Abstract
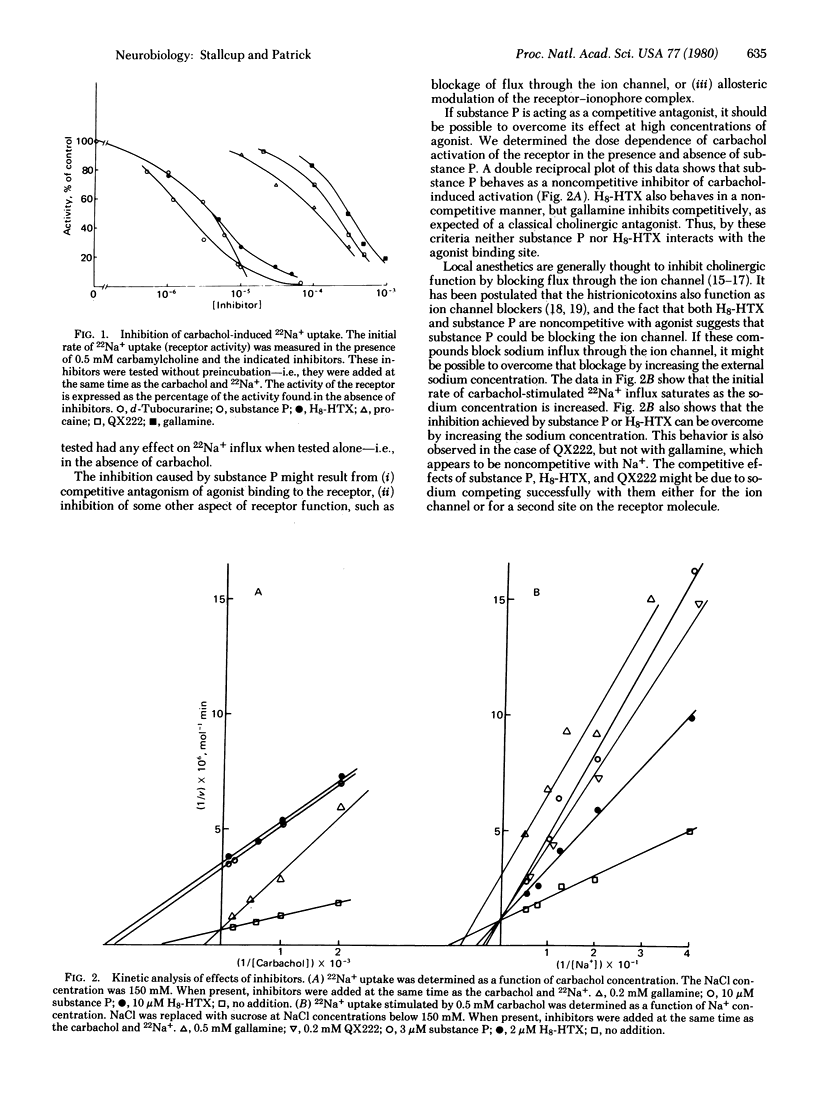
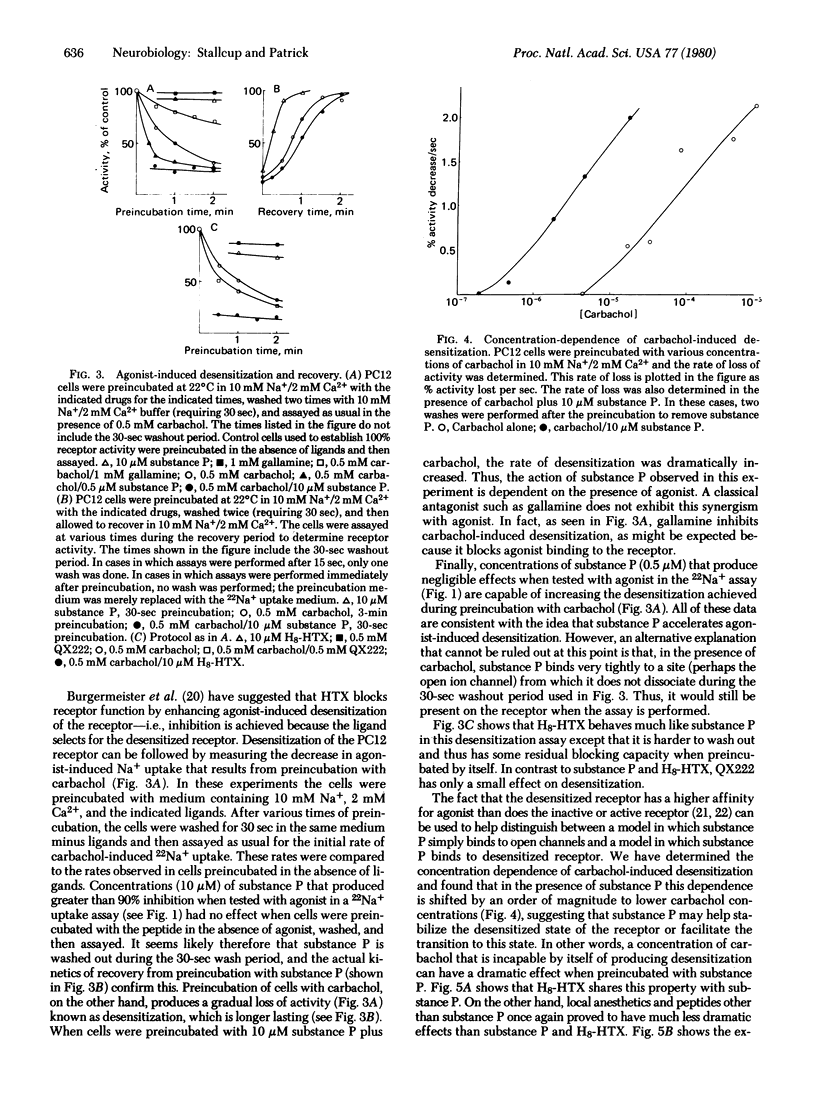
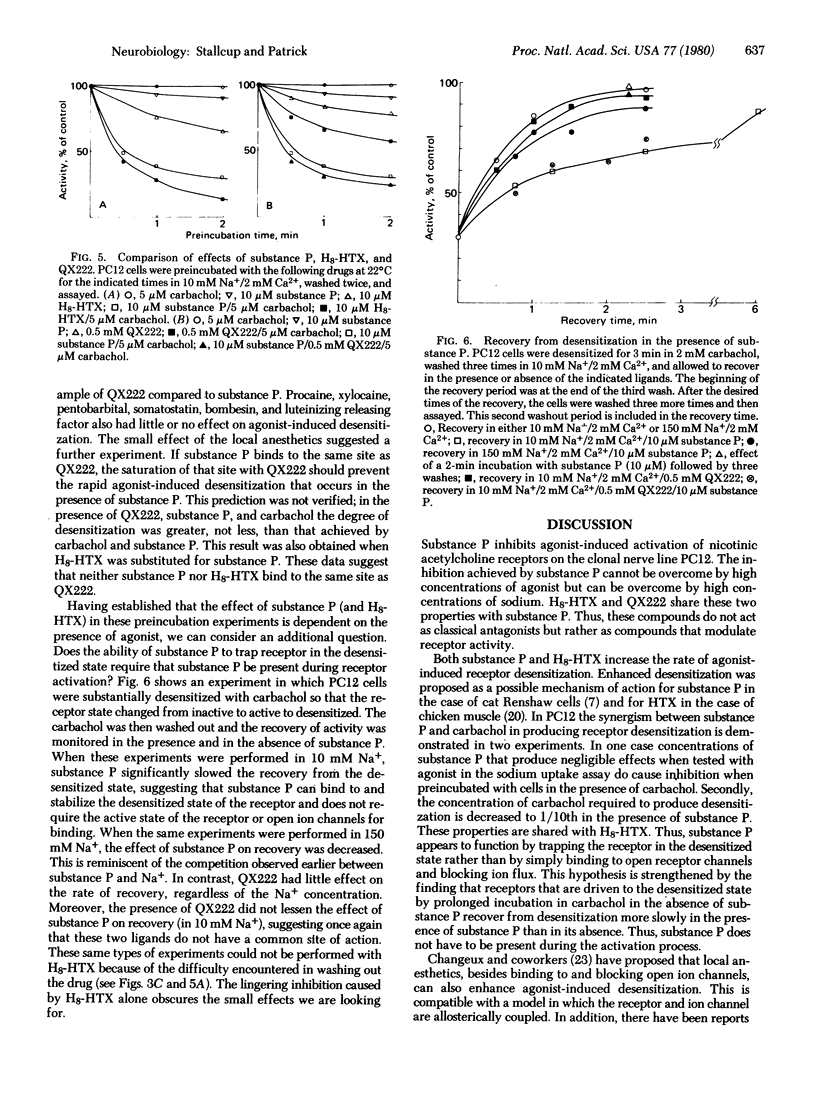
Substance P inhibits carbamylcholine-induced 22Na+ uptake in the clonal cell line PC12. This inhibition is noncompetitive with agonist but competitive with Na+. Octahydrohistrionicotoxin (H8-HTX) also exhibits this same pattern of inhibition. Moreover, both substance P and H8-HTX are very effective in enhancing agonist-induced receptor desensitization. Local anesthetics, such as QX222, also cause inhibition that is competitive with Na+, but they have only marginal effects on desensitization. Because substance P and H8-HTX cannot by themselves cause desensitization, their action is dependent on and synergistic with the action of agonist. Furthermore, substance P and H8-HTX do not appear to compete for the same site as QX222, which is thought to bind to the ion channel. Finally, substance P can stabilize the desensitized state of the receptor even when added subsequent to the actual desensitization and removal of agonist. Thus, substance P does not require open ion channels for binding and may modulate the activity of the receptor-ionophore complex by binding to a distinct regulatory site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Barker J. L., Neale J. H., Smith T. G., Jr, Macdonald R. L. Opiate peptide modulation of amino acid responses suggests novel form of neuronal communication. Science. 1978 Mar 31;199(4336):1451–1453. doi: 10.1126/science.204016. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W. Substance P and Renshaw cells: a new concept of inhibitory synaptic interactions. J Physiol. 1977 Oct;272(1):105–119. doi: 10.1113/jphysiol.1977.sp012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister W., Catterall W. A., Witkop B. Histrionicotoxin enhances agonist-induced desensitization of acetylcholine receptor. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5754–5758. doi: 10.1073/pnas.74.12.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Dray A. Effects of enkephalin and morphine on Renshaw cells in feline spinal cord. Nature. 1976 Aug 12;262(5569):603–604. doi: 10.1038/262603a0. [DOI] [PubMed] [Google Scholar]
- Davies J., Dray A. Substance P and opiate receptors. Nature. 1977 Jul 28;268(5618):351–352. doi: 10.1038/268351a0. [DOI] [PubMed] [Google Scholar]
- Eldefrawi A. T., Eldefrawi M. E., Albuquerque E. X., Oliveira A. C., Mansour N., Adler M., Daly J. W., Brown G. B., Burgermeister W., Witkop B. Perhydrohistrionicotoxin: a potential ligand for the ion conductance modulator of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1977 May;74(5):2172–2176. doi: 10.1073/pnas.74.5.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. L., Krnjevíc K., Morris M. E. Substance P and spinal neurones. Can J Physiol Pharmacol. 1975 Jun;53(3):423–432. doi: 10.1139/y75-061. [DOI] [PubMed] [Google Scholar]
- Kato G., Changeux J. P. Studies on the effect of histrionicotoxin on the monocellular electroplax from Electrophorus electricus and on the binding of (3H)acetylcholine to membrane fragments from Torpedo marmorata. Mol Pharmacol. 1976 Jan;12(1):92–100. [PubMed] [Google Scholar]
- Konishi S., Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974 Dec 20;252(5485):734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lekić D. Substance P selectively blocks excitation of Renshaw cell by acetylcholine. Can J Physiol Pharmacol. 1977 Aug;55(4):958–961. doi: 10.1139/y77-129. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. An excitatory action of substance P on cuneate neurones. Can J Physiol Pharmacol. 1974 Jun;52(3):736–744. doi: 10.1139/y74-094. [DOI] [PubMed] [Google Scholar]
- Leeman S. E., Mroz E. A. Substance P. Life Sci. 1974 Dec 15;15(12):2033–2044. doi: 10.1016/0024-3205(74)90020-4. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Kozousek V., Mizobe F., Dean D. M. Substance P inhibits nicotinic activation of chromaffin cells. Nature. 1979 Mar 15;278(5701):256–257. doi: 10.1038/278256a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., Stallcup B. alpha-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve line. J Biol Chem. 1977 Dec 10;252(23):8629–8633. [PubMed] [Google Scholar]
- Patrick J., Stallcup W. B. Immunological distinction between acetylcholine receptor and the alpha-bungarotoxin-binding component on sympathetic neurons. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4689–4692. doi: 10.1073/pnas.74.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Limacher J. J. Substance P excitation of cerebral cortical Betz cells. Brain Res. 1974 Mar 29;69(1):158–163. doi: 10.1016/0006-8993(74)90383-7. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. A new kind of drug antagonism: evidence that agonists cause a molecular change in acetylcholine receptors. Mol Pharmacol. 1969 Jul;5(4):394–411. [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B. Sodium and calcium fluxes in a clonal nerve cell line. J Physiol. 1979 Jan;286:525–540. doi: 10.1113/jphysiol.1979.sp012635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacker A., Highstein S. M. Pre- and postsynaptic action of substance P at the Mauther fiber-giant fiber synapse in the hatchetfish. Brain Res. 1976 Sep 10;114(1):128–133. doi: 10.1016/0006-8993(76)91013-1. [DOI] [PubMed] [Google Scholar]
- Steinbach A. B. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J Gen Physiol. 1968 Jul;52(1):144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. D., Barker J. L. Substance P: evidence for diverse roles in neuronal function from cultured mouse spinal neurons. Science. 1979 Sep 28;205(4413):1409–1412. doi: 10.1126/science.224464. [DOI] [PubMed] [Google Scholar]
- Weber M., David-Pfeuty T., Changeux J. P. Regulation of binding properties of the nicotinic receptor protein by cholinergic ligands in membrane fragments from Torpedo marmorata. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3443–3447. doi: 10.1073/pnas.72.9.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G., Georgia B., Wee V. T., Chignell C. F., Taylor P. Ligand interactions with cholinergic receptor-enriched membranes from Torpedo: influence of agonist exposure on receptor properties. Mol Pharmacol. 1976 Nov;12(6):1091–1105. [PubMed] [Google Scholar]



