Abstract
Interveinal strips (10 × 1.5 mm) excised from growing tobacco (Nicotiana tabacum L. cv Xanthi) leaves have an auxin-specific, epinastic growth response that is developmentally regulated and is not the result of ethylene induction (C.P. Keller, E. Van Volkenburgh [1997] Plant Physiol 113: 603–610). We report here that auxin (10 μm naphthalene acetic acid) treatment of strips does not result in plasma membrane hyperpolarization or detectable proton efflux. This result is in contrast to the expected responses elicited by 1 μm fusicoccin (FC) treatment, which in other systems mimics auxin growth promotion through stimulation of the plasma membrane H+-ATPase and resultant acid wall loosening; FC produced both hyperpolarization and proton efflux in leaf strips. FC-induced growth was much more inhibited by a strong neutral buffer than was auxin-induced growth. Measurements of the osmotic concentration of strips suggested that osmotic adjustment plays no role in the auxin-induced growth response. Although cell wall loosening of some form appears to be involved, taken together, our results suggest that auxin-induced growth stimulation of tobacco leaf strips results primarily from a mechanism not involving acid growth.
Auxin applied to excised sections from coleoptiles or stems dramatically stimulates cell elongation after a lag of about 10 min (Ray and Ruesink, 1962; Yamagata and Masuda, 1975). The increased growth rate coincides with an efflux of protons (Cleland, 1976; Jacobs and Ray, 1976), as well as a hyperpolarization of the PM (Cleland et al., 1977), both apparently consequences of increased activity of an electrogenic proton pump, the PM H+-ATPase (Senn and Goldsmith, 1988). The correlation between auxin-induced proton efflux and increased growth is the principal support for the acid growth theory for the underlying mechanism of auxin action (Hager et al., 1971; Rayle and Cleland, 1972). The theory states that hormone-stimulated proton pumping results in acid-dependent cell wall loosening (presumably through activation of cell wall enzymes [Cleland, 1981]), which in turn permits cell expansion.
Other evidence supporting the acid-growth theory includes the effect of the fungal toxin FC (Rayle and Cleland, 1992). FC, although unrelated to auxins in structure (Marrè, 1979), is an even stronger stimulant of proton efflux, PM hyperpolarization (Cleland et al., 1977), and cell elongation than auxin. FC is also believed to stimulate the activity of the PM H+ATPase but by an apparently different and more rapid mechanism than auxin. The FC receptor is now believed to be a 14–3–3 protein (de Boer, 1997), which results in PM hyperpolarization and proton efflux within 30 s when bound (Cleland, 1990).
Recently, we have shown that auxin produces an epinastic growth response when applied to excised tobacco (Nicotiana tabacum L.) leaf strips (Keller and Van Volkenburgh, 1997). The response was greatest in intercostal or nonveinal tissues. Although auxin was found to induce growth of all tissues across the leaf, epinasty resulted from relatively greater auxin-induced growth by the adaxial (dorsal) epidermis, as well as by the underlying palisade mesophyll, than by the abaxial (ventral) epidermis. Epinastic sensitivity to auxin in tobacco leaves is also strongly developmentally regulated, with responsiveness correlating with the cell-expansion phase of growth. This last observation appears to explain a conflict in the auxin literature. Some of the oldest auxin studies assert that exogenous auxin has no effect on tobacco leaf mesophyll growth (Avery, 1935; Went and Thimann, 1937). These studies, however, appear to have been carried out using only very young leaves in which cell division had not given way to cell expansion (Avery, 1933; Poethig and Sussex, 1985). Recent investigations using more mature leaf material, however, have reported that ATPase and proton-pumping activities of isolated and purified PM from tobacco leaves are stimulated by auxin (Santoni et al., 1990, 1991; Masson et al., 1996). The Em of protoplasts prepared from older tobacco leaf mesophyll has also been found to be sensitive to auxin (Ephritikhine et al., 1987; Barbier-Brygoo et al., 1989, 1991; Venis et al., 1990, 1992). It appears that leaves develop responsiveness to auxin as they mature.
In this study we investigated whether the auxin-induced epinastic growth of tobacco leaf tissues involved a similar acid-growth mechanism to that seen in the auxin-induced growth responses of other organs.
MATERIALS AND METHODS
Plant Material
Batches of approximately 200 tobacco (Nicotiana tabacum L. cv Xanthi) plants were grown individually in soil-filled pots under greenhouse conditions as previously described (Keller and Van Volkenburgh, 1997), except that new batches of plants were sown monthly and growing leaves (8–10 cm in length) were harvested only from plants 2 to 3 months old. Strips of interveinal leaf tissues 10 mm long (or 13 mm as indicated) and 1.5 mm wide (or 0.7 mm as indicated) were cut from the distal one-third of the leaves as previously described (Keller and Van Volkenburgh, 1997).
Electrophysiology
Individual leaf strips (adaxial side exposed) were secured against a plexiglass stage with strands of therostat, preincubated between 1 and 7 h in a solution containing 0.1 mm KCl, 1.0 mm CaCl2, and 1.0 mm Mes/BTP, pH 6.0, and mounted in a perfusion chamber on a microscope stage. The mounted strip was perfused with preincubation solution and the Em of microelectrode-impaled cells was continuously recorded as previously described (Keller and Van Volkenburgh, 1996a). Microelectrode tip potentials did not exceed ±10 mV and tip resistances ranged from 10 to 30 MΩ. Successful recordings (evident as a steadily decreasing potential initially at least as negative as −80 mV) were of the second cell encountered in each impalement (assumed to be palisade mesophyll cells). Recordings were allowed to rest for at least 10 min, until the recorded potential was changing no more than 0.5 mV min−1. The perfusion solution was then changed to include 10 μm auxin (NAA) or 1.0 μm FC. One-millimolar NAA stock solutions were prepared by dissolving in 100 μL of EtOH, diluting with 50 mL of equimolar KOH, and heating briefly to 80°C, and 100 μm FC stock solutions were prepared by dissolving first in ethanol, and then diluting the ethanol to 10% (v/v).
Proton Efflux Assays
The abaxial epidermis was peeled away from the interveinal regions of the distal portion of 8- to 10-cm-long leaves using fine forceps. Peeled regions were trimmed to approximately 3- × 3-mm squares and placed peeled side up on a thin bed of petroleum jelly on top of rubber wafers. A 5-μL droplet of a solution containing 10 mm KCl and 0.1 mm Mes/BTP, pH 6.0, was placed on the exposed mesophyll surface. The wafer bearing the peeled leaf fragment and the droplet was then enclosed in a small chamber lined with moist tissue paper.
For continuous recording experiments, the tip of a small-volume combination pH electrode (model MI-410, Microelectrodes, Inc., Bedford, NH) was then lowered through a small aperture in the humidity chamber to make contact with the 5-μL droplet. After the pH of the droplet had been continuously recorded for 2 h, the droplet was wicked away with a laboratory tissue and replaced with a fresh 5-μL droplet of the same solution, or with one also including 10 μm NAA or 1 μm FC. The pH was then monitored for a further 2 h.
For the noncontinuous recording experiment, a system of staggered starts was used with wafers bearing peeled leaf fragments (18 per humidity chamber) being incubated, and the solution was changed as above without the pH being monitored. After fragments had been in contact with the second test solution for 4 h, individual wafers were transferred to a separate humidity chamber, the droplet was then contacted with the pH electrode as above, and after 2 min the pH was recorded. This experiment was repeated twice with similar results.
Curvature and Elongation Measurement
Two types of curvature assays were performed: short- term and long-term. In the short-term experiments longer (13-mm) leaf strips were prepared under room lighting. The terminal 0.3 mm of one end of each strip was clamped by a slit cut in a small rubber block. Blocks with their attached strips were arrayed as prepared in a 60- × 15-mm Petri dish containing 5 mL of a control solution consisting of 10 mm Suc, 10 mm KCl, and 0.5 mm Mes/BTP, pH 6.0. Each block was placed in the Petri dish so that (viewed from above) the strips were oriented on their sides (i.e. in profile; see fig. 1 in Keller and Van Volkenburgh [1997]). Once 10 strips were in place (5–10 min), the Petri dish was placed on a slide/transparency viewer (model 2020/2131 Portaview, Logan Electric, Chicago, IL) under the camera lucida arm of a microscope. The solution in the Petri dish was then gently removed and replaced by Pasteur pipette with fresh control solution or the same solution also containing 10 μm NAA. A time-0 image of the strips was captured using the camera lucida and a digitizing camera (model MTI CCD-72SX, Dage, Michigan City, IN) and NIH Image 1.41 software (National Institutes of Health, Bethesda, MD). Images were captured at 15-min intervals and thereafter for 5.5 h, the light box being turned off between Image captures. In one set of experiments, the control solution was replaced with the auxin-containing solution after 2 h. The curvature of each strip (i.e. the angle created by the interception of the tangent to the two terminal portions of the strip) was measured in degrees at each time point from printed copies of the stored images using a protractor.
For long-term curvature measurements, strips (10 mm long) were placed immediately into Petri dishes containing 10 mL of either the control, auxin (10 μm NAA)-containing, or FC (1.0 μm)-containing solution. Incubations were conducted in dim-green light, although curvature assays conducted under room lights were found to yield very similar results. The results appeared to be unaffected by gentle agitation. Strips were incubated for 20 h and then gently removed from solution, placed onto the light box, and profile images were captured. Images were used to assess curvature as described above.
Changing length of interveinal strips following 20-h incubations was also assessed from the digitized images. The length of the adaxial surface of each strip was determined by tracing the outline of strips on printed copies of captured images using a digitizing tablet (Kurta) using SigmaScan software (Jandel Scientific, Corte Madera, CA). Strips were repeatedly removed from solution and their length was estimated to the nearest 0.1 mm using a fine-scale ruler to determine the time course of FC-induced elongation.
Determination of Osmotic Concentration
Leaf strips (20–30 per treatment) were incubated for 20 h in solutions containing 0.5 mm Mes/BTP, pH 6.0, with or without 10 mm Suc and 10 mm KCl and with or without either 10 μm NAA or 1.0 μm FC. After 20 h, the strips from each treatment were patted dry on laboratory tissues, enclosed in aluminum, frozen, and thawed. Sap was expressed and its osmotic concentration was determined using a vapor-pressure osmometer (model 5100 C, Wescor, Logan, UT).
To confirm the results, curvature, elongation, and osmotic concentration experiments were repeated twice with similar results.
RESULTS
Effects of NAA and FC on Em
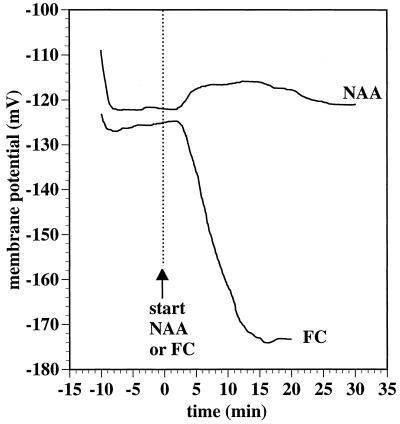
Ten-micromolar NAA, a concentration determined to be optimal for inducing epinastic curvature of strips excised from growing tobacco leaves (Keller and Van Volkenburgh, 1997), caused a slight depolarization of the Em of palisade mesophyll cells (Fig. 1). Approximately 2 min after the inclusion of NAA in the perfusion stream (including an estimated 20–30 s lag before the solution change reached the point of microelectrode impalement), the Em gradually and transiently depolarized approximately 5 mV over a 25-min period. Results were similar in 11 experiments, with a mean Em at time 0 (the start of NAA inclusion in the perfusion stream) of −133 mV (sd = 7.9). The auxin-induced depolarization, measured relative to Em at time 0 in each recording, reached a maximum after 10 to 16 min and measured on average 6.9 mV (95% confidence interval for the mean 3.3 was 10.5). The depolarization was no longer significant after 22 min.
Figure 1.
Effect of 10 μm NAA and of 1.0 μm FC on the Em of tobacco mesophyll cells (sample recordings). Leaf strips (1.5 mm wide) were prepared from the interveinal regions of the apical one-third of growing, 8- to 10-cm-long leaves. Palisade mesophyll cell Em was monitored using a microelectrode inserted through the overlying adaxial epidermal cell and into a mesophyll cell. Strips were initially perfused with a solution containing 0.1 mm KCl, 1.0 mm CaCl2, and 1.0 mm Mes/BTP, pH 6.0. At time 0 (vertical dashed line), the perfusion source was switched to one also containing NAA (10 μm) or FC (1 μm) as indicated.
Following NAA-induced depolarization, the Em of the mesophyll cells tended to repolarize back only to approximately the time-0 value (Fig. 1). Tobacco mesophyll Em recordings tended to fail over time, but 40 min following the start of NAA treatment, three of the five recordings that still survived were slightly hyperpolarized relative to their time-0 values (−2 or −1 mV), whereas the other two recordings had values that were still slight depolarizations (3 and 4 mV).
In contrast to the response to NAA, mesophyll cells strongly hyperpolarized in response to FC (Fig. 1). Following the start of FC treatment and a 2-min lag the Em hyperpolarized approximately 48 mV before failing at 20 min. Two additional tests of the effect of FC also produced strong hyperpolarization responses.
Effects of NAA and FC on Proton Efflux
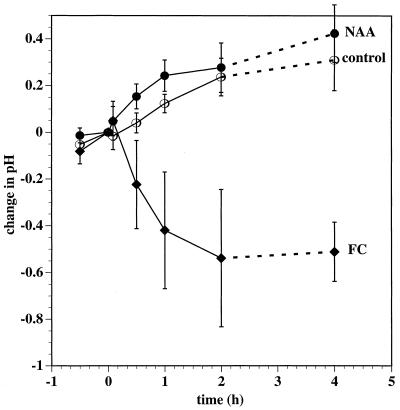
The ability of NAA and FC to induce an efflux of protons from tobacco leaf tissues was tested. Using a small-volume pH electrode, we monitored the pH of a 5-μL droplet of a solution placed onto the exposed mesophyll surface of leaf fragments from which the abaxial epidermis had been peeled away. Microscopic examination of free-hand sections of peeled leaf fragments showed that peeling removed most spongy mesophyll tissue with the epidermal layer. The palisade cells remained intact (data not shown). When the droplet was replaced by one that included 1 μm FC, a sustained measurable acidification of the droplet resulted (Fig. 2). In contrast, NAA produced no detectable acidification, and there was a small but significant alkalization evident after 30 and 60 min.
Figure 2.
Influence of NAA and FC on proton efflux by tobacco leaf mesophyll fragments. Data shown are from both continuous and noncontinuous recording experiments. In each continuous experiment, the tip of a small-volume combination pH electrode was placed in contact with a 5-μL droplet of control solution (10 mm KCl and 0.1 mm Mes/BTP, pH 6.0) on the exposed surface of a 3- × 3-mm square of a tobacco leaf fragment from which the abaxial epidermis had been peeled away. After 1 h (at time 0) the droplet was wicked away and replaced by another 5 μL of fresh control solution (○) or by the same solution augmented with either 10 μm NAA (•) or 1 μm FC (♦). The pH was monitored for a further 2 h. Data at 4 h were determined once without previous continuous monitoring. Data are expressed as change in pH from time 0. pH at time 0 was 6.02 (sd = 0.37; •), 5.99 (sd = 0.36; ○), and 5.93 (sd = 0.29; ♦) and the noncontinuous samples were assumed to have been pH 6.0 at time-0 pH. Error bars indicate the 95% confidence limits for the mean (Student's t test); in continuous recording experiments, n = 9 (○), n = 10 (•), and n = 12 (♦), and in the noncontinuous experiment, n = 18.
Time Course of the Initiation of NAA- and FC-Induced Growth
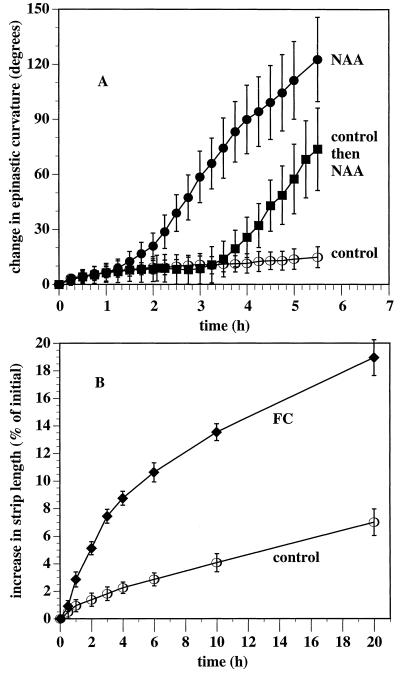
In earlier experiments auxin-induced leaf-strip curvature was evident by 4 h (Keller and Van Volkenburgh, 1997). In a new series of experiments designed to complement the electrical and pH experiments, we more clearly resolved the initiation of the growth response (Fig. 3A). Strips incubated in a control solution were observed to curl slowly over the course of the experiment. NAA-induced curvature began slowly after 1 h of incubation, with the increased curvature significant relative to the controls by 2 h. The maximum rate of curvature increase was achieved within 2.5 h. When the strips were incubated first in control solution for 2 h, and then in NAA, the same 1-h lag was evident before the start of auxin-induced curvature.
Figure 3.
Short-term effect of NAA on tobacco leaf-strip curvature (A) and longer-term effect of FC on strip length (B). In A, 10 strips were each anchored by one end to small rubber blocks in a Petri dish and incubated in control solution (○) (10 mm Suc, 10 mm KCl, and 0.5 mm Mes/BTP [pH 6.0]) or the same solution also including 10 μm NAA (•), or first in control solution for 2 h followed by NAA (▪). Images of the strips were captured every 15 min and curvature was recorded relative to curvature at time 0. In B, 18 strips per treatment were incubated in the same control solution (○) or the same solution plus FC (♦) for 20 h. At each time point they were gently removed from solution, their lengths measured, and then returned to the incubation solution. Error bars indicate 95% confidence limits for mean change (Student's t test) from time 0.
When strips were incubated in 1 μm FC, very little curvature response was evident over the course of 20 h; however, the strips were observed to elongate. The elongation response to FC was initiated much more rapidly than was NAA-induced curvature (Fig. 3B). Short-term measurements of the length of NAA-treated strips showed them to elongate no faster than the control strips for at least the first 2 h of auxin treatment (data not shown). Unlike control strips, which were observed to elongate slowly over the course of 6 h, FC-treated strips elongated more rapidly within 30 min and were significantly longer by 1 h. FC-induced elongation was also sustained over many hours (Fig. 3B). Although the increased elongation rate of the FC-treated strips began slowing after the 1st h, they grew more than the controls between 10 and 20 h.
Effect of Neutral Buffering on NAA- and FC-Induced Growth
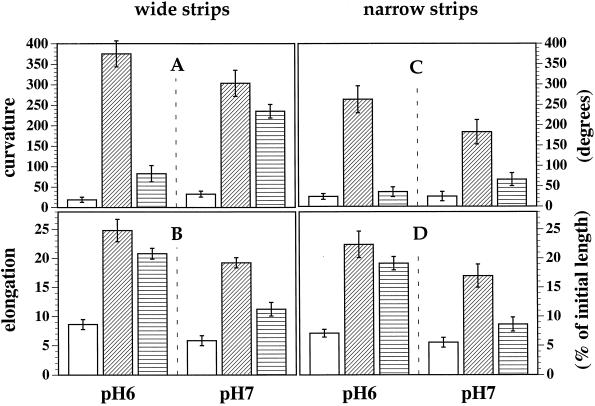
The absence of an auxin-induced acidification response (Fig. 2) that might match the kinetics of the initiation of the curvature response (Fig. 3) suggested that acid growth is not involved in the auxin-induced growth by tobacco leaf cells. To further test this possibility, we looked at the effect of a strong neutral buffer (20 mm Mops/BTP, pH 7.0) on both auxin- and FC-induced strip curvature and on the length of the adaxial surface. Raising the buffer concentration and pH from 0.5 mm and pH 6.0 to 20 mm and pH 7.0 had a small but statistically significant depressing effect on curvature in NAA, which dropped from 375° to 303° (Fig. 4A). Curvature in FC, however, increased from 83° to 235°.
Figure 4.
Effect of strong neutral buffering on NAA- and FC-induced curvature and elongation growth of strips prepared either 1.5 mm in width (wide strips) or 0.7 mm in width (narrow strips). In the control condition (open columns) the strips were incubated for 20 h in 10 mm Suc and 10 mm KCl with 0.5 mm Mes/BTP, pH 6.0, or 20 mm Mops/BTP, pH 7.0, as indicated. Other strips were incubated in the same solutions augmented by either 10 μm NAA (diagonally striped columns) or with 1 μm FC (horizontally striped columns). After 20 h strips were removed from solution and images of the strips, in profile view, were captured using a video camera. Strip curvature and the length of the adaxial surface were then measured from printed copies of the images. Error bars indicate the 95% confidence limits for the mean curvature or elongation (Student's t test; n = 20).
Figure 4B shows the effect of NAA and FC on the elongation of strips in both pH 6.0 and 7.0. In the dilute (0.5 mm), pH 6.0 buffer, strip elongation measured along the longer adaxial surface was more strongly induced by 10 μm NAA than by 1 μm FC. The concentrated (20 mm), pH 7.0 buffer had the effect of depressing both NAA- and FC-induced elongation. However, the inhibition by neutral buffer was much less for NAA-induced than for FC-induced elongation. The mean NAA-induced elongation in the 20 mm, pH 7.0 buffer (calculated as the elongation in NAA minus the control elongation) was 13.4% versus 16.2% in the 0.5 mm, pH 6.0 buffer. Thus, more than 80% of the NAA growth response was not inhibited by the neutral pH conditions. The mean FC-induced elongation in the 20 mm pH 7.0 buffer, however, was 5.4% versus 12.2% in the 0.5 mm pH 6.0 buffer; only 45% of the FC growth response remained in the high pH.
There is some question, however, as to how well the neutral buffer permeated and neutralized the entire apoplast of the incubated strips described in Figure 4. The plant epidermal cuticle forms an effective barrier (Durand and Rayle, 1973). Fixed carboxyl groups within cell wall polymers will also tend to increase the proton and other cation concentrations within the apoplast in equilibrium with the bathing solution (for review, see Grignon and Sentenac, 1991). In Figure 4 (as in all earlier experiments) the strips were 1.5 mm wide so that cells were as much as one-half that of the distance from a cut surface. Perhaps an incomplete perfusion of the apoplast, especially to the adaxial side, by the neutral buffer might explain its incomplete inhibition of both NAA- and FC-induced growth. This possibility was tested by retesting the effect of neutral buffer on growth of narrower strips (0.7 mm).
The inhibitory effect of pH 7.0 was much less for NAA- than for FC-induced elongation; 74% of the NAA elongation response seen in pH 6.0 was not inhibited by pH 7.0, whereas only 25% of the FC response survived in pH 7.0. These results suggest that although some degree of wall acidity may be required for tobacco leaf cell enlargement, most of the auxin-induced growth response does not involve an increase in wall acidity.
Osmotic Concentration
Plant growth is driven by cell turgor pressure in excess of wall yield stress and, under most circumstances, is limited by cell wall extensibility (Cleland, 1981; Cosgrove, 1986). It is possible, however, that cell turgor might be growth limiting if it were not in excess of yield stress. We tested the possibility that auxin-induced leaf-strip growth might result from auxin-induced turgor regulation by looking at the effects of absorbable solutes in the incubation medium. The absence of Suc and KCl had limited effects on both NAA-induced strip curvature and upon FC-induced strip elongation. The NAA-induced curvature of strips incubated for 20 h in only 0.5 mm Mes/BTP, pH 6.0, and 10 μm NAA was not significantly less than the curvature of strips incubated in medium also including 10 mm Suc and 10 mm KCl. FC-induced elongation was reduced approximately 20% (data not shown).
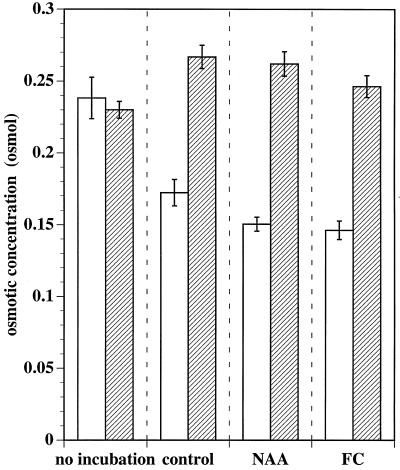
The presence or absence of Suc and KCl did, however, have a substantial effect on the osmotic concentration of sap expressed from strips. The osmotic concentration of strips incubated in Suc and KCl increased over 20 h regardless of treatment, although the increase was significantly greater in control and NAA-treated strips than in FC-treated strips (Fig. 5, striped columns). As might be expected in tissues where uptake of water during growth dilutes preexisting solutes, osmotic concentration was found to decrease in the absence of absorbable solutes especially in the NAA- and FC-treated tissue (Fig. 5, open columns). The osmotic concentration of sap from the variously treated tissue correlated poorly with the magnitude of growth by similar treatment (compare Fig. 4B with Fig. 5, striped columns). Since the osmotic concentration of sap serves as an indicator of cell turgor (Cleland, 1981; Cosgrove, 1986), these data suggest that cell growth of excised leaf tissue is limited by cell wall properties and not by cell turgor.
Figure 5.
Effect of absorbable solutes on the osmotic concentration of tobacco leaf strips. Strips (20–30 per sample) were either sampled immediately (no incubation) or first incubated for 20 h in 0.5 mm Mes/BTP, pH 6.0 (open bars, no solutes), alone (control), or also with 10 μm NAA or 1 μm FC. Alternatively, strips were incubated in 0.5 mm Mes/BTP, pH 6.0, augmented by 10 mm Suc and 10 mm KCl (striped bars, solutes) alone, with NAA, or with FC. The strips were patted dry, frozen, and thawed, and the osmotic concentration of their expressed sap was determined. Error bars indicate the 95% confidence limits for mean osmotic concentration (Student's t test; n = 20 samples per treatment).
DISCUSSION
In our experiments with tobacco leaf strips, we observed an initial NAA-induced depolarization similar to that seen with oat coleoptiles in response to auxin (Cleland et al., 1977; Bates and Goldsmith, 1983; Keller and Van Volkenburgh, 1996a). We did not observe, however, a subsequent pronounced Em hyperpolarization that was evident with auxin-treated oat coleoptile cells (Fig. 1). We were also unable to detect any NAA-induced acidification over the course of 2 h or after 4 h (Fig. 2). FC, however, effectively hyperpolarized the PM and induced an efflux of protons. Auxin-induced growth proved to be much less sensitive to inhibition by neutral buffering than was FC-induced growth (Fig. 4), despite FC being a much more effective stimulator of cell wall acidification than NAA (Fig. 2). Together, these data indicate that auxin-induced growth of tobacco leaf strips cannot be primarily explained by any auxin-induced apoplast-acidification mechanism.
The growth induced by auxin in tobacco leaf strips takes the form of epinastic curvature (Fig. 4; Keller and Van Volkenburg, 1997), whereas strips treated with FC grew by elongation with little epinasty (Fig. 4). This may be further evidence that the mode of action for induced growth differs between these two compounds. FC is believed to stimulate plant tissue growth entirely as a consequence of PM H+-ATPase activation and consequent acid wall loosening (Cleland, 1990; Aducci et al., 1995; de Boer, 1997). Uniform PM H+-ATPase activation by FC or diffusion of excreted protons throughout the lamina presumably produced a uniform pattern of cell growth and elongation of the tobacco leaf strips. Auxin-induced epinasty results from relatively greater growth response by the adaxial cell layers (Keller and Van Volkenburg, 1997). Perhaps the wall-loosening mechanism induced by auxin is not diffusible within the apoplast. It is interesting that the FC-induced growth of wide strips became epinastic in the strong neutral buffer (Fig. 4). This may be an indication that the buffer more effectively neutralized the lower abaxial halves of the strips, where the cells are more widely spaced and a more complete buffer infusion is likely. When the leaf strips were prepared only 0.7 mm wide, the epinasty induced in FC-treated strips in the strong neutral buffer was greatly reduced, an indication that the buffer more uniformly neutralized the entire apoplast.
It is possible that auxin-induced changes to ion-channel conductances mask an increase in PM H+-ATPase activity because the Em is a function of all electrical currents across the PM (Hille, 1992). Much direct and indirect evidence exists indicating that auxin treatment modulates other PM membrane conductances in addition to the activity of PM H+-ATPase in various systems (Marten et al., 1991; Lohse et al., 1992; Rück et al., 1993; Blatt and Theil, 1994; Zimmermann et al., 1994; Keller and Van Volkenburgh, 1996a, 1996b).
Changes to other proton or hydroxyl conductances may also mask evidence of NAA-induced activation of the PM H+-ATPase in the pH of the extracellular solution. Furthermore, increased H+ efflux by corn coleoptile segments requires the presence of K+ (Claussen et al., 1997), presumably because an influx of K+ is required as a charge balance for the electrogenic proton efflux. Consistent with the acid growth theory, in the absence of K+ and detectable proton efflux, no auxin-induced growth occurs (Claussen et al., 1997). We were unable, however, to detect any auxin-induced acidification by tobacco leaf tissues in the presence of K+ ions over the course of 2 h or after 4 h (Fig. 2), although auxin-induced growth of tobacco leaf strips was evident after about 1 h (Fig. 3).
For the leaf cell growth rate to increase following auxin application cell turgor must increase or some form of cell wall loosening must occur; either cell wall extensibility must increase, or (conceivably) cell wall yield threshold must decrease (Cleland, 1981; Cosgrove, 1986). The lack of an effect by auxin on the osmotic concentration of cell sap (Fig. 5) indicates that turgor is unchanged. This implies that NAA-induced growth by tobacco leaf tissues must be the result of some wall-loosening mechanism.
Partial inhibition of auxin-induced strip elongation by pH 7.0 shows that the auxin-induced wall-loosening mechanism in tobacco leaf strips is not completely pH independent. Possibly the apoplast of tobacco leaf strips is normally sufficiently acidic to permit growth, and auxin serves to maintain the capacity of the walls to undergo acid-induced wall loosening (Cleland, 1983), perhaps through the production of wall-loosening enzymes. An obvious possibility is that expansins are induced (McQueen-Mason, 1995). The 1-h lag before the start of auxin-induced curvature (Fig. 3) by tobacco leaf strips leaves plenty of time for a response involving de novo gene induction and protein synthesis.
An alternate possibility suggested by the lack of complete pH independence of auxin-induced growth is that some cell wall acidification, undetected as a lowering of pH of the extracellular solution (Fig. 2), does occur and that this accounts for approximately one-quarter of the growth response. Our results do not rule out this possibility.
Our results suggesting that auxin does not induce increased PM H+ATPase activity (Figs. 1, 2, and 4) in intact tobacco mesophyll appear to be in conflict with results collected with systems derived from similar source materials. ATPase and proton-pumping activities of isolated and purified PM from tobacco leaves have been reported to be stimulated by auxin (Santoni et al., 1990, 1991; Masson et al., 1996) but the effect is quite small relative to baseline activities. Also, treatment with auxin of protoplasts derived from tobacco mesophyll has been reported to result in a rapid hyperpolarization of the Em of approximately 5 mV within 1 min, as measured by microelectrode impalement (Ephritikhine et al., 1987; Barbier-Brygoo et al., 1989, 1991; Venis et al., 1990, 1992). These scientists reported that antibodies directed against yeast ATPase blocked the response (Barbier-Brygoo et al., 1989), which they considered to be evidence that the hyperpolarization was a consequence of PM H+-ATPase activation. These reports remain controversial, however, because of the relatively small Em reported either with or without auxin (i.e. in the range of 0 to −10 mV). Small values have been reported for the Em of other plant protoplasts (Pantoja and Willmer, 1986), but these are difficult to reconcile with the much more negative potentials generally found for intact cells (i.e. between −100 and −200 mV) (Higinbotham, 1973). Van Duijn and Heimovaara-Dijkstra (1994) have also reported that low protoplast Em values are an artifact of microelectrode impalement and that initial, very negative potential transients lasting a few milliseconds following impalement indicate that the membrane is much more polarized before impalement.
Our experiments suggest that the mechanism of auxin-induced growth of tobacco leaf strips is similar to the mechanism by which other nonauxin plant growth hormones have been found to induce growth of bean leaf tissues (Brock and Cleland, 1989, 1990). Both GA3 and BA initiated bean leaf growth after a lag of 30 to 50 min by a mechanism that did not involve osmotic adjustment and increased cell wall extensibility in some manner not involving acid growth.
ACKNOWLEDGMENTS
We would like to thank Doug Ewing and his staff of the Botany Department Greenhouses at the University of Washington for their care of the plants used in these experiments.
Abbreviations:
- BTP
1,3-bis(Tris[hydroxymethyl]methylamino)propane
- Em
membrane potential
- FC
fusicoccin
- PM
plasma membrane
Footnotes
This work was supported by National Science Foundation grant no. MCB-9316947 to E.V.V.
LITERATURE CITED
- Aducci P, Marra M, Fogliano V, Fullone MR. Fusicoccin receptors: perception and transduction of the fusicoccin signal. J Exp Bot. 1995;46:1463–1478. [Google Scholar]
- Avery GS. Structure and development of the tobacco leaf. Am J Bot. 1933;20:513–564. [Google Scholar]
- Avery GS. Differential distribution of a phytohormone in the developing leaf of Nicotiana, and its relation to polarized growth. Bull Torr Bot Club. 1935;62:313–330. [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Ghislain M, Guern J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci USA. 1989;86:891–895. doi: 10.1073/pnas.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Maurel C, Palme K, Schell J, Guern J. Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J. 1991;1:83–93. doi: 10.1042/bst0200059. [DOI] [PubMed] [Google Scholar]
- Bates GW, Goldsmith MHM. Rapid response of the plasma membrane potential in oat, Avena sativa, coleoptiles to auxin and other weak acids. Planta. 1983;159:231–237. doi: 10.1007/BF00397530. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Theil G. K+ channels of stomatal guard cells: bimodal control of K+ inward-rectifier evoked by auxin. Plant J. 1994;5:55–68. doi: 10.1046/j.1365-313x.1994.5010055.x. [DOI] [PubMed] [Google Scholar]
- Brock TG, Cleland RE. Role of acid efflux during growth promotion of primary leaves of Phaseolus vulgaris L. by hormones and light. Planta. 1989;177:476–482. doi: 10.1007/BF00392615. [DOI] [PubMed] [Google Scholar]
- Brock TG, Cleland RE. Biophysical basis of growth promotion in primary leaves of Phaseolus vulgaris L. by hormones versus light. Planta. 1990;182:427–431. doi: 10.1007/BF02411395. [DOI] [PubMed] [Google Scholar]
- Claussen M, Lüthen H, Blatt M, Böttger M. Auxin-induced growth and its linkage to potassium channels. Planta. 1997;201:227–234. [Google Scholar]
- Cleland RE. Kinetics of hormone-induced H+ excretion. Planta. 1976;58:210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE. Wall extensibility: hormones and wall extension. In: Loewus FA, Tanner W, editors. Encyclopedia of Plant Physiology, New Series, Vol 13B Plant Carbohydrates. II. Extracellular Carbohydrates. Berlin: Springer-Verlag; 1981. pp. 255–273. [Google Scholar]
- Cleland RE. The capacity for acid-induced wall loosening as a factor in the control of Avena coleoptile cell elongation. J Exp Bot. 1983;34:676–680. [Google Scholar]
- Cleland RE (1990) Proton export, ATPase and hormone action. In M Kutacek, MC Elliot, I Machackova, eds, Molecular Aspects of Hormonal Regulation of Plant Development, Proceedings of the 14th Biochemical Congress. Academic Publishing, The Hague, The Netherlands, pp 185–194
- Cleland RE, Prins HBA, Harper JR, Higinbotham N. Rapid hormone-induced hyperpolarization of the oat coleoptile transmembrane potential. Plant Physiol. 1977;59:395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DL. Biophysical control of plant cell growth. Annu Rev Plant Physiol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- de Boer AH. Fusicoccin: a key to multiple 14–3–3 locks? Trends Plant Sci. 1997;2:60–66. [Google Scholar]
- Durand H, Rayle DL. Physiological evidence for auxin-induced hydrogen-ion secretion and the epidermal paradox. Planta. 1973;114:185–193. doi: 10.1007/BF00387475. [DOI] [PubMed] [Google Scholar]
- Ephritikhine G, Barbier-Brygoo H, Muller J-F, Guern J. Auxin effect on the transmembrane potential difference of wild-type and mutant tobacco protoplasts exhibiting a differential sensitivity to auxin. Plant Physiol. 1987;83:801–804. doi: 10.1104/pp.83.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignon C, Sentenac H. pH and ionic gradients in the apoplast. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:103–128. [Google Scholar]
- Hager A, Menzel H, Krauss A. Versuche und hypothese zur primärwirkung des auxins beim streckungswachstum. Planta. 1971;100:47–75. doi: 10.1007/BF00386886. [DOI] [PubMed] [Google Scholar]
- Higinbotham N. Electropotentials of plant cells. Annu Rev Plant Physiol. 1973;24:25–46. [Google Scholar]
- Hille B (1992) Ionic Channels of Excitable Membranes, Ed 2. Sinauer Associates Inc., Sunderland, MA
- Jacobs M, Ray PM. Rapid auxin-induced decrease in free space pH and its relationship to auxin-induced growth in maize and pea. Plant Physiol. 1976;58:203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CP, Van Volkenburgh E. The electrical response of Avena coleoptile cortex to auxins: evidence in vivo for activation of a Cl− conductance. Planta. 1996a;198:404–412. [Google Scholar]
- Keller CP, Van Volkenburgh E. Osmoregulation by oat coleoptile protoplasts. Effect of auxin. Plant Physiol. 1996b;110:1007–1016. doi: 10.1104/pp.110.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CP, Van Volkenburgh E. Auxin-induced epinasty of tobacco leaf tissues. A non-ethylene mediated response. Plant Physiol. 1997;113:603–610. doi: 10.1104/pp.113.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse G, Hedrich R. Characterization of the plasma-membrane H+-ATPase from Vicia faba guard cells: modulation by extracellular factors and seasonal changes. Planta. 1992;188:206–214. doi: 10.1007/BF00216815. [DOI] [PubMed] [Google Scholar]
- Marrè E. Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol. 1979;30:273–288. [Google Scholar]
- Marten I, Lohse G, Hedrich R. Plant growth hormones control voltage-dependent activity of anion channels in plasma membrane of guard cells. Nature. 1991;353:758–762. [Google Scholar]
- Masson F, Szponarski W, Rossignol M. The heterogeneity of the plasma membrane H+-ATPase response to auxin. Plant Growth Reg. 1996;18:15–21. [Google Scholar]
- McQueen-Mason SJ. Expansins and cell wall expansion. J Exp Bot. 1995;46:1639–1650. [Google Scholar]
- Pantoja O, Willmer CM. Pressure effects on membrane potentials of mesophyll cell protoplasts and epidermal cell protoplasts of Commelina communis L. J Exp Bot. 1986;37:315–320. [Google Scholar]
- Poethig RS, Sussex IM. The cellular parameters of leaf development in tobacco: a clonal analysis. Planta. 1985;165:170–184. doi: 10.1007/BF00395039. [DOI] [PubMed] [Google Scholar]
- Ray PM, Ruesink AW. Kinetic experiments on the nature of the growth mechanism in oat coleoptile cells. Dev Biol. 1962;4:377–397. [Google Scholar]
- Rayle DL, Cleland RE. The in-vitro acid-growth response: relation to in-vitro growth responses and auxin action. Planta. 1972;104:282–296. doi: 10.1007/BF00386312. [DOI] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle HH. Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J. 1993;4:41–46. [Google Scholar]
- Santoni V, Vansuyt G, Rossignol M. Differential auxin sensitivity of proton translocation by plasma membrane H+-ATPase from tobacco leaves. Plant Sci. 1990;68:33–38. [Google Scholar]
- Santoni V, Vansuyt G, Rossignol M. The changing sensitivity to auxin of the plasma-membrane H+-ATPase: relationship between plant development and ATPase content of membranes. Planta. 1991;185:227–232. doi: 10.1007/BF00194065. [DOI] [PubMed] [Google Scholar]
- Senn AP, Goldsmith MHM. Regulation of electrogenic proton pumping by auxin and fusicoccin as related to the growth of Avena coleoptiles. Plant Physiol. 1988;88:131–138. doi: 10.1104/pp.88.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijn B, Heimovaara-Dijkstra S. Intracellular microelectrode membrane potential measurements in tobacco cell-suspension protoplasts and barley aleurone protoplasts: interpretation and artifacts. Biochim Biophys Acta. 1994;1193:77–84. doi: 10.1016/0005-2736(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Venis MA, Napier RM, Barbier-Brygoo H, Perrot-Riechenmann C, Guern J. Antibodies to a peptide from the maize auxin-binding protein have auxin agonist activity. Proc Natl Acad Sci USA. 1992;89:7208–7212. doi: 10.1073/pnas.89.15.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venis MA, Thomas H, Barbier-Brygoo H, Ephritikhine G, Guern J. Impermeant auxin analogues have auxin activity. Planta. 1990;182:232–235. doi: 10.1007/BF00197116. [DOI] [PubMed] [Google Scholar]
- Went FW, Thimann KV (1937) Phytohormones. Macmillan, New York
- Yamagata Y, Masuda Y. Comparative studies on auxin and fusicoccin actions on plant growth. Plant Cell Physiol. 1975;16:41–52. [Google Scholar]
- Zimmermann S, Thomine S, Guern J, Barbier-Brygoo H. An anion current at the plasma membrane of tobacco protoplasts shows ATP-dependent voltage regulation and is modulated by auxin. Plant J. 1994;6:707–716. [Google Scholar]