Abstract
Hyperpolarization-activated, cyclic nucleotide–sensitive (HCN) channels produce the If and Ih currents, which are critical for cardiac pacemaking and neuronal excitability, respectively. HCN channels are modulated by cyclic AMP (cAMP), which binds to a conserved cyclic nucleotide–binding domain (CNBD) in the C terminus. The unliganded CNBD has been shown to inhibit voltage-dependent gating of HCNs, and cAMP binding relieves this “autoinhibition,” causing a depolarizing shift in the voltage dependence of activation. Here we report that relief of autoinhibition can occur in the absence of cAMP in a cellular context- and isoform-dependent manner: when the HCN4 isoform was expressed in Chinese hamster ovary (CHO) cells, the basal voltage dependence was already shifted to more depolarized potentials and cAMP had no further effect on channel activation. This “pre-relief” of autoinhibition was specific both to HCN4 and to CHO cells; cAMP shifted the voltage dependence of HCN2 in CHO cells and of HCN4 in human embryonic kidney (HEK) cells. The pre-relief phenotype did not result from different concentrations of soluble intracellular factors in CHO and HEK cells, as it persisted in excised cell-free patches. Likewise, it did not arise from a failure of cAMP to bind to the CNBD of HCN4 in CHOs, as indicated by cAMP-dependent slowing of deactivation. Instead, a unique ∼300–amino acid region of the distal C terminus of HCN4 (residues 719–1012, downstream of the CNBD) was found to be necessary, but not sufficient, for the depolarized basal voltage dependence and cAMP insensitivity of HCN4 in CHO cells. Collectively, these data suggest a model in which multiple HCN4 channel domains conspire with membrane-associated intracellular factors in CHO cells to relieve autoinhibition in HCN4 channels in the absence of cAMP. These findings raise the possibility that such ligand-independent regulation could tune the activity of HCN channels and other CNBD-containing proteins in many physiological systems.
INTRODUCTION
Hyperpolarization-activated, cyclic nucleotide–sensitive (HCN) channels produce the If and Ih currents, which are critical determinants of cardiac pacemaker activity and neuronal excitability. There are four mammalian HCN isoforms (HCN1–4), each with distinct tissue distributions and biophysical properties. All four isoforms are expressed in the central nervous system, and HCN4 is the predominant isoform in the sinoatrial node of the heart. Structurally, HCN channels are tetramers, with each subunit composed of six transmembrane-spanning domains with intracellular amino (N) and carboxyl (C) terminals. The transmembrane pore domain and the proximal portions of the N and C terminals are conserved among HCN1–4, whereas the distal N and C terminals are unique to each isoform. The sinoatrial HCN4 isoform is characterized by extremely long N and C terminals (260 and 682 residues, respectively), which contain numerous consensus protein interaction and phosphorylation motifs. We previously demonstrated that PKA phosphorylation of a site in the distal C terminus of HCN4 causes a depolarizing shift in the voltage dependence of heterologously expressed channels, and that the PKA activity is required for the depolarizing shift in native If currents in sinoatrial myocytes in response to β-adrenergic stimulation (Liao et al., 2010).
The conserved proximal C-terminal domain of all four mammalian HCN channels contains a cyclic nucleotide–binding domain (CNBD) as well as a “C-linker” domain, which couples the CNBD to the transmembrane pore domains. The CNBD is conserved among a large and diverse family of proteins, such as PKA, the G protein exchange factor, EPAC, and several other ion channels, including CNG, ether-a-go-go (EAG), EAG-related gene (ERG), and EAG-like (ELK) ion channels. In HCN channels, cAMP binding to the CNBD regulates several distinct properties of voltage-dependent gating: cAMP binding accelerates HCN channel activation, shifts the voltage dependence of activation to more positive potentials, and slows HCN channel deactivation. A structural distinction among these effects is revealed by deletion of the CNBD, which mimics the effect of cAMP binding on the voltage dependence and kinetics of activation but not on the rate of deactivation (Wicks et al., 2011). Thus, it is thought that the unliganded conformation of the CNBD functions as an autoinhibitory domain that impedes voltage-dependent channel opening (Wainger et al., 2001), whereas the liganded conformation of the CNBD mediates cAMP-dependent slowing of channel deactivation (Wicks et al., 2011).
In this study, we set out to examine the interaction between PKA- and cAMP-dependent regulation of HCN4 channels. However, we unexpectedly found that HCN4 was insensitive to cAMP when expressed in Chinese hamster ovary (CHO) cells. Rather, autoinhibition of HCN4 was relieved even in the absence of cAMP in CHO cells. This effect required both the CHO cell background and the HCN4 isoform, and was independent of soluble intracellular factors. We found that a unique region of the distal C terminus of HCN4 was necessary, but not sufficient, for the ligand-independent relief of autoinhibition of HCN4 in CHOs. Thus, it appears that cellular factors and multiple channel domains apart from the CNBD interact functionally to tune the basal voltage dependence and cAMP responsiveness of HCN4.
MATERIALS AND METHODS
Molecular biology
Mutant and chimeric HCN channels were produced by overlapping PCR mutagenesis. Construction of HCN4-Δ719 and HCN4-Δ1012 were described previously (Liao et al., 2010). The chimeric HCN2–4tail and HCN4–2tail channels were cloned by swapping the entire C terminals of HCN2 and HCN4: HCN2–4tail consists of HCN2 residues 1–441 plus HCN4 residues 519–1201, and HCN4–2tail consists of HCN4 residues 1–518 plus HCN2 residues 442–863. Sequence alignments of the distal C-terminal regions of mouse HCN1–4 (GenBank accession nos., respectively: 255760033, 6680189, 6680191, and 124487125) were aligned using ClustalW software.
Cell culture and transfection
CHO (CHO-K1) or human embryonic kidney (HEK; HEK293) cells were purchased from American Type Culture Collection and maintained in culture at 37°C and 5% CO2 in a humidified incubator in Hams F12 medium (CHOs) or DMEM (HEKs), which were supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum. Cells from passages ∼3–20 were plated onto glass coverslips for electrophysiology. Transient transfection of cDNA encoding HCN channels (2–6 µg/35-mm dish) along with either CD8 or GFP (1 µg/dish) was performed using Fugene6 (Roche) according to the manufacturer’s directions. Transiently transfected cells were identified by green fluorescence or by binding of anti-CD8 antibody–coated beads (Invitrogen; Jurman et al., 1994). Cells were used for electrophysiology 24–48 h after transfection.
For some experiments, stable CHO cell lines expressing HCN4 or HCN2 under the control of a tetracycline-inducible promoter were used (Liao et al., 2010). In brief, cDNA encoding the channels was cloned into the pcDNA4/TO vector (Invitrogen) and transfected into TREX-CHO cells (which stably express the tetracycline repressor protein from the pcDNC4/TR plasmid; Invitrogen). Cells containing HCN expression vectors and the tetracycline repressor protein vectors were selected using 250 µg/ml Zeocin (Invitrogen) and 10 µg/ml blasticidin (Invitrogen), respectively. Expression of channels was induced by the addition of 1–10 µg/ml tetracycline to the culture medium 24–48 h before recording. Automated sequencing of the entire coding sequence of HCN4 in DNA isolated from the CHO-HCN4 stable line did not reveal any mutations.
Electrophysiology
For electrophysiological recordings, fragments of glass coverslips plated with HCN-expressing cells were transferred to a recording chamber (200 µl) on the stage of an inverted microscope. All experiments were conducted at room temperature. Currents were recorded >2 min after break-in or patch excision, when intracellular dialysis and/or rundown were complete. Whole cell patch pipettes had resistances of ∼1.5–3 MΩ when filled with an intracellular solution that contained (mM) 130 K-aspartate, 10 NaCl, 1 EGTA, 5 HEPES, 0.5 MgCl2, and 2 MgATP, with pH adjusted to 7.2 with KOH, and cells for whole cell experiments were constantly perfused (∼1–2 ml/min) with “30 K+” extracellular solution consisting of (mM) 115 NaCl, 30 KCl, 1 MgCl2, 1.8 CaCl2, 5.5 glucose, and 5 HEPES, with pH adjusted to 7.4 with NaOH. Excised patch experiments were conducted in a symmetrical solution containing (mM) 160 KCl, 1 MgCl2, and 5 HEPES, with pH adjusted to 7.2 with KOH, after formation of cell-attached seals in the 30 K+ extracellular solution. In some experiments as noted, cAMP was included in the pipette (for whole cell recordings; 1 mM) or the perfusing extracellular solution (for patch recordings; 100 µM).
The voltage dependence of activation for HCN4 was estimated from tail currents, which were plotted as a function of the preceding 3-s hyperpolarizing test potentials (corrected for a +14-mV junction potential error in whole cell recordings). The resulting conductance–voltage plots were fit with a Boltzmann equation,
to yield midpoint activation voltages (V1/2). As has been noted previously (Seifert et al., 1999; Liao et al., 2010), steady-state activation for HCN4 was not achieved in response to the 3-s hyperpolarizing test pulses, which we used as an experimentally feasible means to estimate the voltage dependence of activation for the extremely slowly activating HCN4 isoform. Deactivation rates were estimated by fitting tail currents at −50 mV with a single-exponential function, after the initial lag in the current traces.
cAMP binding assays
cDNA encoding the CNBD plus most of the C-linker of HCN4 (residues 528–737) or HCN2 (residues 443–645), or encoding the distal C terminus of HCN4 (C2, residues 917–1201), was subcloned into a pGEX vector (GE Healthcare). Constructs were expressed in BL21-competent cells (Agilent Technologies), which were grown at 37°C to an OD of 0.5–0.7 before induction for 3–4 h at 37°C with 0.1 mM IPTG. Cells were then collected by centrifugation, resuspended in ice-cold PBS with protease inhibitors (Promega), and lysed by five freeze/thaw cycles. The resulting cell suspensions were incubated at 4°C in 10 µg/ml DNase I with gentle rocking and were then centrifuged at 12,000 g for 30 min at 4°C. Supernatants were collected and incubated at 4°C with a 50% slurry of Sepharose 4B beads (GE Healthcare). Glutathione S-transferase (GST) fusion proteins were eluted from the beads, dialyzed into PBS, and stored in single-use aliquots at −80°C. Fluorescence intensity resulting from binding of 8-NBD-cAMP was measured on a spectrophotometer (PerkinElmer). Samples were excited at 470 nm, and emission spectra were collected over the range from 480 to 650 nm.
Statistics
Data are plotted as mean ± SEM. Comparisons were made by Student’s t test (for whole cell population data) or paired t tests (to compare effects before and after cAMP in excised patches). Results were considered significant if the p-value was <0.05.
RESULTS
cAMP does not shift the voltage dependence of HCN4 in CHO cells
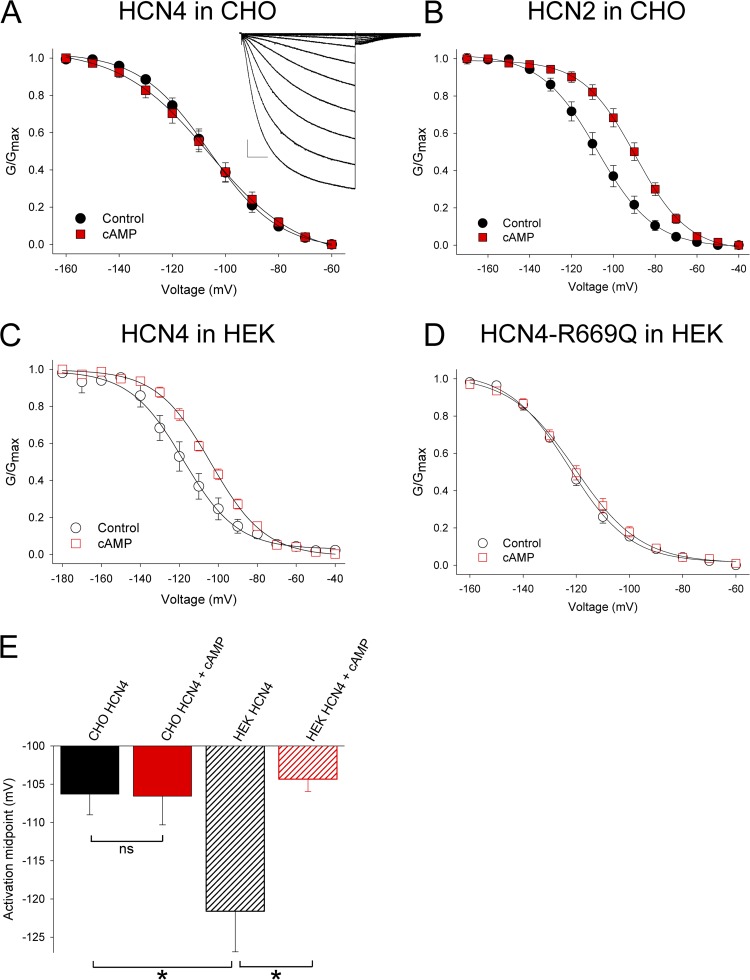
As part of our ongoing studies of HCN4 channel regulation, we set out to describe the effects of cAMP on the voltage-dependent activation of full-length mouse HCN4 channels. Whole cell hyperpolarization-activated currents were recorded from CHO cells expressing HCN4 in the absence or presence of 1 mM cAMP in the patch pipette. To our surprise, cAMP had no effect on the midpoint activation voltage of HCN4 (Fig. 1 A and Table 1; V1/2 = −106.3 ± 2.7, n = 9 without cAMP, and −106.6 ± 3.8 mV, n = 7 with cAMP). The lack of response of HCN4 to cAMP did not appear to result from technical difficulties such as limited intracellular perfusion of cAMP in the CHO cells or problems with the reagents, as the same lot of cAMP produced an ∼16-mV depolarizing shift in the voltage dependence of mouse HCN2 channels in CHO cells in parallel experiments (Fig. 1 B; V1/2 = −107.7 ± 3.4 mV, n = 9 without cAMP, and −91.8 ± 2.7 mV, n = 10 with cAMP).
Figure 1.
HCN4 channels have depolarized voltage dependence and are insensitive to cAMP in CHO cells. Average conductance–voltage relationships plotted from tail currents in whole cell patch-clamp recordings for (A) HCN4 in CHO cells, (B) HCN2 in CHO cells, (C) HCN4 in HEK cells, or (D) HCN4-R669Q in HEK cells. Data were acquired in the absence (black) or presence (red) of 1 mM cAMP in the patch pipette. The inset shows representative CHO-HCN4 currents elicited by 3-s hyperpolarizing voltage steps ranging from −50 to −160 mV, with tail currents at −50 mV. Bars, 200 pA, 500 ms. (E) Average activation midpoints from whole cell recordings for HCN4 channels in CHO or HEK cells in the absence or presence of cAMP. Asterisks indicate statistical significance (P < 0.05) compared with control in t tests, and ns indicates no significant difference from control (P > 0.05).
Table 1.
Voltage-dependent activation of HCN channels
| Cell type, channel, recording configuration | Activation midpoint | ΔV1/2 | |
| Control | cAMP | ||
| mV | mV | mV | |
| CHO, HCN4, whole cell | −106.3 ± 2.7 (9) | −106.6 ± 3.8 (7)a | −0.3 |
| CHO, HCN2, whole cell | −107.7 ± 3.4 (9) | −91.8 ± 2.7 (10)b | +15.9 |
| HEK, HCN4, whole cell | −121.6 ± 5.3 (8)c | −104.3 ± 1.6 (7)b | +17.3 |
| HEK, HCN4-R669Q, whole cell | −123.5 ± 1.2 (7) | −120.1 ± 2.3 (11)a | +3.4 |
| CHO, HCN4, patches | −120.2 ± 3.7 (8) | −118.7 ± 2.8 (8)a | +1.5 ± 1.8 (8) |
| HEK, HCN4, patches | −137.2 ± 2.2 (6)c | −126.1 ± 2.8 (6)b | + 11.1 ± 1.0 (6) |
| CHO, HCN2, patches | −118.2 ± 2.9 (5) | −101.1 ± 2.7 (5)b | +17.1 ± 0.4 (5) |
| CHO, HCN4-2Tail, whole cell | −118.9 ± 2.5 (4) | −105.4 ± 3.0 (5)b | +13.5 |
| CHO, HCN2-4Tail, whole cell | −110.2 ± 2.9 (6) | −78.9 ± 4.3 (4)b | +31.3 |
| CHO, HCN4-Δ719, whole cell | −122.9 ± 2.2 (7)c | −106.7 ± 2.9 (8)b | +16.2 |
| CHO, HCN4-Δ1012, whole cell | −109.2 ± 1.5 (7) | −106.0 ± 1.5 (7)a | +3.2 |
Not significantly different (P > 0.05) compared to corresponding control.
Significantly different (P < 0.05) compared to corresponding control.
Significantly different (P < 0.05) compared to CHO-HCN4 control in same recording configuration.
The cAMP insensitivity of HCN4 in CHO cells was quite surprising because other studies have unequivocally demonstrated that cAMP can shift the voltage dependence of heterologously expressed HCN4 channels (cf. Ludwig et al., 1999; Seifert et al., 1999; Viscomi et al., 2001; Altomare et al., 2003; Harzheim et al., 2008). However, in each of the previous reports, HCN4 was expressed in a different cell type (HEK293 cells or Xenopus laevis oocytes) and/or as a truncated or mutant protein to facilitate expression and cloning. To directly assess the effects of cellular background on the cAMP sensitivity of full-length HCN4 channels, we next expressed HCN4 in HEK293 cells. As shown in Fig. 1 C, cAMP shifted the voltage dependence of HCN4 by approximately +17 mV when the channels were expressed in HEK cells, similar to the response seen in the previous studies (V1/2 = −121.6 ± 5.3 mV, n = 8 in control, and −104.3 ± 1.6 mV, n = 7 in cAMP). This depolarizing shift in voltage dependence appeared to result from direct binding of cAMP to HCN4 because it was eliminated by a point mutation (R669Q) at a conserved arginine in the CNBD that has been shown to disrupt cAMP binding to HCN4 and other cyclic nucleotide–sensitive ion channels (Tibbs et al., 1998; Chen et al., 2001; Harzheim et al., 2008; Fig. 1 D; V1/2 for HCN4-R669Q = −123.5 ± 1.2 mV, n = 7 in control vs. −120.1 ± 2.3 mV, n = 11 in cAMP).
Diffusible intracellular factors cannot explain the cAMP insensitivity of HCN4 in CHO cells
We noticed that the basal V1/2 (i.e., in the absence of cAMP) was significantly more positive for HCN4 in CHO cells than in HEK cells (Fig. 1 E and Table 1). In fact, the basal V1/2 in CHOs was remarkably similar to the V1/2 in the presence of cAMP in HEKs (V1/2 of approximately −106 in CHOs without cAMP vs. approximately −104 mV in HEKs with cAMP; Fig. 1 E). Thus, one possible explanation for our results is simply that the cAMP concentration in CHO cells is higher than that in HEK cells (although the robust cAMP responsiveness of HCN2 in CHOs makes this scenario somewhat unlikely).
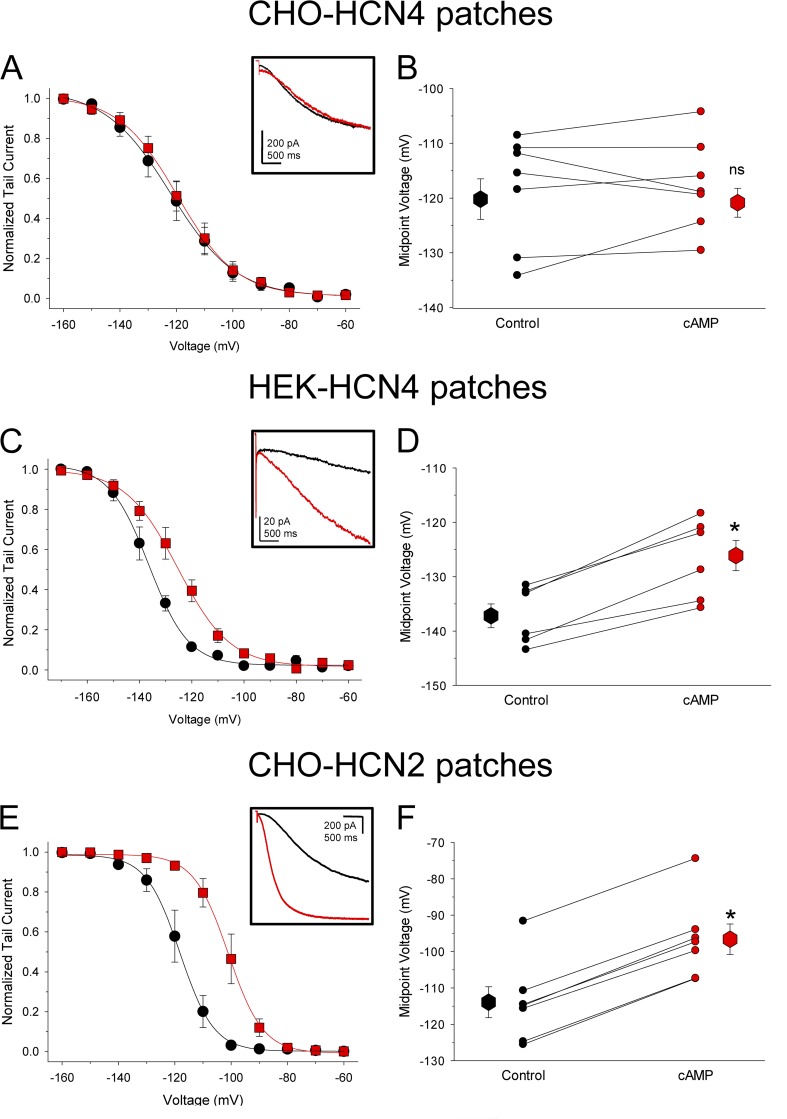
To eliminate any possible effects of endogenous cAMP or other soluble intracellular factors on the channels, we next recorded currents from excised inside-out membrane patches from CHO and HEK cells expressing full-length HCN4 or HCN2 channels. As shown in Fig. 2, the cell context- and isoform-dependent differences in cAMP sensitivity persisted in the patches. Application of 100 µM cAMP directly onto the intracellular face of HCN4-expressing patches from CHO cells had little or no effect on the kinetics or voltage dependence of channel activation (Fig. 2, A and B; ΔV1/2 = 1.5 ± 1.8 mV, n = 8; P = 0.45; paired t test). In contrast, cAMP dramatically accelerated the kinetics and shifted the voltage dependence of activation in HCN4 patches from HEK cells (Fig. 2, C and D; ΔV1/2 = 11.1 ± 1.0 mV, n = 6; P = 0.0001; paired t test) and in HCN2 patches from CHO cells (Fig. 2, E and F; ΔV1/2 = 17.1 ± 0.4 mV, n = 5; P < 0.00001; paired t test). Moreover, the basal voltage dependence remained significantly more positive for HCN4 in CHO cells than in HEK cells (Table 1; V1/2 of approximately −120 mV for CHO-HCN4 patches vs. −137 mV for HEK-HCN4 patches; P = 0.004; note that the absolute V1/2 values were more negative in patches than in whole cell mode for all conditions, consistent with previous reports).
Figure 2.
Diffusible intracellular factors cannot account for the depolarized voltage dependence and cAMP insensitivity of HCN4 in CHO cells. Average conductance–voltage relationships determined from tail currents in excised inside-out patches from CHO cells expressing HCN4 (A), HEK cells expressing HCN4 (C), or CHO cells expressing HCN2 (E) before (black) and after (red) wash-on of 100 µM cAMP. The insets show representative current traces elicited by test pulses to −120 mV in individual patches before and after cAMP. (B, D, and F) Activation midpoints determined from excised patches from CHO cells expressing HCN4 (B), from HEK cells expressing HCN4 (D), and from CHO cells expressing HCN2 (F). V1/2 values for individual cells (small symbols) are plotted before (black) and after (red) wash-on of 100 µM cAMP. Lines connect midpoint values from the same patches. Large symbols represent the average midpoint values (±SEM). Asterisks indicate statistical significance compared with control (P < 0.05) in paired t tests; ns, not significantly different from control (P > 0.05).
cAMP can bind to the CNBD of HCN4 in CHO cells
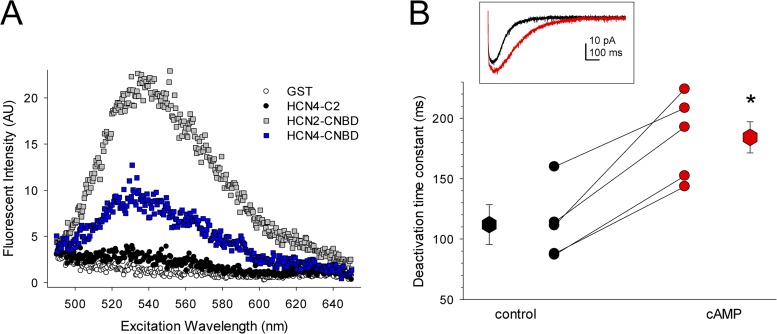
Another potential mechanism for the lack of cAMP responsiveness of HCN4 in CHO cells is that cAMP may not be able to bind to the CNBD of HCN4 in CHO cells, perhaps because of occupation of the binding site by an intrinsic or alternative ligand (analogous to the situation recently described for the crystal structure of zElk channels by Brelidze et al., 2012; see Discussion). To address this possibility, we first confirmed that cAMP can bind to the isolated CNBD of HCN4 (as suggested by the elimination of cAMP modulation of HCN4 in HEK cells by the R669Q mutation; Fig. 1 D). Isolated CNBDs from HCN2 and HCN4 were expressed as GST fusion proteins and were incubated with 3 µM 8-NBD-cAMP, a cAMP analogue that fluoresces in hydrophobic environments, such as when bound to a protein (Kraemer et al., 2001; Cukkemane et al., 2007; Brelidze et al., 2009). As shown in Fig. 3 A, 8-NBD-cAMP was indeed able to bind to the isolated CNBD of HCN4, albeit with lower apparent affinity than to the CNBD of HCN2. In control experiments, 8-NBD-cAMP did not bind appreciably to a downstream region of the HCN4 C terminus (HCN4–C2) or to unconjugated GST.
Figure 3.
cAMP can bind to the CNBD of HCN4 in CHO cells. (A) Fluorescence emission spectra for binding of 3 µM 8-NBD-cAMP to GST fusion proteins. GST, unconjugated glutathione S-transferase; HCN4-C2, residues 917–1201 of the distal C terminus of HCN4 (distal to the CNBD); HCN4-CNBD, residues 528–737 of the C terminus of HCN4, which include the C-linker and CNBD; HCN2-CNBD, residues 443–645 of HCN2, which include the C-linker and CNBD. Spectra are representative of three independent trials. (B) Deactivation time constants for HCN4 in excised patches from CHO cells before (black) and after (red) perfusion with 100 µM cAMP. Asterisk indicates statistical significance compared with control (large symbols; P = 0.009; paired t test). Small symbols represent data from individual patches, and lines connect time constants acquired in the same patches. (Inset) Representative tail currents at −50 mV after voltage steps to −150 mV for a patch expressing HCN4 before (black) and after (red) perfusion of 100 µM cAMP.
To directly assess the ability of cAMP to bind to functional, full-length HCN4 channels in CHO cells, we next measured channel deactivation rates. It has previously been shown that, in addition to shifting the voltage dependence of activation, cAMP binding to the CNBD also slows deactivation of HCN channels (Pian et al., 2006; Wicks et al., 2009). Wicks et al. (2011) further demonstrated that cAMP-dependent regulation of voltage dependence and deactivation in HCN channels can result from structurally distinct mechanisms: cAMP-dependent regulation of voltage dependence is controlled by the unliganded conformation of the CNBD, whereas cAMP-dependent slowing of deactivation is thought to depend on the liganded conformation of the CNBD. Consistent with this model, we found that deactivation of HCN4 in excised patches from CHO cells was significantly slowed by the addition of cAMP, despite the lack of effect of cAMP on the voltage dependence. Single-exponential fits of tail currents at −50 mV after 1-s prepulses to −150 mV before and after wash-on of 100 µM cAMP yielded time constants of 111.9 ± 29.6 ms and 184.2 ± 15.7 ms, respectively (Fig. 3 B; n = 5; P = 0.009; paired t test). These data indicate that the CNBD of HCN4 remains able to bind cAMP when the channels are expressed in CHO cells, thereby suggesting a mechanism in which CNBD-mediated autoinhibition is relieved in a ligand-independent manner for HCN4 channels in the CHO cell background.
A domain in the distal C terminus of HCN4 is required for pre-relief of autoinhibition in CHO cells
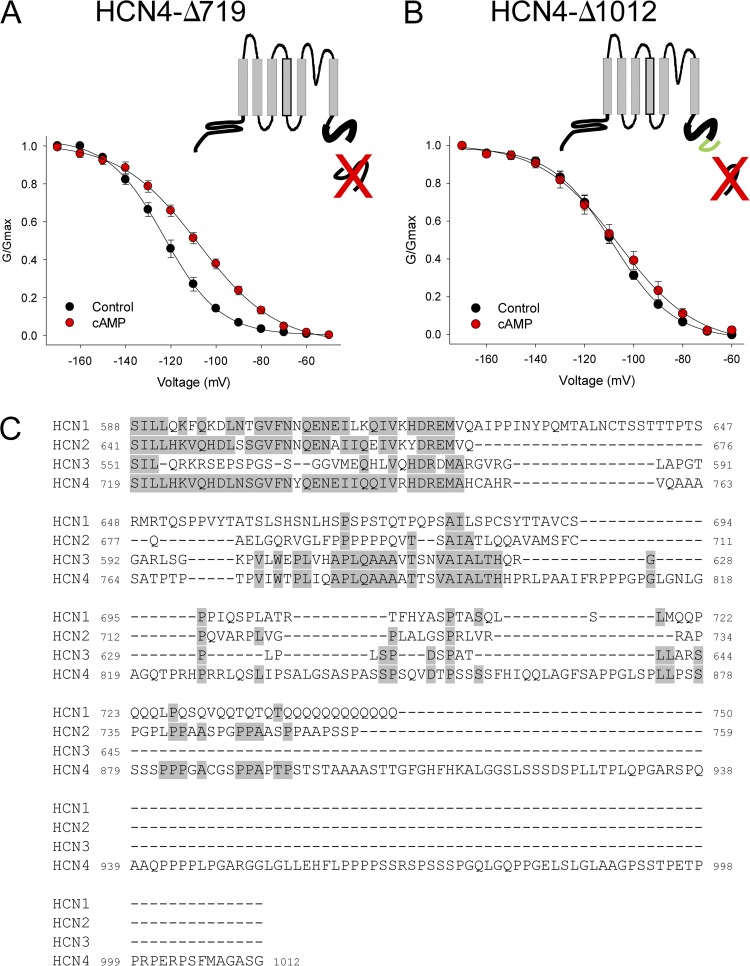
We previously observed a significant negative shift in basal voltage dependence for the truncated HCN4-Δ719 channel, in which the ∼500 residues distal to the CNBD are deleted (Liao et al., 2010). In the context of the present study, we postulated that this negative voltage dependence may correspond to a restoration of autoinhibition in HCN4-Δ719 channels in the CHO cell background. Consistent with this idea, we found that cAMP significantly shifted the voltage dependence by ∼16 mV, similar to the shift observed for wild-type HCN4 channels in HEK cells (Fig. 4 A; V1/2 = −122.9 ± 2.2 mV, n = 7 for HCN4-Δ719 in control, and −106.7 ± 2.9 mV, n = 8 in cAMP). In our earlier study, we also described a shorter truncation of the distal C terminus, HCN4-Δ1012, in which the basal voltage dependence of activation was similar to that of wild-type HCN4. As shown in Fig. 4 B, we found that HCN4-Δ1012 was insensitive to cAMP (V1/2 = −109.2 ± 1.5 mV, n = 7 in control, and −106.0 ± 1.5 mV, n = 7 in cAMP). These observations suggest that residues 719–1012 are critical for voltage- and cAMP-dependent regulation of HCN4, and that this domain of HCN4 may interact, at least functionally, with an endogenous component of CHO cells.
Figure 4.
A region of the HCN4 C terminus distal to the CNBD mediates ligand-independent relief of autoinhibition in CHO cells. (A and B) Schematics and voltage dependence of HCN4-Δ719 and HCN4-Δ1012 channels. In the schematic illustrations, the thick bold lines represent the CNBD, the red “X’s” indicate the deleted portion of the distal C terminus, and the green line illustrates the region between 719 and 1012. The voltage dependence of activation was determined from tail currents after 3-s hyperpolarizing steps to the indicated voltages in whole cell recordings from CHO cells expressing HCN4-Δ719 (A) or HCN4-Δ1012 (B) in the absence (black) or presence (red) of 1 mM cAMP in the patch pipette. (C) Amino acid sequence alignment of the distal C terminals of HCN1-HCN4, beginning just after the CNBD. Residues shown are HCN1 588–750, HCN2 641–758, HCN3 551–680, and HCN4 719–1012. Gray shading indicates identical residues.
We used ClustalW to align the regions corresponding to HCN4 719–1012 in all four mammalian HCN isoforms (Fig. 4 C). The corresponding regions of HCN1, HCN2, and HCN3 were all considerably shorter than that of HCN4 (162, 218, and 93 residues for HCN1-3 vs. 293 residues for HCN4), resulting in large gaps in the alignment. Within these gaps, the unique sequence of HCN4 719–1012 notably contained multiple proline-rich domains (the hallmark of several protein interaction motifs) as well as a strong consensus PKA phosphorylation site (S831), which we found to be phosphorylated by PKA in our previous study (Liao et al., 2010).
Pre-relief of autoinhibition in CHO cells may also require other domains of HCN4
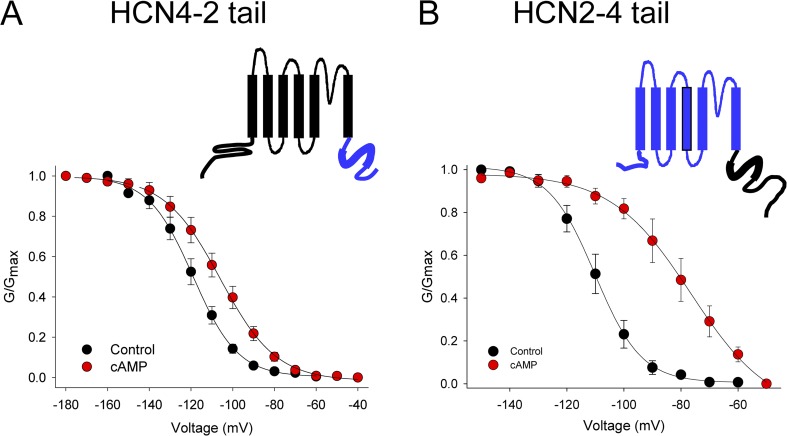
To explore the molecular basis for the different cAMP sensitivity of HCN2 and HCN4 in CHO cells, we constructed chimeric channels in which the entire C-terminal domains (including the C-linker and CNBD) of HCN2 and HCN4 were exchanged to form “HCN2–4tail” and “HCN4–2tail” channels (Fig. 5). Expression of these chimeric channels in CHO cells produced whole cell hyperpolarization-activated currents that were qualitatively similar to those produced by the parent channels. Substitution of the HCN2 C terminus (residues 442–863) onto the HCN4 channel body in the HCN4–2tail chimera resulted in a hyperpolarizing shift in the basal voltage dependence (compared with wild-type HCN4) and in restored cAMP sensitivity, suggesting that the C terminus of HCN2 is sufficient to confer autoinhibition to the HCN4 channel in the CHO cell background (V1/2 = −118.9 ± 2.5 mV, n = 4 in control, and −105.4 ± 3.0 mV, n = 5 with cAMP; Fig. 5 A). However, substitution of the HCN4 C terminus (residues 528–1201) onto the HCN2 channel body did not produce the opposite effect. Rather, the basal voltage dependence of HCN2–4tail was similar to that of wild-type HCN2 channels, and HCN2–4tail was particularly sensitive to cAMP, which shifted the voltage dependence by ∼30 mV (V1/2 = −110.2 ± 2.9, n = 6 in control, and −78.9 ± 4.3, n = 4 with cAMP; Fig. 5 B). These results show that the 719–1012 region in the C terminus of HCN4 is necessary, but not sufficient, for ligand-independent relief of autoinhibition in CHO cells, and thereby suggest that other domains specific to HCN4 may also be required for ligand-independent relief of autoinhibition in CHOs.
Figure 5.
The HCN4 C terminus is necessary but not sufficient for ligand-independent relief of autoinhibition in CHO cells. Schematics and voltage dependence for HCN4–2tail and HCN2–4tail chimeric channels. In the schematic illustrations, HCN4 sequence is indicated in black, and HCN2 in blue. Thick lines indicate the CNBD. The voltage dependence of activation was determined from tail currents after 3-s hyperpolarizing steps to the indicated voltages in whole cell recordings from CHO cells expressing HCN4–2tail (A) or HCN2–4tail (B) in the absence (black) or presence (red) of 1 mM cAMP in the patch pipette.
DISCUSSION
The unliganded CNBD of HCN channels constitutively inhibits voltage-dependent gating, and this “autoinhibition” is relieved by a conformational change associated with cyclic nucleotide binding. Our present results show that relief of autoinhibition can also occur independently of ligand binding for HCN4 channels expressed in CHO cells. This “pre-relief” behavior requires a domain in the distal C terminus of HCN4 that is not conserved in other HCN isoforms. In addition, at least one other region of HCN4 and an endogenous factor from CHO cells appear to contribute to the relief of autoinhibition. Collectively, the data suggest the presence of a multipartite regulatory mechanism that can significantly alter the function of HCN4 channels. Given the important role of HCN4 channels in cardiac pacemaking and neuronal excitability, it will be important in future work to determine whether cAMP-independent relief of autoinhibition can occur in sinoatrial myocytes and/or in sensory and thalamic neurons, and, if so, whether it is dynamically regulated to alter HCN currents in response to physiological stimuli. More generally, the data presented here may also portend the existence of similar mechanisms for cyclic nucleotide–independent regulation of other members of the large family of proteins and ion channels that contain the conserved CNBD.
Potential mechanisms for differences in voltage-dependent gating of HCN4 in CHO versus HEK cells
What are the differences between CHO and HEK cells that cause the ligand-independent relief of autoinhibition for HCN4 but not HCN2? Previous studies have demonstrated differences in behavior of other ion channels in CHO versus HEK cells that arise from differences in translation initiation sites, differences in expression of interacting proteins, and differences in posttranslational modifications. We consider here each of these possibilities in the context of known regulatory mechanisms for HCN channels.
It has recently been reported that an alternative translation initiation site that omits the initial 26 amino acids of the N terminus of HCN4 renders the channels insensitive to cAMP in HEK cells (Liu and Aldrich, 2011). Because a similar translation initiation variance was found to account for differences in inactivation of Kv3.3 channels in CHO versus HEK cells (Fernandez et al., 2003), we wondered whether alternative translation initiation could account for the CHO versus HEK differences in cAMP sensitivity and basal voltage dependence of HCN4. However, this possibility seems unlikely because, although the alternative start site reported by Liu and Aldrich (2011) eliminated the cAMP sensitivity of HCN4 in HEKs, it did not relieve autoinhibition. Instead, the basal voltage dependence was similar for the long and short forms of HCN4, suggesting that the underlying mechanism may be distinct from that reported here.
Differences in expression of endogenous interacting proteins have been shown to underlie differences in the inactivation and voltage dependence of Kv1.5 and KvLQT1 channels in CHO versus HEK cells. In the case of Kv1.5, the differences are caused by the presence of endogenous Kv β subunits in CHO but not HEK cells (Uebele et al., 1996; Plante et al., 2006). Similarly, KvLQT2 and minK express functional currents only in CHO cells, not in HEK cells, because of the presence of the LIM domain protein, fhl2, in CHOs but not HEKs (Kupershmidt et al., 2002). We considered whether differences in the HCN-interacting protein, Trip8b/PEX5R, could account for the different behaviors of HCN4 in CHO versus HEK cells because Trip8b/PEX5R has been shown to antagonize cAMP-dependent modulation of voltage dependence for HCN channels (Santoro et al., 2009; Zolles et al., 2009). However, the Trip8b/PEX5R interaction domains are shared between HCN2 and HCN4, and cAMP sensitivity of both isoforms is reduced to a similar degree. Furthermore, the Trip8b/PEX5R reduction in cAMP sensitivity of HCN channels occurs without alteration of the basal voltage dependence. Thus, it appears that Trip8b/PEX5R may work by a different mechanism than the one that alters autoinhibition of HCN4 in CHO cells. Thus, if an interacting protein mediates the cell type–dependent behavior of HCN4, it may well be a novel interactor.
As discussed above, our data from excised membrane patches excludes the possibility that soluble intracellular factors could cause the relief of autoinhibition of HCN4 in CHO cells. It should be noted that this finding specifically excludes a role for phosphatidylinositol 4,5-bisphosphate (PIP2) in the relief of autoinhibition. Although PIP2 profoundly shifts the voltage dependence of HCN channel activation and, at least partially, interacts with cAMP-dependent regulation (Zolles et al., 2006; Pian et al., 2007), it is well-known that PIP2 levels run down in excised patches (e.g., Hilgemann and Ball, 1996), whereas the differences we observed in HCN4 behavior in CHOs versus HEKs persisted in cell-free membrane patches. Moreover, PIP2 has been shown to regulate both the HCN2 and HCN4 isoforms (Zolles et al., 2006). Thus, it seems quite improbable that PIP2 could account for the isoform- and cell context–specific differences in HCN channel behavior that we report here.
Differences in posttranslational modification of HCN4 in CHO versus HEK cells are another potential mechanism that could explain our data. Such differential phosphorylation has been shown to underlie differences in the voltage dependence of Kv2.1 channels in COS versus HEK cells (Mohapatra and Trimmer, 2006). However, if cell type–specific phosphorylation were to account for the differences in HCN4 behavior in CHOs versus HEKs, it must occur at a site distinct from the PKA site in the extreme distal C terminus of HCN4 that we described previously (Liao et al., 2010), as this site is lacking in both HCN4-Δ719 and HCN4-Δ1012 channels, whereas cAMP sensitivity was restored only in HCN4-719 channels. It will be interesting in future work to examine the possibility that other phosphorylation sites may cause cAMP-independent relief of autoinhibition of HCN4 in CHO cells. Of particular note is the presence of a strong consensus PKA phosphorylation site within the 719–1012 region (S831), which would be a candidate for such regulation.
Biophysical considerations for ligand-independent relief of autoinhibition of HCN4
The above discussion considers various cellular mechanisms that may account for the differences in HCN4 properties in CHO versus HEK cells. For any of the above mechanisms, it is also informative to contemplate the biophysical basis by which they might affect the voltage dependence and cAMP sensitivity of HCN4. We consider two questions here: (1) is the CNBD of HCN4 occupied in CHO cells, and (2) does the N terminus of HCN4 interact functionally with the C terminus to regulate gating?
A recent structural study reported that the cAMP insensitivity of the CNBD-containing zELK channel results from binding of an “intrinsic ligand,” composed of a portion of the β9 helix of the CNBD. Although this specific domain cannot account for our data—the β9 helices of the CNBDs of HCN2 and HCN4 are completely conserved, and differ from that of zELK—we have considered the possibility that an alternative ligand may occupy the CNBD of HCN4 in CHO cells. We do not presently favor this explanation because (a) the slowing of HCN4 deactivation in response to cAMP (Fig. 3) suggests that CNBD maybe unoccupied in CHOs (a coincidence of affinities would be required to explain displacement of an alternative ligand by cAMP); and (b) we did not observe qualitative differences in deactivation kinetics between CHOs and HEKs, which would be predicted if the ligand-binding site were occupied in a manner similar to cAMP binding.
As discussed above, alternative translation initiation between CHO and HEK cells alone probably cannot account for the relief of autoinhibition of HCN4 in CHOs. However, several lines of evidence suggest a functional interaction between the N and C terminals of HCN4: (a) deletion of the N-terminal 26 amino acids from HCN4 renders the channels insensitive to cAMP (Liu and Aldrich, 2011); (b) substitution of the HCN4 C terminus onto the HCN2 channel was not sufficient to mediate preshift/cAMP insensitivity (Fig. 5); and (c) HCN4 channels lacking the distal N terminus did not express, but channels lacking both the distal N and distal C terminals did express (Liao et al., 2010). Collectively, these results suggest the possibility of a functional interaction between the N and C terminals of HCN4, similar to the interactions observed between the N and C terminals of the related hERG channels (Gustina and Trudeau, 2011). Thus, the N terminus remains a potential candidate for an additional domain in HCN4 that may participate with an endogenous factor in CHO cells and with the 719–1012 domain to regulate channel gating.
Acknowledgments
We thank Roger Bannister and Kristen M.S. O’Connell for critical reading of the manuscript and Bill Zagotta for the GST-HCN2 clone and for advice on 8-NBD-cAMP experiments.
This work was supported by the National Institutes of Health (grant HL 088427 to C. Proenza).
Author contributions: Z. Liao, D. Lockhead, E.D. Larson, J.R. St. Clair, C.E. Wilson, and C. Proenza conducted experiments and analyzed data, and C. Proenza wrote the manuscript.
Footnotes
Abbreviations used in this paper:
- CHO
- Chinese hamster ovary
- CNBD
- cyclic nucleotide–binding domain
- EAG
- ether-a-go-go
- GST
- glutathione S-transferase
- HCN
- hyperpolarization-activated, cyclic nucleotide–sensitive
- HEK
- human embryonic kidney
- PIP2
- phosphatidylinositol 4,5-bisphosphate
References
- Altomare C., Terragni B., Brioschi C., Milanesi R., Pagliuca C., Viscomi C., Moroni A., Baruscotti M., DiFrancesco D. 2003. Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J. Physiol. 549:347–359 10.1113/jphysiol.2002.027698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., Carlson A.E., Zagotta W.N. 2009. Absence of direct cyclic nucleotide modulation of mEAG1 and hERG1 channels revealed with fluorescence and electrophysiological methods. J. Biol. Chem. 284:27989–27997 10.1074/jbc.M109.016337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., Carlson A.E., Sankaran B., Zagotta W.N. 2012. Structure of the carboxy-terminal region of a KCNH channel. Nature. 481:530–533 10.1038/nature10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang J., Siegelbaum S.A. 2001. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 117:491–504 10.1085/jgp.117.5.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukkemane A., Grüter B., Novak K., Gensch T., Bönigk W., Gerharz T., Kaupp U.B., Seifert R. 2007. Subunits act independently in a cyclic nucleotide-activated K(+) channel. EMBO Rep. 8:749–755 10.1038/sj.embor.7401025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F.R., Morales E., Rashid A.J., Dunn R.J., Turner R.W. 2003. Inactivation of Kv3.3 potassium channels in heterologous expression systems. J. Biol. Chem. 278:40890–40898 10.1074/jbc.M304235200 [DOI] [PubMed] [Google Scholar]
- Gustina A.S., Trudeau M.C. 2011. hERG potassium channel gating is mediated by N- and C-terminal region interactions. J. Gen. Physiol. 137:315–325 10.1085/jgp.201010582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D., Pfeiffer K.H., Fabritz L., Kremmer E., Buch T., Waisman A., Kirchhof P., Kaupp U.B., Seifert R. 2008. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J. 27:692–703 10.1038/emboj.2008.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W., Ball R. 1996. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 273:956–959 10.1126/science.273.5277.956 [DOI] [PubMed] [Google Scholar]
- Jurman M.E., Boland L.M., Liu Y., Yellen G. 1994. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 17:876–881 [PubMed] [Google Scholar]
- Kraemer A., Rehmann H.R., Cool R.H., Theiss C., de Rooij J., Bos J.L., Wittinghofer A. 2001. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. J. Mol. Biol. 306:1167–1177 10.1006/jmbi.2001.4444 [DOI] [PubMed] [Google Scholar]
- Kupershmidt S., Yang I.C., Sutherland M., Wells K.S., Yang T., Yang P., Balser J.R., Roden D.M. 2002. Cardiac-enriched LIM domain protein fhl2 is required to generate I(Ks) in a heterologous system. Cardiovasc. Res. 56:93–103 10.1016/S0008-6363(02)00498-4 [DOI] [PubMed] [Google Scholar]
- Liao Z., Lockhead D., Larson E.D., Proenza C. 2010. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J. Gen. Physiol. 136:247–258 10.1085/jgp.201010488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Aldrich R.W. 2011. Tissue-specific N terminus of the HCN4 channel affects channel activation. J. Biol. Chem. 286:14209–14214 10.1074/jbc.M110.215640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Zong X., Stieber J., Hullin R., Hofmann F., Biel M. 1999. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 18:2323–2329 10.1093/emboj/18.9.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra D.P., Trimmer J.S. 2006. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J. Neurosci. 26:685–695 10.1523/JNEUROSCI.4620-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian P., Bucchi A., Robinson R.B., Siegelbaum S.A. 2006. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J. Gen. Physiol. 128:593–604 10.1085/jgp.200609648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian P., Bucchi A., Decostanzo A., Robinson R.B., Siegelbaum S.A. 2007. Modulation of cyclic nucleotide-regulated HCN channels by PIP(2) and receptors coupled to phospholipase C. Pflugers Arch. 455:125–145 10.1007/s00424-007-0295-2 [DOI] [PubMed] [Google Scholar]
- Plante I., Fournier D., Ricard G., Drolet B., O’Hara G., Champagne J., Mathieu P., Baillot R., Daleau P. 2006. Electrophysiological characterization of three non-synonymous single nucleotide polymorphisms (R87Q, A251T, and P307S) found in hKv1.5. Pflugers Arch. 452:316–323 10.1007/s00424-005-0031-8 [DOI] [PubMed] [Google Scholar]
- Santoro B., Piskorowski R.A., Pian P., Hu L., Liu H., Siegelbaum S.A. 2009. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron. 62:802–813 10.1016/j.neuron.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Scholten A., Gauss R., Mincheva A., Lichter P., Kaupp U.B. 1999. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proc. Natl. Acad. Sci. USA. 96:9391–9396 10.1073/pnas.96.16.9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbs G.R., Liu D.T., Leypold B.G., Siegelbaum S.A. 1998. A state-independent interaction between ligand and a conserved arginine residue in cyclic nucleotide-gated channels reveals a functional polarity of the cyclic nucleotide binding site. J. Biol. Chem. 273:4497–4505 10.1074/jbc.273.8.4497 [DOI] [PubMed] [Google Scholar]
- Uebele V.N., England S.K., Chaudhary A., Tamkun M.M., Snyders D.J. 1996. Functional differences in Kv1.5 currents expressed in mammalian cell lines are due to the presence of endogenous Kv beta 2.1 subunits. J. Biol. Chem. 271:2406–2412 10.1074/jbc.271.5.2406 [DOI] [PubMed] [Google Scholar]
- Viscomi C., Altomare C., Bucchi A., Camatini E., Baruscotti M., Moroni A., DiFrancesco D. 2001. C terminus-mediated control of voltage and cAMP gating of hyperpolarization-activated cyclic nucleotide-gated channels. J. Biol. Chem. 276:29930–29934 10.1074/jbc.M103971200 [DOI] [PubMed] [Google Scholar]
- Wainger B.J., DeGennaro M., Santoro B., Siegelbaum S.A., Tibbs G.R. 2001. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 411:805–810 10.1038/35081088 [DOI] [PubMed] [Google Scholar]
- Wicks N.L., Chan K.S., Madden Z., Santoro B., Young E.C. 2009. Sensitivity of HCN channel deactivation to cAMP is amplified by an S4 mutation combined with activation mode shift. Pflugers Arch. 458:877–889 10.1007/s00424-009-0687-6 [DOI] [PubMed] [Google Scholar]
- Wicks N.L., Wong T., Sun J., Madden Z., Young E.C. 2011. Cytoplasmic cAMP-sensing domain of hyperpolarization-activated cation (HCN) channels uses two structurally distinct mechanisms to regulate voltage gating. Proc. Natl. Acad. Sci. USA. 108:609–614 10.1073/pnas.1012750108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolles G., Klöcker N., Wenzel D., Weisser-Thomas J., Fleischmann B.K., Roeper J., Fakler B. 2006. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 52:1027–1036 10.1016/j.neuron.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Zolles G., Wenzel D., Bildl W., Schulte U., Hofmann A., Müller C.S., Thumfart J.O., Vlachos A., Deller T., Pfeifer A., et al. 2009. Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN channels to cAMP and adrenergic stimulation. Neuron. 62:814–825 10.1016/j.neuron.2009.05.008 [DOI] [PubMed] [Google Scholar]