Abstract
Aging is associated with increased retrograde and oscillatory shear in peripheral conduit arteries of humans. Although the mechanisms responsible for these age-related changes are not completely understood, augmented downstream α-adrenergic tone likely plays a significant role in this phenomenon. Therefore, in Protocol 1 brachial artery diameter and blood velocity were measured via Doppler ultrasound during 1) rest (control), 2) endogenous norepinephrine release via intra-arterial infusions of tyramine, and 3) α-adrenergic blockade via infusions of phentolamine in young healthy humans (n=12). Tyramine increased brachial artery retrograde (−4.0±1.4 to −9.5±1.4 s−1) and oscillatory shear (0.05±0.02 to 0.18±0.05 arbitrary units), whereas, phentolamine abolished retrograde and oscillatory shear (P<0.05). Additionally, in Protocol 2 we examined brachial artery shear patterns in young (n=12; 29±2 years) and older (n=13; 69±2 years) healthy adults during 1) rest (control), 2) sympathetic activation via lower body negative pressure, and 3) infusion of phentolamine. At rest older adults exhibited greater brachial artery retrograde and oscillatory shear (−9.9±2.7 s−1 and 0.11±0.03 arbitrary units, respectively) compared with younger adults (−3.1±1.0 s−1 and 0.05±0.02 arbitrary units, respectively; P<0.05 for both). Lower body negative pressure increased retrograde and oscillatory shear in young (P<0.05), but not older adults (P=0.85–0.97), such that differences between young and older were eliminated (P>0.05). During infusion of phentolamine, retrograde and oscillatory shear were abolished in young adults (P<0.05) and markedly reduced, yet still persistent, in older adults (P<0.01). Our data indicate that α-adrenergic vasoconstriction is a major contributor to age-related discrepancies in conduit artery shear rate patterns at rest.
Keywords: α-adrenergic receptors, aging, shear rate, sympathetic activation
INTRODUCTION
Blood flow patterns within large peripheral conduit arteries (i.e. brachial and femoral) commonly present with a triphasic appearance that is characterized by an initial large antegrade flow during systole, followed by a brief episode of retrograde flow early in diastole and subsequent antegrade flow in mid- to late diastole.1, 2 The pattern of blood flow and the resulting oscillatory (bidirectional blood flow) shear patterns in conduit vessels are thought to influence endothelial cell function. Indeed, in vitro studies using endothelial cell culture and isolated perfused arteries indicate that increased retrograde and oscillatory shear can induce profound pro-atherogenic effects on endothelial cells, including increased production of NADPH oxidase-and mitochondria-derived reactive oxygen species, augmented production of endothelin-1, and enhanced expression of adhesion molecules.3–12 Moreover, although limited, there are also data available in humans suggesting that brachial artery endothelium-dependent dilation is impaired following an acute bout of increased retrograde and oscillatory shear.13.
Evidence in humans suggests that aging is associated with greater retrograde and oscillatory shear in peripheral conduit arteries.14–16 Although the mechanisms responsible for these age-related changes are not completely understood, alterations in downstream vascular resistance likely play a significant role in the increased retrograde and oscillatory shear in conduit arteries of older adults. Indeed, brachial artery retrograde flow is reduced as a result of experimentally induced decreases in downstream resistance in young adults,17, 18 whereas, conditions that elevate vascular resistance provoke an increase in retrograde flow.13, 17, 19, 20 Of particular interest to the current study, acute elevations in muscle sympathetic nerve activity (MSNA) are associated with an increase in conduit artery retrograde and oscillatory shear in young males.20 Since MSNA increases with aging,21, 22 it is possible that an increased α-adrenergic vasoconstriction in resistance vessels contributes to the age-related differences in resting shear rate patterns in the upstream conduit arteries. Therefore the aim of the current study was to examine the contribution of α-adrenergic vasoconstriction in resistance vessels on shear rate profiles in upstream conduit arteries of young and older adults. We hypothesized that postjunctional α-adrenergic vasoconstriction in young adults and/or local α-adrenergic blockade in older adults would eliminate age-related differences in resting conduit artery retrograde and oscillatory shear.
METHODS
Study Design
Two separate protocols were performed in which brachial artery blood velocity and diameter were measured in order to calculate blood flow and shear rate. In protocol 1, we examined the influence of α-adrenergic vasoconstriction on conduit artery shear rate patterns in young healthy adults. In protocol 2 we investigated whether α-adrenergic vasoconstriction in resistance vessels contributes to age-related discrepancies in conduit artery shear rate patterns at rest. All study protocols were approved by the Mayo Clinic Institutional Review Board and were performed according to the Declaration of Helsinki. Each subject gave written informed consent prior to participation.
Subjects
A total of 37 subjects volunteered to participate in one of two protocols. Studies were performed between 0700 and 1100 after an overnight fast and refraining from exercise and caffeine for at least 24 h. All studies were performed while the subjects rested in the supine position. Protocol 1 included 12 young healthy males (24–40 years). Protocol 2 included a separate group of 12 young (6 male and 6 female; 19–37 years) and 13 older (7 male and 6 female; 61–81 years) healthy subjects. Subjects from both protocols underwent a standard screening and were healthy, non-obese (BMI ≤ 30 kg m−2), non-smokers, not taking any vasoactive medications and were sedentary to moderately active. In protocol 2, four older subjects were taking Omeprazole (proton pump inhibitor) to treat gastroesophageal reflux (withheld for a minimum of 3 days prior to study), one older subject was taking aspirin (withheld for 1 week prior to study), and two older subjects were taking Synthroid to treat hypothyroidism (withheld 3 days prior to study). Five older subjects reported taking a daily vitamin. Young female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives.23 All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy.
Arterial catheterization
A 20 gauge, 5 cm (Model RA-04020, Arrow International, Reading, PA, USA) catheter was placed in the brachial artery of the experimental arm under aseptic conditions after local anesthesia (2% lidocaine) for administration of study drugs. The catheter was connected to a three-port connector in series, as previously described in detail.24 One port was linked to a pressure transducer positioned at heart level (Model PX600F, Edwards Lifescience, Irvine, CA, USA) to allow measurement of arterial pressure and was continuously flushed (3 ml h−1) with saline with a stop-cock system to enable arterial blood sampling. The remaining two ports allowed arterial drug administration.
Heart rate and systemic blood pressure
Heart rate (HR) was recorded via continuous 3 lead ECG. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure (Cardiocap/5, Datex-Ohmeda, Louisville, CO, USA)
Forearm blood flow
Brachial artery mean blood velocity and brachial artery diameter were determined with a 12 MHz linear-array Doppler probe (Model M12L, Vivid 7, General Electric, Milwaukee, WI, USA). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole. Forearm blood flow was calculated as the product of mean blood velocity (cm s−1) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml min−1).
Pharmacologic infusions
In protocol 1 tyramine (Sigma-Aldrich, St. Louis, MO, USA) was administered intra-arterially (12 μg (dl forearm volume)−1 min−1 for 3 min) to evoke endogenous norepinephrine release and stimulate both α1- and α2-adrenorecpetors. In protocols 1 and 2 phentolamine mesylate (Bedford Laboratories, Bedford, Ohio, USA), a non-selective α-adrenergic receptor antagonist, was administered to the experimental forearm via brachial artery catheter as a loading dose (10 μg (dl forearm volume)−1 min−1 for 5 min) followed by a continuous maintenance dose (25–50μg min−1). This dose of phentolamine has been shown to effectively inhibit α-adrenergic receptor vasoconstriction.25
Lower body negative pressure (LBNP)
LBNP was used to examine the influence of increased sympathetic outflow on conduit artery shear patterns. While supine the lower body of each subject was sealed in an airtight box above the level of the iliac crests. The box was attached to a vacuum source, which allowed for rapid reductions in pressure. During the LBNP trials the pressure was reduced to −20mmHg. All variables were continuously recorded for 3 minutes while at the target LBNP pressure (−20mmHg). Application of low levels of LBNP (−20 mmHg) increases MSNA without concurrent changes in heart rate and arterial pressure.26 Moreover, this level of LBNP increases MSNA in young adults to similar levels observed in older adults at rest (i.e. without LBNP).21
Experimental protocol
Protocol 1: Measurements were collected at rest during control (no drug), α-adrenergic receptor vasoconstriction (via intra-arterial infusion of tyramine), and local α-adrenergic blockade (via intra-arterial infusion of phentolamine) conditions. Protocol 2: Measurements were collected at rest during control (no drug), sympathetic stimulation (via LBNP), and local α-adrenergic blockade (via intra-arterial infusion of phentolamine) conditions. Due to the long half-life of phentolamine, α-adrenergic blockade trials were always performed last in both protocols. A rest period of 15 min was allowed between conditions to ensure reestablishment of baseline hemodynamic variables.
Data analysis and statistics
Data were collected at 250Hz, stored on a computer and analyzed off-line with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was determined from the brachial artery pressure waveform and HR was determined from the electrocardiogram. Time average mean blood velocity, antegrade mean velocity, and retrograde mean velocity were calculated. Diameter and velocity measures were used to estimate brachial artery shear rates. Mean shear rate (s−1) was defined as 4 · Vm/D, where Vm is mean blood velocity (cm · s−1) and D is arterial diameter (cm).27, 28 For calculations of antegrade and retrograde shear rate, antegrade and retrograde mean blood velocities were used, respectively. Oscillatory shear index is a dimensionless parameter that can be used as an indicator of the magnitude of oscillation and can be defined as follows:|Retrograde shear|/(|Antegrade shear|+|Retrograde shear|).20, 27, 29 Note that the values for oscillatory shear range from 0 to 0.5, where a value of 0 corresponds to a unidirectional shear rate throughout the cardiac cycle, and a value of 0.5 represents pure oscillation with a time-average shear equal to 0. Forearm vascular conductance was calculated as the ratio between forearm blood flow (Vm·π· D2/4 · 60) and mean arterial pressure and expressed as ml·min−1 (100 mm Hg)−1. All measurements were averaged over the last minute of data collection.
All values are expressed as means ± SE. Protocol 1: Hemodynamic and shear profile variables were compared via repeated measures analysis of variance (ANOVA) to detect differences across conditions. Protocol 2: Descriptive statistics were used to characterize the younger and older groups of subjects. Hemodynamic and shear profile variables were compared via repeated measures ANOVA to detect differences between age groups and across conditions. Appropriate post hoc analysis determined where statistical differences occurred. Statistical difference was set a priori at P < 0.05.
RESULTS
Protocol 1
All 12 subjects completed the study. The subjects were 29 ± 2 years of age, 179 ± 2 cm in height, and weighed 81 ± 3 kg (BMI: 25 ± 1 kg m−2).
Contribution of α-adrenergic receptors in conduit artery shear patterns
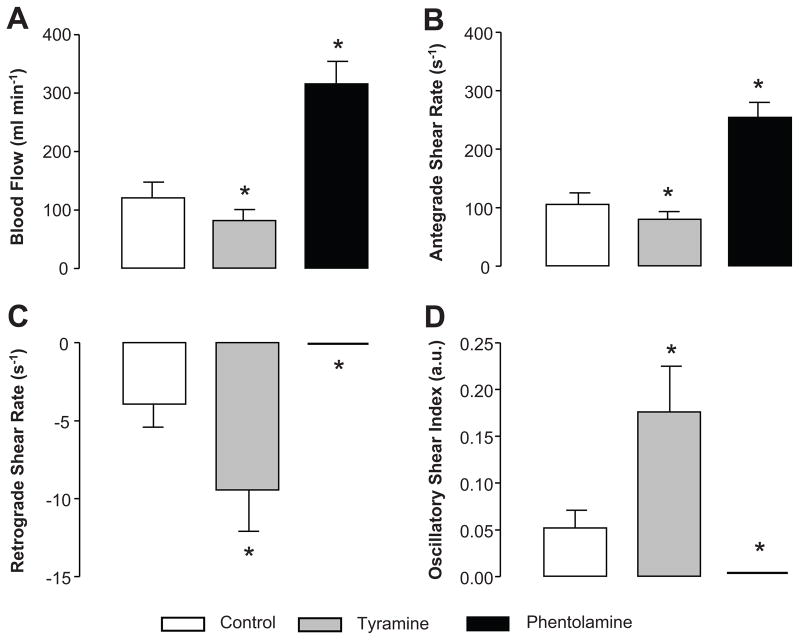
Endogenous norepinephrine release in the experimental forearm via administration of tyramine resulted in drastic changes in brachial artery hemodymanics (Table 1 and Figure 1). As illustrated in Figure 1, during infusion of tyramine, forearm blood flow and antegrade shear rate were substantially decreased, while retrograde and oscillatory shear were increased, compared to control conditions (P<0.05). α-adrenergic blockade via administration of phentolamine reduced MAP and resulted in a small, yet significant increase in brachial artery diameter (P < 0.01, Table 1). Infusion of phentolamine substantially elevated forearm blood flow and antegrade shear rate, while abolishing retrograde and oscillatory shear (P < 0.05, Figure 1).
Table 1.
Forearm Hemodynamics and Shear Rate Patterns at Rest During Control, Tyramine, Phentolamine in Young Men (n=12)
| Variable | Condition
|
||
|---|---|---|---|
| Control | Tyramine | Phentolamine | |
| Mean arterial pressure (mmHg) | 90 ± 1 | 91 ± 2 | 87 ± 2* |
| Forearm vascular conductance (mL · min−1 · 100mmHg−1) | 136 ± 31 | 90 ± 20* | 369 ± 48* |
| Brachial artery diameter (cm) | 0.46 ± 0.01 | 0.46 ± 0.01 | 0.47 ± 0.01* |
| Mean velocity (cm · s−1) | 11.8 ± 2.4 | 8.1 ± 1.7* | 29.8 ± 3.1* |
| Mean shear rate (s−1) | 101.4 ± 20.1 | 70.3 ± 13.9* | 254.2 ± 25.7* |
| Antegrade velocity (cm · s−1) | 12.2 ± 2.4 | 9.1 ± 1.6† | 29.8 ± 3.1* |
| Retrograde velocity (cm · s−1) | −0.4 ± 0.1 | −1.0 ± 0.3* | 0.0 ± 0.0* |
Values are means ± SE.
P < 0.01 vs. control;
P < 0.05 vs. control
Figure 1.
Brachial artery blood flow (A), antegrade shear (B), retrograde shear (C), and oscillatory shear index (D) at rest during control (no drug), α-adrenergic vasoconstriction via tyramine, and α-adrenergic blockade via phentolamine in young adults (Protocol 1). Values are means ± SE. *P < 0.05 vs. control
Protocol 2
Subject characteristics are summarized in Table 2. Young and older subjects were of similar height, weight, and body mass index (P > 0.05). Older subjects demonstrated a greater systolic and mean arterial pressure, total cholesterol, and low density lipoprotein than their younger counterparts (P < 0.05).
Table 2.
Subject Characteristics for Protocol 2
| Variable | Young (n = 12) | Older (n = 13) |
|---|---|---|
| Age | 27 ± 2 | 69 ± 2* |
| Male/Female | 6/6 | 7/6 |
| Height, cm | 174 ± 2 | 170 ± 3 |
| Weight, kg | 75 ± 3 | 76 ± 4 |
| BMI, kg/m2 | 24.7 ± 0.9 | 26.1 ± 0.9 |
| SBP, mmHg | 129 ± 3 | 142 ± 3* |
| DBP, mmHg | 79 ± 2 | 84 ± 2 |
| MAP, mmHg | 96 ± 2 | 103 ± 2* |
| Total cholesterol, mg/dL | 143 ± 5 | 186 ± 6* |
| LDL, mg/dL | 71 ± 3 | 110 ± 6* |
| HDL, mg/dL | 53 ± 4 | 53 ± 6 |
| Triglycerides, mg/dL | 89 ± 8 | 90 ± 6 |
Values are means ± SE. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; LDL, low density lipoprotein, HDL, high density lipoprotein.
P < 0.05 vs. young.
Sympathetic influence and the age-related discrepancies in shear patterns
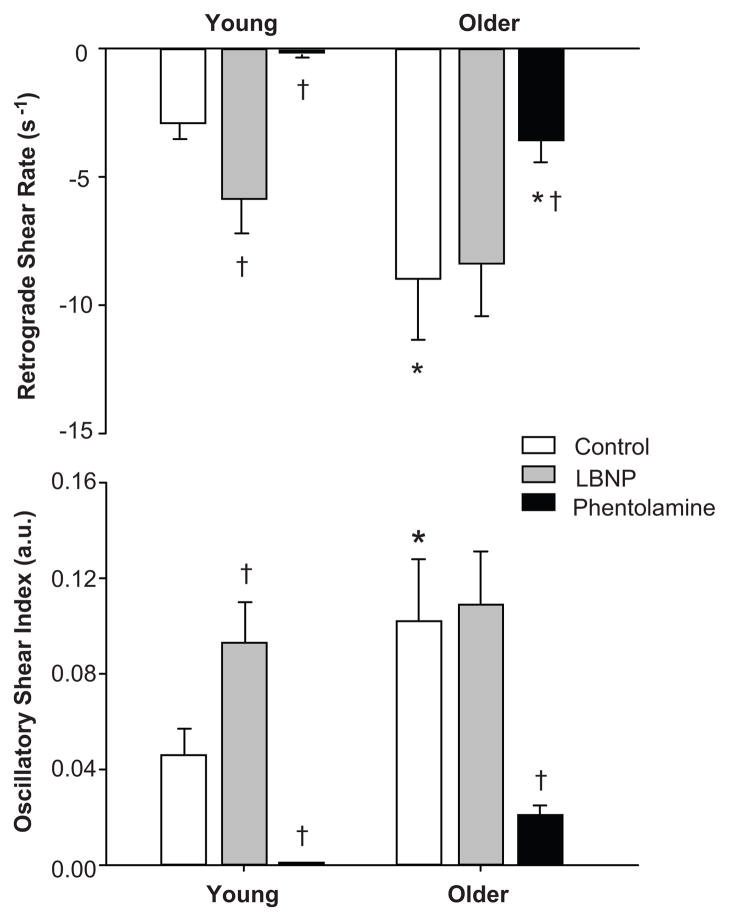
Under control conditions, older adults demonstrated greater retrograde and oscillatory shear (−9.0 ± 2.4 s−1 and 0.10 ± 0.03 arbitrary units [a.u.], respectively) compared with younger adults (−2.9 ± 0.6 s−1 and 0.05 ± 0.01 a.u., respectively; P < 0.05 for both; Figure 2). Sympathetic activation via LBNP in young, but not older, adults increased retrograde (−2.9 ± 0.6 to −5.9 ± 1.3 s−1; P < 0.05) and oscillatory (0.05 ± 0.01 to 0.09 ± 0.02 a.u., P < 0.05) shear, such that differences between young and older were eliminated (P = 0.24 for retrograde and 0.42 for oscillatory shear).
Figure 2.
Brachial artery retrograde shear rate and oscillatory shear index at rest during control, sympathetic activation via lower body negative pressure (LBNP), and intra-arterial administration of phentolamine (non-specific α-adrenergic antagonist) in young and older subjects (Protocol 2). Values are means ± SE. *P < 0.05 vs. young; †P <0.01 vs. control
α-adrenergic blockade via phentolamine resulted in a substantial increase in forearm blood flow and conductance in young and older adults (Table 3). In turn, mean and antegrade shear were greater in young and older adults during phentolamine infusion compared to control conditions (P < 0.01, Table 3). As depicted in Figure 2, during α-adrenergic blockade, retrograde and oscillatory shear were abolished in young adults (P<0.05) and markedly reduced, yet still persistent, in older adults (P<0.01). Indeed, retrograde shear was greater in older compared to young adults during infusion of phentolamine (P <0.05, Figure 2).
Table 3.
Systemic and Forearm Hemodynamics at Rest During Control, LBNP, Phentolamine conditions in Young and Older Subjects
| Variable | Young (n=12) | Older (n = 13) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | LBNP | Phentolamine | Control | LBNP | Phentolamine | |
| Heart rate (beats · min−1) | 59 ± 2 | 61 ± 2† | 59 ± 2 | 57 ± 2 | 59 ± 2 | 56 ± 2 |
| Mean arterial pressure (mmHg) | 96 ± 1 | 97 ± 2 | 98 ± 1 | 104 ± 2* | 105 ± 1* | 105 ± 1* |
| Forearm blood flow (mL · min−1) | 52 ± 8 | 43 ± 7 | 121 ± 20† | 67 ± 9 | 57 ± 7 | 143 ± 21† |
| Forearm vascular conductance (mL · min−1 · 100mmHg−1) | 53 ± 8 | 45 ± 7 | 123 ± 20† | 62 ± 8 | 53 ± 6 | 132 ± 19† |
| Brachial artery diameter (cm) | 0.37 ± 0.02 | 0.37 ± 0.02 | 0.37 ± 0.02 | 0.40 ± 0.02 | 0.40 ± 0.02 | 0.40 ± 0.02 |
| Mean velocity (cm · s−1) | 7.2 ± 0.7 | 6.1 ± 0.7 | 18.5 ± 1.8† | 8.8 ± 0.7 | 7.1 ± 0.6 | 18.3 ± 2.1† |
| Mean shear rate (s−1) | 77.9 ± 7.8 | 67.3 ± 7.3 | 201.2 ± 21.1† | 89.5 ± 7.1 | 73.9 ± 6.9 | 185.8 ± 20.2† |
| Antegrade velocity (cm · s−1) | 7.4 ± 0.7 | 6.6 ± 0.7 | 18.5 ± 1.8† | 9.6 ± 0.7 | 7.9 ± 0.6 | 18.7 ± 2.1† |
| Antegrade shear rate (s−1) | 80.8 ± 7.7 | 73.1 ± 7.7 | 201.2 ± 21.1† | 98.5 ± 7.9 | 82.2 ± 8.2 | 189.3 ± 20.5† |
| Retrograde velocity (cm · s−1) | −0.3 ± 0.1 | −0.5 ± 0.1† | 0.0 ± 0.0† | −0.8 ± 0.2* | −0.8 ± 0.2 | −0.3 ± 0.1*† |
Values are means ± SE. LBNP, lower body negative pressure
P < 0.05 vs. young;
P < 0.05 vs. control
DISCUSSION
The primary novel findings of the present study are as follows: 1) endogenous norepinephrine release in the forearm of young men increased upstream conduit artery retrograde and oscillatory shear, 2) sympathetic activation increased retrograde and oscillatory shear in the brachial artery of young, but not older, adults, such that age-related differences were no longer apparent, 3) brachial artery retrograde and oscillatory shear were abolished in younger adults and reduced in older adults during α-adrenergic blockade, and 4) despite drastic changes in brachial artery blood flow and shear rate patterns (increased antegrade and decreased retrograde shear) during α-adrenergic blockade, retrograde shear remained significantly higher in older vs. younger adults. Taken together our data indicate that α-adrenergic vasoconstriction contributes, in part to age-related increases in conduit artery retrograde and oscillatory shearat rest.
The finding that low levels of LBNP (−20mmHg) can increase retrograde and oscillatory shear in young adults is in agreement with previous data.20 Padilla and colleagues20 demonstrated that progressive increases in MSNA associated with graded LBNP (−10 to −40 mmHg) elicit commensurate increases in retrograde and oscillatory shear in young men. Herein, taking a pharmacological approach, our data further supports the role of the sympathetic nervous system in dictating conduit artery blood flow profiles. We demonstrated in young men that increased forearm resistance (via endogenous norepinephrine release) promotes enhanced retrograde and oscillatory shear at the brachial artery; whereas, reciprocally, decreased resistance (via α-adrenergic blockade) completely abolishes retrograde and oscillatory shear (Figure 1).
Importantly, our current results suggest that sympathetic activation increases retrograde and oscillatory shear in young men, to the extent that age-related discrepancies were abolished by tyramine. Of interest, in the present study retrograde and oscillatory shear were unaltered during LBNP in older adults. The lack of change in the conduit artery shear rate patterns in older adults with LBNP might be related to an already elevated retrograde and oscillatory blood flow present at rest prior to sympathetic activation. That is, it is possible that with aging the influence of sympathetic nerve activity on conduit artery retrograde and oscillatory flow has reached a ceiling effect such that further sympatho-excitation does not produce additional changes in flow profiles. Alternatively, it is also plausible that α-adrenergic responsiveness is diminished in the resistance vasculature of older adults. Indeed, it has been well characterized that aging is associated with a reduction in forearm and leg postjunctional α-adrenergic responsiveness to endogenous norepinephrine release.30, 31
Our initial hypothesis was that α-adrenergic blockade with phentolamine would reduce retrograde and oscillatory shear in young and older adults and thus eliminate any age-related differences. The current findings demonstrate that retrograde and oscillatory shear are abolished in young adults and substantially reduced in older adults during α-adrenergic blockade (Figure 2). However, the age-related difference in retrograde shear persisted despite the removal of α-adrenergic vasoconstrictor tone. This is an important observation as it suggests that other factors, independent of α-adrenergic vasoconstrictor tone, likely contribute to the age-related increases in conduit artery retrograde shear. Along these lines, we recently found that nitric oxide (NO) synthase inhibition in the forearm circulation of young, but not older, subjects increased retrograde and oscillatory shear, thus suggesting that reduced NO bioavailability in the resistance vessels contributes, in part, to age-related discrepancies in resting shear patterns.15 Taken together, we propose that with aging increased retrograde shear in conduit arteries during rest is due to combined increased α-adrenergic vasoconstriction and reduced NO bioavailability in the downstream resistance vasculature. However it is possible that alterations in β-adrenergic mediated vasodilation might also play a role in the age-related differences in retrograde shear during the phentolamine trials. In this context, age-associated reductions in β-adrenergic vasodilation have been observed in rat skeletal muscle arterioles32 and human limbs.33, 34 An attenuated β-adrenergic vasodilation may serve to increase downstream resistance and partially explain the retrograde shear that persists in older adults during α-adrenergic blockade. Further research needs to examine the contribution of the β-adrenergic receptors as well as other vasoactive factors that are influenced by aging (e.g., endothelin-1, angiotensin II).
Experimental considerations
In the present study we did not directly assess MSNA under control or LBNP conditions. Therefore we were not able to quantify the magnitude of sympathetic activation in the young and older adults during LBNP. However, low levels of LBNP (−20 mmHg) have consistently been shown to be effective in increasing MNSA in men and women.20, 21, 26, 35 Of particular interest to the current study, LBNP applied at −20 mmHg increases MSNA in young adults to similar levels observed in older adults at rest.21 Moreover, the change in MSNA in response to LBNP is not different in young and older adults.21
The use of a non-specific α-adrenergic antagonist (phentolamine) in the current study did not allow us to discern the relative roles of α1- and α2-adrenergic receptors in the age-related differences in resting shear patterns. In this regard, it has been reported in young healthy men that α2-adrenergic receptors have a greater contribution to basal forearm vascular tone compared to α1-adrenergic receptors.36 Further research needs to establish the relative contribution of α1-and α2-adrenergic receptors in controlling shear rate patterns with aging.
Lastly, previous data from our laboratory37 and others38 demonstrate that, in young women, high MSNA and/or exogenous infusion of norepinephrine does not necessarily translate into elevated peripheral vascular resistance as it does in men. Therefore it is plausible that sex-specific differences exist in conduit artery shear patterns of young men and women. Along these lines, there was a trend for a lower oscillatory shear in the young women (n = 6) compared to the young men (n = 6) under control conditions in protocol 2 (0.03 ± 0.01 vs. 0.06 ± 0.02 a.u., P = 0.09). To this point, future studies should address the interactions between sex, vascular resistance, and conduit shear rate patterns in humans.
Perspectives
Aging in healthy humans is characterized by a progressive activation of the sympathetic nervous system. In turn, chronically elevated sympathetic nerve activity is associated with several cardiovascular diseases.39 Our findings highlight the influence that sympathetic activation and α-adrenergic vasoconstriction have on conduit artery hemodynamics and shear patterns. Importantly, our data suggest that α-adrenergic vasoconstriction substantially contributes to the increased magnitude of retrograde and oscillatory shear present in peripheral conduit arteries of older adults, which in turn may contribute to the deleterious alterations in endothelial function commonly observed in this population.40, 41 Further research needs to evaluate whether increased cardiac sympathetic activity with aging leads to alterations in blood flow profiles in the coronary arteries where the clinically significant lesions are most likely to occur.
CONCLUSIONS
In young adults, acute sympathetic stimulation via LBNP and/or norepinephrine release via tyramine administration increased conduit artery retrograde and oscillatory shear to similar levels observed in older adults. During α-adrenergic blockade retrograde and oscillatory were abolished in young adults and markedly reduced, however, still persistent, in older adults. Collectively, the present investigation provides the first evidence that α-adrenergic vasoconstriction contributes, in part, to age-related increases in conduit artery retrograde and oscillatory shearat rest.
NOVELTY AND SIGNIFICANCE: 1) WHAT IS NEW? 2) WHAT IS RELEVANT?
What is new?
Pharmacological stimulation of α-adrenergic receptors in the forearm of young men increased upstream conduit artery retrograde and oscillatory shear.
Sympathetic activation increased retrograde and oscillatory shear in the brachial artery of young, but not older, adults, such that age-related differences were no longer apparent.
Brachial artery retrograde and oscillatory shear were abolished in younger adults and
reduced in older adults during α-adrenergic blockade.
Despite drastic changes in brachial artery blood flow and shear rate patterns (increased antegrade and decreased retrograde shear) during α-adrenergic blockade, retrograde shear remained significantly higher in older vs. younger adults.
What is Relevant?
Increased α-adrenergic vasoconstriction in the resistance vessels contributes, in part, to age-related discrepancies in resting shear patterns, thus identifying a potential mechanism for increased risk of atherosclerotic disease in conduit arteries.
Summary
α-adrenergic vasoconstriction contributes, in part, to age-related discrepancies in conduit artery shear rate patterns at rest.
Acknowledgments
The authors are grateful to the study volunteers for their participation. We also thank Branton Walker, Essa Mohamed, Christopher Johnson, Pam Engrav, Sarah Wolhart, and Shelly Roberts for their technical assistance.
Funding Sources
This research was supported by the NIH research grants HL-105467 (to D.P. Casey) and HL-46493 (to M.J. Joyner) and by CTSA RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research. J.P. is supported by American Heart Association 11POST5080002.
Footnotes
Disclosures: None
References
- 1.Blackshear WM, Jr, Phillips DJ, Strandness DE., Jr Pulsed doppler assessment of normal human femoral artery velocity patterns. J Surg Res. 1979;27:73–83. doi: 10.1016/0022-4804(79)90113-6. [DOI] [PubMed] [Google Scholar]
- 2.McDonald DA. The relation of pulsatile pressure to flow in arteries. J Physiol. 1955;127:533–552. doi: 10.1113/jphysiol.1955.sp005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: Low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol. 2006;290:H2320–2328. doi: 10.1152/ajpheart.00486.2005. [DOI] [PubMed] [Google Scholar]
- 7.Godbole AS, Lu X, Guo X, Kassab GS. Nadph oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol. 2009;296:H152–158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 10.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates nadph oxidase subunit expression: Implication for native ldl oxidation. Circulation research. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of o2- from p47phox-dependent nad(p)h oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 12.Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol. 2004;561:575–582. doi: 10.1113/jphysiol.2004.075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 14.Credeur DP, Dobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: Relationship to physical function. Eur J Appl Physiol. 2009;107:219–225. doi: 10.1007/s00421-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: Role of nitric oxide. Hypertension. 2011;57:484–489. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–392. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: Role of thermoregulatory vasodilation. J Appl Physiol. 2011;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54:278–285. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baccelli G, Pignoli P, Corbellini E, Pizzolati PL, Bassini M, Longo T, Zanchetti A. Hemodynamic factors changing blood flow velocity waveform and profile in normal human brachial artery. Angiology. 1985;36:1–8. doi: 10.1177/000331978503600101. [DOI] [PubMed] [Google Scholar]
- 20.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol. 2010;298:H1128–1135. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- 22.Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, Charkoudian N. Hysteresis in the sympathetic baroreflex: Role of baseline nerve activity. J Physiol. 2011;589:3395–3404. doi: 10.1113/jphysiol.2011.208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 24.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480 ( Pt 2):361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eklund B, Kaijser L. Effect of regional alpha- and beta-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol. 1976;262:39–50. doi: 10.1113/jphysiol.1976.sp011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol. 1989;66:2778–2781. doi: 10.1152/jappl.1989.66.6.2778. [DOI] [PubMed] [Google Scholar]
- 27.Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1833–1839. doi: 10.1152/ajpheart.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: A limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297:H1103–1108. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 29.Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: An in vivo mri study. J Magn Reson Imaging. 2004;19:188–193. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
- 30.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- 31.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579:115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford GA, Dachman WD, Blaschke TF, Hoffman BB. Effect of aging on beta 2-adrenergic receptor-stimulated flux of k+, po4, ffa, and glycerol in human forearms. J Appl Physiol. 1995;78:172–178. doi: 10.1152/jappl.1995.78.1.172. [DOI] [PubMed] [Google Scholar]
- 34.van Brummelen P, Buhler FR, Kiowski W, Amann FW. Age-related decrease in cardiac and peripheral vascular responsiveness to isoprenaline: Studies in normal subjects. Clinical science. 1981;60:571–577. doi: 10.1042/cs0600571. [DOI] [PubMed] [Google Scholar]
- 35.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009;297:E85–91. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: Implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- 39.Hart EC, Charkoudian N. Sympathetic neural mechanisms in human blood pressure regulation. Current hypertension reports. 2011;13:237–243. doi: 10.1007/s11906-011-0191-1. [DOI] [PubMed] [Google Scholar]
- 40.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]