Abstract
Salicylic acid (SA) is an important component of systemic-acquired resistance in plants. It is synthesized from benzoic acid (BA) as part of the phenylpropanoid pathway. Benzaldehyde (BD), a potential intermediate of this pathway, was found in healthy and tobacco mosaic virus (TMV)-inoculated tobacco (Nicotiana tabacum L. cv Xanthi-nc) leaf tissue at 100 ng/g fresh weight concentrations as measured by gas chromatography-mass spectrometry. BD was also emitted as a volatile organic compound from tobacco tissues. Application of gaseous BD to plants enclosed in jars caused a 13-fold increase in SA concentration, induced the accumulation of the pathogenesis-related transcript PR-1, and increased the resistance of tobacco to TMV inoculation. [13C6]BD and [2H5]benzyl alcohol were converted to BA and SA. Labeling experiments using [13C1]Phe in temperature-shifted plants inoculated with the TMV showed high enrichment of cinnamic acids (72%), BA (34%), and SA (55%). The endogenous BD, however, contained nondetectable enrichment, suggesting that BD was not the intermediate between cinnamic acid and BA. These results show that BD and benzyl alcohol promote SA accumulation and expression of defense responses in tobacco, and provide insight into the early steps of SA biosynthesis.
It has long been observed that plants display a battery of defense mechanisms in response to the presence of pathogens (Chester, 1933). In Xanthi-nc tobacco (Nicotiana tabacum L.) plants containing the N gene, the spread of TMV is contained by localized cell death known as the HR. In addition to cell death, HR is characterized by an increase in the production of cell wall phenolics, the release of active oxygen species, the production of phytoalexins, the induction of PR proteins, and the accumulation of SA. HR leads to subsequent local resistance and SAR, which depends in part on the production and transport of SA (Malamy et al., 1990; Métraux et al., 1990; Enyedi et al., 1992; Gaffney et al., 1993; Lee et al., 1995; Shulaev et al., 1995, Lee and Raskin, 1997).
According to the currently accepted model, the de novo biosynthesis of SA, which occurs after inoculation and promotes resistance to subsequent inoculation, results from the 2-hydroxylation of BA (Léon et al., 1995) (Fig. 1). BA is produced from CA as a part of general phenylpropanoid metabolism. BA2H, a soluble monooxygenase that is responsible for the conversion of BA to SA, is induced by either the presence of TMV or the application of BA (Léon et al., 1995). Therefore, the rate-limiting step in the biosynthesis of SA could be in the production of BA rather than the conversion of BA to SA. Since the production of BA from CA has not been well elucidated, this proposed model may be more complex.
Figure 1.
Possible pathways of SA biosynthesis in tobacco. The conversion of CA to BA could occur either by β-oxidation or by a non-β-oxidative route. CA is derived from Phe as the product of PAL, and BA2H catalyzes the conversion of BA to SA.
The accumulation of free SA is also associated with the formation of SA conjugates such as the SA glucoside and the Glc ester (Edwards, 1994; Lee and Raskin, 1997), as well as methyl salicylate (Seskar et al., 1997; Shulaev et al., 1997). Methyl salicylate vapor from inoculated tobacco may serve as an airborne signal that activates resistance in nearby plants (Shulaev et al., 1997).
Two proposed routes for the conversion of CA, the product of PAL, to BA are shown in Figure 1. The side chain of CA could be oxidatively shortened in a manner analogous to the β-oxidation of fatty acids followed by hydrolysis of the thioester. This route would produce trans-cinnamoyl-CoA as an intermediate. Support for the existence of this pathway comes from early studies showing that acetyl-CoA stimulated the conversion of CA to BA (Alibert and Ranjeva, 1971). The side chain of CA could also be shortened by a “nonoxidative” mechanism in a manner analogous to the formation of p-hydroxybenzoic acid from p-coumaric acid. Such a nonoxidative mechanism for the formation of p-hydroxybenzoic acid, an important intermediate in the biosynthesis of lignin, has been demonstrated in cell cultures of carrot (Schnitzler et al., 1992) and in cell-free extracts of Lithospermum erythrorhizon (Yazaki et al., 1991) and potato (French et al., 1976), and is characterized by the presence of p-hydroxybenzaldehyde as an important metabolic intermediate. In addition, the formation of vanillic acid from ferulic acid may occur nonoxidatively and is characterized by the aldehyde intermediate of vanillin (French et al., 1976). The formation of BA from CA could, by analogy, be characterized by the presence of BD as a metabolic intermediate if the conversion were nonoxidative and not via a CoA-derived intermediate. In this study we measured BD as both an endogenous compound and as a volatile released from tobacco tissue, and we investigated the possible involvement of BD in SA metabolism with BD application. We also investigated the role of benzyl alcohol in SA biosynthesis, as benzyl alcohol has been shown to be involved in BA metabolism in cranberry (Croteau, 1977).
MATERIALS AND METHODS
Isolation and Quantification of Endogenous BD
Tobacco (Nicotiana tabacum L cv Xanthi nc) leaf samples (200–500 mg) were ground in liquid nitrogen and extracted in 5 mL of 100% ethanol. Five-hundred nanograms of [13C6]BD (Cambridge Isotope Laboratories, Andover, MA) dissolved in ethyl acetate was added to each sample as an internal standard. The samples were allowed to extract and equilibrate for 1 h at 4°C and diluted to 50 mL with 10 mm phosphate buffer, pH 7.0. The extracts were then passed through two consecutive 3-mL quaternary amine SPE columns (J.T. Baker, Philipsburg, NJ) to remove many of the pigments. The columns were conditioned with 2 mL each of methanol and 100 mm phosphate buffer, pH 7.0, and rinsed with 5 mL of water. The samples were then applied to a conditioned (rinsed with 2 mL each of ethyl acetate, methanol, and water) 3-mL C18 SPE column (J.T. Baker), rinsed with 5 mL of water, and eluted with 4 mL of ethyl acetate. Residual water was removed from the ethyl acetate eluent by drying over magnesium sulfate for 1 h at 4°C. The ethyl acetate eluent was reduced to approximately 500 μL in vacuo and then further reduced to 20 μL with a stream of dry nitrogen.
The BD contained within the ethyl acetate was quantified by GC-MS-selected ion monitoring on the basis of the ratio of the m/z 105/106 combined ion abundance with the m/z 111/112 combined ion abundance using stable isotope-dilution analysis similar to that described by Cohen et al. (1986) for IAA. The samples were manually injected in the splitless mode into a gas chromatograph (model 5890, Hewlett-Packard)/mass spectrometer (model 5971, Hewlett-Packard) equipped with a 30-m × 0.25-mm DB-5MS fused silica capillary column (J&W Scientific, Folsom, CA). Chromatographic parameters were as follows: injector temperature at 150°C, initial oven temperature at 40°C for 3 min, followed by a ramp at 40°C/min to 280°C. The monitored ions for BD (native, enriched, and internal standard) were: (m/z) 77, 83, 105, 106, 107, 110, 111, and 112. In addition, ions for methyl salicylate, which often copurified with BD, were also monitored at: (m/z) 92, 120, 121, 126, 152, and 158. Full scans of each compound were also performed to provide precise chemical identity.
Isolation and Analysis of BA, Cinnamic Acid, and SA
Tobacco leaf samples (200–500 mg) were ground in liquid nitrogen and extracted in 5 mL of 90% methanol. Two micrograms of [2H5]BA (Sigma) dissolved in methanol was added during sample grinding to serve as an internal standard. The sample was extracted at 4°C for 1 h in a 13- × 100-mm test tube, centrifuged for 10 min at 10,000g. The pellet was rinsed once with 1 mL of 100% methanol and centrifuged again. The pooled sample was divided equally for those experiments in which both free and total pools were analyzed. Each sample was reduced in vacuo to near dryness.
The sample to be analyzed for free acids was resuspended in 2 mL of 1% acetic acid and centrifuged for 10 min at 10,000g to pellet any particulates and force oils to the surface. The aqueous portion of the sample was transferred to a fresh test tube and partitioned twice with 2 mL of ethyl acetate:cyclopentane:2-propanol (100:99:1, v/v). The sample to be analyzed for total acids was resuspended in 1 mL of 2 n NaOH and heated at 70°C for 2 h (Enyedi et al., 1992); it was then acidified with 250 μL of 36.9% HCl and heated at 70°C for an additional 1 h. After cooling to 4°C, the organic acids were partitioned into the same organic solvent mixture used for the free portion of the sample. The sample was reduced in vacuo to dryness, resuspended in 2 mL of 1% acetic acid, applied to a conditioned (rinsed with water, methanol, and 0.5% acetic acid) C18 SPE column (J.T. Baker), rinsed with 5 mL of water and eluted with 3 mL of acetonitrile, reduced in vacuo to dryness, resuspended in 100 μL of methanol, and methylated with ethereal diazomethane. The sample was then reduced to near dryness after 5 min and resuspended in 25 μL of ethyl acetate for analysis by GC-MS-selected ion monitoring.
The samples were analyzed by GC-MS as described above. Chromatographic parameters were as follows: injector temperature at 280°C, initial oven temperature at 50°C for 3 min, followed by a ramp at 30°C/min to 280°C. The ions monitored for methyl cinnamate were (m/z): 103, 104, 131, 132, 162, and 163; for methyl benzoate were (m/z): 77, 82, 105, 106, 110, 136, 137 and 141; and for methyl salicylate, see above.
The concentration of BA was calculated based on the ratio of the major ion (105) of native BA and the comparable ion (110) from the 2H-labeled standard using the stable isotope-dilution equation as for BD. Hydrolysis conditions can promote exchange of deuterium atoms, which can affect measurement precision. This was investigated by mixing labeled and unlabeled standards of BA and subjecting one-half of these mixtures to the conditions of hydrolysis. Some exchange was observed but was always less than 4%.
Volatile BD Treatment of Tobacco Plants
Four week-old plants were grown in small plastic cups (142 mL), wrapped in aluminum foil, and placed in 4-L airtight jars (Wheaton, Millville, NJ). BD was dissolved in ethanol and applied as 0-, 0.01-, 0.1-, or 1.0-mg doses in 20 μL to vertically fixed cotton swabs each day for 6 d. BD vapor from the treatment would then permeate the air within the jar. Before each BD application, the gases within the jar were replaced with the ambient air. After BD treatment, samples were harvested from some plants and stored at −80°C. Other plants were kept moist for 24 h and then infected with TMV at the rate of about 2 μg/leaf. After 3 d lesion size on the leaves from each of the treatments was measured and used as an indicator of relative resistance.
Measurement of PR Protein RNA
Total RNA was extracted as described by Chomczynski and Sacchi (1987), loaded onto a 1% agarose gel with 37% formaldehyde (v/v) at 30 μg per lane, and transferred onto a Zeta-Probe membrane (Bio-Rad). Tobacco PR-1 mRNA was detected with a radioactive probe made from PR-1 cDNA.
Measurement of Volatile BD
Seven-week-old tobacco plants selected to be of uniform size were inoculated with 5 μg of TMV/leaf on every expanded leaf or mock inoculated with water only, as described by Shulaev et al. (1997). After 72 h the volatile organic compounds were collected from individual plants by a modified purge and trap method, as described by Patt et al. (1992). The top portion of the tobacco plant consisting of eight leaves was enclosed in a custom-made, 4-L split flask (Kontes, Vineland, NJ). The spherical flask was split through one of three ground-glass necks, which held the plant stem. Laboratory film was used to seal the opening between the plant stem and the neck of the flask. The two other necks were located symmetrically on each half of the split flask. One of the necks was the entrance for clean air from a 20.3-L Tedlar bag (Fisher Scientific); the other was the exit for the exposed air, which was passed through a silylated, glass-lined, stainless-steel desorption tube (3.0 mm i.d. × 10 mm; Scientific Instrument Services, Ringoes, NJ) filled with 100 mg of Tenax-TA resin (60/80 mesh; Scientific Instrument Services).
Air was pulled through the system by a vacuum pump at the rate of 100 mL/min for a collection time of 3 h. Water vapor was removed from the Tenax column by a purge of dry nitrogen (40 mL/min) for 30 min. Organic volatiles from the Tenax columns were analyzed using a short-path thermal desorption system (Scientific Instrument Services) as described by Hartman et al. (1993). Volatile compounds were separated on a gas chromatograph (Varian, Sugarland, TX) equipped with a DB-5MS fused silica capillary column and analyzed on a high-resolution double-focusing magnetic sector mass spectrometer (model MAT 8230, Finnigan, Madison, WI). Methyl salicylate and BD were quantified based on the response factor relative to a [2H8]toluene (Aldrich) internal standard and given as total amount collected per plant. The plants were of very similar age, size, leaf number, and dry weight.
Labeling of SA Biosynthetic Intermediates 3-[13C1]Phe
Six-week-old tobacco plants were inoculated with TMV (5 μg/leaf) and held at 32°C for 4 d followed by a temperature shift to 23°C. Six hours following the temperature shift, leaf sections without the major veins were cut from the inoculated leaf and vacuum infiltrated with 1 mm 3-[13C1]Phe in 5 mm phosphate buffer, pH 5.8. Samples were then blotted and frozen 2, 4, and 6 h after infiltration. In other experiments, 1 mm [13C6]BD or [2H5]benzyl alcohol (Aldrich) in 5 mm phosphate buffer, pH 5.8, was infiltrated into 24-h-inoculated leaf discs and incubated at 24°C for 6 h. All values given with se are the averages of three to five replicate measurements.
RESULTS
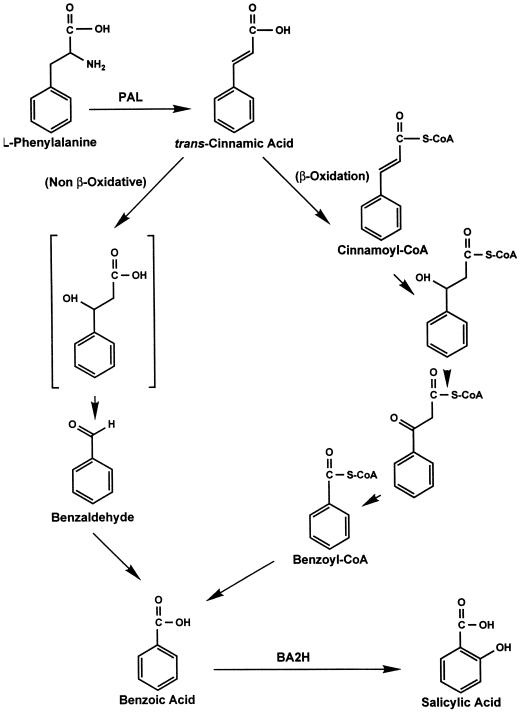
Healthy tobacco plants contained measurable amounts of endogenous BD that averaged about 100 ng/g fresh weight, which did not change significantly after TMV inoculation at 23°C (Fig. 2). The endogenous production of BD was also reflected in the release of BD as a volatile organic compound as shown in Table I. The amount of volatilized BD was nearly 3-fold higher in TMV-inoculated plants than in mock-inoculated control treatments. The amount of gaseous methyl salicylate evolved from inoculated leaves was 402 ± 53 ng/plant, compared with nondetectable amounts produced by control plants (Table I). These results are comparable to those of Shulaev et al. (1997). Even though the amount of gaseous methyl salicylate was much greater than the amount of gaseous BD (Table I), it is possible that BD could contribute to an airborne signaling between plants. Application of BD vapor to tobacco plants did not promote a significant increase in the endogenous BD concentration when measured 12 h after treatment (data not shown), which agrees with the nearly constant endogenous levels shown in Figure 2. The BD concentration within leaf tissue reached concentrations of 390 ± 48 ng/g fresh weight when measured at 2 h after treatment (data not shown), suggesting that volatile BD is rapidly metabolized by the plant tissue.
Figure 2.
Endogenous BD concentration in TMV-inoculated tobacco. BD concentrations were measured by GC-MS using stable isotope-dilution analysis with a [13C6]BD internal standard in tobacco leaves at various time points after TMV inoculation. fw, Fresh weight.
Table I.
Release of volatile compounds in mock and TMV-inoculated tobacco
| Volatile Compound | Mock | TMV |
|---|---|---|
| ng | ||
| Methyl salicylate | n.d.a | 402 ± 53 |
| BD | 3.6 ± 0.5 | 8.7 ± 1.1 |
Seven-week-old tobacco plants were inoculated with TMV on every expanded leaf and 72 h later were placed in a split-flask apparatus. Air was pulled through the apparatus at a flow rate of 100 mL/min. Volatile organics were collected onto a Tenax column at the exit port of the flask. Volatile BD and methyl salicylate were measured by thermal desorption GC-MS. Values (±se/plant) are given as total amounts collected during 3 h. Each plant was of equal age and size. The lowest detectable level for methyl salicylate and BD was less than 1 ng.
n.d., Signifies nondetectable amounts.
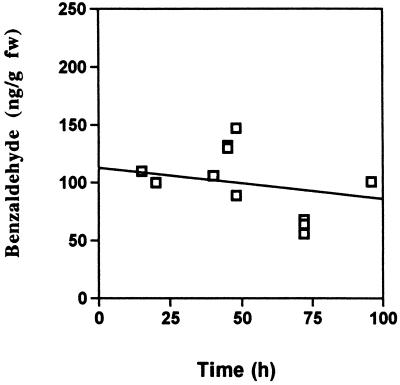
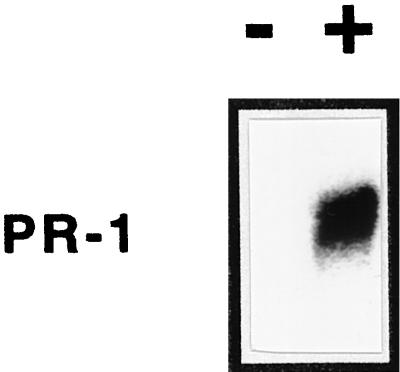
BD treatment had no obvious visual toxic effects on the plants, even after extended treatment periods of 6 d; however, it promoted a large increase in SA from 61 ± 9 to 803 ± 153 ng/g fresh weight over a 5-d period (data not shown). In addition to promoting an increase in SA, which has been shown to induce PR-1 expression (Yalpani et al., 1991, 1993b), BD treatment also caused an increase in PR-1 mRNA, as shown in Figure 3. To determine whether BD treatment promoted an increase in resistance, tobacco plants were treated with various BD concentrations and inoculated with TMV (Fig. 4). The results showed that BD treatment caused a dose-dependent decrease in lesion size, which reached 60% at the 1-mg dose compared with the control.
Figure 3.
PR-1 gene expression in control (−) and BD (+)-treated tobacco. RNA was extracted from plants treated with volatile BD (1-mg dose rate) and from control treatments. PR-1 gene expression was measured with a radioactive probe prepared from PR-1 cDNA. Equal amounts of RNA were loaded in each lane. This experiment was repeated with similar results.
Figure 4.
TMV resistance of tobacco treated with volatile BD as measured by a decrease in average lesion size. Plants were treated with various doses of volatile BD and inoculated with TMV 24 h after treatment. Average lesion diameters were determined 3 d after inoculation for each of the treatments.
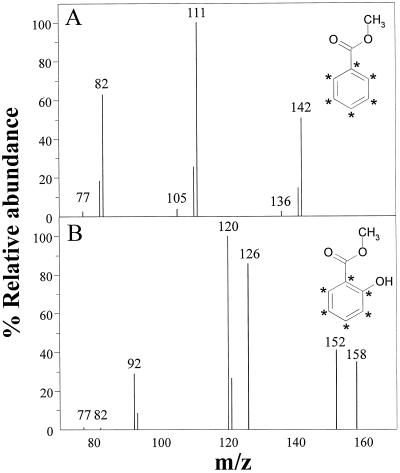
Infiltration of TMV-inoculated tobacco leaves with [13C6]BD led to mass enrichment of both SA and BA (Fig. 5). The total hydrolyzed BA pool was almost completely labeled, with an enrichment of 96 ± 1%, whereas the total SA pool was only 41 ± 2% enriched over a 24-h period. Enrichment was measured as a shift of 6 mass units of the major ions of both BA and SA, as shown in Figure 5. The structures of the methylated molecules showing the location of enrichment from 13C are also given in Figure 5. Methylation of the organic acids was necessary for GC-MS analysis. The three major ions of methylated BA at m/z 77, 105, and 136 were shifted to m/z 83, 111, and 142, respectively, and the major ions of methylated SA at m/z 120 and 152 were shifted to m/z 126 and 158, respectively. Therefore, the exogenous BD was directly converted to BA and SA.
Figure 5.
Selected ion mass spectra of methyl benzoate (A) and methyl salicylate (B) from BD-treated tobacco showing enrichment from [13C6]BD. Major ions are shown and enrichment was determined by a shift in the mass to M+ 6. Methylated structures are shown and the location of enrichment is indicated with an asterisk.
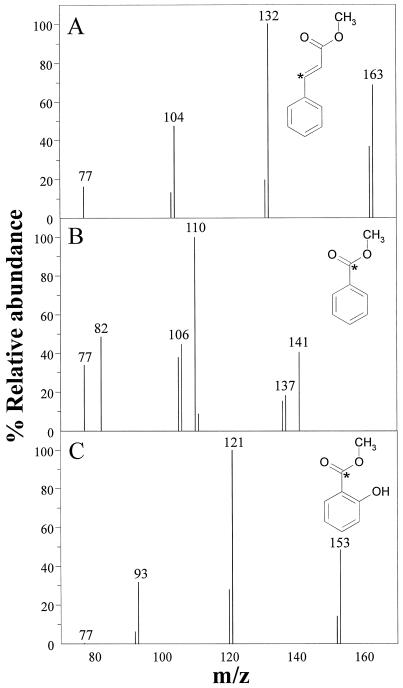
3-[13C1]Phe was used as a precursor of SA to label all of the key intermediates of the biosynthetic pathway as depicted in Figure 1. Enrichment for each of the examined intermediates was calculated based on the abundance of the major ion with 1 extra unit of mass (m/z + 1) divided by the total abundance of that ion (m/z + [m/z + 1]) (Table II). Methyl benzoate has a major ion of m/z 105, which was used as the basis for the calculation of enrichment (106/[105 + 106]). Enrichment of CA was also calculated on the basis of the shift in mass of the major ion (132/[131 + 132]). For SA, enrichment was determined using the values for the molecular ion (153/[152 + 153]) because the major ion of 120 is typically associated with substantial m/z + 1 enrichment, which is characteristic of the typical electron-impact fragmentation pattern of SA. The native ions for each of these phenolic compounds and their enriched forms are depicted in Figure 6, which also shows the structures of the methylated compounds and the position of 13C. Enrichment of each of the acids was also verified based on the mass enrichment of another major ion for each of the examined compounds. Each of the values was adjusted to account for the natural abundance of 13C.
Table II.
Mass enrichment of proposed intermediates of SA biosynthesis from [13C1]phenylalanine
| Time | Pool | CA | BD | BA | SA |
|---|---|---|---|---|---|
| h | % | ||||
| 2 | F | 74 ± 1 | n.d.a | 17 ± 7 | 58 ± 9 |
| T | 72 ± 2 | 34 ± 7 | 55 ± 12 | ||
| 4 | F | 73 ± 3 | n.d. | 12 ± 4 | 57 ± 4 |
| T | 71 ± 9 | 29 ± 7 | 58 ± 7 | ||
| 24 | F | 47 ± 13 | n.d. | 3 ± 1 | 17 ± 4 |
| T | 70 ± 5 | 29 ± 4 | 52 ± 10 | ||
Leaf discs from TMV-inoculated plants held at 32°C for 96 h were infiltrated with a 1 mm solution of 3-[13C1]phenylalanine 6 h after a temperature shift to 24°C. BD was analyzed for enrichment at 2, 4, and 24 h after infiltration, as well as for the free (F) and total (T) pools of CA, BA, and SA. The enrichment value is the percentage of 13C-labeled compound from its total pool ± se.
n.d., Signifies nondetectable amounts of enrichment.
Figure 6.
Selected ion mass spectra of methyl cinnimate (A), methyl benzoate (B), and methyl salicylate (C) showing isotopic enrichment from [13C1]Phe. Tobacco plants were inoculated with TMV, held at 32°C for 96 h, shifted to 23°C for 6 h, and infiltrated with 1 mm [13C1]Phe. The phenolics were then analyzed by GC-MS 6 h after infiltration. Major ions are shown and enrichment was determined by a shift in the mass to M+ 1. Methylated structures are shown and the location of enrichment is indicated with an asterisk.
Inoculated plants were kept at 32°C for 96 h and shifted to 23°C for 6 h prior to infiltration to promote a maximal induction of SA biosynthesis (Malamy et al., 1992). Excised leaf discs were used in this experiment because preliminary measurements suggested that infiltrated Phe was translocated rapidly from the infiltrated, attached leaf (data not shown). Both free and total chemically hydrolyzed extracts (Table II) were analyzed for enrichment of CA, BA, and SA. The free and total CA pools were enriched to approximately the same extent at 73% and did not begin to show a decline in enrichment until 24 h, at which time the labeled Phe pool was likely to be depleted. The free BA pool was labeled at nearly 15% at both 2 and 4 h after infiltration but decreased after 24 h. The total BA pool was consistently more enriched than the free BA and did not decrease substantially from 2 to 24 h. The enrichment of SA from Phe was slightly lower than that of CA. However, SA was labeled to a greater extent than BA. The BD pool showed no detectable amounts of enrichment throughout the experiment.
The relative ability of exogenous BA and benzyl alcohol to label the endogenous BA and SA pools was determined, as benzyl alcohol is a closely related compound and has been shown to be a precursor of both BD and BA (MacKintosh and Fewson, 1988a, 1988b). Both BA and benzyl alcohol were able to almost completely label the total endogenous BA pool in 24-h-inoculated plants, as shown in Table III. The SA pool was, in contrast, enriched to a greater extent from benzyl alcohol (77.3 ± 2.3%) than from BA (27.5 ± 3.5%).
Table III.
Mass enrichment of the total BA and SA pools from [13C1]BA or [2H5]benzyl alcohol
| Infiltrated Compound | BA | SA |
|---|---|---|
| % | ||
| BA | 98.2 ± 0.8 | 27.5 ± 3.5 |
| Benzyl alcohol | 96.3 ± 0.2 | 77.3 ± 2.3 |
Leaf discs from 24-h-inoculated tobacco were infiltrated with either 1 mm [13C1]BA or [2H5]benzyl alcohol. BA and SA were analyzed for enrichment 6 h after infiltration. The enrichment value is the percentage of 13C-labeled compound from its total pool ±se.
DISCUSSION
BD as a Precursor of BA and SA
Tobacco leaf tissue contains low basal levels of BD, which did not increase after TMV inoculation (Fig. 2). Tobacco plants also emit small amounts of gaseous BD. This emission is stimulated by TMV inoculation (Table I). Therefore, in addition to being a metabolic intermediate of SA biosynthesis, BD may also be a component of the airborne signaling mechanism between plants, similar to methyl salicylate (Shulaev et al., 1997). When inoculated leaves were infiltrated with BD, the label from the [13C6]BD efficiently enriched both the BA and SA pools (Table II). The enrichment of the BA pool was almost 2-fold greater than the enrichment of the SA pool. This enrichment pattern is consistent with the directional conversion of BA to SA. The differential labeling of the two phenolic acids also suggests that the conversion of BD to BA occurs more quickly than the conversion of BA to SA. This difference could also result from a much larger initial pool of SA into which the label is diluted. It is known that the oxidation of BD to BA may occur enzymatically and nonenzymatically and that both processes can proceed rapidly under favorable conditions (MacKintosh and Fewson, 1988a, 1988b). The observations that BD accumulated in tobacco leaves, was emitted as a volatile from TMV-inoculated plants, and was readily converted to BA and SA raise the possibility that BD could be a natural intermediate of SA biosynthesis. It is possible, however, that the observations made with exogenous BD are an artifactual result of the nonspecific oxidation of BD to BA rather than being a reflection of the presence of an active intermediate.
However, the labeling studies using 3-[13C1]Phe (Table II) discount the possibility that BD was the key intermediate between CA and BA and raise questions about the source of the BD in tobacco. It is known that BD can be released from the cyanogenic monoglycoside (R)-prunasin present in cherry (Swain and Poulton, 1994). The cyanogenic process is thought to be a defense mechanism to deter herbivory and pathogens (Nahrstedt, 1985) and may serve as a storage form of reduced nitrogen (Selmar et al., 1988), but there is no indication that such compounds exist in tobacco. BD could also be released from lignin as a breakdown product. Both hydroxybenzoic acids and BA have been reported to be released during the chemical degradation of lignin (Wise, 1944). It is possible that these phenolics could be the oxidation products of their aldehyde counterparts, but the direct release of BD from lignin has not been well documented (Wise, 1944). BD is, however, a common contaminant produced by the paper-pulping process and, therefore, can be chemically released from lignin. The chemical release of BD from lignin may have no relevance to the living plant, but suggests that the enzymatic release of BD is chemically possible (although such activity has not been demonstrated).
Phe Enrichment of Phenolics
Tracer experiments with [13C1]Phe showed that label was incorporated into CA, BA, and SA but that no label was incorporated into BD (Table II). The conversion of CA to BA has been demonstrated (Yalpani et al., 1993b) but how this conversion occurs remains unclear. There are two basic mechanisms by which BA could be formed from CA (Fig. 1): either an oxidative pathway, which requires the presence of CoA, or a nonoxidative pathway, which may include BD as a key intermediate. Our data indicate that BD was not enriched from the 13C1 of Phe, whereas CA and BA contained substantial amounts of label 2, 4, and 24 h after infiltration. Therefore, it is unlikely that BD is the intermediate between CA and BA in TMV-inoculated tobacco. However, BD could be a precursor of BA if the endogenous BD were derived from a source other than CA. Since the oxidation of BD to BA occurs so readily, it would be likely that some BA is made from the endogenous BD, but it would be difficult to determine how significant such a conversion would be in the overall biosynthesis of SA. Our data indicate that the oxidative conversion of CA to BA is the most likely pathway leading to SA biosynthesis.
The synthesis of SA from BA has been well documented in tobacco (Yalpani et al., 1993a), and radioactive Phe has also been used to label SA in healthy and infected cucumber (Meuwly et al., 1995). However, labeling of all of the intermediates of SA biosynthesis with Phe has previously not been successful. Phe is an important precursor for many biosynthetic pathways. Therefore, the label from applied Phe would be expected to be diluted into many pools. However, the use of leaf discs from TMV-inoculated tobacco grown under temperature-shifted conditions created a condition in which the biosynthesis of SA from Phe was enhanced. Under these conditions [13C1]Phe enriched CA and BA, as well as SA. The mass spectra of each of these compounds from different time points provide quantification of enrichment and a clear identity of each compound (Fig. 6). It has been suggested that the production of SA in Arabidopsis may be a major function of PAL (Mauch-Mani and Slusarenko, 1996) and, therefore, it is not surprising for the SA pool, as well as the CA pool, to be greatly enriched after only 2 h (Table II). This indicates that the turnover of CA under these conditions is very rapid.
The time course for the formation of SA from Phe was rapid, with maximum labeling of all measured phenolics observed after 2 h. Thereafter, the percentage of enrichment declined. The greatest decrease was observed for the free pools, consistent with the expected formation of stable metabolic conjugates from the relatively mobile pools of free acids. For all time points the percentage of enrichment of free and total BA was smaller than that of its suggested product, SA. This could be explained by the presence of several BA pools, with only one being effectively converted to SA. Alternatively, some of the SA could be produced by an additional pathway that does not include BA as an intermediate. For example, it is possible that SA could be produced directly from benzoyl-CoA, which has not been investigated as a possible substrate for BA2H (Léon et al., 1995) or via a completely different route from Phe or CA. The basic principles of stable isotope labeling of intermediates in a biosynthetic pathway predict successively lower amounts of enrichment within each proceeding intermediary pool due to dilution of the isotope within each pool. The fact that BA has a lower percentage of enrichment than SA, which has also been observed by other investigators, presents an interesting question that has yet to be resolved (Meuwly et al., 1995).
Benzyl alcohol, a close relative of BD, is a common constituent of the essential oils from jasmine, hyacinth, and ylang-ylang and can be chemically synthesized from BD. Benzyl alcohol has also been shown to be a precursor of BD in Acinetobacter calcoaceticus (MacKintosh and Fewson, 1988a, 1988b). Experiments with ripe cranberry tissue slices demonstrated the conversion of BD to benzyl alcohol and benzyl benzoate, as well as the conversion of BA to all three of these compounds (Croteau, 1977). Such conversions, however, did not occur in unripe tissues, suggesting a developmental component to this metabolism (Croteau, 1977). The ability of infiltrated benzyl alcohol to label BA and SA was therefore investigated. Table III shows that both benzyl alcohol and BA were able to label the total BA pools, but that benzyl alcohol was able to label the SA pool to a greater extent. Our results may be explained by the more efficient uptake of applied benzyl alcohol into the cells compared with that of BA, which is a more polar compound. The conversion of benzyl alcohol into BA and SA did occur in inoculated tobacco leaves, but labeling experiments suggested that this conversion did not involve BD as an intermediate (data not shown). The possible conversion of BD to benzyl alcohol was not investigated.
In summary, these results provide the following new insights into the biosynthesis and action of SA and its precursors: (a) We have obtained direct evidence that in tobacco, SA can be synthesized from Phe via CA and BA; (b) we have detected BD in tobacco leaves and observed that TMV inoculation leads to an increase in the evolution of BD vapor from the inoculated plant; and (c) whereas BD treatment was very effective in increasing the tissue content of SA, PR-1 mRNA, and TMV resistance, this compound is not likely to be an intermediate in SA biosynthesis from Phe. Therefore, at least in tobacco, β-oxidation is the most likely mechanism for the biosynthesis of BA from CA. The relatively low labeling of BA compared with SA (Table II) may suggest the presence of an additional route of SA synthesis from CA that does not involve BA. The SA biosynthetic pathway, therefore, may be more complex than pictured in Figure 1.
ACKNOWLEDGMENTS
The authors wish to thank Tom Hartman, Bin Khong Khoo, Eric Butrym, and Joe Lech for their assistance and advice needed for the measurements of volatile BD; Mirjana Seskar, Hyung-il Lee, and Sergey Smirnov for experimental suggestions and helpful discussion; John Ryals for the PR-1 cDNA clone; and Peter Day for a critical reading of the manuscript.
Abbreviations:
- BA
benzoic acid
- BA2H
BA 2-hydroxylase
- BD
benzaldehyde
- CA
trans-cinnamic acid
- HR
hypersensitive response
- PAL
Phe ammonia-lyase
- PR
pathogenesis-related
- SA
salicylic acid
- SAR
systemic-acquired resistance
- SPE
solid-phase extraction
- TMV
tobacco mosaic virus
Footnotes
This work was supported by a grant from Pioneer Hi-Bred International to I.R.
LITERATURE CITED
- Alibert G, Ranjeva R. Recherches sur les enzymes catalysant la biosynthese des acides ph 130 noliques chez Quercus pedunculata (ehrh.): I-formation des premiers termes des series cinnamique et benzoique. FEBS Lett. 1971;19:11–14. doi: 10.1016/0014-5793(71)80593-8. [DOI] [PubMed] [Google Scholar]
- Chester KS. The problem of acquired physiological immunity in plants. Q Rev Biol. 1933;8:129–154. [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Baldi BG, Slovin JP. 13C6-[Benzene ring]-indole-3-acetic acid. Plant Physiol. 1986;80:14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R. Biosynthesis of benzaldehyde, benzylalcohol and benzyl benzoate from benzoic acid in cranberry (Vaccinium macrocarpon) J Food Biochem. 1977;1:317–326. [Google Scholar]
- Edwards R. Conjugation and metabolism of salicylic acid in tobacco. J Plant Physiol. 1994;143:609–614. [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA. 1992;89:2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CJ, Vance CP, Towers GHN. Conversion of p-coumaric acid to p-hydroxybenzoic acid in cell free extracts of potato tubers and Polysporus hispidus. Phytochemistry. 1976;15:564–566. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Ucknes S, Ward E, Kessman H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Hartman TG, Lech J, Salinas J, Rosen RT, Ho CT (1993) Flavor characterization using adsorbent trapping thermal desorption or direct thermal desorption-gas chromatography and gas chromatography-mass spectrometry. In C-T Ho, CH Manly, eds, Flavor Measurements. Dekker, New York, pp 37–60
- Lee H, Léon J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Raskin I (1997) Biochemistry of salicylic acid and its role in plant disease resistance. Kor J Plant Tiss Cult (in press)
- Léon J, Shulaev V, Yalpani N, Lawton MA, Raskin I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci USA. 1995;92:10413–10417. doi: 10.1073/pnas.92.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh RW, Fewson CA. Benzyl alcohol dehydrogenase and benzaldehyde dehyrogenase II from Acintobacter calcoaceticus, purification and preliminary characterization. Biochem J. 1988a;250:743–751. doi: 10.1042/bj2500743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh RW, Fewson CA. Benzyl alcohol dehydrogenase and benzaldehyde dehyrogenase II from Acintobacter calcoaceticus, substrate specificities and inhibition studies. Biochem J. 1988b;255:653–661. [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to tobacco mosaic virus. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J, Hennig J, Klessig DJ. Temperature-dependent induction of salicylic acid and its conjugate during the resistance response to tobacco mosaic virus infection. Plant Cell. 1992;4:1002–1004. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Meuwly P, Molders W, Buchala A, Métraux JP. Local and systemic biosynthesis of salicylic acid in infected cucumber plants. Plant Physiol. 1995;109:1107–1114. doi: 10.1104/pp.109.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naharstedt A. Cyanogenic compounds as protecting agents for organisms. Plant Syst Evol. 1985;150:35–47. [Google Scholar]
- Patt JM, Hartman TG, Creekmore RW, Elliott JJ, Schal C, Lech J, Rosen RT. The floral odour of Peltandra virginica contains novel trimethyl-2,5-dioxabicyclo[3.2.1]nonanes. Phytochemistry. 1992;31:487–491. [Google Scholar]
- Schnitzler JP, Madlung J, Rose A, Seitz HU. Biosynthesis of p-hydroxybenzoic acid in elicitor-treated carrot cell cultures. Planta. 1992;188:594–600. doi: 10.1007/BF00197054. [DOI] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B. Mobilization and utilization of cyanogenic glycosides. Plant Physiol. 1988;86:711–716. doi: 10.1104/pp.86.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seskar M, Shulaev V, Raskin I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1997;116:387–392. [Google Scholar]
- Shulaev V, Léon J, Raskin I. Is salicylic acid a translocated signal of systemic acquired resistance in tobacco? Plant Cell. 1995;7:1691–1701. doi: 10.1105/tpc.7.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Swain E, Poulton JE. Immunocytochemical localization of prunasin hydrolase and mandelonitrile lyase in stems and leaves of Prunus serotina. Plant Physiol. 1994;106:1285–1291. doi: 10.1104/pp.106.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE (1944) The chemistry of lignin. In LE Wise ed, Wood Chemistry. Reinhold Publishing, New York, pp 318–319
- Yalpani N, Léon J, Lawton MA, Raskin I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993a;103:315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Shulaev V, Raskin I. Endogenous salicylic acid levels correlate with accumulation of pathogenesis-related proteins and virus resistance in tobacco. Phytopathology. 1993b;83:702–708. [Google Scholar]
- Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K, Heide L, Tabata M. Formation of p-hydroxybenzoic acid from p-coumaric acid by cell free extract of Lithospermum erythrorhizon cell cultures. Phytochemistry. 1991;30:2233–2236. [Google Scholar]