Abstract
The South American tomato pinworm (Tuta absoluta) has recently invaded Europe and is rapidly spreading in the Afro-Eurasian continent where it is becoming a major pest on tomato crops. Laboratory tests were undertaken to evaluate the potential of 29 European strains of Trichogramma parasitoids to control T. absoluta. In addition to the host itself, the host plant (tomato) was used during the laboratory tests in order to increase the chance of selecting the best parasitoid strains. Trichogramma females were placed with T. absoluta eggs on a tomato leaflet in tubes. We compared the parasitism of T. absoluta by the various Trichogramma species tested to the Trichogramma species currently commercially available for the pest control in Europe, i.e. Trichogramma achaeae. Thereafter, the more promising strains were tested on a larger scale, in mesocosm (i.e. cages in greenhouses) and in greenhouse compartments to evaluate efficiency of laboratory selected strains under cropping conditions. The most efficient strain from the laboratory screening trials did not perform as efficiently under the greenhouse conditions. We discuss differences in parasitism levels among species and strains and among the different scales tested in the experiments, as well as implications of these results for further screening for biocontrol agents.
Introduction
Some of the most serious arthropod pests in agricultural landscapes are invasive species [1]–[3]. In the USA, for example, introduced arthropod pests have been estimated to cause losses of around $20 billion each year [4]. For most of the invasive pests, chemical pesticides are the dominant pest management method, contributing to additional production costs and negative side effects on non-target organisms and human health [4]–[7]. The tomato leafminer, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae), is one of the most devastating tomato pests in South America [8]. This pest has recently invaded European and Mediterranean basin countries and in few years has become a major pest in both greenhouse and outdoor tomato crops [9], [10]. Biological control is a key component of integrated pest management programs in tomato greenhouses in Europe, but its sustainability is threatened due to the extensive use of pesticides to control T. absoluta and possible well known associated side effects of these products on biocontrol agents [6], [11], [12].
The possible use of parasitic wasps of the Trichogramma genus (Hymenoptera: Trichogrammatidae) as biological control agents of T. absoluta is currently considered in Europe because of the natural parasitism of T. absoluta by various Trichogramma species reported in South America and in Europe (e.g. in Italy) [13], [14] and the effective use of Trichogramma pretiosum Riley for inundative releases against T. absoluta in South American tomato crops [15]–[18]. Trichogramma parasitoids have already been successfully used in biological control of various lepidopteran agricultural pests [2], [16], [19]. They are easy to rear [20] and to release in open fields or protected crops. Every year, more than 32 million hectares are treated worldwide using Trichogramma spp. [21], [22], mostly through seasonal inundative releases [19], [23]. The success of the Trichogramma releases depends on the knowledge of the biological characteristics of the parasitoid species or strains used, and on their interactions with a specific host [24]–[26]. Selecting the Trichogramma species with the highest affinity for the target pest and for characteristics of the agro-ecosystem is crucial to the success of the biological control program [19], [27]. Trichogramma achaeae Nagaraja and Nagarkatti, is currently available in some European and North African countries for inundative biological control of T. absoluta [28]. T. achaeae was shown to be efficient in pilot experiments and lowered T. absoluta infestation levels in experimental and commercial tomato greenhouses [9], [29]. However, the efficiency of this parasitoid depends on the use of high quantities of parasitoids per release (as indicated by biocontrol companies; e.g. 250,000–1,000,000/ha per week [30]), the level of infestation by T. absoluta, and upon presence of other natural enemies on the crop. In addition, it is expensive to produce T. achaeae because the absence of diapause causes difficulty in storage and handling. Identifying a more efficient Trichogramma species would allow establishing an optimized economically-sound biological control program against T. absoluta.
The aim of the present study was to compare the efficiency of 29 Trichogramma strains in parasitizing T. absoluta eggs on tomatoes on three different scales: laboratory, mesocosm (cages in greenhouse) and in greenhouse compartments. We tested European Trichogramma strains from 11 different species. They were selected because (i) they were representative of the biodiversity of the European Trichogramma species, (ii) they were collected on hosts similar (size and/or ecology) to T. absoluta or on hosts present on tomato plants, and (iii) they showed characteristics (e.g. diapause, thelytoky, etc.) which make mass rearing easier i.e. cost-effective industrial production. A strain of T. pretiosum was also tested to compare other strains to one from the area of origin of T. absoluta. T. achaeae was chosen as the control species as it is already commercially available. Only the most promising strains under laboratory conditions were tested on a larger scale to assess the effectiveness of these selected Trichogramma strains under cropping conditions.
Materials and Methods
Biological Materials
The plants used in the experiments were five-week old tomato plants, Solanum lycopersicum L. cv. Marmande. They were grown in climatic chambers (24±1°C, HR: 65%, photoperiod 16L:8D) and a nutrient solution was applied daily. A colony of T. absoluta was set up using greenhouse-collected individuals in July 2009 at INRA, Alenya, France (initial number of individuals = 190). T. absoluta were reared in growth chambers (25±1°C, RH 70±10%, 16L:8D). Adults were kept in cages (55×75×80 cm), containing tomato plants. Adult moths were fed on honey placed on one wall inside the cages. The eggs used in the screening were between zero and 12 h old. Parasitoids used for the experiments originated from collections in various countries (Table 1). Dr. B. Pintureau from the French National Institute for Agricultural Research in Lyon (France) identified all species before the experiments. Stock colonies of parasitoids were reared on UV-irradiated eggs of a substitute host, Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) (18±1°C, RH 70±10%, 12L:12D). Rearing was carried out in glass tubes (length: 4.5 cm; diameter: 0.7 cm) and the parasitoids were fed on honey. Trichogramma species were maintained for at least three generations at the temperature of 25°C on E. kuehniella eggs before experimentation. The parasitoids used in the screening were between 12 and 24 h old.
Table 1. Year of collection, initial host and host plant, country of origin and Thelythoky status (females produced from unfertilized) of the 29 Trichogramma strains studied.
| Species | Geographic origin | Host plant (family) | Host moth or butterfly (family) | Thelytoky | Year of Collection |
| T. achaeae | Canaries Island | Tomato (Solanaceae) | Chrysodeixis chalcites (Noctuidae) | no | 2010 |
| T. buesi | Southern France | Cabbage (Brassicaceae) | Mamestra brassicae (Noctuidae) | no | 2009 |
| T. cacoeciae 1 | Southern France | Carnation (Caryophyllaceae) | Epichoristodes acerbella (Tortricidae) | yes | 2002 |
| T. cacoeciae 2 * | Northern France | Vine (Vitaceae) | Lobesia botrana (Tortricidae) | yes | 1989 |
| T. cordubensis 1 | Spain | Blackthorn (Rosaceae) | Iphiclides podalirius (Papilionidae) | yes | 1999 |
| T. cordubensis 2 | Egypt a | Olive tree (Oleaceae) | Palpita unionalis (Pyralidae) | yes | 2005 |
| T. cordubensis 3 | Portugal | – | – (Noctuidae) | yes | 1994 |
| T. daumalae 1 | Southern France | Apple tree (Rosaceae) | Cydia pomonella (Tortricidae) | no | 2009 |
| T. daumalae 2 | Bulgaria | Apple tree (Rosaceae) | Cydia pomonella (Tortricidae) | no | 1998 |
| T. dendrolimi 1 | China a | – | Palpita unionalis (Pyralidae) | no | 1998 |
| T. dendrolimli 2 | Italy | Vine (Vitaceae) | Lobesia botrana (Tortricidae) | no | 1991 |
| T. euproctidis 1 * | Switzerland | – | – | no | – |
| T. euproctidis 2 | Egypt a | Sugar cane (Poaceae) | Chilo sacchariphagus (Crambidae) | no | 1999 |
| T. euproctidis 3 | Southern France | Carnation (Caryophyllaceae) | Olethreutes arcuella (Tortricidae) | no | 2002 |
| T. evanescens 1 * | Northern France | Vine (Vitaceae) | Lobesia botrana (Tortricidae) | no | 1990 |
| T. evanescens 2 | Northern France | Cauliflower (Brassicaceae) | Argyrotaenia sphaleropa (Tortricidae) | no | 2002 |
| T. evanescens 3 | Turkey | Maize (Poaceae) | Ostrinia nubilalis (Crambidae) | no | 2003 |
| T. evanescens 4 | Southern France | Geranium (Geraniaceae) | Cacyreus marshalli (Lycaenidae) | no | 2005 |
| T. evanescens 5 * | Southern France | Tomato (Solanaceae) | Chrysodeixis chalcites (Noctuidae) | yes | 1982 |
| T. evanescens 6 | Germany | Maize (Poaceae) | Ostrinia nubilalis (Crambidae) | no | 2009 |
| T. evanescens 7 | Southern France | Vine (Vitaceae) | Lobesia botrana (Tortricidae) | no | 1990 |
| T. evanescens 8 | Southern France | Cabbage (Brassicaceae) | Ephestia kuehniella (Pyralidae) | no | 1998 |
| T. evanescens 9 | Southern France | Cabbage (Brassicaceae) | Ephestia kuehniella (Pyralidae) | yes | 1998 |
| T. evanescens 10 | Southern France | Tomato (Solanaceae) | Ephestia kuehniella (Pyralidae) | yes | 2010 |
| T. oleae | Yugoslavia | Olive tree (Oleaceae) | Glyphodes unionalis (Pyralidae) | yes | 1972 |
| T. pretiosum | Uruguay | Vine (Vitaceae) | Argyrotaenia sphaleropa (Tortricidae) | no | 1995 |
| T. semblidis 1 | Southern France | Rice (Poaceae) | Ephestia kuehniella (Pyralidae) | no | 1997 |
| T. semblidis 2 | Northern France | Cabbage (Brassicaceae) | Plutella xylostella (Plutellidae) | no | 2002 |
| T. semblidis 3 | Southern France | Apple tree (Rosaceae) | Cydia pomonella (Tortricidae) | yes | 2009 |
Asterisks indicate the strains for which diapause or quiescence capacity has been identified in our laboratory.
strain not collected in Europe but species is present in Europe.
Laboratory Screening
The experiments were conducted in growth chambers at the temperature of 25°C (RH 70±10%, 16L:8D). Twenty-nine strains (among 11 species) were tested on T. absoluta eggs (designated as strains thereafter). Mated Trichogramma females were placed individually for 24 h with 30 T. absoluta eggs on a tomato leaflet (length: 7 cm ±0.5 SD) in a plastic tube sealed with a mesh to ensure ventilation (length: 14 cm × diameter: 4 cm), five drops of honey were deposited on the internal wall of the tube as food source for parasitoids. The 30 eggs per leaflet were obtained by releasing 15 T. absoluta (mixed males and females) on each leaflet in a tube overnight. Then, T. absoluta adults were removed and extra eggs were discarded (using a brush) to have 30 eggs per leaflet. The leaflet stem, sticking out of the tube, was planted into floral foam for watering. This design ensured that the leaflet stayed in good shape for the whole duration of the experiment. Between 10 and 15 replicates were conducted per parasitoid strain, and the replicates were carried out in a randomized order at different times. The tubes containing parasitized T. absoluta eggs were kept in the climatic chamber and maintained during five days. We counted the number of parasitized eggs (black eggs) and the number of aborted eggs (yellow non-hatched eggs). The proportion of females that parasitized at least one egg was also recorded.
In parallel to the tests using T. absoluta as host, experiments were also done using the rearing host E. kuehniella in order to compare biological characteristics of the various Trichogramma strains between the rearing and the targeted hosts. Mated parasitoid females were placed individually in glass tubes (containing honey as food) with 30–40 E. kuehniella eggs for 24 h in climatic chambers (25±1°C, RH 70±10%, 16L:8D). E. kuehniella eggs were glued on a strip of cardboard (3×10 mm) with 10% arabic gum. On each day of experiment, parasitoid strains were tested with the order of strains randomized (14–15 replicates per strain). The proportion of females that parasitized at least one E. kuehniella egg was recorded in the same way as tests using T. absoluta as tested host.
Cage Experiments
The experiments were conducted in cages placed in a greenhouse located in Valbonne (French Riviera, France). Two species were compared to T. achaeae in cages: Trichogramma euproctidis 1 which showed the highest parasitism of T. absoluta eggs in laboratory trial (see Results section), and Trichogramma evanescens 5 which showed a similar level of parasitism as T. achaeae but that can be easily reared because of its thelytoky. The cages were placed in a glass greenhouse with semi-controlled temperatures, and the temperature was recorded in the cages with data-loggers during the whole experiment (min < mean temperature < max: 16.3°C <25.3°C <33.7°C; min < mean RH < max, 27.8% <70.8% <98.3%; natural ambient light: May-June 2011). Eight tomato plants (development stage from seven to 10 leaves) were put into cages (145×70×80 cm) covered by an insect-proof mesh. Twenty-five two-day old T. absoluta adults (mixed males and females) were released in the cages simultaneously with one of the Trichogramma strains. To release the parasitoids, small cardboard strips containing about 400 parasitized eggs of E. kuehniella from which parasitoids were just beginning to emerge, were placed in an open tube (drops of honey were provided as food source). The experiment lasted three days which is nearly the mean longevity of Trichogramma adults in tomato greenhouse (Chailleux A. and Desneux N., unpublished data). Then leaflets were collected (20–30 leaflets collected on upper, medium and lower part of the plant) until 100 eggs were found. Eggs were kept on the leaflets and placed into boxes in environmental cabinets (25°C, RH 70±10%, 16L:8D). Under these conditions, parasitized eggs become black in five days. The parasitized eggs were counted under a binocular microscope and the percentage of parasitism was calculated. We conducted seven to 12 randomized replicates for each Trichogramma strain (cage and order).
Greenhouse Experiments
The most promising strain, T. euproctidis 1 (see Results section), was tested under greenhouse conditions and compared to T. achaeae. Experiments were conducted in two 60 m2 glass greenhouse compartments (min < mean temperature < max: 17.2°C <26.2°C <37.6°C; min < mean RH < max, 27.3% <71% <94.1%; natural ambient light: June-July 2011) located in Valbonne, French Riviera, France. Each greenhouse compartment contained three double rows of tomato plants under hydroponics cropping conditions. Forty adult moths (mixed males and females) were released simultaneously with 2,000 Trichogramma individuals. This situation mimicked a high level of infestation by the pest and a commercial release of Trichogramma for management of T. absoluta in tomato greenhouse (2,000 parasitoids for 25–100 m2). Parasitoids were released using the same method used in cages (i.e. on cardboard strips) at one central point of the greenhouse. A sample of 50 eggs was collected randomly in each compartment three days after the release and kept for incubation as described in the previous section (cage experiments). Six replicates were conduced for each Trichogramma strain, three in each compartment of the greenhouse.
Statistical Analysis
All statistical analyses were performed using R software (R Development Core Team 2009) with the packages multcomp and DTK. For the laboratory experiments, the number of parasitized eggs (per female and per strain) were analysed using a generalized linear model based on Poisson distributed data with a log link function. Multiple comparisons were done using a Dunnett’s post-hoc test (comparison to the reference species T. achaeae). In addition, the effect of Trichogramma species, along with host plant family, host moth family and year of collection (for each strain) was also tested using a similar generalized linear model. Differences between the proportions of females parasitizing T. absoluta eggs on tomato compared to E. kuehniella (control host, i.e. rearing host) were tested using a generalized linear model designed for modelling binomial data with a logit link function. The assessment of preference-performance relationship [31] may be an important factor in choosing biological control agents [32]. Therefore, a linear regression analysis was used to assess the relationship between the mean number of parasitized eggs (log-transformed data) and the proportion of females that accepted T. absoluta eggs as host for each Trichogramma strain (i.e. proportion of females stinging [aborted eggs] or parasitizing [black eggs] at least one egg of T. absoluta). Finally, for the cages and greenhouse experiments, the percent parasitism was analyzed using a generalized linear model designed for modelling binomial data.
Results
Screening of 29 Strains on T. absoluta
The mean number of eggs parasitized in tubes varied significantly depending on the strain (F28, 338 = 10.907, P<0.001) (Fig. 1). The level of parasitism was significantly linked to Trichogramma species (F10, 335 = 8.296, P<0.001). The characteristics of the habitat of origin also had a significant effect on parasitism of T. absoluta by the parasitoids tested (host moth family: F6, 329 = 4.318, P<0.001, and host plant family: F5,324 = 7.328, P<0.001); strains originally collected from Noctuidae, Plutellidae and Crambidae, as hosts, and from Solanaceae, Oleaceae and Vitaceae, as host plants, parasitized the most T. absoluta eggs. In contrast, the year of collection was not significant (F1,323 = 0.146, P = 0.702). Only the strain T. euproctidis 1 was significantly more efficient than T. achaeae (Z = 3.379, P = 0.019). Another relevant strain was T. evanescens 5 because of its thelytoky, and because it showed a similar level of parasitism to T. achaeae (Z = −1.104, P = 0.999). Moreover, T. pretiosum was not significantly different from T. achaeae (Z = −0.428, P = 0.999) although it came from the same area as T. absoluta. Altogether sixteen strains were not significantly different from T. achaeae.
Figure 1. Parasitism of Tuta absoluta eggs under laboratory conditions.
Mean (±SEM) number of parasitized T. absoluta eggs per Trichogramma strain in laboratory screening tubes on tomato leaflets. Strains with an asterisk are significantly different from Trichogramma achaeae at P<0.05 level (GLM analysis). One strain of Trichogramma, Trichogramma euproctidis 1, was significantly more efficient than T. achaeae.
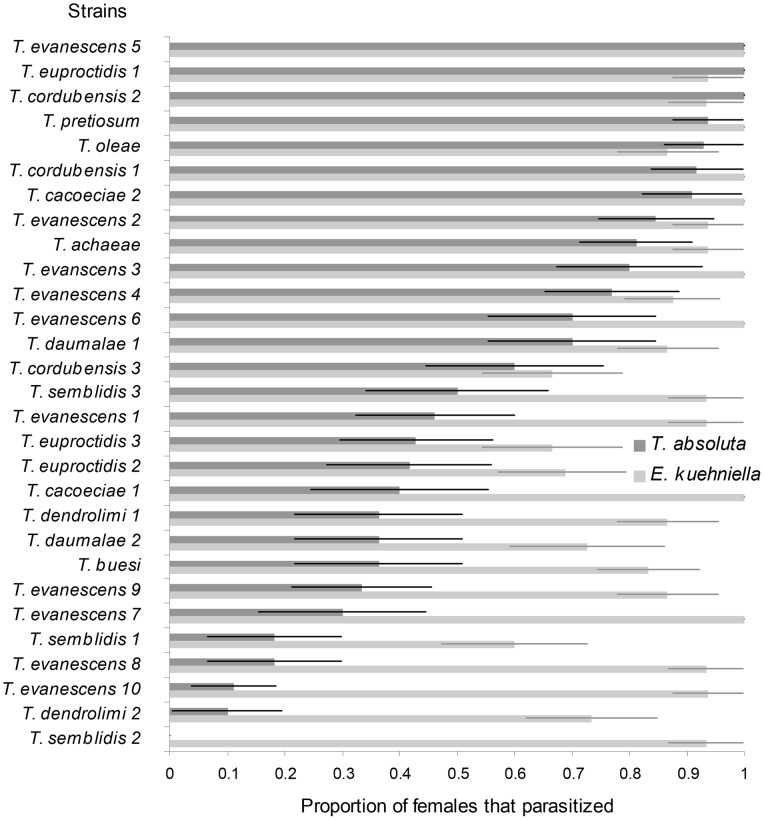
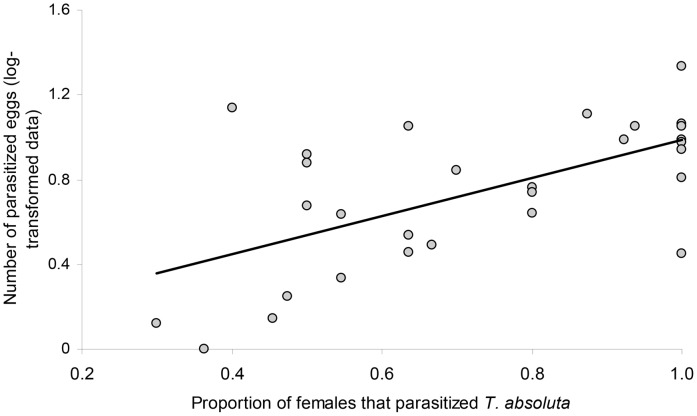
The proportion of females that parasitized the host varied across the 29 strains tested: in three strains all females parasitized T. absoluta, and in one strain (Trichogramma semblidis 2) none of the females parasitized the host. The proportion of females that parasitized T. absoluta on tomatoes was significantly different than on E. kuehniella (F1, 56 = 29.101, P<0.001) (Fig. 2). For a majority of the strains tested, the proportion of females that parasitized at least one egg was lower on T. absoluta on tomatoes than on E. kuehniella on cardboard. The linear regression analysis between the mean numbers of T. absoluta parasitized eggs and the proportion of females that accepted T. absoluta eggs, i.e. preference-performance assessment [31], [32], showed that strains parasitizing the most eggs also showed highest number of females accepting T. absoluta as host (R2 = 0.37, F1, 27 = 16.14, P<0.001) (Fig. 3).
Figure 2. Acceptance of two hosts, Tuta absoluta and Ephestia kuehniella, by Trichogramma females.
Data are presented as proportions (mean±SEM) of Trichogramma females that parasitized at least one egg on T. absoluta and on E. kuehniella in laboratory screening tubes.
Figure 3. Preference-performance relationship.
Data are presented as proportions of parasitoid females (for a given Trichogramma strain) attacking the host (T. absoluta) under laboratory conditions and the mean numbers of T. absoluta eggs parasitized (log-transformed data) (regression line: y = 0.9001×−0.0856).
Cage Tests and Greenhouse Tests
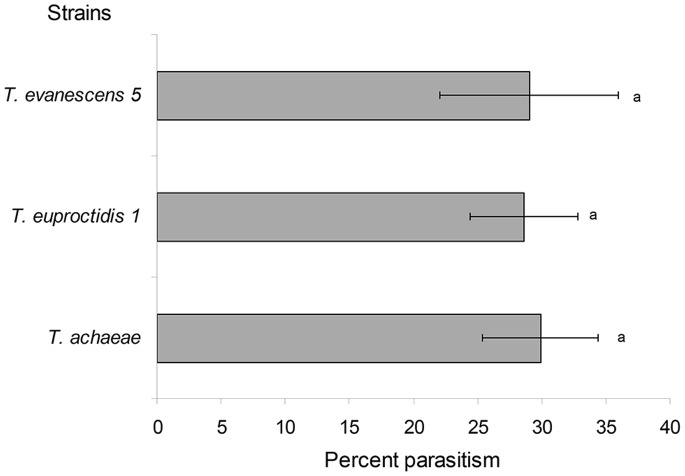
Differences previously observed under laboratory conditions were no longer observed in cages (Fig. 4). The three strains (T. achaeae, T. euproctidis 1 and T. evanescens 5) showed similar efficiency against T. absoluta in cages; they all parasitized ∼30% of the eggs (F2, 25 = 0.019, P = 0.981). In greenhouse compartments, both Trichogramma strains tested (T. achaeae and T. euproctidis 1) were able to parasitize T. absoluta. T. achaeae showed the highest efficiency: 65.9±7.77% (mean ± SEM) as opposed to 19.4±2.73% (mean ± SEM) for T. euproctidis. In this case, the difference was significant (F 1, 11 = 50.49, P<0.001).
Figure 4. Parasitism of Tuta absoluta eggs in cages in greenhouse.
Percentage (mean±SEM) of parasitized T. absoluta eggs per Trichogramma strain in cages in the greenhouse. Histograms bearing the same letter do not differ at P<0.05 (GLM analysis).
Discussion
Twenty-nine Trichogramma species-strains were tested under laboratory conditions and one strain of the T. euproctidis species (1) appeared promising (68.7% parasitism vs. 35.4% for parasitism for T. achaeae i.e. the species already commercialized in Europe and North Africa). Consequently this T. euproctidis strain was further tested on a larger scale i.e. in cages and greenhouses. However, the results of these later experiments did not corroborate the results obtained under laboratory conditions. Indeed the most efficient Trichogramma parasitoid against T. absoluta was still T. achaeae. Under cropping conditions, e.g. greenhouses, T. euproctidis was twofold less efficient against T. absoluta than T. achaeae. Our results showed that despite the fact that most Trichogramma strains did accept T. absoluta eggs on tomato leaflets, at least to some extent under laboratory conditions; the design was not sufficiently realistic to enable us to foresee their efficacy as a natural enemy of T. absoluta under real conditions.
The levels of parasitism of T. absoluta observed among the strains tested in the laboratory varied significantly. The low parasitism recorded for some strains may be attributed to two factors. First, in parasitoids, host specificity is mediated in part by host recognition and acceptance by the adult female parasitoid [25], [32], [33]. It has been shown that Trichogramma parasitoids prefer hosts with relatively big eggs [34] but the eggs of T. absoluta are, by comparison, three times smaller than the eggs of E. kuehniella used in the rearing. Furthermore, we found a positive relationship between the number of parasitized eggs and the proportion of females accepting T. absoluta as host, demonstrating that parasitism levels were directly linked to the willingness of females to attack the host (as demonstrated in other parasitoid systems [25], [32], [35], [36]). Second, low parasitism may result not only from rejection of T. absoluta eggs as host but rather from the poor capacity of some Trichogramma species/strains to cope with specific tomato plant characteristics. Various Trichogramma species have been reported to be highly susceptible to plant trichomes [37]–[41]. Although our study did not specifically assess the effect of trichomes on Trichogramma parasitoids, the experimental design was successful in identifying species having very little affinity for T. absoluta/tomato as host/host-plant complex.
Laboratory results also showed that the level of parasitism can differ greatly among strains of the same species. Variation within species of Trichogramma has already been encountered in other screenings [42]–[44]. Chassain and Bouletreau [45] studied the inter-strain variability of the main traits involved in Trichogramma parasitoid efficiency in host exploitation i.e. longevity, fecundity, progeny viability, progeny sex ratio and progeny allocation. They reported great differences among strains of the same species coming from different habitats, as well as between two different species coming from the same habitat. Consistent with these findings, our results showed that the characteristics of the original host and host plant of a given parasitoid strain, had an effect on its efficiency in parasitizing T. absoluta eggs, with strains originally collected from Solanaceae, Oleaceae and Vitaceae showing the best performance. Therefore, it is important to consider both initial host species and habitats when selecting strains of Trichogramma parasitoids for biological control programs.
The results from the cages/greenhouses did not match those from the laboratory; higher efficacy of T. euproctidis on T. absoluta disappeared when the scale of the experiments was increased. Differences between results under laboratory and greenhouse conditions may be due to both biotic and abiotic parameters. First, Trichogramma are known to be able to avoid plants bearing trichomes [46]. For some strains, females may have attacked T. absoluta eggs when constrained on tomato leaves in tubes in the laboratory but may have been able to avoid foraging on tomato leaves when released into cages or greenhouses. In addition, the oviposition pattern of hosts is a key factor for Trichogramma efficiency; T. absoluta does not lay egg masses but most of the time isolated eggs which thus increases the energy cost of foraging for hosts. Second, high temperatures, that are typical of greenhouses in summer, may impact differentially the various strains/species of Trichogramma [43], [47]. Moezipour et al. [48] indicated that there is a significant difference in the functional response of Trichogramma brassicae when tested at 20 or 30°C, and previous studies have shown that temperature and relative humidity can affect biological traits in Trichogramma spp. [43], [47], [49].
On the other hand, we could assume that differences between parasitoid efficiency recorded under laboratory and greenhouse conditions may result from the time that the different strains have spent under rearing conditions (year of collection). Efficiency under cropping conditions of one Trichogramma strain could be modified by the length of time spent under the rearing conditions in the laboratory, i.e. in tubes on alternative hosts such as E. kuehniella eggs, and at optimal temperature and humidity i.e. 25°C and 70% HR [50]. As the life cycle of Trichogramma parasitoids is usually short (egg to adult in about 11 days), adaptation to rearing conditions (humidity, temperature, mass-rearing host, confined environment, etc.) may occur. On the contrary, previous adaptations to given field conditions could disappear after a long spell of rearing under optimal laboratory conditions. Despite this, the collection year did not affect Trichogramma efficiency when assessed under laboratory conditions (no significant year of collection factor, P = 0.702). However it may impact parasitism only at a larger experimental scale (e.g. in greenhouse) where, for example, the foraging and dispersal capacities are key components of the parasitoid efficacy [33], [51]. Therefore, time spent in optimal rearing conditions may likely also partly explain some of the differences among strains tested in cages and greenhouse compartments.
We recorded the potential of various Trichogramma strains for biological control of T. absoluta in Europe. Nevertheless, our results did not identify that other Trichogramma strains showed better biocontrol traits than T. achaeae, i.e. higher fertility, high proportion of females/thelytoky and the capacity of diapause in cold storage in biocontrol company facilities. Further screening of Trichogramma parasitoids for potential management of T. absoluta would have to be based on the assessment of parasitoids collected on the targeted host in tomato crops under standard greenhouse cropping conditions in Europe. Doing this would increase the chances of assessing species that show greater likelihood of affinity within the cropping conditions. Colonies should be initiated with high number of field-collected individuals and new parasitoids should be added periodically. During laboratory screening, strains that showed very low parasitism levels were identified and removed; nonetheless, the efficacy of Trichogramma parasitoids under cropping conditions was not easily predictable from laboratory experiments. Studies could also be conducted directly in large cages (i.e. with multiple plants) in greenhouses when there are few strains to be tested. This way, all relevant criteria for strain selection could be taken into account and laboratory screening steps may be bypassed. Further studies would aim to (i) identify efficient parasitoids on T. absoluta, notably to prevent overuse of insecticides in tomato crops (and therefore ensure sustainability of current biological control and integrated pest management programs on this crop), and (ii) define new criteria that allow research and development programs at biocontrol companies to select accurately and quickly new Trichogramma strains (and more generally parasitoids) in the framework of biological control.
Acknowledgments
The authors thank Dr. Bernard Pintureau (INRA Lyon, France) and Emily Mohl (University of Minnesota, USA) for helpful comments on the manuscript, Philippe Bearez, Etty-Ambre Colombel and Cecile Thomas from INRA Sophia Antipolis for technical assistance.
Funding Statement
This work was funded by ANRT (Association Nationale de la Recherche et de la Technologie) (PhD fellowship 167/2010 to AC), Invivo AgroSolutions & Biotop and the French ministry of agriculture (CASDAR project 10063 to AC, ND and ET). The funders had no role in data analysis and preparation of the manuscript but were involved in the study design, data collection and decision to publish.
References
- 1. Haack RA, Herard F, Sun JH, Turgeon JJ (2010) Managing invasive strains of asian longhorned beetle and citrus conghorned beetle: A Worldwide Perspective. Annual Review of Entomology 55: 521–546. [DOI] [PubMed] [Google Scholar]
- 2. Suckling DM, Brockerhoff EG (2010) Invasion biology, ecology, and management of the light Brown apple moth (Tortricidae). Annual Review of Entomology 55: 285–306. [DOI] [PubMed] [Google Scholar]
- 3. Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of the soybean aphid in North America. Annual Review of Entomology 56: 375–399. [DOI] [PubMed] [Google Scholar]
- 4. Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52: 273–288. [Google Scholar]
- 5. Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, et al. (2000) Biotic invasions: Causes, epidemiology, global consequences, and control. Ecological Applications 10: 689–710. [Google Scholar]
- 6. Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52: 81–106. [DOI] [PubMed] [Google Scholar]
- 7. Lu YH, Wu KM, Jiang YY, Guo YY, Desneux N (2012) Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487: 362–365. [DOI] [PubMed] [Google Scholar]
- 8. Barrientos ZR, Apablaza HJ, Norero SA, Estay PP (1998) Temperatura base y constante térmica de desarrollo de la polilla del tomate, Tuta absoluta (Lepidoptera: Gelechiidae). Ciencia e Investigación Agraria 25: 133–137. [Google Scholar]
- 9. Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, et al. (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. Journal of Pest Science 83: 197–215. [Google Scholar]
- 10. Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. Journal of Pest Science 84: 403–408. [Google Scholar]
- 11. Arno J, Gabarra R (2011) Side effects of selected insecticides on the Tuta absoluta (Lepidoptera: Gelechiidae) predators Macrolophus pygmaeus and Nesidiocoris tenuis (Hemiptera: Miridae). Journal of Pest Science 84: 513–520. [Google Scholar]
- 12. Biondi A, Desneux N, Siscaro G, Zappalà L (2012) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus . Chemosphere 87: 803–812. [DOI] [PubMed] [Google Scholar]
- 13. Polaszek A, Rugman-Jones PF, Stouthamer R, Hernandez-Suarez E, Cabello T, et al. (2012) Molecular and morphological diagnoses of five species of Trichogramma: biological control agents of Chrysodeixis chalcites (Lepidoptera: Noctuidae) and Tuta absoluta (Lepidoptera: Gelechiidae) in the Canary Islands. Biocontrol 57: 21–35. [Google Scholar]
- 14. Zappalà L, Bernardo U, Biondi A, Cocco A, Deliperi S, et al. (2012) Recruitment of native parasitoids by the exotic pest Tuta absoluta (Meyrick) in Southern Italy. Bulletin of Insectology 65: 51–61. [Google Scholar]
- 15. Parra JRP, Zucchi RA (2004) Trichogramma in Brazil: Feasibility of use after twenty years of research. Neotropical Entomology 33: 271–281. [Google Scholar]
- 16. Pratissoli D, Thuler RT, Andrade GS, Zanotti LCM, da Silva AF (2005) Estimate of Trichogramma pretiosum to control Tuta absoluta in stalked tomato. Pesquisa Agropecuaria Brasileira 40: 715–718. [Google Scholar]
- 17. de Medeiros MA, Boas GLV, Vilela NJ, Carrijo OA (2009) A preliminar survey on the biological control of South American tomato pinworm with the parasitoid Trichogramma pretiosum in greenhouse models. Horticultura Brasileira 27: 80–85. [Google Scholar]
- 18. de Medeiros MA, Sujii ER, de Morais HC (2011) Mortality factors at egg stage of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on organic tomato system and on conventional tomato system. Bragantia 70: 72–80. [Google Scholar]
- 19. Smith SM (1996) Biological control with Trichogramma: Advances, successes, and potential of their use. Annual Review of Entomology 41: 375–406. [DOI] [PubMed] [Google Scholar]
- 20. Mansour M (2010) Effects of gamma radiation on the Mediterranean flour moth, Ephestia kuehniella, eggs and acceptability of irradiated eggs by Trichogramma cacoeciae females. Journal of Pest Science 83: 243–249. [Google Scholar]
- 21. Li SY, Henderson DE, Myers JH (1994) Selection of suitable Trichogramma (Hymenoptera, Trichogrammatidae) species for potential control of the blackheaded fireworm (Lepidoptera, Tortricidae) infesting cranberries. Biological Control 4: 244–248. [Google Scholar]
- 22. Van Lenteren JC, Bueno VHP (2003) Augmentative biological control of arthropods in Latin America. Biocontrol 48: 123–139. [Google Scholar]
- 23.Mills N (2010) Egg parasitoids in biological control and integrated pest management. In: Consoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, Dordrecht, 389–411.
- 24. Bourchier RS, Smith SM (1996) Influence of environmental conditions and parasitoid quality on field performance of Trichogramma minutum . Entomologia Experimentalis et Applicata 80: 461–468. [Google Scholar]
- 25. Desneux N, Blahnik R, Delebecque CJ, Heimpel GE (2012) Host phylogeny and host specialization in parasitoids. Ecology Letters 15: 453–460. [DOI] [PubMed] [Google Scholar]
- 26.Pizzol J, Wajnberg E, Desneux N, Thiery D (2012) Parasitoid age and host egg age have independent impact on various biological traits in a Trichogramma species. Journal of Pest Science doi:10.1007/s10340-012-0434-1.
- 27. Agamy E (2010) Field evaluation of the egg parasitoid, Trichogramma evanescens West. against the olive moth Prays oleae (Bern.) in Egypt. Journal of Pest Science 83: 53–58. [Google Scholar]
- 28. Desneux N, Pizzol J, Thomas C, Pautrat E, Bearez P, Poncet C, Tabone E, Kabiri F, Frandon J (2011) Potential for direct interference between natural enemies of Tuta absoluta on tomato. Acta Horticulturae 917: 31–37. [Google Scholar]
- 29. Cabello T, Gallego JR, Vila E, Soler A, del Pino M, et al. (2009) Biological control of the South American tomato pinworm, Tuta absoluta (Lep.: Gelechiidae), with releases of Trichogramma achaeae (Hym.: Trichogrammatidae) in tomato greenhouses of Spain. IOBC/WPRS Bulletin 49: 225–230. [Google Scholar]
- 30. Frandon J, Séguret J, Desneux N, Tabone E (2010) Un nouvel auxiliaire contre Tuta absoluta . Phytoma 634: 9–12. [Google Scholar]
- 31. Jaenike J (1978) On optimal oviposition behavior in phytophagous insects. Theoretical Population Biology 14: 350–356. [DOI] [PubMed] [Google Scholar]
- 32. Desneux N, Barta RJ, Hoelmer KA, Hopper KR, Heimpel GE (2009) Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 160: 387–398. [DOI] [PubMed] [Google Scholar]
- 33.Godfray HCJ (1994) Parasitoids: behavioural and evolutionary ecology. Princeton University Press, Chichester.
- 34. Roriz V, Oliveira L, Garcia P (2006) Host suitability and preference studies of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae). Biological Control 36: 331–336. [Google Scholar]
- 35. Kraaijeveld AR, Nowee B, Najem RW (1995) Adaptive variation in host-selection behaviour of Asobara tabida, a parasitoid of Drosophila larvae . Functional Ecology 9: 113–118. [Google Scholar]
- 36. Chau A, Mackauer M (2001) Preference of the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae) for different aphid species: female choice and offspring survival. Biological Control 20: 30–38. [Google Scholar]
- 37. Romeis J, Shanower TG, Zebitz CPW (1999) Why Trichogramma (Hymenoptera: Trichogrammatidae) egg parasitoids of Helicoverpa armigera (Lepidoptera: Noctuidae) fail on chickpea. Bulletin of Entomological Research 89: 89–95. [Google Scholar]
- 38. Kennedy GG (2003) Tomato, pests, parasitoids, and predators: Tritrophic interactions involving the genus Lycopersicon . Annual Review of Entomology 48: 51–72. [DOI] [PubMed] [Google Scholar]
- 39. Kauffman WC, Kennedy GG (1989) Relationship between trichome density in tomato and parasitism of Heliothis spp (Lepidoptera, Noctuidae) eggs by Trichogramma spp (Hymenoptera, Trichogrammatidae). Environmental Entomology 18: 698–704. [Google Scholar]
- 40. Kashyap RK, Kennedy GG, Farrar RR (1991) Mortality and inhibition of Helicoverpa zea egg parasitism rates by Trichogramma in relation to trichome methyl ketone-mediated insect resistance of Lycopersicon-hirsutum-f-glabratum, accession PI-134417. Journal of Chemical Ecology 17: 2381–2395. [DOI] [PubMed] [Google Scholar]
- 41. Kashyap RK, Kennedy GG, Farrar RR (1991) Behavioral-response of Trichogramma pretiosum Riley and Telenomus sphingis (Ashmead) to trichome/methyl ketone mediated resistance in tomato. Journal of Chemical Ecology 17: 543–556. [DOI] [PubMed] [Google Scholar]
- 42. Bueno R, Parra JRP, Bueno AD, Haddad ML (2009) Performance of Trichogrammatids as biocontrol agents of Pseudoplusia includens Walker (Lepidoptera: Noctuidae). Neotropical Entomology 38: 389–394. [DOI] [PubMed] [Google Scholar]
- 43. Pizzol J, Pintureau B, Khoualdia O, Desneux N (2010) Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Journal of Pest Science 83: 447–452. [Google Scholar]
- 44. Tabone E, Bardon C, Desneux N, Wajnberg E (2010) Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. Journal of Pest Science 83: 251–256. [Google Scholar]
- 45. Chassain C, Bouletreau M (1991) Genetic-variability in quantitative traits of host exploitation in Trichogramma (Hymenoptera, Trichogrammatidae). Genetica 83: 195–202. [Google Scholar]
- 46. Romeis J, Shanower TG, Zebitz CPW (1998) Physical and chemical plant characters inhibiting the searching behaviour of Trichogramma chilonis . Entomologia Experimentalis et Applicata 87: 275–284. [Google Scholar]
- 47. Andrade GS, Pratissoli D, Dalvi LP, Desneux N, dos Santos HJG (2011) Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. Journal of Pest Science 84: 313–320. [Google Scholar]
- 48. Moezipour M, Kafil M, Allahyari H (2008) Functional response of Trichogramma brassicae at different temperatures and relative humidities. Bulletin of Insectology 61: 245–250. [Google Scholar]
- 49. Kalyebi A, Overholt WA, Schulthess F, Mueke JM, Sithanantham S (2006) The effect of temperature and humidity on the bionomics of six African egg parasitoids (Hymenoptera: Trichogrammatidae). Bulletin of Entomological Research 96: 305–314. [DOI] [PubMed] [Google Scholar]
- 50.Pintureau B, (2009) La lutte biologique et les Trichogrammes: Application au contrôle de la pyrale du maïs. Paris, Le Manuscrit.
- 51. Tabone E, Bardon C, Desneux N (2012) Dispersal study as Trichogrammatidae selection criteria for biological control in cauliflower greenhouses. Acta Horticulturae 927: 227–235. [Google Scholar]