Abstract
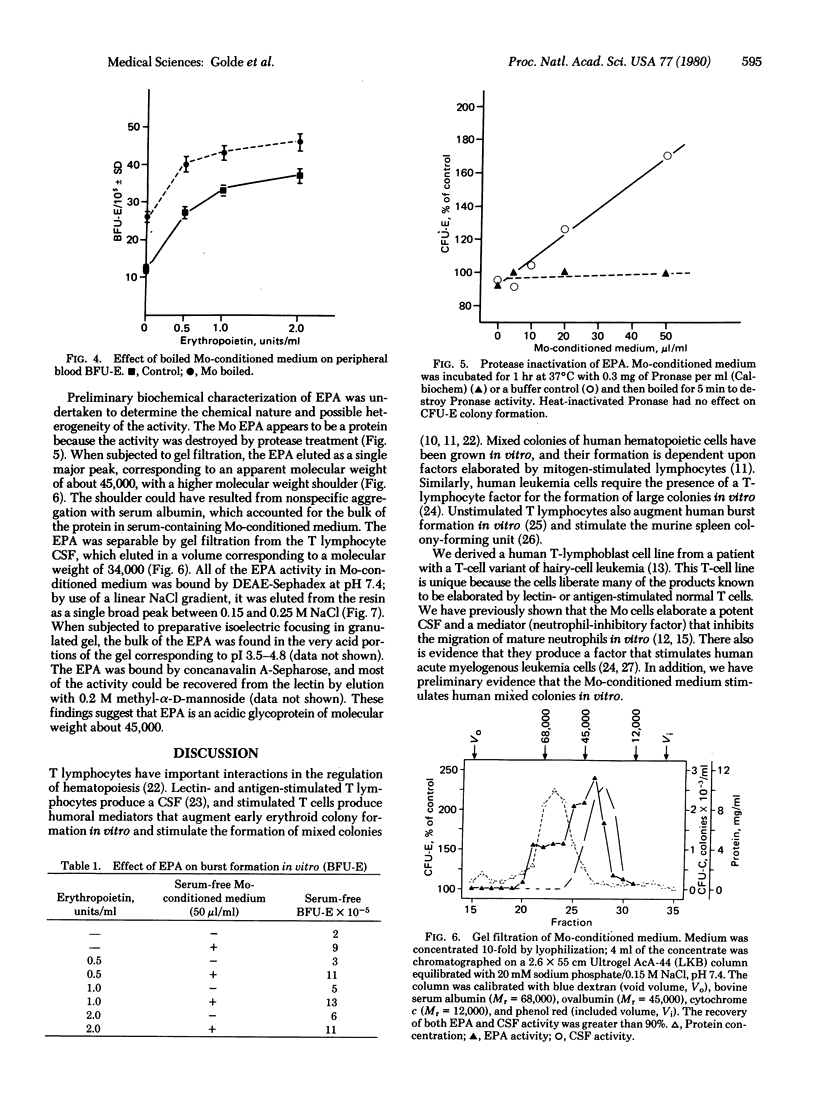
We derived a human T-lymphoblast cell line (Mo) that constitutively elaborates certain lymphokines. The Mo cells produce a colony-stimulating factor necessary for the growth of human granulocyte-monocyte precursors in vitro as well as an erythroid-potentiating activity (EPA) that enhances the proliferation of human erythroid progenitors in vitro. In the presence of serum, the EPA in Mo-conditioned medium stimulated the growth of small and large erythroid colonies almost 2-fold. EPA was also produced in serum-free medium, and, when assayed in serum-free cultures of human erythroid progenitors, it stimulated colony growth about 3-fold. The EPA produced by the Mo cell line did not stimulate normal murine erythroid progenitors (CFU-E) or Friend erythroleukemia cell growth in vitro. EPA was inactivated by protease treatment but was remarkably heat stable, with most of the activity recovered after boiling for 15 min. Preliminary biochemical characterization suggests that EPA is an acidic glycoprotein with molecular weight approximately 45,000. EPA is clearly separable from colony-stimulating factor on the basis of heat stability and gel-filtration chromatography. The present observations provide strong support for the concept that activated T cells produce humoral factors important in the regulation of erythropoiesis. The availability of a cell line producing human EPA should facilitate the characterization of the protein and permit definitive studies of its biologic effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aye M. T. Erythroid colony formation in cultures of human marrow: effect of leukocyte conditioned medium. J Cell Physiol. 1977 Apr;91(1):69–77. doi: 10.1002/jcp.1040910108. [DOI] [PubMed] [Google Scholar]
- Cerny J. Stimulation of bone marrow haemopoietic stem cells by a factor from activated T cells. Nature. 1974 May 3;249(452):63–66. doi: 10.1038/249063a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Cellular interactions in haematopoiesis. Nature. 1979 Jan 18;277(5693):177–181. doi: 10.1038/277177a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Production of colony-stimulating activity by human lymphocytes. Nature. 1974 Apr 19;248(5450):703–704. doi: 10.1038/248703a0. [DOI] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Ghio R., Bianchi G., Löwenberg B., Dicke K. A., Ajmar F. Effects of fibroblasts on the growth of erythroid progenitor cells in vitro. Exp Hematol. 1977 Sep;5(5):341–347. [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Li C. H. Growth hormone modulation of murine erythroleukemia cell growth in vitro. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3437–3439. doi: 10.1073/pnas.75.7.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Li C. H. Growth hormone: species-specific stimulation of erythropoiesis in vitro. Science. 1977 Jun 3;196(4294):1112–1113. doi: 10.1126/science.870971. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Identification of the colony-stimulating cell in human peripheral blood. J Clin Invest. 1972 Nov;51(11):2981–2983. doi: 10.1172/JCI107124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Quan S. G., Cline M. J. Human T lymphocyte cell line producing colony-stimulating activity. Blood. 1978 Nov;52(5):1068–1072. [PubMed] [Google Scholar]
- Guilbert L. J., Iscove N. N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoietic cell cultures. Nature. 1976 Oct 14;263(5578):594–595. doi: 10.1038/263594a0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Iscove N. N. The role of erythropoietin in regulation of population size and cell cycling of early and late erythroid precursors in mouse bone marrow. Cell Tissue Kinet. 1977 Jul;10(4):323–334. doi: 10.1111/j.1365-2184.1977.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Minden M. D., Buick R. N., McCulloch E. A. Separation of blast cell and T-lymphocyte progenitors in the blood of patients with acute myeloblastic leukemia. Blood. 1979 Jul;54(1):186–195. [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Golde D. W. T-lymphocyte variant of hairy-cell leukemia. Ann Intern Med. 1978 Mar;88(3):323–326. doi: 10.7326/0003-4819-88-3-323. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Quan S. G., Golde D. W. Immunologic characterization of hairy cell leukemias in continuous culture. J Immunol. 1978 Mar;120(3):777–782. [PubMed] [Google Scholar]
- Weisbart R. H., Billing R., Golde D. W. Neutrophil migration-inhibition activity produced by a unique T lymphoblast cell line. J Lab Clin Med. 1979 Apr;93(4):622–626. [PubMed] [Google Scholar]



