Abstract
Background
The whitefly Bemisia tabaci is cryptic species complex composed of numerous species. Individual species from the complex harbor a diversity of bacterial endosymbionts including Wolbachia. However, while Wolbachia is known to have a number of different roles, its role in B. tabaci is unclear. Here, the antibiotic rifampicin is used to selectively eliminate Wolbachia from B. tabaci so as to enable its roles in whitefly development and reproduction to be explored. The indirect effects of Wolbachia elimination on the biology of Encarsia bimaculata, a dominant parasitoid of B. tabaci in South China, were also investigated.
Methodology/Principal Finding
qRT-PCR and FISH were used to show that after 48 h exposure to 1.0 mg/ml rifampicin, Wolbachia was completely inactivated from B. tabaci Mediterranean (MED) without any significant impact on either the primary symbiont, Portiera aleyrodidarum or any of the other secondary endosymbionts present. For B. tabaci MED, Wolbachia was shown to be associated with decreased juvenile development time, increased likelihood that nymphs completed development, increased adult life span and increased percentage of female progeny. Inactivation was associated with a significant decrease in the body size of the 4th instar which leads us to speculate as to whether Wolbachia may have a nutrient supplementation role. The reduction in nymph body size has consequences for its parasitoid, E. bimaculata. The elimination of Wolbachia lead to a marked increase in the proportion of parasitoid eggs that completed their development, but the reduced size of the whitefly host was also associated with a significant reduction in the size of the emerging parasitoid adult and this was in turn associated with a marked reduction in adult parasitoid longevity.
Conclusions/Significance
Wolbachia increases the fitness of the whitefly host and provides some protection against parasitization. These observations add to our understanding of the roles played by bacterial endosymbionts.
Introduction
Endosymbiotic, mutualistic and parasitic bacterial endosymbionts that have either obligate or facultative relationships with insects, play an important role in many aspects of insect biology and ecology [1]. Knowledge drawn from the study of these associations is already leading to novel means by which to control insects and the pathogens they transmit [2], [3]. A large number of herbivorous insects, especially the phloem feeding insects of the Hemiptera suborder Sternorrhyncha (aphids, whiteflies, psyllids, scales and mealybugs) harbour these bacteria [4], which are defined as being either primary or secondary endosymbionts. Primary endosymbionts are confined to specialized host cells called bacteriocytes that together form an organ called a bacteriome [5]. They have an obligatory relationship with the host, are transmitted vertically from mother to offspring and are thought to synthesize essential non-dietary metabolites [1], [6]–[8]. Secondary endosymbionts can also occur in the bacteriocytes, but are often also found in the cells of tissues throughout the host [5], [9]. These bacteria usually have a facultative relationship with the host and while primarily transmitted vertically from mother to offspring, may also be transmitted horizontally through direct and indirect contact with other infected individuals [1], [2], [9], [10]. In many cases their function is unclear, but they are perhaps best known for the ability to act as reproductive manipulators [2], [11].
Among the secondary endosymbionts, Wolbachia is perhaps the most widely distributed within arthropods and has been estimated to infect as many as 70% of all species [12], [13]. Wolbachia can be either mutualistic or parasitic. Mutualistic functions vary and examples include the provision of essential nutrients to bedbug hosts [14], increasing fitness in a leaf-mining moth [15], uzi fly [16] and mosquito [17] although these effects are at times linked to environmental conditions [18]. Wolbachia can also suppress the replication of a range of RNA viruses in Drosophila, Culex quinquefasciatus and Aedes aegypti [19]–[22]. In addition, Wolbachia has also been shown to be required for oogenesis in the parasitoid Asobara tabida as its removal prevented the production of mature oocytes [23]. As a parasite, Wolbachia acts as a reproductive manipulator either by reducing the reproductive output of uninfected females via cytoplasmic incompatibility (CI), or by shifting the sex ratio of offspring in favour of females by either killing male offspring, inducing feminisation in genetic males or thelytokous parthenogenesis [12].
The whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), is a cryptic species complex composed of numerous species [24]–[26]. All members of the complex are known to be infected with endosymbionts [27]–[30]. In China, B. tabaci was first recorded in 1949 [31], but it was not considered an important pest until the invasions by Middle East-Asia Minor 1 (commonly referred to as the B biotype, hereon MEAM1) and Mediterranean (commonly referred to as the Q biotype, hereon MED) [24]–[26]. As well as these two invaders, China is home to a number of indigenous members of the complex [26]. MEAM1 and MED now cause considerable damage to a wide range of crops in 30 of China’s 34 provinces [32]. In the last decade the endosymbiont community within B. tabaci has been studied extensively. Across the complex, one primary endosymbiont, Portiera aleyrodidarum as well as six secondary endosymbionts, Arsenophonus, Cardinium, Fritschea, Hamiltonella, Rickettsia and Wolbachia have been found in various populations [7], [27], [33]–[39]. In China, infection by Wolbachia in B. tabaci has been previously surveyed and reported. Ahmed et al. [28] collected 350 individuals from 29 different populations across China and the PCR detection revealed that 89.4% were infected, 33.6% with supergroup A, 50.9% with supergroup B, and 4.8% with both. In 2005–2008 Chu et al. [30] analysed 373 MEAM1 and 1830 MED individuals collected from 15 locations across 11 provinces. Infection in MEAM1 ranged from 5.9–36.4% while in MED ranged from 1.0–8.4%. Both studies indicated that Wolbachia prevalence varied considerably in regards to host plants, geographical location and whitefly species.
The primary endosymbiont provides its whitefly host with essential nutrients that cannot be obtained from the nutrient deficient phloem [33], while the function of the different secondary endosymbionts varies. Several of the secondary endosymbionts appear to affect the capacity of the host to be a pest. For example, Hamiltonella facilitates the transmission of plant viruses [40] while Rickettsia confers heat tolerance, increases susceptibility to some insecticides [29], [41] and also leads to the increased fecundity, greater survival to adulthood, faster development times and an increased proportion of daughters [42]. However, the full range of functions is still unknown and in many cases the perceived role is the result of correlative rather than experimental studies.
In order to identify the function of one endosymbiont in its arthropod host, two methods have been used. The first is through the use of microinjection to introduce a new endosymbiont and then make comparisons against an uninfected control [43]–[45], but this has not yet been applied to whiteflies. The second, undertaken for a number of different insect species, is through the elimination or inactivation of a specific endosymbiont, often through the use of selective antibiotics [46], [47]–[49]. However, very few studies have used the latter method for B. tabaci and/or its parasitoids. Ruan et al. [50] used three antibiotics (tetracycline, ampicillin trihydrate and rifampicin) to eliminate endosymbionts. They showed that any of the three antibiotics at 50 ug/ml and 48 h exposure, eliminated Hamiltonella defensa, Wolbachia and Arsenophonus, but failed to remove the primary endosymbiont P. aleyrodidarum. Ahmed et al. [27] compared the efficiencies of the same antibiotics in removing the secondary endosymbionts in three species of B. tabaci, Middle East- Asia Minor 1, Mediterranean and AsiaII_7 and showed differential responses between the antibiotics and the different species and their endosymbionts. The antibiotic rifampicin has also been used to remove Rickettsia and Hamiltonella from two aphelinid parasitoids, Eretmocerus sp. nr. emiratus and Eretmocerus eremicus [48].
Despite this research the physiological roles of Wolbachia in whitefly B. tabaci remains poorly defined. To better define the role of Wolbachia in B. tabaci we use an antibiotic to eliminate it selectively from its host and then compared the biology of infected and uninfected individuals. Furthermore, aphelinid parasitoids belonging to the genera Encarsia and Eretmocerus are important parasitoids of B. tabaci [51]. A rich complex of at least 19 species of aphelinid parasitizing B. tabaci has been reported from surveys across South China, among which Encarsia bimaculata and Eretmocerus sp. nr. furuhashii (Hymenoptera: Aphelinidae) are the most abundant [52], [53]. Previous research has shown that endosymbionts in the insect host may influence interactions with its parasitoid [43], [44], and we explored this for B. tabaci and the parasitoid E. bimaculata.
Materials and Methods
Plant Rearing
Cowpea, variety Yuefeng was chosen due to its economic value in South China. Seeds were grown in 1.3 L plastic pots containing a soil-sand mixture (40% sands, 5% clay and 55% peat) in a greenhouse under ambient conditions. Plants were watered as needed and were used when they were three weeks old and had 4–6 expanded true leaves.
Insect Cultures
Bemisia tabaci MED was collected from eggplant in May of 2006 in Nantong, Jiangsu and then maintained in a glasshouse on hibiscus (Hibiscus rosa-sinensis) under ambient conditions. It was identified using mitochondrial cytochrome oxidase I [54] and then alignment against the consensus sequences determined by Dinsdale et al. [24].The parasitoid, Encarsia bimaculata, was collected from parasitized B. tabaci nymphs on hibiscus in Tianhe, Guangzhou; the species was identified by J. Huang (Fujian Agriculture and Forestry University, China). Voucher specimens have been deposited into the Insect Museum of the Department of Entomology, South China Agricultural University, Guangzhou. It is a solitary, arrhenotokous, heteronomous, autoparasitoid where the mated female lays female eggs internally into unparasitised nymphs whereas male eggs are placed into parasitized nymphs [55]. The parasitoid culture was maintained on B. tabaci feeding on hibiscus at 26.0±0.5°C, 70–80% relative humidity, 14∶10 (L:D) photoperiod, and a light intensity of approximately 3000 Lux.
Detection of Endosymbionts
To extract DNA, individual whiteflies were first washed with double distilled water to remove alcohol and then homogenized in 200 µl lysis buffer (1% SDS, 10 mM Tris-HCl, pH 8.0, 25 mM EDTA, 25 mM NaCl, proteinase K 200 mg/ml) in a 0.5 ml microcentrifuge tube. The homogenate was incubated at 55°C for 2–3 h in a water bath and then at 95°C for 10 min to inactivate the proteinase K. After incubation, samples were centrifuged for 1 minute and then either used directly for PCR amplification or stored at −20°C for later use. The primers and PCR conditions used to detect the primary endosymbiont, P. aleyrodidarum and secondary endosymbionts, Arsenophonus, Cardinium, Fritschea Hamiltonella, Rickettsia and Wolbachia were from Zchori-Fein & Brown [33], Thao & Baumann [34], Everett et al. [35], Weeks & Breeuwer [56], Gottlieb et al. [57] and Li et al. [58] (Table S1).
For each of 150 individual whiteflies selected at random, PCR was undertaken using a 25 µl reaction volume and included: 2.5 mM MgCl2, 200 mM for each dNTPs, 1 µM of each primer, 1 unit DNA Taq polymerase (Invitrogen). Samples were amplified using a thermocycler (Bioer xpcycler, TC-XP-D). After amplification, 5 µl of the reaction mix was electrophoresed using 1.0% agarose gels with a DL2000 bp DNA marker in 1×TAE at 8 V/cm for 2 h; after electrophoresis the gel was then stained using 10 mg/ml ethidium bromide for 30 min. Bands were visualized using UV light with expected size were visible in the gels. Five products were sequenced for each endosymbiont using an ABI 3730XL automated DNA sequencer (PE Applied Biosystems). The resulting sequences were compared to the known sequences in GenBank using BLAST to verify their identity. The sequences of P. aleyrodidarum (AY429619), Arsenophonus (FJ766370), Cardinium (FJ766339), Fritschea (AY140910), Hamiltonella (JF795506), Rickettsia (JF795501) and Wolbachia (FJ545748) were used as the references for identification.
The Inactivation of Endosymbionts with Rifampicin
To enable us to explore the possible roles of endosymbionts in B. tabaci it was necessary to have lines that were either infected or uninfected by a particular endosymbiont. The antibiotic rifampicin has been used to inactivate endosymbionts in whiteflies, but the level of reported inactivation varies across different studies [27], [50]. Artificial feeding through a parafilm membrane was used to deliver rifampicin to adult whiteflies so as to determine whether it could be used to selectively eliminate one or more species of endosymbiont. The method involved stretching three layers of parafilm (American National Can, Chicago, IL, USA) across the open end of a glass tube, 5 cm diameter and 10 cm height. The tube was then set upright on a tray in a controlled environment insect chamber with 26.0±0.5°C, 70–80% relative humidity, 14∶10 (L:D) photoperiod. The feeding solution containing 20% sucrose and 1.0 mg/ml rifampicin in double distilled water was injected between the bottom and middle layers of parafilm while a fresh cowpea leaf was inserted between the middle and upper layers so as to attract the whiteflies in the tube to the diet. Forty pairs of whitefly adults were released into the tube through the lower open end and were then allowed to feed for either 12, 24, 36 or 48 h. The control for each exposure period was the same as the above except that the feeding solution did not contain rifampicin. There were four replicates for each exposure period and control.
PCR, as described above, was followed by both fluorescence in situ hybridization (FISH) for Wolbachia and real-time PCR (qRT-PCR) for both the primary and secondary endosymbionts. FISH was used to confirm the results for Wolbachia elimination, while qRT-PCR was used to determine (a) whether time influenced inactivation and (b) which of the endosymbionts was inactivated by the antibiotic. Real-time PCR enables the activity of the endosymbionts to be measured as an inactive bacterium may still yield a positive result using PCR. For FISH, 10 whitefly pairs were selected at random from Wolbachia positive populations; five were then introduced into separate leaf cages to lay eggs. The eggs, except for 10, were left to develop to 3rd instars. The 10 selected eggs were used for FISH detection of Wolbachia. The other 5 whitefly pairs were first treated with 1.0 mg/ml rifampicin for 48 h as above and were then also introduced into separate leaf cages for oviposition. All of the eggs, except for 10, were left to develop to 3rd instars. Then the treated adults, 10 of their F1 eggs and 10 of the 3rd instar nymphs were analysed for the presence of Wolbachia using FISH. The FISH procedure followed the method of Sakurai et al. [59] with slight modifications (Method S1). All the samples were observed using an inverted fluorescence microscope (Nikon Eclipse Ti-U). Specificity of the detection was confirmed using the Wolbachia negative whiteflies as the controls.
For qRT-PCR, total RNA from the whiteflies was extracted and the related cDNAs were then transcribed. Ten pairs of the whiteflies from each exposure period were collected at random and placed into a 0.5 ml microcentrifuge tube and then homogenized in 500 µl Trizol buffer. The total RNA was extracted using the Trizol RNA Extraction Kit (Omega) according to the method provided by the manufacturer. The RNA was then reverse-transcribed to cDNA using M-MLV (Invitrogen) and random hexamer primers (Invitrogen) as recommended by the manufacturer. qRT-PCR was performed using the cDNAs as targeted transcripts with QuantiTect primers in the CFX-96 PCR system (Applied Bio-Rad). The primers and qRT-PCR protocols are listed in Table S1, and the qRT-PCR analysis was repeated three times for each endosymbiont.
As Wolbachia was shown by the above to be the only endosymbiont inactivated by rifampicin under inactive conditions of 1.0 mg/ml for 48 h, we then explored whether the inactivation of Wolbachia influenced the biology of B. tabaci and parasitisation of B. tabaci by E. bimaculata.
Effect of Rifampicin on the Biology of B. tabaci
Five pairs of adults from each of exposure period and their positive control (Wolbachia positive adults without inactivation) were collected at random from each of the four replicates and each pair then placed separately into one of five leaf cages on cowpea plants. As an additional negative control, 25 pairs of Wolbachia negative whitefly adults were selected at random and divided into 5 equal groups for the 0, 12, 24, 36 and 48 h exposures to rifampicin and released into leaf cages on cowpea plants as negative control experiment. Both the positive and negative controls were repeatedly screened using PCR throughout the culturing process to ensure that were either 100% infected or uninfected. The females were then allowed to lay eggs for 24 h after which the adults were removed, the eggs left to hatch and the nymphs to complete their development. All the experiments were undertaken at 26.0±0.5°C, 70–80% relative humidity and 14∶10 (L:D) photoperiod. There were four replicates. The size of the whitefly 4th instar in each of the treatments and the controls was determined by first photographing the nymph (AxioCam, HRc and Coolsnap-Procf & CRI Micro*Color connected to a dissecting microscope, Discovery V20 Zeiss) and then measuring its length and width using Axio Vision Rel 4.8 software; 20 individuals from each treatment and the control were measured. The developmental time, survivorship, sex ratio and longevity of the F1 generation B. tabaci were determined.
Effect of Rifampicin on Parasitisation by E. bimaculata
The 2nd-3rd instar whiteflies from the 48 h inactivation treatment and control were prepared as above and then exposed to adult parasitoids. They were observed with the aid of a binocular microscope and when oviposition took place, the nymph was then marked. A total of 20 marked nymphs from the treatment and control were then selected at random and dissected to check for the presence of parasitoid eggs and their number. The parasitized nymphs that were not selected for dissection were left to allow the parasitoids to develop to pupa stage at which time 20 were selected at random from the treatment and controls. Each parasitoid pupa was then dissected from the whitefly host, photographed and the length and width of the head capsule then measured using the same methodology described above for the whitefly nymphs. The size of the head capsule is a reliable measure of parasitoid size.
In a parallel experiment, 20 pairs of adult B. tabaci were selected at random, divided into 5 equal groups and then treated with 1.0 mg/ml rifampicin for 0,12, 24, 36 and 48 h, respectively. In addition to the treated whiteflies, 20 pairs of Wolbachia positive and 20 pairs of Wolbachia negative whitefly adults were selected at random. Each adult pair was then introduced into a leaf cage on a cowpea plant and allowed to oviposit for 24 h. Once all the eggs had hatched in one leaf cage, all nymphs except 10 at the 2nd–3rd instar stage were removed and a single mated E. bimaculata female (3–4 day old) was then released into the cage for 24 h and allowed to parasitize the nymphs. The wasp was observed ovipositing with the aid of a binocular microscope and the position of each nymph into which an egg was placed was then marked. When the F1 generation of E. bimaculata was visible (the first visible stage is the 2nd instar which appears as a characteristic ‘C’ shape) in a whitefly nymph, the survivorship of parasitoid F1 juveniles (from egg to 2nd instar, and from 2nd instar to adult) was then calculated based on the numbers of eggs, visible 2nd instars and the numbers of emerged adults. Additionally, 20 newly emerged E. bimaculata adults from each of the treatments as well as the controls were selected at random and released individually into one of 20 leaf cages with an abundance of eggs and 1st to 3rd instars. The parasitoids were observed daily until death so as to determine adult longevity. All the experiments were undertaken at 26.0±0.5°C, 70–80% relative humidity, 14∶10 (L:D) photoperiod, and each experiment was repeated four times.
Data Analysis
To confirm whether inactivation of each of the endosymbionts had occurred, the expression levels of the different target genes were compared between the control and treatments and calculated using the Bio-Rad CFX software. Differences in developmental times, survivorship of whitefly and parasitoids juveniles, the body size of whitefly 4th instar and the sex ratio and longevity of the whitefly and parasitoid adults were analysed using analysis of variance (multiple comparisons, PROC ANOVA, SAS Institute 2003) [60]; the differences in the head capsule size of E. bimaculata pupa and in the number of eggs that were oviposited between the control and the 48 h exposure treatment were analysed using a t-test (SAS Institute 2003) [60]. The normality and homoscedasticity of the percentage survivorship and adult sex ratio data were first tested and arcsine transformed before the analysis of variance. Means were separated using Tukey’s Studentized range (HSD) test at a significant level of a = 0.05 (SAS Institute 2003) [60].
Results
The Detection of Endosymbionts in the MED B. tabaci
PCR detection indicated that all seven endosymbionts, the primary obligate endosymbiont P. aleyrodidarum and the six secondary endosymbionts Arsenophonus, Cardinium, Fritschea, Hamiltonella, Rickettsia as well as Wolbachia were present in the populations sampled. All products were sequenced to confirm identity (GenBank accession numbers JQ009297 to JQ009303). All individuals were infected with the primary endosymbiont. The number of different endosymbionts in any one individual whitefly varied. Wolbachia was the most prevalent (78.6%) followed by Arsenophonus (59.4%), Rickettsia (35.5%), Hamiltonella (17.2%), Cardinium (13.7%) and Fritschea (8.3%) (Table 1); the identity of each was confirmed through sequencing of the amplicon. Of the individuals screened, 14.7% were uninfected with secondary endosymbionts while 10% of individuals were infected with Wolbachia only. No other secondary endosymbionts were present as single infections. 38.7% were infected by two endosymbionts, 21.3% with three and 15.3% with four (Table 1). No individuals were infected with more than four secondary endosymbionts (Table 1). Most, 91.2%, had an infection with Wolbachia plus at least one other secondary endosymbiont. Only 6.7% involved infections with secondary endosymbionts that were not Wolbachia (Table 1). The full details are presented in Table 1.
Table 1. The percentage infections by Wolbachia, Arsenophonus, Rickettsia, Hamiltonella, Cardinium and Fritschea in a sample of 150 individuals.
| Endosymbiont code | Endosymbiont combinations | Percentage | Individuals |

|
Wolbachia | 78.6 | 118 |

|
Arsenophonus | 59.4 | 89 |

|
Rickettsia | 35.5 | 53 |

|
Hamiltonella | 17.2 | 26 |

|
Cardinium | 13.7 | 21 |

|
Fritschea | 8.3 | 12 |
| nil infection | 22 | ||

|
15 | ||
|
38 | ||
|
7 | ||
|
1 | ||
|
2 | ||
|
4 | ||
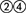
|
1 | ||
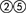
|
1 | ||
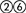
|
1 | ||
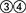
|
2 | ||
|
1 | ||

|
13 | ||

|
4 | ||
|
3 | ||

|
1 | ||

|
5 | ||

|
4 | ||

|
1 | ||

|
1 | ||
|
7 | ||
|
5 | ||
|
4 | ||
|
3 | ||
|
2 | ||
|
2 | ||
| n | 150 |
The combinations of different secondary endosymbiont infections detected and the number of individuals in which each combination was present are also shown. The endosymbiont codes  to
to  are used to identify the respective endosymbionts.
are used to identify the respective endosymbionts.
The Inactivation of Various Endosymbionts with Rifampicin
PCR amplification indicated that only Wolbachia was eliminated after 48 h exposure to rifampicin (figure not shown). FISH analysis indicated that exposure to 1.0 mg/ml rifampicin for 48 h, eliminated Wolbachia from whitefly adults and their progeny (eggs and nymphs) (Fig. S1). The qRT-PCR gene expression for the primary endosymbiont, P. aleyrodidarum and the six secondary endosymbionts are shown in Fig. S2A–G, which also confirmed the initial PCR based detection in that the primary endosymbiont and each of secondary endosymbionts, except Wolbachia, were positive for each duration of exposure, and the expression levels of the target genes (16S/23S rDNA) were not significantly different regardless of the duration of exposure to rifampicin (Fig. S2 A–F). In contrast, while positive for exposure durations up to and including 36 h, Wolbachia was negative after 48 h exposure indicating there was no activity (Fig. S2G).
Effects of Wolbachia Inactivation on the Development of B. tabaci Juveniles
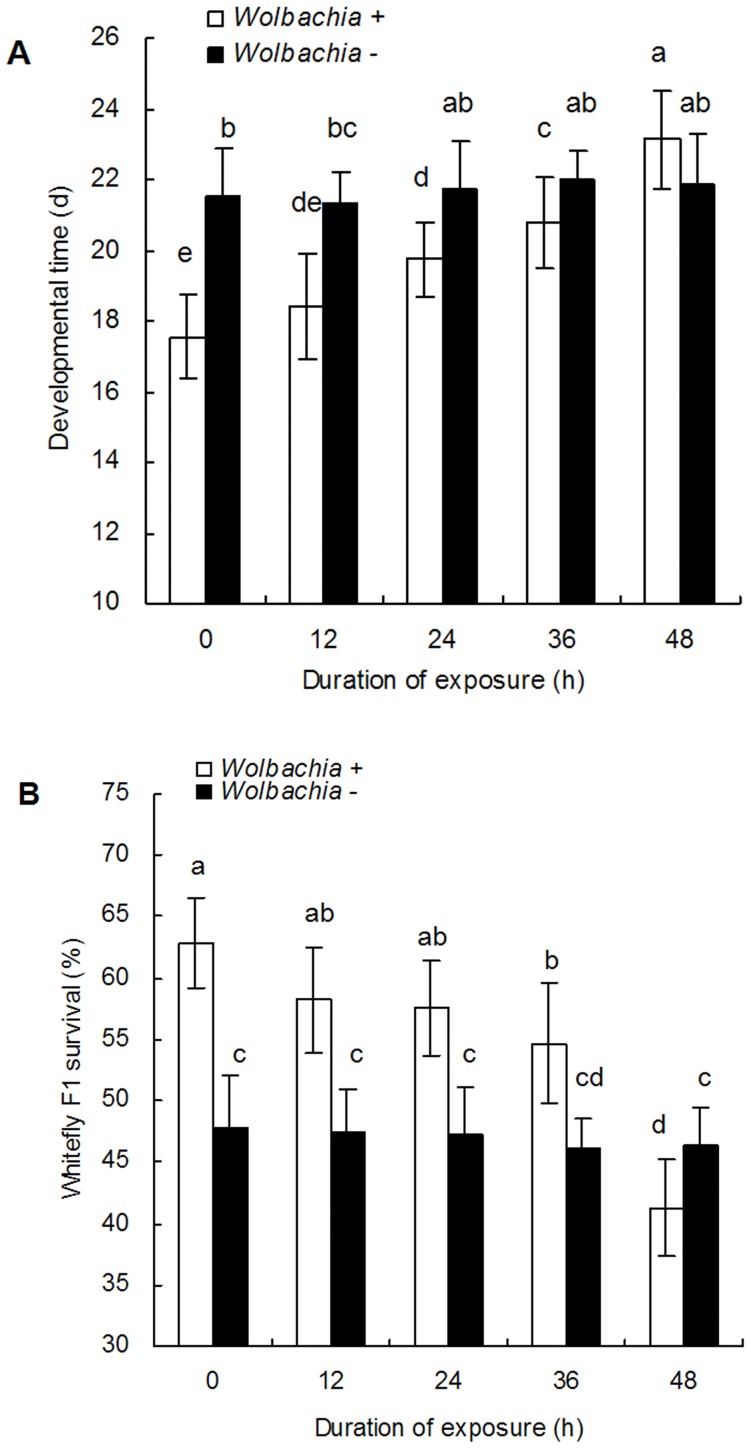
Compared with the mean development duration of 17.6±1.2 days in the Wolbachia positive controls, the developmental time of the F1 progeny increased the longer the parents were exposed to rifampicin (Fig. 1A, F 9,190 = 22.4, P<0.0001). Similarly, the mean percentage of F1 juveniles that completed their development declined as exposure to rifampicin in the parents increased (Fig. 1B, F 9,190 = 28.4, P<0.0001). All exposure durations lead to significant declines in the percentage of nymphs that completed their development relative to the untreated control with the lowest percentage survival, 41.3±2.2%, being observed after 48 h exposure. On the other hand, the mean developmental time of the F1 progeny from Wolbachia negative whitefly adults was longer (21.3 to 22.0 days) and mean survivorship lower (46.2% to 47.9%) than that observed in the Wolbachia infected treatment, but no significant differences were found between different exposure periods (Fig. 1A, B).
Figure 1. The development time (M±SE) and survival of the F1 progeny of Wolbachia positive (+) and negative (−) whitefly adults after different rifampicin treatments A: development time, B: survival.
The exposed time to 1.0 mg/ml rifampicin is either 12, 24, 36 or 48 h. 0 is the untreated control. The different letters indicate a significant difference using Tukey HSD.
Effects of Wolbachia Inactivation on the Sex Ratio and Longevity of B. tabaci Adults
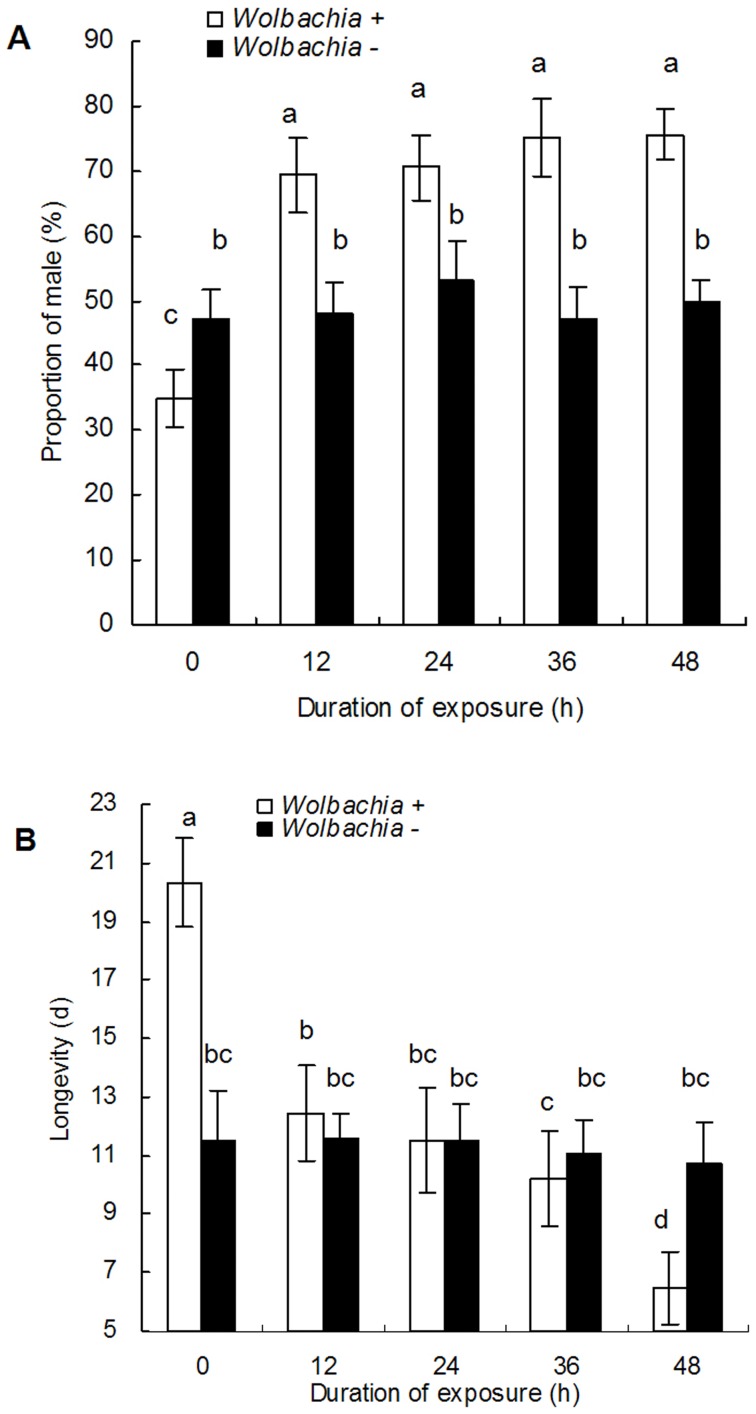
As the duration of adult exposure to rifampicin increased, the mean percentage of male progeny increased from an average of 35.0±2.5% in the untreated control to 75.6±5.3% after 48 h of exposure (Fig. 2A, F 9,190 = 85.7, P<0.0001), but there was no significant differences among the F1 progeny from various antibiotic exposures. The duration of exposure to rifampicin was also associated with a significant decline in the average longevity of F1 adults (Fig. 2B, F 9,190 = 55.5, P<0.0001), with the lowest longevity resulting occurring after 48 h of exposure in the parents. However, results also indicated that different antibiotic treatments had no significant effects on either the proportion F1 males or the longevity of F1 adults from Wolbachia negative whitefly parents (Fig. 2A, B).
Figure 2. The averaged longevity (M±SE) of the F1 adults and the proportion of male F1 progeny from whitefly adults.
A: male proportion, B:longevity. The exposed time to 1.0 mg/ml rifampicin is either 12, 24, 36 or 48 h. 0 is the untreated control. The different letters indicate a significant difference using Tukey HSD.
Effects of Wolbachia Inactivation on the Body Size of Whitefly and Head Capsule Size of the Parasitoid
Inactivation of Wolbachia significantly affected the development of both the whitefly nymph and the parasitoid. The length of whitefly 4th instar declined as exposure to rifampicin in the parent increased (F4,95 = 79.9, P<0.0001, Table 2,) as did the width (F4,95 = 128.9, P<0.0001, Table 2, Fig. S3). In particular, the length in the control was 0.81±0.01 mm which was 39.5% longer than that observed after 48 h exposure, 0.58±0.01 mm. Similarly, the width of the 4th instar in the control was 0.63±0.01 mm which was 76.7% wider than that observed after 48 h exposure to the antibiotic, 0.36±0.01 mm (Table 2). In the case of the parasitoid, the head capsule in the control was significantly wider (43.4%, T = 9.4, P<0.0001) and longer (21.7%, T = 9.5, P<0.0001) (Table 3, Fig. S4) than those pupae removed from nymphs that lacked a Wolbachia infection.
Table 2. The length and width (±SE) of whitefly 4th instars from parents exposed to 1.0 mg/ml rifampicin for 12, 24, 36 or 48 h; 0 is the untreated control.
| Duration of exposure to rifampicin (h) | ||||||
| Size(mm) | 0 | 12 | 24 | 36 | 48 | |
| Length | 0.81±0.01 a | 0.75±0.01 a | 0.71±0.01b | 0.64±0.0 b | 0.58±0.01 c | F4,95 = 79.9, p<0.0001 |
| Width | 0.63±0.01 a | 0.59±0.01 a | 0.56±0.01 a | 0.48±0.01 b | 0.36±0.01 c | F4,95 = 128.9, p<0.0001 |
Table 3. Comparison of the length and width of the head capsule (±SE) of Encarsia bimaculata pupa from the untreated control (0 h) with pupa after parental exposure for 48 h to 1.0 mg/ml rifampicin.
| Duration of exposure (h) | |||
| Size(mm) | 0 | 48 | |
| Length | 0.12±0.002 | 0.08±0.003 | t = 9.4, p<0.0001 |
| Width | 0.23±0.003 | 0.19±0.003 | t = 9.5, p<0.0001 |
Effects of Wolbachia Inactivation on the Development and Survival of E. bimaculata Juveniles
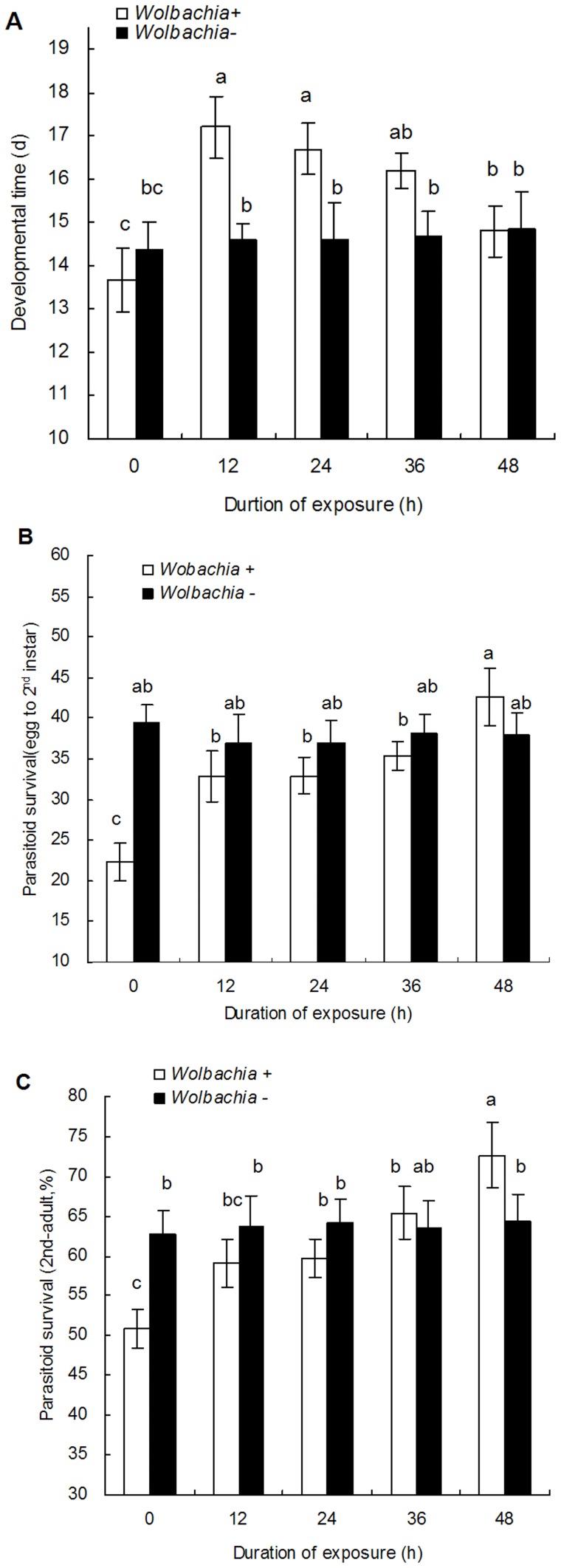
Wolbachia inactivation in B. tabaci also had significant effects on the development and survival of the parasitoid. The development time of E. bimaculata was significantly longer in the nymphs from parents which had been exposed to rifampicin (Fig. 3A, F 9,190 = 11.8, P<0.0001) for either 12, 24 or 36 h. Here, the mean developmental time of the untreated whiteflies was 13.7±0.7 days, while for the nymphs from parents exposed to rifampicin for either 12, 24 or 36 was 17.2±0.7, 16.7±0.6 and 16.2±0.4 days, respectively. However, in the nymphs in which Wolbachia had been completely inactivated, the development time of 14.8±0.6 days was not significantly different to that observed in the untreated nymphs.
Figure 3. The developmental time and survival (M±SE) of E. bimaculata in F1 B. tabaci nymphs from different rifampicin treatments.
A: developmental time, B: survival of egg to 2nd instar larvae, C: survival of 2nd instar larvae to adults. The exposed time to 1.0 mg/ml rifampicin is either 12, 24, 36 or 48 h. 0 is the untreated control. The different letters indicate a significant difference using Tukey HSD.
Dissection of the nymphs after oviposition showed that in each case at least one egg was deposited. In the case of the control, 19 nymphs had one egg present and the remaining nymph had two whereas in the nymphs where Wolbachia had been eliminated, all parasitized nymphs had a single egg present. There were no significantly differences in the number of eggs oviposited by E. bimaculata in nymphs positive for Wolbachia and those that were negative.
As for the survival, both the survivorship of E. bimaculata from egg to 2nd instar and 2nd instar to adult from parents exposed to rifampicin increased with the increasing duration of exposure to the antibiotic (Fig. 3B, F 9,190 = 23.9, P<0.0001; Fig. 3C, F 9,190 = 9.55, P<0.0001). The overall survivorship of E. bimaculata in the F1 whitefly progeny that were not infected with Wolbachia (48 h exposure), was significantly higher than that observed following shorter periods of exposure or no exposure at all. Most of the mortality was observed in the period from egg to 2nd instar. In contrast, the development time of E. bimaculata in F1 progeny from Wolbachia negative whiteflies was significantly shorter (14.35±0.67 to14.85±0.87 days) and survivorship significantly higher for both egg-2nd instar (36.97±2.74 to 39.49±2.09%) and 2nd instar to adult (62.68±2.99 to 64.38±3.44%) than those parasitoids that developed from Wolbachia infected hosts, but no significant differences were found between the different antibiotic exposures for each set of treatments.
Effects of Wolbachia Inactivation on the Longevity of E. bimaculata
The longevity of E. bimaculata females that emerged from the nymphs is shown in Fig. 4. There was no significant difference between the longevity of parasitoids from positive control nymphs, 5.9±0.4 days, and the longevities of parasitoids exposed to rifampicin for 12, 24 and 36 h, here longevities were 5.8±0.3, 5.7±0.3, 5.6±0.2 days, respectively. However, the longevity of parasitoids that emerged from nymphs where the parents had been exposed to rifampicin for 48 h was significantly shorter, 5.2±0.12 days (F9,190 = 14.2, P<0.0001). In this treatment Wolbachia was completely inactivated which indicates that the presence of Wolbachia significantly reduces the longevity of these parasitoids. Furthermore, parasitoids that develop in the progeny from Wolbachia negative females were significantly longer than that observed for parasitoids that developed in Wolbachia positive nymphs.
Figure 4. The longevity (M±SE) of E. bimaculata female adults developing in F1 B. tabaci nymphs.
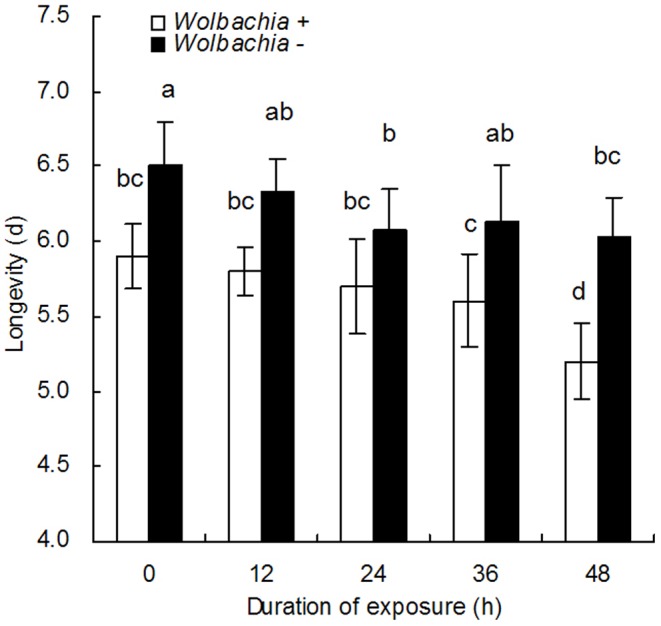
The whitefly nymphs from parents exposed to 1.0 mg/ml rifampicin for either 12, 24, 36 or 48 h. 0 is the untreated control. The different letters indicate a significant difference using Tukey HSD.
Discussion
Symbiotic associations between bacteria and insects are common in nature [48], but disentangling the roles of different endosymbionts is challenging when one host contains more than one endosymbiont and so studies that selectively remove an endosymbiont are one way to infer the roles played by different endosymbionts. While FISH is usually used to confirm the elimination of the endosymbiont [29], [49], [61], [62], we found that qRT-PCR can be used to determine the level of activity of Wolbachia. We used both and through this approach showed that exposure to 1.0 mg/ml rifampicin for 48 h completely inactivated Wolbachia in B. tabaci without reducing the activity of either the primary or non-Wolbachia secondary endosymbionts. We also show that the duration of exposure was critical as exposure times shorter than 48 h meant that some Wolbachia activity remained.
The biological contribution of bacteria endosymbionts to their hosts have been studied in detail [11], [12], [29], [43], [63]–[68], but the experimental evidence for the biological and ecological roles of Wolbachia in many of its insect hosts has remained largely unexplored [14], [46] with perhaps the exception of studies on host reproduction (reviewed in [69]–[72]). In addition to host reproduction, Fytrou et al. [46] observed that the presence of Wolbachia was associated with significant increases in the susceptibility of D. simulans to parasitisation by L. hererotoma. Here, Wolbachia infected D. simulans were less able to encapsulate parasitoid eggs, compared to those free of infection. Here, the removal of Wolbachia from the parasitoid was beneficial as its eggs suffered significantly lower encapsulation rates. In the case of the bedbug, Cimex lectularius, infections with Wolbachia increased both the rate of growth and egg viability. Furthermore, the negative effects associated with the removal of Wolbachia could be reversed by oral administration of B vitamins indicating that Wolbachia was also associated with the synthesis of essential non-dietary nutrients [14]. Wolbachia is also known to protect Drosophila melanogaster from infections of the Drosophila C viruses [19], [20].
In our study we showed that in MED, Wolbachia decreased the development time of the nymphs, increased the likelihood that nymphs completed their development, increased the life span of the adult whitefly and increased the proportion of progeny that developed into females. These indicate that the Wolbachia infection increases the fitness of the whitefly relative to its uninfected competitors. The faster development, higher survival and larger body size of the infected 4th instar relative to the uninfected nymphs suggests that Wolbachia may confer some nutritional and immune benefits on the host, however, the physiological roles of Wolbachia may vary in other B. tabaci species since its phenotypic effects can be affected by the genotype of its host. More work is needed on this and the overall ecological roles and interactions played by Wolbachia as well as the other secondary endosymbionts.
The reduction in nymph body size also appears to have had several consequences for the parasitoid. Firstly, there was a complex interaction between duration of exposure to the antibiotic and parasitoid development time. Initially, shorter parental exposures (12 to 36 h) to rifampicin lead to a marked increase in the time taken for the parasitoid to complete development in the F1 nymphs. The developmental time of the whitefly increased along with the increasing duration of exposure whereas the developmental time of the parasitoid decreased. These opposing responses maybe due to the nonlinear effects of Wolbachia inactivation in the two different trophic levels. This is not unexpected as cause-and-effect relationships in ecological food-webs are often nonlinear [73]–[76]. In the case of the whitefly, the removal of Wolbachia has a direct effect on the host whereas the removal in the whitefly is an indirect effect in regards to the parasitoid. Alternatively, the slow-growth, high-mortality hypothesis suggests that the shorter development time of E. bimaculata in smaller and therefore lower quality hosts, may reduce the risk of not completing development [77]. The acceleration of development on small hosts is a common phenomenon in parasitoids [78]. Furthermore, as in our case, Sequeira & Mackauer [79], [80] observed an initial slowing of the rate of development on intermediate sized hosts followed by a marked increase on the smallest hosts.
Secondly, the survival of parasitoid larvae increased as the duration of parental whitefly exposure to rifampicin increased, i.e. the percentage of parasitoids that completed their development in whitefly nymphs that lacked Wolbachia was considerably higher than that observed in infected nymphs. However, the longevity of the adult parasitoids emerging from nymphs lacking Wolbachia was considerably shorter than that observed for adults emerging from infected nymphs. This reduced longevity was also associated with the observation that the parasitoids developing in nymphs free of infection, were significantly smaller. The relationship between host size and size of the emerging parasitoid is also well known and it is not unexpected that a small host will lead to a small parasitoid adult with reduced fitness [81], [82]. Finally, while there was no difference in the number of eggs oviposited into infected and uninfected nymphs, parasitoid eggs inserted into nymphs that were uninfected by Wolbachia were more likely to complete their development than those inserted into infected nymphs suggesting that Wolbachia provided some protection against parasitisation.
In summary, our findings showed that Wolbachia is involved in many aspects of whitefly host biology including development duration, reproduction and defense. Our results lead us to speculate that Wolbachia may play a role in nutrient supplementation as its removal was associated with a decline in nymph body size and not surprisingly, smaller nymphs resulted in smaller, less fit parasitoids. We know from previous research [14] that Wolbachia can play this role and so more work is needed here to determine whether this is the case. Wolbachia also plays a protective role as eggs inserted into infected nymphs were less likely to complete development. Finally, Wolbachia also plays a role in the manipulation of whitefly sex ratio as a greater proportion of female progeny are produced by infected rather than uninfected females. Our observations therefore suggest that Wolbachia in B. tabaci is playing a role similar to that observed in other species.
Our results show that the elimination of Wolbachia in whitefly can result in delayed development, increased mortality, shortened lifespan, weakened ability to counter parasitism, and potentially, a slower rate of population increase due to the bias towards the production of male offspring. These suggest that novel technologies that target endosymbionts may provide new tools with which to manage these pests. However, much more research is needed to unravel the various and complex interactions that occur between the host, its endosymbionts and the environment before such tools are likely to provide effective control options.
Supporting Information
Fluorescence in situ hybridization of B. tabac i using a Wolbachia specific probe (red). A: FISH of Wolbachia positive B. tabaci adult (a, b), its egg (c, d) and 3rd nymph (e, f). The left panels are under natural light, the right panels under fluorescence. Wolbachia was found in the main body of the adult, egg and nymph. B: FISH of Wolbachia positive B. tabaci adult (a, b), its egg (c, d) and nymph (e, f) after 48 exposure to 1.0 mg/ml rifampicin. The left panels are under natural light, the right panels are under fluorescence. The absence of red fluorescence showed that Wolbachia can be completely eliminated in the B. tabaci adult, egg and third instar.
(TIF)
The relative expression levels of target genes used to measure activity in each of the different endosymbionts in the qRT-PCR. 0 is the untreated control and 12, 24, 36 and 48 h indicate the duration of exposure to 1.0 mg/ml rifampicin. The lower the level of expression the greater the level of inactivation. A–G were P. aleyrodidarum, Arsenophonus, Cardinium, Fritschea, Hamiltonella, Rickettsia and Wolbachia, respectively.
(DOC)
The body size of the Bemisia tabaci 4th instar. 0 is the untreated control and 12, 24, 36 and 48 h indicate the duration of exposure to 1.0 mg/ml rifampicin. The body size was first photographing using a microscope (Discovery V20 Zeiss) connected to a camera (AxioCam, HRc and Coolsnap-Procf & CRI Micro*Color) and then measured using Axio Vision Rel 4.8 software.
(TIF)
Head capsule size of Encarsia bimaculata pupa. The wasps developed from either whitefly nymphs infected with Wolbachia or those that were uninfected as a result of 48 h exposure to 1.0 mg/ml rifampicin. The head size was first photographing using a microscope (Discovery V20 Zeiss) connected to a camera (AxioCam, HRc and Coolsnap-Procf & CRI Micro*Color) and then measured using Axio Vision Rel 4.8 software.
(TIF)
The primers and PCR programs used for the detection of the various endosymbionts. Protocol-1 refers to standard PCR detection; Protocol-2 refers to the measurement of target gene expression using qRT-PCR.
(DOC)
Fluorescence in situ hybridization of Bemisia tabaci samples.
(DOC)
Acknowledgments
The authors thank the anonymous reviewers for their constructive comments on the previous version of this manuscript; thank XM Wang (SCAU) for his assistance in photograph.
Funding Statement
This research was funded by the National Basic Research Program of China (2009CB119203), the Program for New Century Excellent Talents in University (NCET-0917–2011) and the China National Natural Science Foundation (31071732). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Clark EL, Karley AJ, Hubbard SF (2010) Insect endosymbionts: manipulators of insect herbivore trophic interactions? Protoplasma 244: 25–51. [DOI] [PubMed] [Google Scholar]
- 2. Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36: 533–543. [Google Scholar]
- 3. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476(7361): 454–U107. [DOI] [PubMed] [Google Scholar]
- 4. Moran NA (2001) The coevolution of bacterial endosymbionts and phloem-feeding insects. Ann Missouri Bot Garden 88: 35–44. [Google Scholar]
- 5. Moran NA, Telang A (1998) Bacteriocyte-associated symbionts of insects. Bioscience 48: 295–304. [Google Scholar]
- 6. Houk EJ, Griffiths GW (1980) Intracellular symbiotes of the Homoptera. Ann Rev Entomol 25: 161–187. [Google Scholar]
- 7. Baumann P (2005) Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Ann Rev Microbiol 59: 155–189. [DOI] [PubMed] [Google Scholar]
- 8. Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23: 38–47. [Google Scholar]
- 9.Buchner P (1965) Endosymbiosis of animals with plant microorganisms. John Wiley and Sons Interscience, New York, N.Y. 332–338.
- 10. Baumann P, Moran NA, Baumann L (2006) Bacteriocyte-associated endosymbionts of insects. Prokaryotes. 1: 403–438. [Google Scholar]
- 11. Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Ann Rev Genet 42: 165–190. [DOI] [PubMed] [Google Scholar]
- 12. Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751. [DOI] [PubMed] [Google Scholar]
- 13. Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia? – a statistical analysis of current data. FEMS Microbiol Lett 2: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA 107: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaiser W, Huguet E, Casas J, Commin C, Giron D (2010) Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc R Soc Lond [Biol] 277: 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guruprasad NM, Mouton L, Puttaraju HP (2011) Effect of Wolbachia infection and temperature variations on the fecundity of the uzi fly Exorista sorbillans (Diptera: Tachinidae). Symbiosis 54: 151–158. [Google Scholar]
- 17. Dobson SL, Marsland EJ, Rattanadechakul W (2002) Mutualistic Wolbachia infection in Aedes albopictus: Accelerating cytoplasmic drive. Genetics 160: 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brownlie JC, Johnson KN (2009) Symbiont-mediated protection in insect hosts. Trends Microbiol 17: 348–354. [DOI] [PubMed] [Google Scholar]
- 19. Hedges LM, Brownlie JC, O’Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322: 702–702. [DOI] [PubMed] [Google Scholar]
- 20. Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biol 6: 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glaser RL, Meola MA (2010) The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile Virus infection. PLoS ONE 5(8): e11977 doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 23. Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg M, et al. (2001) Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA 98: 6247–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dinsdale A, Cook L, Riginos C, Buckley YM, De Barro PJ (2010) Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann Entomol Soc Am 103: 196–208. [Google Scholar]
- 25. De Barro PJ, Liu SS, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Ann Rev Entomol 56: 1–19. [DOI] [PubMed] [Google Scholar]
- 26. Hu J, De Barro PJ, Zhao H, Wang J, Nardi F, et al. (2011) An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS ONE 6(1): e16061 doi: 10.1371/journal.pone.0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmed MZ, Ren SX, Xue X, Li XX, Jin GH, et al. (2010a) Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr Microbiol 61: 322–328. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed MZ, Ren SX, Mandour NS, Greeff JM, Qiu B-L (2010b) Prevalence of Wolbachia supergroups A and B in Bemisia tabaci and some of its natural enemies. J Econ Entomol 103: 1848–1059. [DOI] [PubMed] [Google Scholar]
- 29. Brumin M, Kontsedalov S, Ghanim M (2011) Rickettsia influences thermo-tolerance in the whitefly Bemisia tabaci B biotype. Insect Sci 18: 57–66. [Google Scholar]
- 30. Chu D, Gao CS, De Barro P, Zhang YJ, Wan FH, et al. (2011) Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: Comparison of secondary symbionts from biotypes B and Q in China. Bull Entomol Res 101: 477–486. [DOI] [PubMed] [Google Scholar]
- 31. Chou I (1949) List of whitefly species in China. J Chin Entomol 3: 1–18. [Google Scholar]
- 32. Qiu B-L, Mandour NS, Xu CX, Ren SX (2008) Evaluation of entomopathogenic nematode, Steinernema feltiae as a biological control agent against the whitefly Bemisia tabaci . Int J Pest Manag 54: 247–253. [Google Scholar]
- 33. Zchori-Fein E, Brown JK (2002) Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann Entomol Soc Am 95: 711–718. [Google Scholar]
- 34. Thao ML, Baumann P (2004) Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl Environ Microbiol 70: 3401–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Everett KDE, Thao ML, Horn M, Dyszynski GE, Baumann P (2005) Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int J Syst Evol Microbiol 55: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 36. Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, et al. (2007) Biotype-dependent secondary symbionts communities in sympatric populations of Bemisia tabaci. . Bull Entomol Res 97: 407–413. [DOI] [PubMed] [Google Scholar]
- 37. Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, et al. (2008) Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB Journal 22: 2591–2599. [DOI] [PubMed] [Google Scholar]
- 38. Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M (2010) Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol 10: no.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, et al. (2010) Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol 19: 4365–4378. [DOI] [PubMed] [Google Scholar]
- 40. Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, et al. (2010) The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol 84: 9310–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kontsedalov S, Zchori-Fein E, Chiel E, Gottlieb Y, Inbar M, et al. (2008) The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag Sci 64: 789–792. [DOI] [PubMed] [Google Scholar]
- 42. Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, et al. (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332 (6026): 254–256. [DOI] [PubMed] [Google Scholar]
- 43. Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA 100: 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oliver KM, Moran NA, Hunter MS (2005) Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102: 12795–12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie J, Vilchez I, Mateos M (2010) Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma . PLoS ONE 5(8): e12149 doi:10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF (2006) Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc R Soc Lond [Biol] 273: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O’Neill SL, Karr TL (1990) Bidirectional incompatibility between conspecific populations of Drosophila simulans . Nature 348: 178–180. [DOI] [PubMed] [Google Scholar]
- 48. Chiel E, Zchori-Fein E, Inbar M, Gottlieb Y, Adachi-Hagimori T, et al. (2009) Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS ONE 4(3): e4767 doi:10.1371/journal.pone.0004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuriwada T, Hosokawa T, Kumano N, Shiromoto K, Haraguchi D, et al. (2010) Biological role of Nardonella endosymbiont in its weevil host. PloS ONE 5(10): e13101 doi:10.1371/journal.pone.0013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruan YM, Xu J, Liu SS (2006) Effects of antibiotics on fitness of the B biotype and a non-B biotype of the whitefly Bemisia tabaci . Entomol Exp Appl 121: 159–166. [Google Scholar]
- 51. Gerling D, Alomar S, Arno J (2001) Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot 20: 779–799. [Google Scholar]
- 52. Ren SX, Wang ZZ, Qiu BL, Xiao Y (2001) The pest status of Bemisia tabaci in China and non-chemical control strategies. Entomol Sinica 18: 279–288. [Google Scholar]
- 53. Qiu BL, Ren SX, Lin L, Wang XM (2004) Species and dynamics of aphelinid parasitoids of Bemisia tabaci in Guangdong area. Entomol Knowl 41(4): 18–22. [Google Scholar]
- 54. Qiu BL, Chen YP, Liu L, Peng WL, Li XX, et al. (2009) Identification of three major Bemisia tabaci biotypes in China based on morphological and DNA polymorphisms. Prog Nat Sci 19: 713–718. [Google Scholar]
- 55. Antony B, Palaniswami MS, Kirk AA, Henneberry JT (2004) Development of Encarsia bimaculata (Heraty & Polaszek) (Hymenoptera: Aphelinidae) in Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) nymphs. Biol Control 30: 546–555. [Google Scholar]
- 56.Weeks AR, Breeuwer JAJ (2003) A new bacterium from the cytophaga-flavobacterium- bacteroides phylum that causes sex ratio distortion. In: Bourtzisn K, Miller T, ed. Insect Symbiosis II. Florida CRC Press. pp165–176.
- 57. Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, et al. (2006) Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 72: 3646–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li ZX, Lin HZ, Guo XP (2007) Prevalence of Wolbachia infection in Bemisia tabaci . Cur Microbiol 54: 467–471. [DOI] [PubMed] [Google Scholar]
- 59. Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T (2005) Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropis, effect on host fitness, and interaction with the essential symbiont Buchnera . Appl Environ Microbiol 71(7): 4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.SAS Institute (2003) SAS/STAT Version 8.2. SAS Institute, Cary, NC.
- 61. Mahadav A, Gerling D, Gottlieb Y, Czosnek H, Ganim M (2008) Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune response and symbiotic bacteria proliferation in the whitefly Bemisia tabaci . BMC Genomics 9: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stahlhut JK (2010) The endosymbiont community as taxonomic character: a novel approach to resolving the Bemisia tabaci complex. Mol Ecol 19: 4102–4104. [DOI] [PubMed] [Google Scholar]
- 63. Nogge G (1981) Significance of symbionts for the maintenance of an optimal nutritional state for successful reproduction in haematophagous arthropods. Parasitology 82: 101–104. [Google Scholar]
- 64. Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera . Ann Rev Entomol 43: 17–37. [DOI] [PubMed] [Google Scholar]
- 65. Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27: 189–195. [Google Scholar]
- 66. Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303: 1989–1989. [DOI] [PubMed] [Google Scholar]
- 67. Feldhaar H, Straka J, Krischke M, Berthold K, Stoll S, et al. (2007) Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia . BMC Biol 5: 48 doi:10.1186/1741-7007-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bourtzis K, Miller T (2008) Insect symbiosis. volume 3. Boca Raton: CRC Press. 384.
- 69. Werren JH (1997) Biology of Wolbachia . Ann Rev Entomol 42: 587–609. [DOI] [PubMed] [Google Scholar]
- 70. Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Ann Rev Microbiol 53: 71–102. [DOI] [PubMed] [Google Scholar]
- 71. Sasaki T, Massaki N, Kubo T (2005) Wolbachia variant that induces two distinct reproductive phenotypes in different hosts. Heredity 95: 389–393. [DOI] [PubMed] [Google Scholar]
- 72. Landmann F, Orsi GA, Loppin B, Sullivan W (2009) Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathogens 5(3): e1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huxel GR, McCann K (1998) Food web stability: The influence of trophic flows across habitats. Amer Nat 152: 460–469. [DOI] [PubMed] [Google Scholar]
- 74. Schmitz OJ, Sokol-Hessner L (2002) Linearity in the aggregate effects of multiple predators in a food web. Ecol Lett 5: 168–172. [Google Scholar]
- 75. Huxel GR (2007) Antagonistic and synergistic interactions among predators. Bull Math Biol 69: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 76. Qiu BL, Harvey JA, Raaijmakers CE, Vet LEM, van Dam NM (2009) Nonlinear effects of plant root and shoot jasmonic acid application on the performance of Pieris brassicae and its parasitoid Cotesia glomerata . Funct Ecol 23: 496–505. [Google Scholar]
- 77. Benrey B, Denno RF (1997) The slow-growth-high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78: 987–999. [Google Scholar]
- 78. Kouame KL, Mackauer M (1991) Influence of aphid size, age and behaviour on host choice by the parasitoid wasp Ephedrus californicus: a test of host-size models. Oecologia 88: 197–203. [DOI] [PubMed] [Google Scholar]
- 79. Sequeira R, Mackauer M (1992a) Covariance of adult size and development time in the parasitoid wasp Aphidius ervi in relation to the size of its host, Acyrthosiphon pisum . Evol Ecol 6: 34–44. [Google Scholar]
- 80. Sequeira R, Mackauer M (1992b) Variation in selected life-history parameters of the parasitoid wasp, Aphidius ervi: influence of host developmental stage. Entomol Exp Appl 71: 15–22. [Google Scholar]
- 81. Mackauer M (1986) Growth and developmental interactions in some aphids and their hymenopterous parasites. J Insect Physiol 32: 275–280. [Google Scholar]
- 82. Gunasena GH, Vinson SB, Williams HJ (1989) Interrelationships between growth of Heliothis virescens (Lepidoptera: Noctuidae) and that of its parasitoid, Campoletis sonorensis (Hymenoptera: Ichneumonidae). Ann Entomol Soc Am 82: 187–191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence in situ hybridization of B. tabac i using a Wolbachia specific probe (red). A: FISH of Wolbachia positive B. tabaci adult (a, b), its egg (c, d) and 3rd nymph (e, f). The left panels are under natural light, the right panels under fluorescence. Wolbachia was found in the main body of the adult, egg and nymph. B: FISH of Wolbachia positive B. tabaci adult (a, b), its egg (c, d) and nymph (e, f) after 48 exposure to 1.0 mg/ml rifampicin. The left panels are under natural light, the right panels are under fluorescence. The absence of red fluorescence showed that Wolbachia can be completely eliminated in the B. tabaci adult, egg and third instar.
(TIF)
The relative expression levels of target genes used to measure activity in each of the different endosymbionts in the qRT-PCR. 0 is the untreated control and 12, 24, 36 and 48 h indicate the duration of exposure to 1.0 mg/ml rifampicin. The lower the level of expression the greater the level of inactivation. A–G were P. aleyrodidarum, Arsenophonus, Cardinium, Fritschea, Hamiltonella, Rickettsia and Wolbachia, respectively.
(DOC)
The body size of the Bemisia tabaci 4th instar. 0 is the untreated control and 12, 24, 36 and 48 h indicate the duration of exposure to 1.0 mg/ml rifampicin. The body size was first photographing using a microscope (Discovery V20 Zeiss) connected to a camera (AxioCam, HRc and Coolsnap-Procf & CRI Micro*Color) and then measured using Axio Vision Rel 4.8 software.
(TIF)
Head capsule size of Encarsia bimaculata pupa. The wasps developed from either whitefly nymphs infected with Wolbachia or those that were uninfected as a result of 48 h exposure to 1.0 mg/ml rifampicin. The head size was first photographing using a microscope (Discovery V20 Zeiss) connected to a camera (AxioCam, HRc and Coolsnap-Procf & CRI Micro*Color) and then measured using Axio Vision Rel 4.8 software.
(TIF)
The primers and PCR programs used for the detection of the various endosymbionts. Protocol-1 refers to standard PCR detection; Protocol-2 refers to the measurement of target gene expression using qRT-PCR.
(DOC)
Fluorescence in situ hybridization of Bemisia tabaci samples.
(DOC)