Abstract
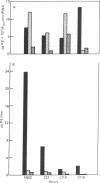
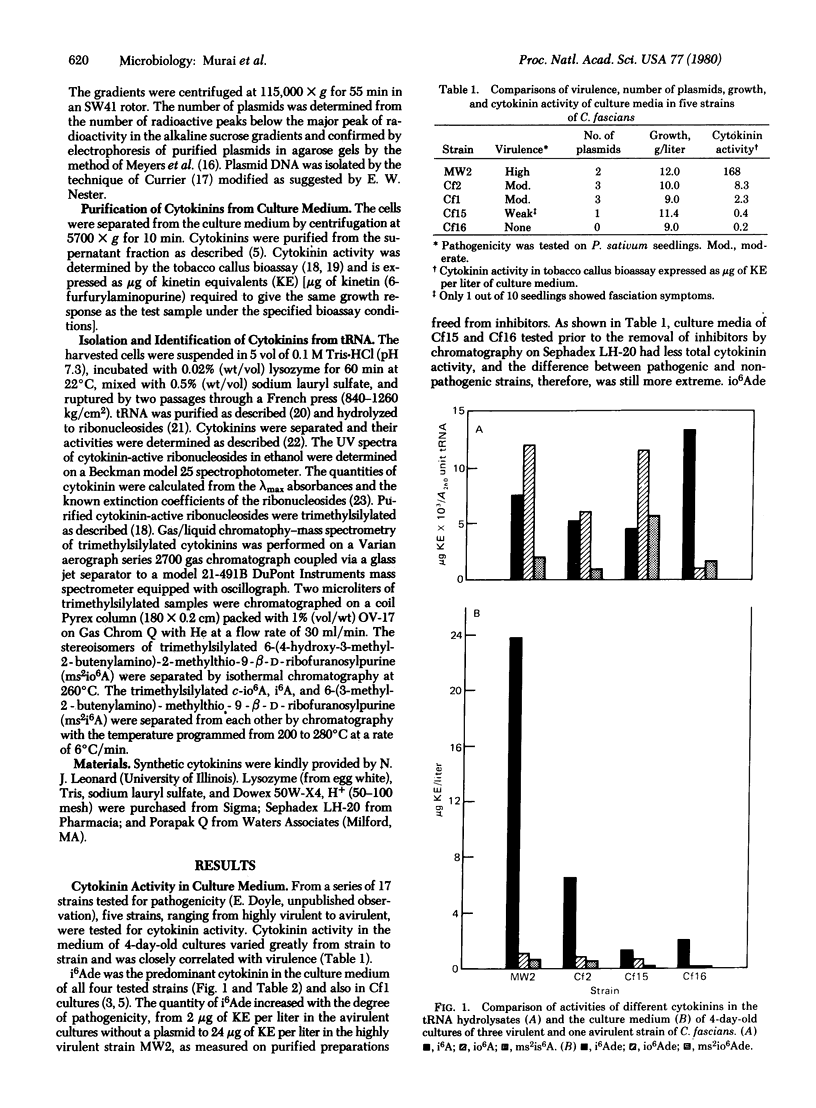
Cytokinin activity in the culture medium of four pathogenic strains of Corynebacterium fascians varied from 168 to 0.4 μg of kinetin (6-furfurylaminopurine) equivalents per liter, as compared to 0.2 in an avirulent control. N6-Isopentenyladenine was the predominant cytokinin in the medium of all five strains, and its increased production was correlated with the degree of pathogenicity; however, the virulent strains also produced 8-13 times more cis-zeatin [6-(4-hydroxy-3-methyl-cis-2-butenylamino)purine] than the avirulent strain. The three most virulent strains (with the higher cytokinin contents in the medium) contained a large (Mr ≈ 108) plasmid. The barely virulent strain contained a smaller plasmid. No plasmid was detected in the avirulent control. The total cytokinin content (mole%) and biological activity (μg of kinetin equivalent per mg of tRNA) in tRNA were about the same in all three virulent strains and in the avirulent control. Five cytokinins were isolated from each strain. Four were rigorously characterized as 6-(4-hydroxy-3-methyl-cis-2-butenylamino)-9-β-D-ribofuranosylpurin e, 6-(3-methyl-2-butenylamino)-9-β-D-ribofuranosylpurine, and their 2-methylthio derivatives. The fifth cytokinin was tentatively identified as 6-(4-hydroxy-3-methyl-trans-2-butenylamino)-9-β-D-ribofuranosylpur ine. Ribosyl-cis-zeatin was 3-fold higher and N6-isopentenyladenosine was correspondingly lower in the plasmid-containing strains than in the plasmidless control. Because the entire syndrome of fasciation caused by infection can be induced with synthetic cytokinins, the disease would appear to be caused by plasmid-induced high rates of cytokinin production by the bacteria.
Keywords: tRNA, 6-(3-methyl-2-butenylamino)purine, cis-zeatin
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Burrows W. J., Evans P. K., Skoog F. Isolation of cytokinins from tRNA. Biochem Biophys Res Commun. 1969 Oct 22;37(3):451–456. doi: 10.1016/0006-291x(69)90936-x. [DOI] [PubMed] [Google Scholar]
- Armstrong D. J., Scarbrough E., Skoog F. Cytokinins in Corynebacterium fascians Cultures: Isolation and Identification of 6-(4-Hydroxy-3-methyl-cis-2-butenylamino)-2-methylthiopurine. Plant Physiol. 1976 Dec;58(6):749–752. doi: 10.1104/pp.58.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. J., Skoog F., Kirkegaard L. H., Hampel A. E., Bock R. M., Gillam I., Tener G. M. Cytokinins: distribution in species of yeast transfer RNA. Proc Natl Acad Sci U S A. 1969 Jun;63(2):504–511. doi: 10.1073/pnas.63.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. W., Morris R. O., Zaerr J. B. Occurrence of trans-ribosylzeatin in Agrobacterium tumefaciens tRNA. Nature. 1976 Jul 8;262(5564):153–154. doi: 10.1038/262153a0. [DOI] [PubMed] [Google Scholar]
- Cherayil J. D., Lipsett M. N. Zeatin ribonucleosides in the transfer ribonucleic acid of Rhizobium leguminosarum, Agrobacterium tumefaciens, Corynebacterium fascians, and Erwinia amylovora. J Bacteriol. 1977 Sep;131(3):741–744. doi: 10.1128/jb.131.3.741-744.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Einset J. W., Skoog F. K. Isolation and identification of ribosyl-cis-zeatin from transfer RNA of Corynebacterium fascians. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1117–1121. doi: 10.1016/0006-291x(77)91121-4. [DOI] [PubMed] [Google Scholar]
- HALL R. H. ISOLATION OF N6-(AMINOACYL)ADENOSINE FROM YEAST RIBONUCLEIC ACID. Biochemistry. 1964 Jun;3:769–773. doi: 10.1021/bi00894a006. [DOI] [PubMed] [Google Scholar]
- Helgeson J. P., Leonard N. J. Cytokinins: identification of compounds isolated from Corynebacterium fascians. Proc Natl Acad Sci U S A. 1966 Jul;56(1):60–63. doi: 10.1073/pnas.56.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiss-Chapman R. W., Morris R. O. Trans-zeatin in culture filtrates of Agrobacterium tumefaciens. Biochem Biophys Res Commun. 1976 May 23;76(2):453–459. doi: 10.1016/0006-291x(77)90746-x. [DOI] [PubMed] [Google Scholar]
- Kline L. K., Fittler F., Hall R. H. N6-(delta-2-isopentenyl) adenosine. Biosynthesis in transfer ribonucleic acid in vitro. Biochemistry. 1969 Nov;8(11):4361–4371. doi: 10.1021/bi00839a021. [DOI] [PubMed] [Google Scholar]
- Klämbt D., Thies G., Skoog F. Isolation of cytokinins from Corynebacterium fascians. Proc Natl Acad Sci U S A. 1966 Jul;56(1):52–59. doi: 10.1073/pnas.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S., Armstrong D. J., Skoog F. Cytokinins in tRNA of Corynebacterium fascians. Plant Physiol. 1968 Mar;43(3):451–453. doi: 10.1104/pp.43.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Zeatin and zeatin riboside from a mycorrhizal fungus. Science. 1967 Sep 1;157(3792):1055–1057. doi: 10.1126/science.157.3792.1055. [DOI] [PubMed] [Google Scholar]
- Murai N., Taller B. J., Armstrong D. J., Skoog F. Kinetin incorporated into tobacco callus ribosomal RNA and transfer RNA preparations. Plant Physiol. 1977 Aug;60(2):197–202. doi: 10.1104/pp.60.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarbrough E., Armstrong D. J., Skoog F., Frihart C. R., Leonard N. J. Isolation of cis-Zeatin from Corynebacterium fascians Cultures. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3825–3829. doi: 10.1073/pnas.70.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Taya Y., Tanaka Y., Nishimura S. 5'-AMP is a direct precursor of cytokinin in Dictyostelium discoideum. Nature. 1978 Feb 9;271(5645):545–547. doi: 10.1038/271545a0. [DOI] [PubMed] [Google Scholar]