 | 0 | 0 | 0 |
| Sweating/diaphoresis | 1 | 5 | 1 | 5 | 0 | 0 | 0 | 0 |
| Weight loss | 1 | 5 | 1 | 5 | 0 | 0 | 0 | 0 |
| Limb edema | 2 | 9 | 1 | 5 | 1 | 5 | 0 | 0 |
| Dermatologic/Skin | ||||||||
| Injection site reaction | 15 | 68 | 15 | 68 | 0 | 0 | 0 | 0 |
| Pruritus/itching | 5 | 23 | 4 | 18 | 1 | 5 | 0 | 0 |
| Rash | 2 | 9 | 1 | 5 | 1 | 5 | 0 | 0 |
| Gastrointestinal | ||||||||
| Anorexia | 2 | 9 | 1 | 5 | 1 | 5 | 0 | 0 |
| Diarrhea | 2 | 9 | 2 | 9 | 0 | 0 | 0 | 0 |
| Nausea | 9 | 41 | 8 | 36 | 1 | 5 | 0 | 0 |
| Taste alteration | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 |
| Vomiting | 7 | 32 | 1 | 5 | 5 | 23 | 0 | 0 |
| Infection | ||||||||
| Mucosal | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 |
| Skin | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 |
| Neuro-Psychiatric | ||||||||
| Psychosis | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 |
| Respiratory | ||||||||
| Cough | 2 | 9 | 2 | 9 | 0 | 0 | 0 | 0 |
| Dyspnea | 1 | 5 | 1 | 5 | 0 | 0 | 0 | 0 |
| Other | ||||||||
| Hypertension | 1 | 5 | 1 | 5 | 0 | 0 | 0 | 0 |
| Hypotension | 1 | 5 | 1 | 5 | 0 | 0 | 0 | 0 |
| Headache | 4 | 18 | 1 | 5 | 3 | 14 | 0 | 0 |
| Pain (muscle/extremity) | 6 | 27 | 6 | 27 | 0 | 0 | 0 | 0 |
DISCUSSION
Cancer vaccination has the unique advantage of targeting the host immune response against melanoma and creating tumor specific immunity while potentially minimizing unwanted non-specific autoimmunity.26 However, tumor vaccination approaches have generally had limited clinical efficacy in melanoma despite solid preclinical data and the novel immunization strategies employed.6 One strategy to improve our immunization outcomes is the testing of new and potent immunization adjuvants such as PF-3512676 and GM-CSF given in combination in oil-adjuvant as tested in this study with the multiepitope peptide vaccine for which significant data exist in the context of E1696 trial.6
In this safety and immunogenicity study we successfully immunized 9 (8 at day 50 and 1 at day 90) out of 20 evaluable patients which approaches our target of at least 9 ELISPOT positive patients based on our original design. Therefore, we consider our vaccination regimen to be potentially worthy of further study. In addition, we consider our adjuvant combination administered locally with the vaccine to be worthy of further testing with this and other vaccines. Our clinical data build upon evidence from preclinical studies supporting this vaccination adjuvant combination.15, 17 Our conclusion is also supported by the data that GM-CSF when administered locally with tumor vaccines has been found to have beneficial effects on vaccine immune responses believed to be due to its effects on DCs 12, 22, 27, 28, including evidence that GM-CSF attracts DCs to the site of vaccine injection. In our combination approach, this would be coupled with the impact of the TLR9 agonist on enhancing plasmacytoid dendritic cell (pDC) maturation, increasing their expression of MHC class I and II molecules and co-stimulatory molecules, and promoting Th1-type immune responses.4
Clinically, 10 out of 21 patients had either a response or stable disease, although in all cases of limited duration (range 2–7 months). Median PFS was 1.9 months and median OS was 13.4 months compared to a historical control of median PFS of 1.7 months (95% CI, 1.6 months to 1.8 months) and median OS of 6.2 months (95% CI, 5.9 months to 6.5 months).29 It is noteworthy that 11/21 patients evaluable for efficacy had M1c disease. In addition, 7/21 had prior treated brain metastases and among these, 6/7 had subsequent disease progression in the brain. In patients with metastatic melanoma, brain metastases have been reported in at least 18%–46% of patients 30, 31, while autopsy series report twice this prevalence31–34. Brain metastases lead directly to death in as many as 95% of melanoma patients with CNS involvement 31, 34, 35. Surgery or stereotactic radiosurgery (SRS) can achieve local control and may prolong survival when used with or without WBRT in carefully selected patients with limited brain metastases36–41. We conclude that the clinical activity observed with this vaccination regimen in this poor prognosis population is notable. However, the overall clinical activity of the proposed regimen in this population is clearly suboptimal. In regards to the safety of this regimen, there were no regimen-related grade 3 or higher AEs. The vaccination regimen was relatively very well tolerated.
In addition, we were interested in looking into evidence of induced autoimmunity against self antigens that has been reported as a potential clinical/serologic correlate or overcoming immune tolerance after treatment high dose IL-2, anti-CTLA4 monoclonal antibodies and IFN-α, possibly resulting from self-antigen cross-presentation.42–46 Patient serum samples were tested at baseline (N=18), day 50 (N=18) and day 90 (N=8) for the presence of the following autoantibodies using ELISA immunoassay kits from (DiaSorin, Inc.) 47: Antinuclear antibody (ANA) screen, Antithyroglobulin antibody (ATGAB), Antithyroperoxidase Antibody (ATPOAB), Anticardiolipin (TOTAL: IgA + IgM + IgG). Among 18 patients tested, none had serologic evidence of induced autoimmunity at day 50 or day 90 against any of the autoantigens tested. This may support the hypothesis that tumor specific vaccination has the potential of focusing the immune response while minimizing non-specific autoimmunity that limits therapy with other non-specific immunotherapeutic agents.
This relatively very good safety as well as immunogenicity profile and the strong rationale for focusing the immune response to melanoma makes this regimen a good candidate for combinations with other immunotherapeutic agents with superior clinical activity in melanoma such CTLA4-blockade with ipilimumab where autoimmunity in the form of immune related AEs are potentially serious AEs limiting continued therapy. Other candidates for future combinations that may enhance the patient’s antitumor response are other monoclonal antibodies (mAbs) that target other immunoregulatory checkpoints that are able to suppress/enhance host responses to tumor associated antigens (TAAs) such as anti-CTLA4, anti-PD1 and anti-PD-L1 blocking mAbs48} as well as CD4049, OX40 and CD137 (4-1BB) agonist mAbs. The use of antibodies that modulate these immunoregulatory mechanisms appear to be among the most promising approaches to amplifying the host’s antitumor response prolonging T-cell activation and restoring T-cell proliferation.50 For example, it has been reported that tumor antigen-specific CD8 T cells infiltrating tumor, including MART-1 melanoma antigen-specific CD8 T cells are functionally impaired and overexpress PD-1, unlike T cells in normal tissues and peripheral blood.51 These findings suggest that factors within the tumor microenvironment contribute to impaired antitumor immune responses partly by inducing up-regulation of PD-1 on anti-tumor T cells.51 Therefore, a vaccination strategy combined with an anti-PD-1 blocking mAb has the potential of improving clinical efficacy to vaccination approaches in melanoma.
Conclusion
Our adjuvant vaccination regimen is safe and is worthy of further testing with these or alternative peptides, potentially in combination with mAbs that target immunoregulatory checkpoints, in an effort to improve clinical efficacy.
Acknowledgments
This investigator initiated study was supported by the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP), Pfizer Oncology and Genzyme. We thank the NCI CTEP, Pfizer Oncology and Genzyme for their support. We also thank Dr Pawel Kalinski and Dr Lisa Butterfield for their valuable critical editorial input.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–28. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura T, Nakui M, Sato M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46 (Suppl):S52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarhini AA, Kirkwood JM, AMK Early development of the Toll-like receptor 9 agonist, PF-3512676, for the treatment of patients with advanced cancers. Expert Opin Drug Discov. 2009;4(5):587–603. doi: 10.1517/17460440902824784. [DOI] [PubMed] [Google Scholar]
- 5.Tarhini AA, Iqbal F. CTLA-4 blockade: therapeutic potential in cancer treatments. Onco Targets Ther. 2010;3:15–25. doi: 10.2147/ott.s4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–51. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci U S A. 1997;94:10833–7. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Davis HL, Suparto, Weeratna RR, et al. CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine. 2000;18:1920–4. doi: 10.1016/s0264-410x(99)00443-0. [DOI] [PubMed] [Google Scholar]
- 10.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21:2461–7. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 11.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–46. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong SP, Enders-Zohr P, Zhou YM, et al. Recombinant human granulocyte macrophage-colony stimulating factor (rhGM-CSF) and autologous melanoma vaccine mediate tumor regression in patients with metastatic melanoma. J Immunother. 1999;22:166–74. doi: 10.1097/00002371-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Yamshchikov GV, Barnd DL, Eastham S, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer. 2001;92:703–11. doi: 10.1002/1097-0215(20010601)92:5<703::aid-ijc1250>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.SlingluffCL, Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–26. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Liu HM, Newbrough SE, Bhatia SK, Dahle CE, Krieg AM, Weiner GJ. Immunostimulatory CpG oligodeoxynucleotides enhance the immune response to vaccine strategies involving granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:3730–6. [PubMed] [Google Scholar]
- 16.Slingluff CL, Jr, Petroni GR, Olson WC, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–44. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandler AD, Chihara H, Kobayashi G, et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Res. 2003;63:394–9. [PubMed] [Google Scholar]
- 18.Marchand M, van Baren N, Weynants P, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–30. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–8. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 20.Lenardo M, Chan KM, Hornung F, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 21.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–7. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 22.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–58. [PubMed] [Google Scholar]
- 23.Herr W, Schneider J, Lohse AW, Meyer zum Buschenfelde KH, Wolfel T. Detection and quantification of blood-derived CD8+ T lymphocytes secreting tumor necrosis factor alpha in response to HLA-A2. 1-binding melanoma and viral peptide antigens. J Immunol Methods. 1996;191:131–42. doi: 10.1016/0022-1759(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 24.Herr W, Linn B, Leister N, Wandel E, Meyer zum Buschenfelde KH, Wolfel T. The use of computer-assisted video image analysis for the quantification of CD8+ T lymphocytes producing tumor necrosis factor alpha spots in response to peptide antigens. J Immunol Methods. 1997;203:141–52. doi: 10.1016/s0022-1759(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 25.Keilholz U, Weber J, Finke JH, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kim CJ, Dessureault S, Gabrilovich D, Reintgen DS, Slingluff CL., Jr Immunotherapy for melanoma. Cancer Control. 2002;9:22–30. doi: 10.1177/107327480200900104. [DOI] [PubMed] [Google Scholar]
- 27.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998;95:13141–6. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 30.Harrison BE, Johnson JL, Clough RW, Halperin EC. Selection of patients with melanoma brain metastases for aggressive treatment. Am J Clin Oncol. 2003;26:354–7. doi: 10.1097/01.COC.0000020963.71379.FE. [DOI] [PubMed] [Google Scholar]
- 31.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660–8. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–10. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 33.Bullard DE, Cox EB, Seigler HF. Central nervous system metastases in malignant melanoma. Neurosurgery. 1981;8:26–30. doi: 10.1227/00006123-198101000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Budman DR, Camacho E, Wittes RE. The current causes of death in patients with malignant melanoma. Eur J Cancer. 1978;14:327–30. doi: 10.1016/0014-2964(78)90201-3. [DOI] [PubMed] [Google Scholar]
- 35.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 36.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 37.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–90. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 38.Smalley SR, Laws ER, Jr, O’Fallon JR, Shaw EG, Schray MF. Resection for solitary brain metastasis. Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg. 1992;77:531–40. doi: 10.3171/jns.1992.77.4.0531. [DOI] [PubMed] [Google Scholar]
- 39.Sause WT, Crowley JJ, Morantz R, et al. Solitary brain metastasis: results of an RTOG/SWOG protocol evaluation surgery + RT versus RT alone. Am J Clin Oncol. 1990;13:427–32. [PubMed] [Google Scholar]
- 40.Kondziolka D, Martin JJ, Flickinger JC, et al. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005;104:2784–91. doi: 10.1002/cncr.21545. [DOI] [PubMed] [Google Scholar]
- 41.Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:809–16. doi: 10.1016/j.ijrobp.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Gogas H, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 43.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675, 206. J Clin Oncol. 2005;23:8968–77. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 44.Atkins MB, Mier JW, Parkinson DR, Gould JA, Berkman EM, Kaplan MM. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318:1557–63. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 45.Krauze MT, Tarhini A, Gogas H, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. Semin Immunopathol. 2011 doi: 10.1007/s00281-011-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan GQ. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci. 2003;2003:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarhini AAMS, Schlesselman JS, Sander C, Kirkwood JM. Preliminary safety and clinical activity results of the combination of high-dose interferon alfa-2b and tremelimumab for recurrent inoperable stage III or stage IV melanoma. J Clin Oncol. 2008 May 20;26(suppl):abstr 9009. In; 2008; Chicago; 2008. [Google Scholar]
- 48.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 49.Law CL, Gordon KA, Collier J, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 50.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]