Abstract
Feeding success of free-living hematophagous insects depends on their ability to be active when hosts are available and to reach places where hosts are accessible. When the hematophagous insect is a vector of pathogens, determining the components of host-seeking behavior is of primary interest for the assessment of transmission risk. Our aim was to describe endo/exophagy and circadian host-seeking activity of Palaearctic Culicoides species, which are major biting pests and arbovirus vectors, using drop traps and suction traps baited with four sheep, as bluetongue virus hosts. Collections were carried out in the field, a largely-open stable and an enclosed stable during six collection periods of 24 hours in April/May, in late June and in September/October 2010 in western France. A total of 986 Culicoides belonging to 13 species, mainly C. brunnicans and C. obsoletus, was collected on animal baits. Culicoides brunnicans was clearly exophagic, whereas C. obsoletus was able to enter stables. Culicoides brunnicans exhibited a bimodal pattern of host-seeking activity with peaks just after sunrise and sunset. Culicoides obsoletus was active before sunset in spring and autumn and after sunset in summer, thus illustrating influence of other parameters than light, especially temperature. Description of host-seeking behaviors allowed us to discuss control strategies for transmission of Culicoides-borne pathogens, such as bluetongue virus. However, practical vector-control recommendations are difficult to provide because of the variation in the degree of endophagy and time of host-seeking activity.
Introduction
Host-parasite systems are subject to opposing selective processes: on the one hand, parasite fitness is increased by a higher frequency of encounters with hosts and on the other hand, host fitness is increased by avoiding these contacts [1]. Feeding success of free-living hematophagous insects, which can be considered parasites of vertebrates with periodic and brief contacts, depends on their ability to be active when hosts are available and to reach places where hosts are accessible [2]. Circadian and seasonal activities of insect are regulated by endogenous oscillators which are initiated by natural diel alternation of light and darkness [3]. These biological clocks enable organisms to anticipate variations of biotic and abiotic factors associated with seasonal or diel progression rather than follow them [3], [4]. Diel timing of host-seeking activity should be selected to ensure temporal encounter of the insect and host and to minimize the risk of dying during host-seeking activities. Similarly, endophagy, defined as the trend of obtaining a blood meal within a man-made structure [3], can be regarded as an adaptation of hematophagous insect to reach their hosts.
The description of circadian activity and endo/exophagy of hematophagous insects could have practical implications for the control of diseases induced by transmitted pathogens. A well-known example is stabling horses at night in South Africa where Culicoides imicola Kieffer is known to be nocturnal and mainly exophagic [5], [6]. This vector-control strategy has proven to be an environmentally-friendly and inexpensive method to prevent African horse sickness (AHS) by reducing bites of the main vector. From 2006 to 2008, Europe faced a huge epizootic of bluetongue virus serotype 8 (BTV8) transmission, leading to disastrous sanitary consequences in domestic ruminant populations and to important disruptions in animal trade [7]. European regulations recommend the stabling of animals during Culicoides activity to reduce BTV transmission (EU Council Directive 2000/75/EC). The direct costs due to indoor housing of animals in The Netherlands in 2006, which was compulsory in restriction zones, were estimated to 18 million Euros, i.e. 55% of total economic impact [8]. However, endo/exophagy of Palaearctic Culicoides was not precisely described and then benefits of this housing strategy were uncertain. The recent emergence of a novel Orthobunyavirus, named Schmallenberg virus [9], in European ruminant populations highlighted the crucial need of address this issue.
Endophagous behavior of Palaearctic species was reported first by Overgaard Nielsen and Christensen [10] in Denmark. In late 2006, comparing UV-light trap collections inside and outside sheds in The Netherlands, Meiswinkel et al. [11] collected threefold more Culicoides outside than inside, where cattle were kept for the night. The species collected mainly inside, i.e. Culicoides obsoletus Meigen/Culicoides scoticus Downes and Kettle, Culicoides dewulfi Goetghebuer and Culicoides chiopterus (Meigen), were suspected to be involved in BTV8 transmission [11]. Using the same method in France at the same period, Baldet et al. [12] collected mainly the same species inside stables, but in greater number inside than outside. Authors conceded that the results were difficult to compare and interpret because of the variation in building openings and in cattle abundance close to the trap in the different collection sites [12]. With a standardized approach, Baylis et al. [13] highlighted that the presence of animals and the opening of stable increased the indoor number of Culicoides collected by UV-light trap. Moreover, authors showed that the decrease of Culicoides number between outside and inside traps is greater in summer (6.5 fold) than in autumn (3 fold) [13]. Unfortunately, all these studies used UV-light traps to assess Culicoides abundance, which do not assess correctly the biting rate on animals [14]–[16]. Indeed, light attraction might lead to an overestimation of the Culicoides endophagy, due to an higher trapping efficiency when light traps are set inside stables, rather than outside [17].
Similarly, circadian cycles of Palaearctic species are poorly described. It is widely assumed that Culicoides are mostly crepuscular and may continue to be active throughout the night. However, many species are also troublesome in the day displaying two biting peaks: one after sunrise and the other close to sunset. Hours of midge attacks can lengthen when low-light overcast conditions prevail, leading to biting throughout the day in both open (Culicoides impunctatus Goetghebuer) and forested environments (C. obsoletus) [17]. However, only a few studies have described rigorously Culicoides nycthemeral cycles with consecutive 24 h collections [18], [19], and only one has utilized host-seeking behavior of European Culicoides [20]. Using horse-baited collections, the latter found that the largest number of C. obsoletus was collected at sunset, far less at sunrise and occasionally in the afternoon and night.
The aim of this study was to describe circadian host-seeking activity and endo/exophagous behavior of Palaearctic Culicoides using host-baited trap collections. As insect behavior is directly affected by geophysical (sun and moon light cycles) and climatic (temperature, humidity, wind) factors, we carried out collections in three different periods of the year.
Results
Climatic Data
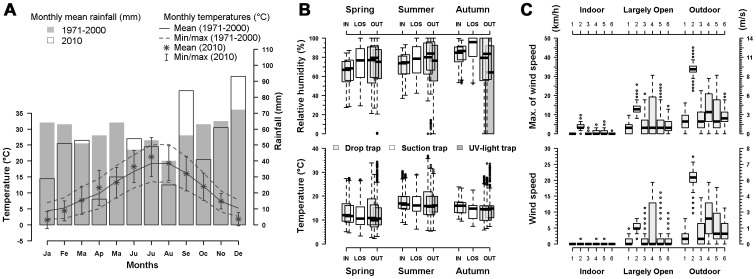
Climate at the study site was oceanic, with a mean annual temperature of 11.4°C, thermal amplitude of 14.9°C and annual rainfall of 694 mm (Météo-France data, 1971–2000). In 2010, annual rainfall was lower than for the 1971–2000 period (586 mm), with deficit in rainfall during the first part of the year (179 mm between January and May 2010 versus 298 mm for the reference period). Temperatures were generally close to normal values in 2010, with colder values in January and December and warmer in June and July (Fig. 1A).
Figure 1. Climatic conditions in the study site.
A. Ombrothermic diagram for comparison between 2010 and the 1971–2000 period (data from Météo-France station of Parcay-Meslay); B. Boxplots of temperature (°C) and relative humidity (%) recorded by data loggers at each location during the 15 collection sessions; C. Boxplots of mean and maximum wind speed (km/h) recorded by the local weather station during each of the 6 summer collections.
During collection sessions, temperatures were higher in summer than in autumn and spring, with smaller amplitude in autumn, whereas humidity was higher in autumn than in summer and spring (Fig. 1B). The temperature decreased gradually from indoors to outdoors, while humidity showed the inverse tendency. In autumn, however, the relative humidity was the highest in the largely-open stable (Fig. 1B). Amplitudes of temperature and humidity were higher outdoors than in other locations, with outlier observations, especially in autumn, probably due to direct exposure of recording devices to the sun. Wind speeds were correlated between locations, with an expected wind gradient from outdoors to indoors, with an intermediate situation for the largely-open stable (Fig. 1C).
Diversity and Seasonality
During the 18 collection sessions, a total of 986 Culicoides (948 females and 38 males) belonging to 13 species was collected in host-baited traps (drop trap and suction trap) and 539 Culicoides (451 females and 88 males) belonging to 19 species in the UV-light/suction trap (Table 1). Molecular assays were performed on 407 Culicoides females morphologically identified as belonging to the Obsoletus Complex. The sample contained 349 C. obsoletus, 25 C. scoticus, 6 C. dewulfi and 1 C. chiopterus; 26 individuals were not identified. Only 4% of Culicoides collected in host-baited traps were males – mainly the dominant species Culicoides brunnicans Edwards collected by the drop trap – whereas 20% of Culicoides were males in UV-light/suction trap. A total of 84 gravid females was collected: all in the suction trap mainly in summer (47%) and most were C. obsoletus (76%). Blood-fed females were collected by all traps, but mainly by the drop trap (197 versus 4 in suction trap and 9 in the UV-light/suction trap). The majority of blood-fed females (196/210) belonged to the dominant species C. brunnicans and C. obsoletus; the engorgement rate in the drop trap was 28% for C. brunnicans and 65% for C. obsoletus.
Table 1. Numbers of Culicoides collected over 18 sessions in spring, summer and autumn by host-baited traps and UV-light/suction trap.
| Location | R1 | Species | Host-baited traps | UV-light/suction trap | ||||||||||||||||||||||||||||
| Total | Suction Trap | Drop Trap | Total | Spring | Summer | Autumn | ||||||||||||||||||||||||||
| Spring | Summer | Autumn | Spring | Summer | Autumn | |||||||||||||||||||||||||||
| F 2 | M | F | P | F | P | M | F | P | M | F | P | M | F | P | M | F | P | F | M | F | P | M | F | P | M | F | P | M | ||||
| OUTDOORS | Field | 1 | C. brunnicans | 592 | 24 | 111 | 11 | 475 | 50 | 24 | 6 | 67 | 140 | 62 | 126 | 5 | 62 | 14 | 93 | |||||||||||||
| 2 | C. obsoletus | 193 | 3 | 31 | 55 | 61 | 54 | 27 | 63 | 2 | 17 | 65 | 42 | 60 | 1 | 15 | 40 | 186 | 16 | 17 | 59 | 95 | 43 | 3 | 74 | 38 | 13 | |||||
| 3 | C. scoticus | 43 | 29 | 81 | 5 | 40 | 2 | 6 | 83 | 1 | 29 | 2 | 18 | 33 | 8 | 75 | 3 | 2 | ||||||||||||||
| 4 | C. punctatus | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 27 | 4 | 7 | 57 | 20 | 80 | 4 | |||||||||||||||||
| 5 | C. festivipennis | 22 | 11 | 100 | 11 | 91 | ||||||||||||||||||||||||||
| 6 | C. pulicaris | 8 | 2 | 1 | 5 | 40 | 12 | 1 | 12 | 50 | 1 | |||||||||||||||||||||
| 7 | C. dewulfi | 16 | 5 | 3 | 6 | 33 | 1 | 1 | ||||||||||||||||||||||||
| 8 | C. chiopterus | 10 | 4 | 5 | 100 | 1 | ||||||||||||||||||||||||||
| 9 | C. poperinghensis | 2 | 2 | 6 | 6 | |||||||||||||||||||||||||||
| 10 | C. achrayi | 4 | 4 | |||||||||||||||||||||||||||||
| 10 | C. lupicaris | 1 | 1 | 3 | 2 | 1 | ||||||||||||||||||||||||||
| 10 | C. vexans | 3 | 2 | 1 | 1 | 1 | ||||||||||||||||||||||||||
| 13 | C. nubeculosus | 3 | 1 | 1 | 1 | |||||||||||||||||||||||||||
| 13 | C. simulator | 3 | 3 | |||||||||||||||||||||||||||||
| 15 | C. kibunensis | 2 | 2 | |||||||||||||||||||||||||||||
| 15 | C. santonicus | 1 | 1 | 1 | 2 | 1 | 2 | |||||||||||||||||||||||||
| 17 | C. fascipennis | 1 | 1 | |||||||||||||||||||||||||||||
| 17 | C. pictipennis | 1 | 1 | |||||||||||||||||||||||||||||
| 17 | C. salinarius | 1 | 1 | |||||||||||||||||||||||||||||
| 17 | C. puncticollis | 1 | 1 | |||||||||||||||||||||||||||||
| Obsoletus Complex | 24 | 3 | 9 | 67 | 2 | 9 | 55 | 1 | 10 | 1 | 6 | 83 | 3 | |||||||||||||||||||
| Pulicaris Group | 1 | 1 | ||||||||||||||||||||||||||||||
| Total Field | 900 | 29 | 189 | 86 | 38 | 4 | 514 | 24 | 55 | 1 | 18 | 451 | 88 | 174 | 64 | 164 | 4 | 113 | 20 | |||||||||||||
| INDOORS | Largely open stable | 1 | C. brunnicans | 1 | 1 | |||||||||||||||||||||||||||
| 2 | C. obsoletus | 13 | 2 | 1 | 3 | 9 | 56 | 2 | ||||||||||||||||||||||||
| 3 | C. scoticus | 4 | 2 | 2 | ||||||||||||||||||||||||||||
| 7 | C. dewulfi | 1 | 1 | |||||||||||||||||||||||||||||
| 17 | C. newsteadi | 1 | 1 | |||||||||||||||||||||||||||||
| Obsoletus Complex | 1 | 1 | ||||||||||||||||||||||||||||||
| Total Largely open stable | 21 | 2 | 4 | 5 | 12 | 2 | – | – | – | ND 3 | – | – | – | – | – | – | – | – | – | ND 3 | – | – | – | – | – | |||||||
| Closed stable | 1 | C. brunnicans | 2 | 1 | 1 | |||||||||||||||||||||||||||
| 2 | C. obsoletus | 18 | 4 | 8 | 25 | 1 | 1 | 7 | 1 | 3 | 1 | |||||||||||||||||||||
| 3 | C. scoticus | 2 | 2 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||||
| 4 | C. punctatus | 1 | 1 | |||||||||||||||||||||||||||||
| 8 | C. chiopterus | 3 | 1 | 3 | 1 | |||||||||||||||||||||||||||
| Obsoletus Complex | 1 | 1 | ||||||||||||||||||||||||||||||
| Total Closed stable | 27 | 7 | 10 | 4 | 2 | 8 | 2 | 1 | 5 | 2 | – | – | – | – | – | ND 3 | – | – | – | – | – | |||||||||||
| TOTAL | 948 | 38 | 203 | 95 | 2 | 58 | 6 | 516 | 24 | 56 | 6 | 20 | 451 | 88 | 174 | 64 | 164 | 4 | 113 | 20 | ||||||||||||
R: species rank calculated with the total number of individuals whatever the trap and the season.
F: females; (P): parity rate as No. parous/No. females (given in percentage if F >5); M: males. For the sake of clarity, 0 were not quoted.
ND: not done. The drop trap was not used in the largely open stable, and only one UV-light/suction trap was used outdoors.
Diversity and abundance of species varied across seasons and traps. Culicoides brunnicans was present almost exclusively in spring (Table 1). Culicoides obsoletus was present during the three periods, mainly in summer (Table 1). The abundance of C. scoticus decreased progressively from the first to the last collection period (Table 1). Culicoides brunnicans was more abundant in host-baited traps than in the UV-light/suction trap, whereas the opposite situation was observed for C. obsoletus. Moreover, the UV-light trap collected some species rarely or not collected in host-baited traps, as Culicoides punctatus Latreille and Culicoides festivipennis Keiffer. On the contrary C. dewulfi and C. chiopterus were found in host-baited traps but not in the UV-light/suction trap (Table 1).
Endo/exophagy
The best model predicting the observed abundance of Culicoides overall (Pearson’s product-moment correlation, r = 0.98) was the complete model including location, season, interactions between both and trap as fixed effects and the session as a random effect, even though season lacked a clear effect on abundance (Table 2). Most Culicoides females were collected outdoors (mean predicted number of Culicoides was 8.5 outdoors versus between 0.4 and 1.0 indoors) and more were collected by the drop trap (4.2 versus 2.4).
Table 2. Mean No. observed (max) and predicted Culicoides for all species and the most abundant species depending on the trap, the location and the season.
| Effect | Value | All species | C. brunnicans | C. obsoletus | C. scoticus | C. dewulfi | |||||
| Observed | Pred3 | Observed | Pred | Observed | Pred | Observed | Pred | Observed | Pred | ||
| Trap | Drop trap | 16.4(249) | 4.18*** | 13.4(237) | 1.94*** | 2.1(19) | 0.91*** | 0.2(4) | 0.04*** | 0.1(1) | 0.04* |
| Suction trap | 6.6(99) | 2.37 | 2.1(63) | 0.45 | 2.7(28) | 1.62 | 0.8(25) | 0.22 | 0.3(3) | 0.26 | |
| Location | Outdoor | 25.0(249) | 8.46*** | 16.4(237) | 3.55*** | 5.4(28) | 3.09*** | 1.2(25) | 0.34*** | 0.4(3) | 0.41 |
| LOS2 | 1.2(6) | 0.99** | 0.1(1) | 0.03 | 0.7(4) | 0.39 | 0.2(2) | 0.04 | 0.1(1) | 0.03 | |
| Indoor | 0.8(6) | 0.38 | 0.1(1) | 0.01 | 0.5(5) | 0.32 | 0.1(1) | 0.02 | 0.0 | 0.00 | |
| Season | Spring | 24.0(249) | 5.62 | 19.6(237) | 3.55 | 1.9(14) | 0.89 | 1.3(25) | – | 0.2(3) | – |
| Summer | 5.0(34) | 2.03 | 0.2(3) | 0.04 | 3.6(28) | 1.53° | 0.2(2) | – | 0.1(0) | – | |
| Autumn | 2.6(18) | 2.17 | 0.0 | 0.00 | 2.0(14) | 1.38 | 0.2(1) | – | 0.2(2) | – | |
| Sea*loc 1 | – | p<0.001 | – | p<0.001 | – | – | |||||
Interaction between season and location.
LOS: largely open stable; Pred: predicted values; NS: not significant.
Predicted values (Pred) are in bold and underlined if analyze of variance between models with and without that effect showed significant differences with for α = 0.05. P-values were given for effect modalities compared to a reference value, i.e. “suction trap” for trap, “indoor” for location and “autumn” for season (***p<0.001; **p<0.01; *p<0.05;o p<0.1, no indication p>0.1).
The best model predicting C. brunnicans abundance (r = 0.99) was the complete model without the interaction term (Table 2). This species was found almost exclusively during the first collection period and outdoors (between 100 and 300 fold more abundant outdoors than indoors) and mainly by the drop trap (1.9 versus 0.5 by the suction trap). Interaction between location and season (p<0.001) was needed to obtain the best prediction of C. obsoletus abundance (r = 0.91). Culicoides obsoletus was collected more abundantly outdoors (between 8 and 10 fold more abundant outdoors than indoors) and by the suction trap (1.6 versus 0.9 by the drop trap). For C. scoticus and C. dewulfi, only trap and location as fixed effect were needed to obtain parsimonious models with good fit (r = 0.98 and r = 0.72). Both species were collected more abundantly by the suction trap (predicted abundance was 0.22 and 0.26 versus 0.04 and 0.04), and C. scoticus was collected more abundantly outdoors (between 9 and 22 fold more abundant outdoor than indoor).
Circadian Host-seeking Activity
During collections, the time of sunrise ranged from 6h49 (GMT +1) the 26th April to 8h06 the 8th October, when the sunset ranged from 21h01 to 19h24 between the same dates.
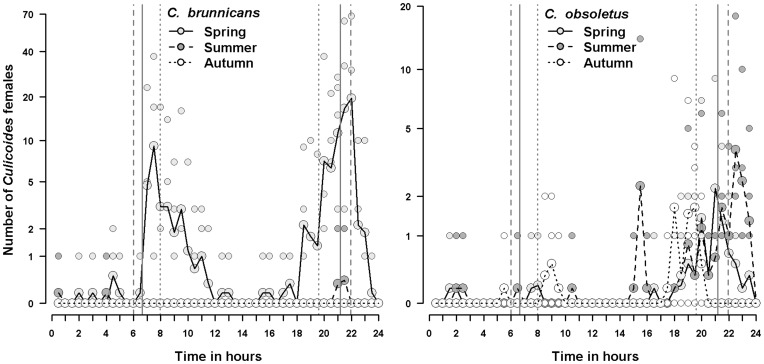
In spring, C. brunnicans exhibited two peaks of diel activity. Host-seeking activity increased rapidly just after sunrise, and then slowly decreased during the next 5 hours. Host-seeking activity started again 5 hours before the sunset, reaching a peak just after sunset and then decreasing rapidly (Fig. 2).
Figure 2. Circadian host-seeking activity of C. brunnicans and C. obsoletus: total number of females collected outdoor by host-baited traps at each session and day time.
Small symbols are single collection and lines with large symbols the means by period. Vertical lines symbolize the time of sunrise and sunset.
Host-seeking activity of C. obsoletus was recorded mainly around sunset. In spring and autumn, host-seeking activity started slowly 2 hours before sunset, peaked just before sunset and then decreased rapidly (Fig. 2). In summer, we observed a similar pattern of host-seeking activity except that the peak occurred just after sunset. Minor host-seeking activity was recorded around sunrise, especially in autumn. Outside these 2 periods, host-seeking activity ceased. The surprising peak observed in the middle of the afternoon (15h20) was the result of a single collection with the drop trap.
The same pattern, i.e. host-seeking activity mainly around sunset and a small amount around sunrise, was observed for C. dewulfi, C. chiopterus and C. scoticus, even if the low number of females collected did not provide details of the daily host-seeking activity for these 3 species.
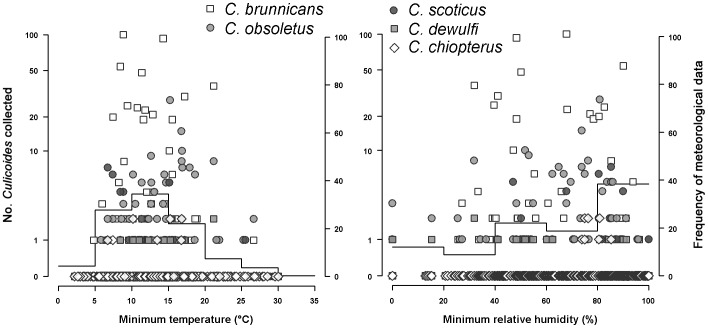
Almost all the collections were carried out without any rainfall. Comparing Culicoides abundance with data logger records, we noted that Culicoides were collected across a large range of temperature (Fig. 3): single individuals were collected at temperatures as lowest as 4.8°C and the first substantial catch (60 Culicoides) with low temperatures was recorded at temperatures between 8.4 and 10.4°C. Low relative humidity did not seem to inhibit host-seeking activity (Fig. 3) as 47 Culicoides were collected between 32 and 52% relative humidity. Moreover, we noted that the first significant catch (16 Culicoides) with strong wind was recorded with a wind speed of 5 m/s, but only 2/3 of collection sessions could be analyzed. Indeed, due to technical problems, local meteorological parameters were missing for some collection sessions.
Figure 3. Number of Culicoides collected outdoor in host-baited traps compared to temperature and relative humidity recorded by data loggers.
Lines are the distribution histogram of meteorological parameters recorded during the collections.
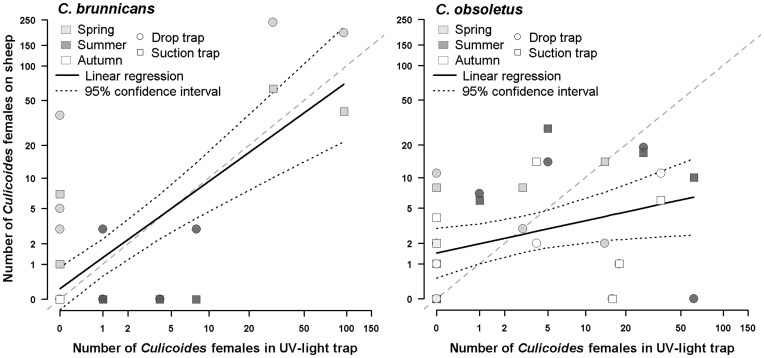
Culicoides brunnicans biting rates in spring assessed by drop trap or suction trap were linearly and positively correlated with the abundance in the UV-light/suction trap (R2 = 0.55, p<0.001), but some collections were positive in host-baited traps but not in UV-light/suction trap, or conversely (Fig. 4). Correlation was much lower for C. obsoletus (R2 = 0.13, p = 0.032), although it seemed that UV-light/suction trap tended to over-estimate biting rates.
Figure 4. Correlation between the numbers of C. brunnicans and C. obsoletus collected outdoor in host-baited traps and in UV-light/suction trap.
Discussion
We described endo/exophagy and circadian host-seeking activity of Palaearctic Culicoides species using drop trap and suction trap collections. The mean number of Culicoides per collection was rather low. It illustrated the highly variable Culicoides abundance in space and time. Indeed, up to 3,000 Culicoides were collected at this site by single UV-light trap night-collections, carried out independently of these collections. The trap, as a fixed effect, was necessary to model abundances of all Culicoides species. The drop trap collected more C. brunnicans than the suction trap – Viennet et al. [16] showed that drop trap collected more C. brunnicans than other host-baited trap methods – but abundances assessed by both methods were highly correlated (Pearson’s r = 0.99). The suction trap collected more individuals of other species than the drop trap, and presence of gravid females suggested that this trap may have collected non host-seeking females. Moreover, differences between traps may reflect spatial variations of Culicoides populations. However, satisfactory concordance of abundance (r = 0.67) for C. obsoletus in both traps suggested that the suction trap could be used as an efficient and easy alternative to drop trap for this species.
To date, investigations of Culicoides endo/exophagy had been carried out always with UV-light/suction traps [5], [6], [11]–[13], [21], [22], with the exception of one study [23] on ornithophilic Culicoides species. But UV-light/suction trap collections were greatly dependent on local conditions, such as presence and number of animals in the vicinity of trap [24], leading to complicated interpretations. For instance, C. imicola was collected in greater numbers by indoor animal-baited light traps than by outdoor unbaited light traps in Spain [21], in apparent contradiction with South-African descriptions, where this species exhibited exophagic behavior [5], [6]. In our study, host-baited collections in outdoor and indoor (with two degrees of opening for the host-baited suction trap) conditions highlighted that Palaearctic Culicoides are primary exophagous insects. Some species are strictly exophagous, such as C. brunnicans, some others show some degree of endophagy, such as C. obsoletus or C. scoticus. Baylis et al. [13] showed that differences between outdoor and indoor catches decreased from summer to autumn. To explain these differences, authors suggested factors difficult to untangle. Colder temperatures and stronger wind in autumn could suppress outdoor Culicoides host-seeking activity – overcast conditions have been shown to decrease outdoor UV-light/suction collections [6], [11]. On the other hand, autumnal conditions could also interfere in light trap efficiency – wind could modify the trap suction efficiency – or host-seeking activity could occur mainly before sunset late in the year, decreasing part of the Culicoides which otherwise would be collected [25], [26]. Even though interaction between season and location was necessary to model C. obsoletus abundance, our data did not allow to clearly confirm this observation. Finally, most of Culicoides females collected indoors in host-baited traps may have been host-seeking females. However, the low number of gravid C. obsoletus indoors suggest than some females may enter into sheds to oviposit, as illustrated by the possibility to find Culicoides larvae indoors [27], [28].
It is widely assumed that Culicoides are mostly crepuscular [29]. However, only few studies have described rigorously Culicoides circadian cycles with consecutive 24 h collections [18], [19], [30], and only one has considered host-seeking behavior of European Culicoides using horse-baited collections [20]. Two patterns of host-seeking activity were observed during our 24 consecutive hours of collection. Culicoides brunnicans showed a bimodal pattern of host-seeking activity with peaks at dawn and dusk, as found by Blackwell et al. [31] for flight activity of C. impunctatus. Other species, C. obsoletus, C. scoticus, C. dewulfi and C. chiopterus, showed a main activity peak around sunset, even if some individuals could be collected around sunrise. We cannot exclude that in context of higher Culicoides abundance, these species present a second, but lower peak of host-seeking activity around sunrise, as highlighted by Van der Rijt et al. [20] for C. obsoletus (84 individuals collected at sunset versus 10 at sunrise). Indeed, Sanders et al. [32] observed two peaks of flight activity for C. obsoletus, C. scoticus, C. dewulfi and C. chiopterus around both sunrise and sunset. Meteorological conditions are known to impact adult midge activity [29], [33]. It seems clear that windy conditions suppress the flight activity of Culicoides [6], [31], [33]. Flight activity is described experimentally as exceptional at temperatures below to 10°C and optimal at temperatures above to 20°C for Culicoides oxystoma Kieffer and Culicoides pictipennis (Staeger) [34]. Finally, Blackwell et al. [31] found a positive correlation between flight activity of C. impunctatus females and relative humidity and rainfall. During our observations, relative humidity was not found to influence host-seeking activity and minimal temperature required seemed in coherence with that described by Tsutsui et al. [34]. We found that the highest host-seeking activity occurred in low sunlight, corresponding to twilight periods. Changes in light intensity during the day could explain “abnormal” host-seeking activity. For instance, 15 C. obsoletus were collected in the middle of the afternoon (at 15h20) one day of June (Fig. 2). During this afternoon, sunlight decreased from 120 (14h00) to 77 (15h00) and to 20 J/cm2 (16h00), compared to means of 226 (14h00), 224 (15h00) and 225 (16h00) the other collection days of June. The first consequence is the possible lengthening of Culicoides attack hours when low-light overcast conditions prevail leading to biting throughout the day, as is known in open pastures for C. impunctatus or C. obsoletus and in forested environments for C. obsoletus [17], [35]. The other consequence is the possible nocturnal host-seeking activity of Culicoides due to moon illumination, as observed for North-American Culicoides species by Barnard and Jones [18] or Lillie et al. [19]. Therefore, it would be interesting to study influence of moon light and moon phases on circadian host-seeking activity of Palaearctic Culicoides. Sunset and sunrise times depend on locality and season, leading to a change in host-seeking activity times throughout the year. Moreover, C. obsoletus was active before sunset during spring and autumn, and after sunset during summer, illustrating the influence of other parameters than sunlight. This could be an adaptation to temperatures as suggested by Lillie et al. [19] who found the same pattern change throughout the year.
Finally, this work allowed the comparison of biting midge abundance in sheep-baited traps and in UV-light/suction traps, operated as a standardized method. Due to experimental design, these methods could not be rigorously compared. A positive and high correlation could be established for C. brunnicans, showing that UV-light collections may be used without correction to follow changes in biting rates. Correlation was worse for C. obsoletus, but the tendency of biting rate over-estimation by the UV-light/suction trap was already recorded by Carpenter et al. [14] and Viennet et al. [16].
To conclude, in farms with grazing animals, keeping valuable animals in closed stables would limit their risk to be bitten by Culicoides and then to be infected by Culicoides-borne pathogens, as biting midges seem to be primarily exophagic insects. However, practical recommendations are difficult to provide because of the variation in the degree of endophagy and time of host-seeking activity. The activity parameters depend on Culicoides species, host abundance, season, stabling conditions and weather. This illustrates the consequence of evolutionary processes selecting the ability of hematophagous insects to ensure host encounter in time and space.
Materials and Methods
Study Site and Culicoides Collections
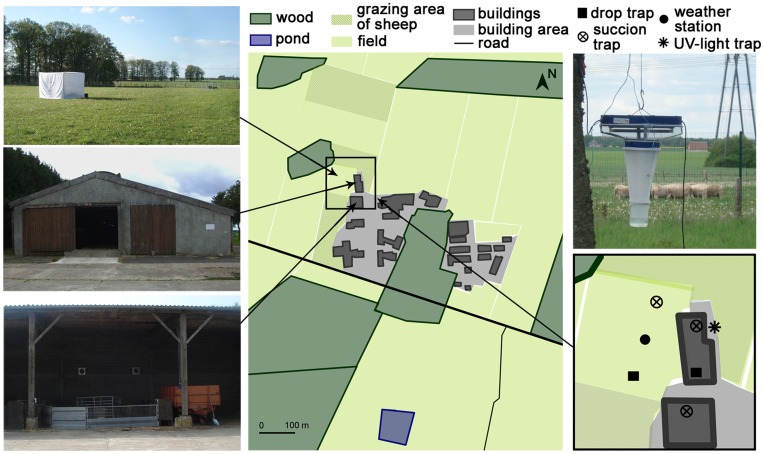
Indoor and outdoor collections were carried out during six collection periods of 24 hours in April/May, in late June and in September/October 2010 on an experimental farm (Institut National de la Recherche Agronomique, INRA, UE1277 PFIE) breeding sheep and located in Nouzilly (47°33′01′′N; 00°47′52′′E), western France. Three areas were investigated: a field (61×103 m), a closed stable (17×45 m) and a largely-open stable (30×10 m) (Fig. 5). The closed stable had closed windows (1×0.64 m; 30 windows west side and 12 east side) and two large doors (3.1×3.4 m) half open, thus about 3% of the walls were open surface. The largely open stable was constituted by 3 walls, 1 roof and 1 open side, and openings constituted about 38% of the wall surface. During collections, meteorological conditions (air temperature, relative humidity, wind speed and direction, solar radiation, rainfall and atmospheric pressure) were recorded every five minutes using a weather station Vantage Pro 2 (Davis Instruments France). Temperature and relative humidity were also recorded by a Tiny Tag TGP-4500 data logger at each trap location. Monthly meteorological data recorded by the national weather station (Météo-France) at Parcay-Meslay (47°26′36′′N; 00°43′36′′E; 13 km from the study site) in 2010 were used to illustrate meteorological conditions during the year of collections.
Figure 5. Sketch map of the study site at Nouzilly (western France) with the location of the sheep-baited traps (drop trap and suction trap) in the three sites (field, large-open-stable, closed stable) and of the UV-light/suction trap.
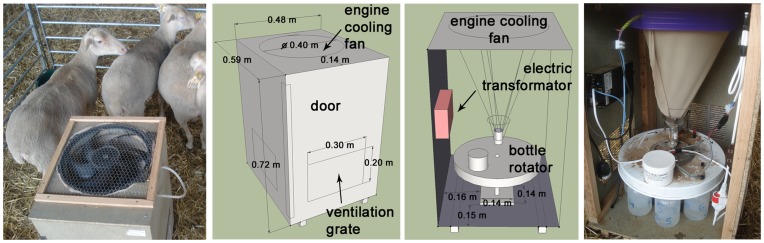
We collected Culicoides using one drop trap and one suction trap in the field and in the closed stable, and one suction trap in the largely-open-stable (Fig. 5). All traps were separated by a minimum of 30 m to minimize interference and to assess the level of endophagy of Culicoides. The drop trap is a host-baited trap and consisted of a rectangular cage (2.5 m wide×3 m long and 2 m high) and made of white cotton netting (<0.25 mm2 mesh size). Its structure and use was described in detail by Viennet et al. [16]. The suction trap allows Culicoides collections without human intervention and consisted of a wooded box (48 cm wide×58 cm long and 73 cm high) equipped with a engine cooling fan at the top and an inner collection bottle rotator (model 1512, John W. Hock Company, Gainesville, FL) (Fig. 6). Culicoides were collected in 0.5 liter plastic collection bottles, which were filled 1/3 full with water and two drops of soap, and then were transferred in 70% ethanol.
Figure 6. Inside and outside schemes and pictures of the suction trap.
Traps were operated during 24 consecutive hours (from 12 pm to 12 pm the next day), giving 48 collections at 30 min intervals, 6 times at each of the three 2-week periods. Each trap was baited with four south-Prealpes sheep females of 3 to 5-years old and 55 to 65 kg weight enclosed in a pen (2.5 m long×2 m wide×1.3 m high). There were no horses or cattle within 200 m from the sites during the collection sessions. The protocol was examined internally in the Plate Forme d’Infectiologie Expérimentale (UE1277 PFIE) by an internal group in charge of animal welfare, including veterinary surgeon and animal keepers. The protocol procedure did not cause any pain or stress, i.e. no injection, no biological sample, no surgery. Thus, according to the Directive 2010/63/EU on the protection of animals used for scientific purposes in Europe, it was not necessary to submit this protocol to an ethics committee and each step of the protocol was conducted with respect to the standard ethical rules: staff were qualified for animal experimentation and premises were licensed for experimentation.
In parallel, a UV-light/suction trap (manufactured by the Onderstepoort veterinary institute in South Africa) was operated during each collection session to provide an overview of Culicoides diversity. It was run with an 8 W UV light tube and on a 12-volt car battery, was placed at 1.5 m height from the ground on a tree and was not visible from the animal baits (Fig. 5). The insects collected with the UV-light/suction trap were stored in 70% ethanol.
Culicoides Identification
All Culicoides were morphologically identified under a stereomicroscope (Stemi 2000 C ZEISS) to species level based on an identification key for the Palaearctic region [36] and sorted by sex. Females were classified as nulliparous, parous [37], freshly blood-fed and gravid. When morphological identification with a stereomicroscope was not possible, individuals were dissected and identified using microscopic slide preparations (ZEISS imager A.1 fluorescence microscope).
Individuals belonging to the Obsoletus Complex (C. obsoletus and C. scoticus) were molecularly identified following the assay developed by Nolan et al. [38]. DNA extraction was done high-throughput using Chelex100® resin (200 µL/Culicoides) before polymerase chain reaction (PCR) [39]. Primers and PCR amplifications conditions were as described by Nolan et al. [38].
Statistical Analysis
Outdoor activity of Culicoides was compared between periods of the year to highlight influence of day length on circadian activity and was compared with climatic conditions to assess influence of meteorological parameters on host-seeking activity.
Abundance of Culicoides species was modeled using a Poisson mixed-effect model fitted with a method providing an adaptive Gauss-Hermite approximation to the log-likehood. We used the session (1 to 18) as a random effect, and the location (field, closed stable and largely-open-stable), the trap (drop trap, suction trap) and the season (spring, summer and autumn) as fixed effects. We considered also interactions between location and season, as endophagic behavior may change between the different seasons [12], [13]. Selection of effects and/or interactions in the abundance model was based on a likehood ratio test, and Pearson’s product-moment correlation was used as an overall test for goodness of fit.
Finally, we tested outdoor biting rates assessed by the host-baited traps with the abundance in UV-light collections using linear models.
All data analyses were performed using the R statistical package [40].
Acknowledgments
We are particularly grateful to the co-operators on whose premises this work was conducted. From the Plate-Forme d’Infectiologie Expérimentale (PFIE) Inra Nouzilly, we would like to thank his director Bertrand Schwartz and P. Sarradin and all people who gave assistance in operating traps on several nights (technicians, animal keepers, shepherds, especially Dany Leguéré, Michel Maillon and Pascal Lallier). We thank Frédéric Simard (IRD-MIVEGEC) for his attentive reading.
Funding Statement
This study was funded by CIRAD, by the Ministère de l’agriculture, de l’alimentation, de la pêche, de la ruralité et de l’aménagement du terroire and by the EU FP7-HEALTH-2010-single-stage grant 261504 EDENext. This paper is catalogued by the EDENext Steering Committee as EDENext032 (http://www.edenext.eu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Combes C (2000) Selective pressure in host-parasite systems. Journal de la Société de Biologie 194: 19–23. [PubMed] [Google Scholar]
- 2.Combes C (2010) L’art d’être parasite: les associations du vivant; Flammarion, editor. Paris.
- 3.Clements AN (1999) The biology of mosquitoes. Sensory reception and behaviour; Oxon, editor. New York: CABI.
- 4. Tauber CA, Tauber MJ (1981) Insect seasonal cycles: genetics and evolution. Annual Review of Ecology and Systematics 12: 281–308. [Google Scholar]
- 5. Barnard BJH (1997) Some factors governing the entry of Culicoides spp. (Diptera : Ceratopogonidae) into stables. Onderstepoort Journal of Veterinary Research 64: 227–233. [PubMed] [Google Scholar]
- 6. Meiswinkel R, Baylis M, Labuschagne K (2000) Stabling and the protection of horses from Culicoides bolitinos (Diptera : Ceratopogonidae), a recently identified vector of African horse sickness. Bulletin of Entomological Research 90: 509–515. [DOI] [PubMed] [Google Scholar]
- 7. Wilson AJ, Mellor PS (2009) Bluetongue in Europe: past, present and future. Philosophical Transactions of the Royal Society B-Biological Sciences 364: 2669–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velthuis AGJ, Saatkamp HW, Mourits MCM (2009) Financial consequences of the dutch bluetongue serotype 8 epidemics of 2006 & 2007. Preventive Veterinary Medicine: 11. [DOI] [PubMed]
- 9. Hoffmann B, Scheuch M, Höper D, Jungblut R, Holsteg M, et al. (2012) Novel Orthobunyavirus in Cattle, Europe, 2011. Emerging Infectious Diseases 18: 469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overgaard Nielsen B, Christensen O (1975) A mass attack by biting midge Culicoides nubeculosus (Mg.) (Diptera, Ceratopogonidae) on grazing cattle in Denmark a new aspect of sewage discharge. Nordisk Veterinaermedicin 27: 365–372. [PubMed] [Google Scholar]
- 11. Meiswinkel R, Goffredo M, Dijkstra EGM, van der Ven IJK, Baldet T, et al. (2008) Endophily in Culicoides associated with BTV-infected cattle in the province of Limburg, South-Eastern Netherlands, 2006. Preventive Veterinary Medicine 87: 182–195. [DOI] [PubMed] [Google Scholar]
- 12. Baldet T, Delécolle JC, Cetre-Sossah C, Mathieu B, Meiswinkel R, et al. (2008) Indoor activity of Culicoides associated with livestock in the bluetongue virus (BTV) affected region of Northern France during autumn 2006. Preventive Veterinary Medicine 87: 84–97. [DOI] [PubMed] [Google Scholar]
- 13. Baylis M, Parkin H, Kreppel K, Carpenter S, Mellor PS, et al. (2010) Evaluation of housing as a means to protect cattle from Culicoides biting midges, the vectors of bluetongue virus. Medical and Veterinary Entomology 24: 38–45. [DOI] [PubMed] [Google Scholar]
- 14. Carpenter S, Szmaragd C, Barber J, Labuschagne K, Gubbins S, et al. (2008) An assessment of Culicoides surveillance techniques in northern Europe: have we underestimated a potential bluetongue virus vector? Journal of Applied Ecology 45: 1237–1245. [Google Scholar]
- 15. Gerry AC, Monteys VSI, Vidal JOM, Francino O, Mullens BA (2009) Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in northeastern spain in relation to midge capture using UV light and carbon dioxide-baited traps. Journal of Medical Entomology 46: 615–624. [DOI] [PubMed] [Google Scholar]
- 16. Viennet E, Garros C, Lancelot R, Allene X, Gardes L, et al. (2011) Assessment of vector/host contact: comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of Orbiviruses. Parasites & Vectors 4: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. EFSA (2008) Bluetongue Scientific Opinion of the Panel on Animal Health and Welfare (Question No EFSA-Q-2007–201) adopted on 19 June 2008. The EFSA Journal 735: 1–70. [Google Scholar]
- 18. Barnard DR, Jones RH (1980) Diel and seasonal patterns of flight activity of Ceratopogonidae in Northeastern Colorado: Culicoides . Environmental Entomology 9: 446–451. [Google Scholar]
- 19. Lillie TH, Kline DL, Hall DW (1987) Diel and seasonal activity of Culicoides spp. (Diptera: Ceratopogonidae) Near Yankeetown, Florida, Monitored with a vehicle-mounted insect trap. Journal of Medical Entomology 24: 503–511. [DOI] [PubMed] [Google Scholar]
- 20. van der Rijt R, van den Boom R, Jongema Y, van Oldruitenborgh-Oosterbaan MM (2008) Culicoides species attracted to horses with and without insect hypersensitivity. The Veterinary Journal 178: 91–97. [DOI] [PubMed] [Google Scholar]
- 21. Calvete C, Estrada R, Miranda MA, del Rio R, Borras D, et al. (2009) Entry of bluetongue vector Culicoides imicola into livestock premises in Spain. Medical and Veterinary Entomology 23: 202–208. [DOI] [PubMed] [Google Scholar]
- 22. Anderson GS, Belton P, Belton EM (1993) A population study of Culicoides obsoletus Meigen (Diptera, Ceratopogonidae), and other Culicoides species in the Fraser Valley of British-Columbia. Canadian Entomologist 125: 439–447. [Google Scholar]
- 23. Votypka J, Synek P, Svobodova M (2009) Endophagy of biting midges attacking cavity-nesting birds. Medical and Veterinary Entomology 23: 277–280. [DOI] [PubMed] [Google Scholar]
- 24. Garcia-Saenz A, McCarter P, Baylis M (2011) The influence of host number on the attraction of biting midges, Culicoides spp., to light traps. Medical and Veterinary Entomology 25: 113–115. [DOI] [PubMed] [Google Scholar]
- 25. Murray MD (1987) Local dispersal of the biting midge Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) in south-eastern Australia. Australian Journal of Zoology 35: 559–573. [Google Scholar]
- 26. Kettle DS, Edwards PB, Barnes A (1998) Factors affecting numbers of Culicoides in truck traps in coastal Queensland. Medical and Veterinary Entomology 12: 367–377. [DOI] [PubMed] [Google Scholar]
- 27. Ninio C, Augot D, Dufour B, Depaquit J (2011) Emergence of Culicoides obsoletus from indoor and outdoor breeding sites. Veterinary parasitology 183: 125–129. [DOI] [PubMed] [Google Scholar]
- 28. Zimmer J-Y, Saegerman C, Losson B, Haubruge E (2010) Breeding sites of bluetongue virus vectors, Belgium [letter]. Emerging infectious diseases 16: 575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mellor PS, Boorman J, Baylis M (2000) Culicoides biting midges: Their role as arbovirus vectors. Annual Review of Entomology 45: 307–340. [DOI] [PubMed] [Google Scholar]
- 30. Kettle DS (1969) The biting habits of Culicoides furens (Poey) and C. barbosai Wirth & Blanton. I. The 24-h cycle, with a note on differences between collectors. Bulletin of entomological research 59: 21–31. [Google Scholar]
- 31. Blackwell A (1997) Diel flight periodicity of the biting midge Culicoides impunctatus and the effects of meteorological conditions. Medical and Veterinary Entomology 11: 361–367. [DOI] [PubMed] [Google Scholar]
- 32. Sanders CJ, Gubbins S, Mellor PS, Barber J, Golding N, et al. (2012) Investigation of diel activity of Culicoides biting midges (Diptera: Ceratopogonidae) in the United Kingdom by using a vehicle-mounted trap. Journal of Medical Entomology 49: 757–765. [DOI] [PubMed] [Google Scholar]
- 33. Kettle DS (1969) The biting habits of Culicoides furens (Poey) and C. barbosai Wirth & Blanton. II. Effects of meteorological conditions. Bulletin of entomological research 59: 241–258. [DOI] [PubMed] [Google Scholar]
- 34. Tsutsui T, Hayama Y, Yamakawa M, Shirafuji H, Yanase T (2010) Flight behavior of adult Culicoides oxystoma and Culicoides maculatus under different temperatures in the laboratory. Parasitology research 108: 1575–1578. [DOI] [PubMed] [Google Scholar]
- 35. Balenghien T, Cetre-Sossah C, Grillet C, Delecolle JC, Mathieu B, et al. (2008) Diurnal activity of potential bluetongue vectors in northern Europe. Veterinary Record 162: 323–324. [DOI] [PubMed] [Google Scholar]
- 36.Delécolle J-C (1985) Nouvelle contribution à l’étude systématique et iconographique des espèces du genre Culicoides (Diptera : Ceratopogonidae) du Nord-Est de la France [Thèse de Doctorat d’Université mention Sciences]. Strasbourg, France: Université Louis Pasteur. 238 p.
- 37. Dyce AL (1969) The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Journal of the Australian Entomological Society 8: 11–15. [Google Scholar]
- 38. Nolan DV, Carpenter S, Barber J, Mellor PS, Dallas JF, et al. (2007) Rapid diagnostic PCR assays for members of the Culicoides obsoletus and Culicoides pulicaris species complexes, implicated vectors of bluetongue virus in Europe. Veterinary Microbiology 124: 82–94. [DOI] [PubMed] [Google Scholar]
- 39. Solano P, Duvallet G, Dumas V, Cuisance D, Cuny G (1997) Microsatellite markers for genetic population studies in Glossina palpalis (Diptera: Glossinidae). Acta Tropica 65: 175–180. [DOI] [PubMed] [Google Scholar]
- 40.R Development Core Team (2008) An Introduction to R: Notes on R, A Programming Environment for Data Analysis and Graphics. In: Computing RFfS, editor. 3.2.1 ed. Vienna, Austria.