Abstract
The art and science of the use of deposition markers for the estimation of blood flow distributions throughout the body and within organs is reviewed. Development of diffusible tracer techniques started 50 years ago. Twenty years later, radioactive 15 micron microspheres became the standard marker. Early studies on small animals, fetal sheep in 1967 and rats in 1976, provoked much of the technical development. Needs for avoiding the use of radioactivity, for having long lasting labels, and for providing higher spatial resolution, are driving the continuing exploration of newer techniques using colored and fluorescent microspheres and molecular deposition markers. Strengths and weaknesses of the various methods are compared.
Keywords: Blood flow, Flow heterogeneity, Cardiac output, Deposition markers, Rats, Mice, Dogs
1. Introduction
Microsphere methods provide information on regional perfusion between and within organs that is more detailed than that from flow probes. They are also easier to use and give higher resolution than methods using molecular tracer washout. For these reasons the radioactive microsphere technique, introduced in 1967 by Rudolph and Heymann [1] for examining regional blood flow in sheep fetuses in utero, became an essential tool in cardiovascular research.
A 1976 paper of McDevitt and Nies [2] was one of a series of papers describing the use of the radioactive microsphere techniques in rats [3,4]. Theirs was the first to demonstrate, in rodents, the potential of the microsphere technique to measure cardiac output in addition to regional blood flow. They injected 80,000 spheres with a single radioactive label in each of 18 rats and used the arterial reference sample method. Blood flow was measured in several organs (kidney, spleen, GI tract, liver, etc., but not in heart and brain). The number of microspheres was sufficient to obtain at least 400 microspheres per sample in these organs and did not cause hemodynamic disturbances. The reliability of the method was judged mainly on the comparability of the observed values with those obtained via other techniques. Estimates of hepatic flow in the McDevitt and Nies paper are lower than those reported in earlier 86Rb studies, because, with the latter, the diffusible tracer technique label arrives in the liver through the hepatic artery as well as the portal vein. Later studies showed that, indeed, the microsphere technique accurately determines hepatic artery flow [5] and does not reflect the portal venous flow contribution to total hepatic flow.
Application of the microsphere method in small animals is, in some regards, more technically demanding than in large animals. In 1971, Buckberg et al. [6] and, later, other investigators [7–11] showed that accurate assessment of tissue blood flow requires a sufficient number of micro spheres per sample, 200 to 400 for 10% accuracy. To obtain this accuracy in small animals the number of microspheres injected per unit body mass must be higher in small animals than in dogs. In dogs, about 100,000 spheres (diameter 15 micron) per kilogram body weight are injected for whole body distributions, whereas at least twice this is needed in small animals, increasing the risk for hemodynamic effects caused by obstruction to local perfusion. Some investigators have used up to 360,000 in rats. Even four injections of this number of spheres (a total of approximately 5 million spheres per kilogram body weight) did not give rise to obvious disturbances [12]. Between 30 and 50 million spheres were used in dogs when a large number of different labels or high resolution was needed [13,14]. Therefore, the number of microspheres which can be injected appears to be quite high when they are prevented from aggregating. Large numbers provide accurate blood flow assessments at a small scale.
In this review we shall cover (1) the requirements for the use of deposition methods for flow, (2) assess the fulfillment of these criteria in standard applications using radioactive microspheres, (3) describe developments of non-radioactive spheres and (4) return to applications in animals even smaller than rats.
2. Requirements for markers of regional blood flow
Perhaps the way to think about the vices and virtues of the various techniques for regional flow measurements is to consider them from the point of view of their faithfulness to the prerequisite principles on which they are based. No method is perfect, so it is a question of minimizing sources of random and systematic error. The basic principle of all deposition techniques for regional flow measurement is that the deposition is proportional to the flow (per unit volume or mass of tissue), i.e. that the fraction of the cardiac output going to a particular region is defined by the fractional deposition of a marker in that region. The idea is that deposited markers give a measure of flow per unit volume of tissue at the level of the capillaries. No deposited marker measures aortic flow or arterial or venular flows or velocities. It is, in principle, best to measure flow per unit of extravascular tissue since the blood fraction of an organ is not the metabolizing fraction of the organ. This refinement in the reporting of tissue blood flows requires additional data that is not usually available. Therefore, we will consider the standard measurement to be ‘flow per gram of whole tissue’. There are six conditions to fulfill [15], which are summarized in Table 1 and will be discussed in more detail below.
Table 1.
The six principles to be fulfilled for valid blood flow measurements with tracer microspheres
| Mixing in central circulation Complete extraction in first pass No separation from the blood (no ‘streaming’ or ‘skinning’) |
Site of injection: atrium or ventricle For microspheres: diameter >12 microns Diameter as small as possible (‘molecular microspheres’) Particle density not important |
| Artifacts with deposited markers | Obstruction of vessels No measurements in capillary beds in series |
| Retention of marker in tissue | Retention of particle in tissue No leakage of tracer from particle |
| Measuring accuracy | Poisson distribution! Detection of label |
These six requirements apply to all types of deposition markers for regional flows. The microsphere method has been evaluated extensively. Heymann et al. [16] provide a most comprehensive review on their use and validation. More recent studies [7,8,11,14,17] provide additional evaluations of the techniques and demonstrate its reliability when used as recommended by Heymann et al.
3. How well are requirements fulfilled?
3.1. Soluble and particulate markers
Deposition methods began with solutes that were taken up into the tissues as the blood passed through the capillaries. Love and Burch [18] used rubidium or potassium, a good idea: transcapillary extraction was substantial and marker was lost from the large intracellular potassium pool slowly, about 1–2% per minute [19]. However, extraction was only 40 to 75%, underestimating flow from first pass extraction. Worse yet, because the fractional extraction of the tracer is higher in low flow regions, the deposited concentrations were more uniform than were the actual regional flows. Incomplete extraction was interpreted as arteriovenous shunting (e.g., Ref. [20]), but in the heart there are no arteriovenous shunts. The unidirectional extraction, prior to any back diffusion of marker from tissue back to blood, is approximately E = 1 − e−PS/F, where PS is capillary wall permeability–surface area product (ml g−1 min−1) and F is flow (ml g−1 min−1). Thus, the degree of extraction varied from region to region and organ to organ. Years later, usable molecular microspheres were found, which come close to 100% extraction and have good retention [14].
Particulate markers have the advantage that they are 100% extracted if they are too large to pass through capillaries. Ueda et al. [21] and Taplin et al. [22] developed macroaggregated albumin (MAA), labeled with 131I, as a marker for the multi-organ distribution of cardiac output. It was not perfect, but extraction was higher than potassium, about 75% in the heart [23]. There was marked patchiness of the regional MAA deposition. This was partly due to the wide range of sizes of the macroaggregates [15] and to size dependent biases, and partly due to a real heterogeneity in intraorgan regional flows [18,24].
After their introduction by Rudolph and Heymann [1], Hoffman’s group [6], in collaboration with Rudolph and Heymann, carefully demonstrated that radioactive microspheres are superior to the ion and albumin macroaggregates in terms of completeness of extraction and length of retention [25]. Small spheres, less than 12 microns in diameter, escaped into venous blood, but few of the 15 micron diameter spheres escaped. The use of the 15 micron diameter spheres established the fact that regional myocardial flows were markedly heterogeneous [26], affirming the impressions gained from diffusible indicator studies using 86Rb [18,27].
3.2. Mixing in the central circulation
Standard checks on the adequacy of mixing prior to distribution to the body have been to determine if flows in left versus right cerebral hemispheres or left versus right kidneys are equal, or if equal numbers of spheres per unit flow are obtained from different arterial sampling sites simultaneously [16]. For systemic flows, injection into the left atrium (preferably) or left ventricular apical region gave satisfactory mixing, and these sites differed little from each other even for coronary flow measurement [28]. In smaller animals, injections in the ascending aorta have been used for studies on flow distribution in abdominal organs [29,30], but this is not adequate for obtaining estimates of cardiac output or of cerebral or myocardial flows.
3.3. Artifacts occurring with deposited markers
3.3.1. Capillary beds in series
Particles or completely extracted molecules cannot be used for measuring regional blood flows in the second of two capillary beds in series, i.e. in organs with portal circulations such as the liver, the renal medulla and the pineal gland. Systemically injected microspheres can be used to estimate the distribution of hepatic arterial flow to regions of the liver [5], but not the portal vein flow. The total portal vein flow can be derived indirectly from the arterial inflow to all of the organs whose effluent enters the portal vein [29], but a well mixed portal vein injection is required for intrahepatic flow distribution. In the kidney, the microspheres lodge in the glomeruli and do not reach the medulla: this causes gross underestimation of medullary flow.
3.3.2. Obstruction of arterioles
Initially, there was much concern that the deposition of a first injection of spheres would create significant arterial blockage and would bias subsequent measures of regional flows. Aggregation of microspheres is a serious problem, which is easily prevented by using macromolecules and detergent to keep them in suspension and ultrasonification prior to injection. The observation of Hales and Cliff [31] that erythrocytes could be seen to flow past microspheres within capillaries reduced the level of concern, and showed that preventing the aggregation of spheres prevents them from lodging in arterioles and obstructing flow. They then deposit in capillaries. Reeves and Rakusan [32] and Cicutti et al. [33] determined the direction of flow in individual capillaries by injecting two colors of microspheres in series, thus showing that a single sphere in a capillary bed does not stop flow. King et al. [28] observed that, in repeated observations with six well-separated microsphere injections, the regional flows in the heart were essentially unchanged relative to the mean flows, again suggesting no biases due to obstruction. This may be due not only to the lack of obstruction of capillary flow by individual spheres, but also to the displacement of the sphere from the capillary lumen to the interstitium, which occurs within hours to days after injection [31,34].
3.4. No separation from the blood
3.4.1. Size effects
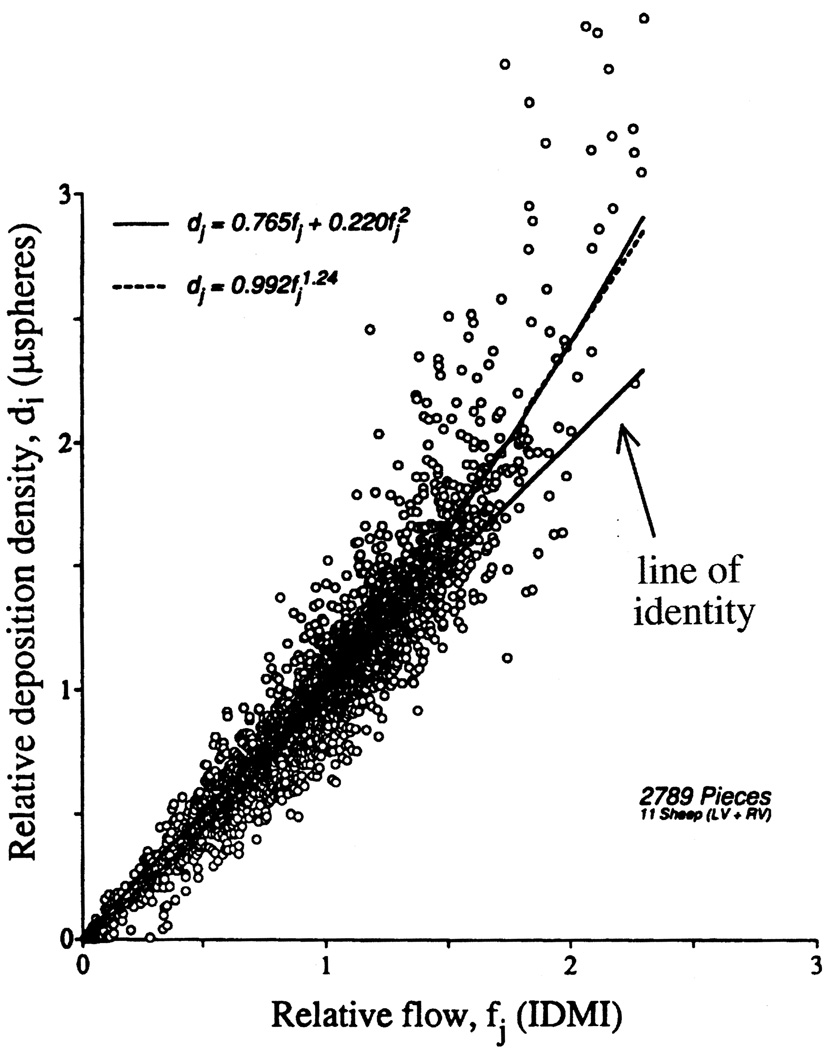
Rheological influences may affect the distribution of spheres so that they are not distributed in proportion to flow. Domenech et al. [25], Yipintsoi et al. [26] and Utley et al. [35] observed that microsphere size had a strong influence on regional deposition density in the heart, larger spheres going preferentially to regions of higher flow. Over the range of 10 to 15 microns diameter this bias appeared unimportant. In any case, because 15-micron spheres are nearly 100% extracted, these have become the standard choice. Ofjord et al. [36] feel that some maldistribution of spheres in the renal cortex is due to the perpendicular branching of the interlobar and arcuate arteries, which results in underestimation of flow to deeper glomeruli. In theory, some separation is expected due to their stiffness and their size [37]. However, the artifact is apparently inconsequential, because a comparison between microspheres and an almost entirely extracted molecular marker, 131I-desmethylimipramine [14,38], showed almost the same distributions in cardiac tissues for both kinds of markers. There was a small tendency for spheres to be deposited preferentially in regions of higher than average flow, as the theory would predict. This artifact appears to cause no bias in low and medium flow regions, but gives 0 to 20% overestimation in the highest flow regions, as seen in Fig. 1. This causes some broadening of the distribution densities of the spheres, as is evident in Fig. 2. This broadening is expected, because when there is any preferential delivery of spheres to high flow regions it is necessarily true that fewer spheres go to low flow regions. The particular example shown in Fig. 2 is the experiment where the greatest separation occurred. Such biases do not occur with respect to measures of flows at the whole organ level in large animals. In small animals such as the shrew and perhaps the mouse such biases are possible, because the major arteries may be of a diameter not much larger than the spheres.
Fig. 1.
Microsphere deposition densities versus 2-iododesmethylimipramine (IDMI) deposition densities in ventricular myocardium of 11 open-chest sheep. The best fitting linear regression line was di = 1.268fi − 0.255, r = 0.925; the best fitting quadratic through the origin (- - -, r = 0.945) and best power fit (———, r = 0.948) are presented. The line of identity is given as a reference. Piece sizes averaged about 220 mg. The relatively higher deposition of microspheres relative to that of the molecular marker IDMI in high flow regions is more than can be accounted for by incompleteness of IDMI extraction and demonstrates a small bias for microspheres to flow in arterioles with higher flow. (Figure reproduced from Ref. [14] with permission from the American Heart Association.)
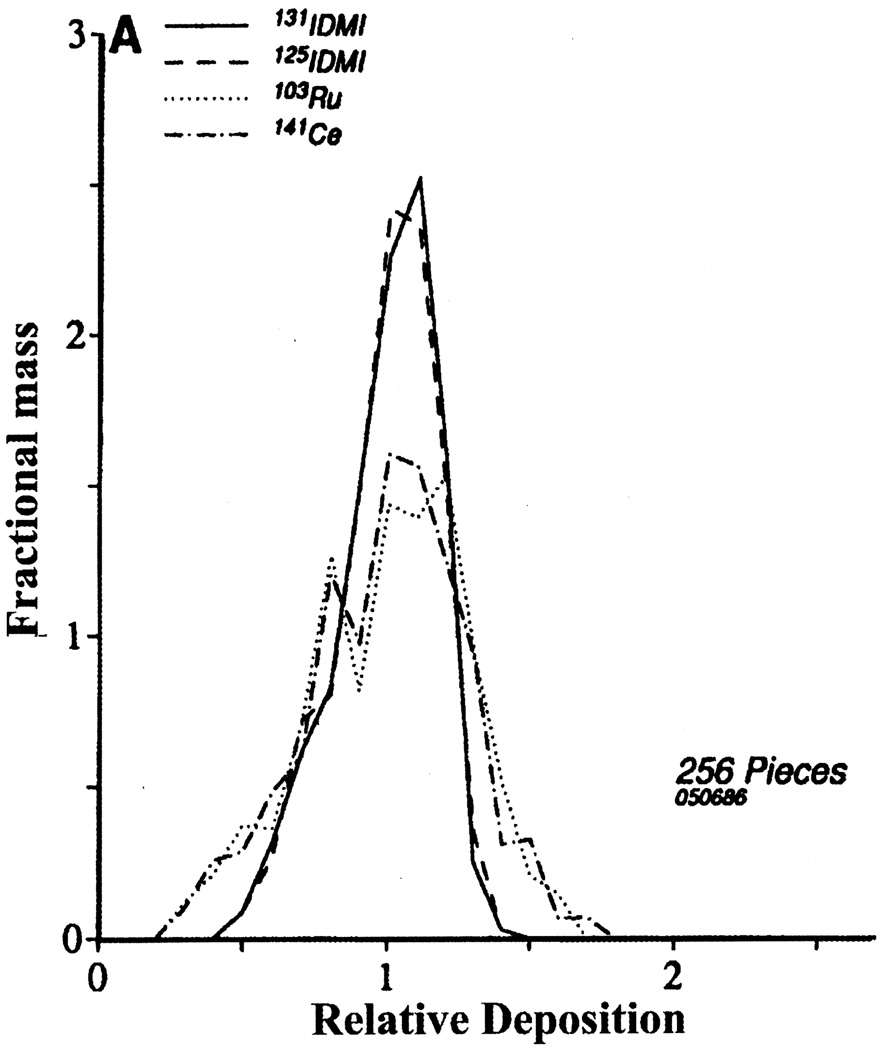
Fig. 2.
Distributions of relative deposition densities for two different IDMIs and two different radioactive microspheres in the ventricular myocardium of an open-chest sheep. The mean for each distribution is, by definition, one. For clarity of presentation, the histograms are presented by polygons joining the midpoints of the classes. The standard deviations of the distributions for the IDMIs were 0.169 and 0.173 and for the microspheres were 0.277 and 0.276. Piece sizes were 250 mg; with smaller piece sizes these distributions would be broader (Figure reproduced from Ref. [67] with permission from the American Heart Association.)
3.4.2. Particle density effects
There is no indication that the difference in density between blood and that of radioactive microspheres (density 1.3 g ml−1) or non-radioactive microspheres (density 1.02–1.05 g ml−1) results in differences in endo-epicardial blood flow ratios [13,39,40]. This implies that rheological properties of the microspheres are minimally affected by differences in their density. The best evidence that buoyancy is not a problem are the observations of Reed and Wood [41], who found no differences in the distributions of microspheres of three densities, 0.2, 1.4 and 3.0 g ml−1, in the lungs of dogs.
3.5. Retention of the marker in the tissue
3.5.1. Retention of spheres in the tissue
Due to the movement of spheres out of the capillaries into the interstitium, retention of microspheres is excellent. Hales and Cliff [31] reported that losses from the rabbit ear or thoracic tissue had stopped within a few minutes after injection and were not detectable for up to 8 weeks. Consigny et al. [34] elegantly demonstrated that, over a period of 5 weeks, some of the microspheres with a diameter of less than 12 microns disappear from the myocardium, but that dislodging of 15-micron microspheres or larger was negligible. To test for long term washout, Medvedev et al. [42] injected 46Sc-labeled radioactive microspheres into donor rats and subsequently implanted their hearts into recipient animals. The donor hearts were retrogradely perfused by attaching the ascending aorta to the abdominal aorta of the recipient animals and the pulmonary artery drained into the caval vein. After 4 weeks they found no accumulation of radioactivity in the lungs of the recipient animal [42].
3.5.2. Retention of label in spheres
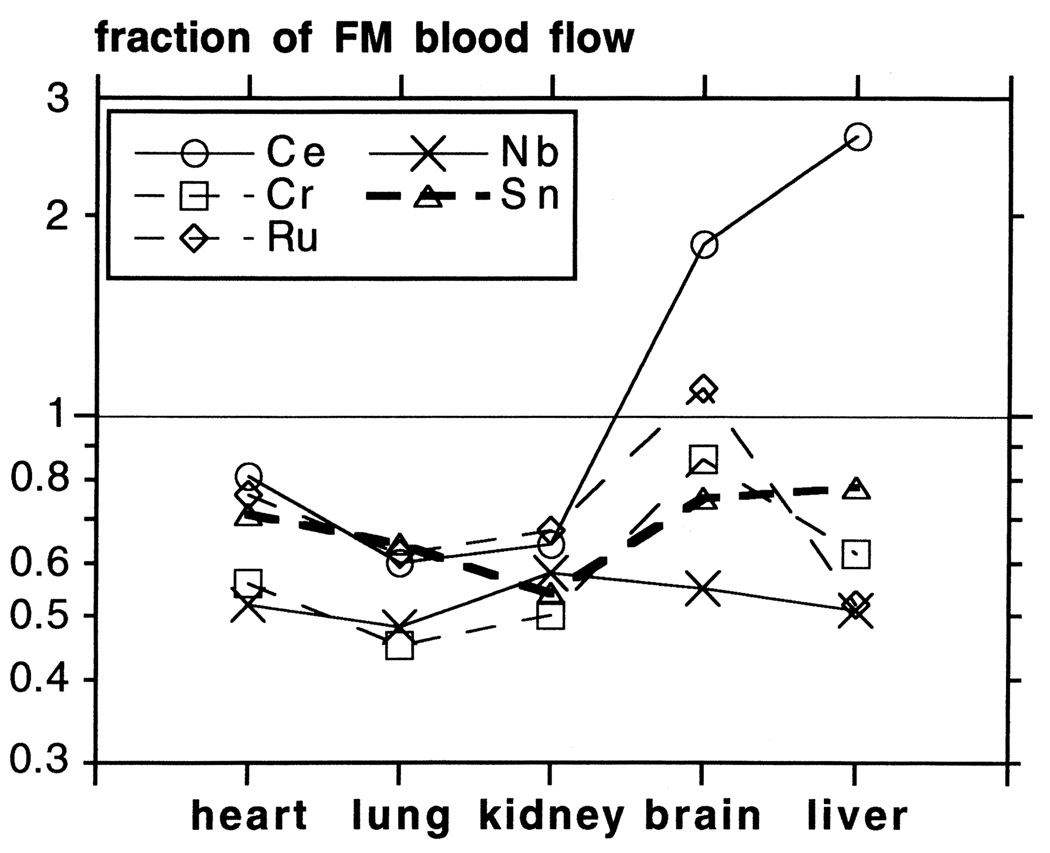
The label can leach from the spheres. Although some studies reported minor loss of label [34], other studies [42] indicated significant leaching of 46Sc from the spheres. Losses of 125I [43] and 113Sn were also reported [44]. The most convincing indication of leaching of radioisotope labels from microspheres was a comparison between radioactive and fluorescent microspheres [45]: two sets of radioactive and two sets of fluorescent spheres were injected simultaneously in rabbits. They were sacrificed at 5 min, and 1 and 2 months later. Even after 2 months, blood flow estimates for the two fluorescent microspheres did not differ, but there was a 25% difference between the estimates from the two radioactive microspheres. Moreover, blood flow estimations were 20–50% lower with the various radioactive microspheres than with the fluorescent microspheres, while brain and liver blood flow values varied even more between isotopes (Fig. 3). Loss of label was evident within 1 day for 51Cr, and within 2 weeks for 141Ce, 95Nb and 85Sr. Thus, radioactive isotopes leach from the spheres. The leaching is different between radioisotopes and between organs. Another problem with the leaching of radioisotopes is that the leached label may bind to certain tissues, causing overestimation of blood flow, as, for example, by Ce in the liver and Ce and Ru in the brain (Fig. 3) [45].
Fig. 3.
Error in blood flow measurements in chronic experiments by radioactive microspheres, specified per organ. Fluorescent and radioactive microspheres were injected simultaneously in rabbits 2 months before the animals were killed. No indications were found for the disappearance of fluorescent labels from the spheres. Radioactive microsphere blood flow values are expressed as a fraction of the fluorescent microsphere (FM) blood flow (mean of two simultaneously injected labels). Note log scale on vertical axis. Each data point is the average of three to five tissue samples from organs of one to two animals. (Figure reproduced from Ref. [45] with permission from the American Physiological Society.)
3.6. Measuring accuracy
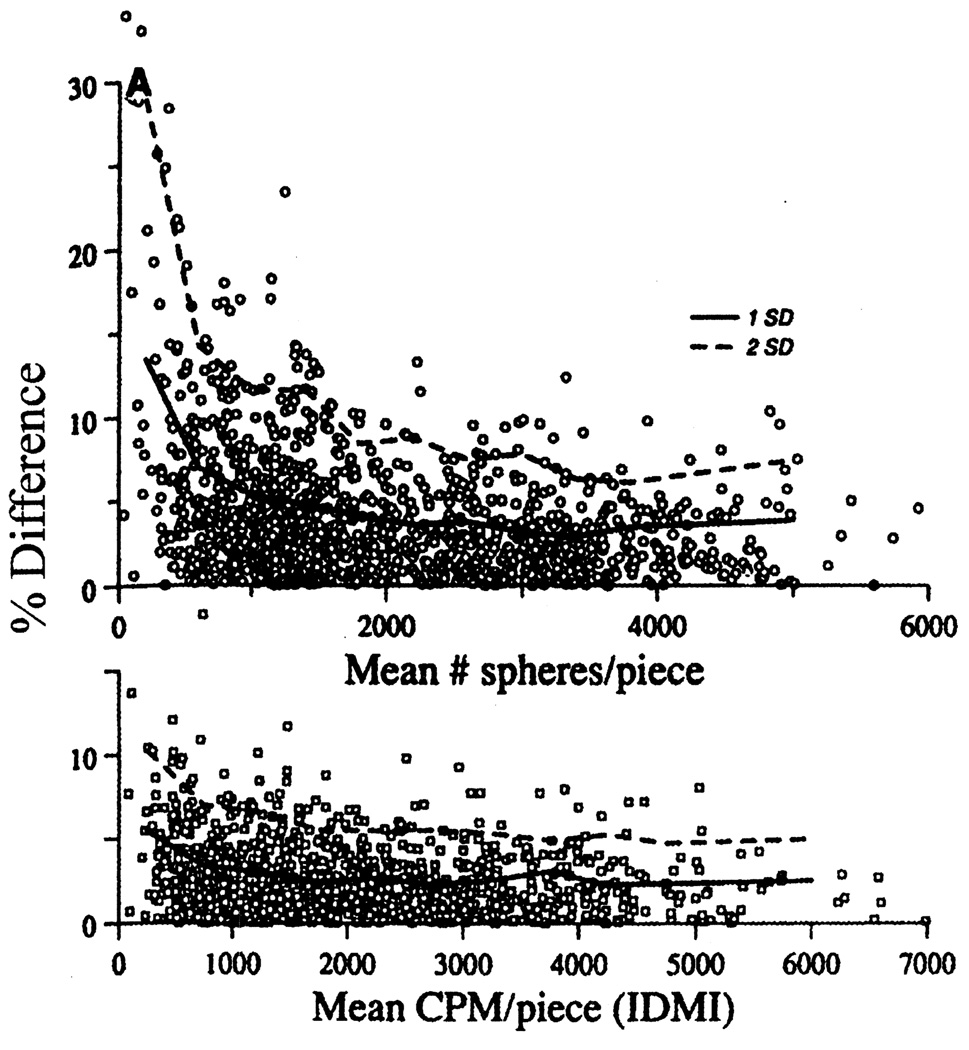
Measurement accuracy has been considered in detail, mathematically and experimentally. Buckberg et al. [6] laid out the idea that one needed at least 400 spheres in a given piece for a 5% statistical accuracy in flow estimation. This was affirmed by Nose et al. [8] and reaffirmed in detailed experiments against a molecular marker by Bassingthwaighte et al. [14], as shown in Fig. 4. The fundamental measure is of the number of spheres per piece, which is what Buckberg et al. considered from the point of view of the Poisson statistics. The important contribution of the Poisson distribution of microspheres to the total error of the microspheres can be observed from the difference in error between the microsphere method and IDMI in Fig. 4. Additional error is always involved when the number of spheres is measured indirectly, e.g. from radioactivity levels, fluorescence, or color intensity. This additional measurement error for radioactivity stemmed from three sources: non-uniform labeling of the spheres, variation in sphere diameter and isotopic counting statistical variation, again a Poisson process. The manufacturing improved after a few years so that labeling was uniform. Because different diameters of microspheres behave differently in the blood stream (see above) and larger spheres contain more label per sphere, a uniform diameter distribution of the microspheres is likely to increase reproducibility of the blood flow measurements. This has been recognized by the companies supplying the radioactive microspheres, resulting in a reduction of the standard deviation of the diameter from 3 microns in the 1970s to less than 0.5 microns in the 1980s. The radioactive count emission error could be reduced to any chosen level by simply counting for a longer period, since the inherent variation is 1/(√N), where N is the total number of counts. The downside of arbitrarily counting until there is 0.1% error, for example, means counting to 1,000,000 counts, which takes a long time for a piece containing few spheres. The counting of the numbers of spheres directly overcomes this source of error, but introduces other issues such as how to count ALL the spheres without losing any. Tabulations of the types of errors associated with each of the various microsphere methods are given by Austin et al. [17] and Prinzen and Glenny [11]. The latter cover five types of measures of sphere depositions: radioactivity, direct counting of numbers of spheres, color extraction, fluorescent dye extraction, and X-ray fluorescence measurement.
Fig. 4.
Assessment of error in the deposition technique as a function of the number of spheres (upper panel) or amount of tracer (lower panel) deposited per sample of myocardium. The ordinates are absolute values of the percent differences between paired observations in five sheep (1258 pieces in total). Confidence limits are shown at 68% (1 SD, ———) and 95% (2 SD, - - -) of the observations in 11 classes encompassing the range. The larger error with the microsphere technique can be largely attributed to the Poisson distribution of the deposition of the microspheres. (Figure reproduced from Ref. [14] with permission from the American Physiological Society.)
4. Development and use of non-radioactive microspheres
Despite the problems described above, blood flow measurement with radioactive microspheres is currently regarded as a ‘gold standard’. Because the use of radioactivity is becoming increasingly problematic due to restrictive legislation and higher costs of storage and disposal and the desire to reduce radiation load to personnel, non-radioactive microspheres have been developed.
4.1. Colored spheres
Hale et al. [46] introduced a method for measuring blood flow with colored microspheres. Blood and tissue samples were digested and the spheres were isolated by centrifugation and then counted in a hemocytometer [46]. As an alternative to manually counting colored microspheres, Kowallik et al. [39] developed a technique where the microspheres are isolated from tissue and blood by digestion with potassium hydroxide and subsequent microfiltration. The dyes are extracted from the colored microspheres and the separation of up to five colors and measurement of their concentration was performed by spectrometry. This method has been further validated [47,48] and employed extensively, although usually with a maximum of three different colors [49].
4.2. Fluorescent microspheres
These were introduced 2 years later [50]. Using five or six different fluorescent labels in the same experiment, good correlations were demonstrated between radioactive and fluorescent microspheres for blood flow in heart and kidneys (r = 0.96–0.98), lungs (r = 0.99) [50] (ischemic and non-ischemic), myocardium [13] and a variety of other organs [13,50]. In most cases the slopes were not significantly different from unity nor the intercept from the origin. By simultaneous injection of two radioisotopes and two fluorescent labels it was shown that the accuracy of the fluorescent microsphere technique was similar to that of the radioactive microsphere technique [13,50].
4.3. Comparison of the types of microspheres
The pros and cons of radioactive, colored and fluorescent microspheres are discussed elsewhere [11] and summarized in Table 2. Apart from the radiation exposure a disadvantage of radioactive microspheres is that they are increasingly more expensive than non-radioactive microspheres and that the costs for disposal of the radioactively contaminated animals may be high. Moreover, radioisotopes decay, limiting their lifetime. Radioisotopes, leaching from the spheres within weeks after injection in the animal, basically preclude their use in chronic animal experiments [45].
Table 2.
Pros and cons of different microsphere types (+, positive virtues; −, negative attributes; 0, absent)
| Radioactive | Colored | Fluorescent | |
|---|---|---|---|
| Radiation load | − − − | 0 | 0 |
| Costs of spheres | − − − | − | − − |
| Costs of equipment | − − − | − | − − |
| Labor | − | − − | − − |
| Decay label | − − | 0 | 0 |
| Sensitivity | + | − | + |
| Spectral separation | + + | + | + + + |
| Retention of label | − | ? | + |
| Optical separation | − | + | + |
A disadvantage of the non-radioactive microspheres is that the time and effort for processing of samples to estimate the number of microspheres deposited is more than measuring the radioactivity by gamma counting. In general, the use of non-radioactive microspheres is economically preferable especially if the number of samples is relatively low (limited time for processing with non-radioactive microspheres) or the size of the animals is relatively large (high disposal costs for radioactive microspheres). Many other considerations may determine the choice of the type of microsphere. For example, if both blood flow and content of a chemical substance is to be determined in the same sample, non-radioactive microspheres offer the advantage that instruments used for the chemical analysis are not contaminated by radioactivity.
There is currently only one study where fluorescent and colored microspheres have been used simultaneously and have been compared with radioactive microspheres [49]. In this study, no differences were found in the accuracy of blood flow values obtained with both types of non-radioactive microspheres. However, studies comparing fluorescent with radioactive microspheres [45,50] consistently find smaller variations than studies comparing colored with radioactive microspheres [39,48]. Also, only fluorescent microspheres have been shown to be as accurate as radioactive microspheres [13] and also to be reliable for chronic experiments [45,50]. Colored microspheres have the disadvantages that the spectra from the different colors overlap [49] and that the sensitivity to detect the colors by spectroscopy is limited [51]. Some investigators have compensated for this by using a larger number of colored microspheres [39,48]. A recent paper showed that the sensitivity of detection of the colors can be improved when using HPLC. Also, separation of the various colors was better [51]. This improvement comes at the expense of increased costs and complexity of the assay.
4.4. Automation
Automation is needed in the use of non-radioactive microspheres. Microspheres can be counted using a fluorescence activated cell sorter (FACS) analyzer [52]. FACS analysis involves detection of particles with a predefined shape and size and has the advantage over manual counting of microspheres in that the entire homogenate is analyzed and that the microspheres do not have to be separated from the homogenate. Because only particles with a predefined size and color are counted by FACS analysis, this method may be less sensitive to spectral interference from tissue or blood than dye extraction methods.
The time consuming and complex filtration of homogenates can be replaced by centrifugal sedimentation [13]. Spectrometry has been automated by interfacing a liquid handling system with a flow cell in the spectrometer [47]. A recently developed robot system even integrates tissue digestion, microsphere isolation and fluorimetry in one system [53]. This appears the most time saving, but also the most expensive, solution.
Another interesting new development is the automated cryomicrotome–video combination by Barlow et al. [54]. By automated sectioning of the organ and recording of the fluorescence using CCD arrays and four different filters, the location of fluorescent microspheres can be determined in 3D space. Off-line, regions can be defined in which the number of spheres can be determined. This method gives a high degree of automation and very high spatial resolution.
4.5. Microsphere flow measurement at the histological level
It should be possible to take advantage of the color or fluorescent quality of the non-radioactive microspheres. New possibilities for blood flow measurements, such as determining the distribution of blood to structures on a microscopic scale, now exist. Using methods of quantitative histology [55–57] it should be possible to quantitate perfusion to whole organs as well as intraorgan structures at a level not previously possible. Fluorescently labeled ‘molecular microspheres’ might replace the radioactive microspheres used for the autoradiography for regional flow measurements in hamster hearts [58] and provide planar images of ‘flow’ distribution using serial section techniques [54], but this will require new agents.
Blood flow in islets of Langerhans was measured by direct microscopy and counting non-labeled microspheres [30]. Taking this approach one step further, Jasper et al. [59] measured blood flow in the capsule, cortex and medulla of the adrenals by counting the number of three different fluorescent microspheres in these structures under the microscope using appropriate fluorescence filters. Other studies have used colored or fluorescent microspheres to study interarterial microvascular anastomoses in the myocardium of dogs [33], the direction of flow in the myocardial microcirculation [32] and the separation of pulmonary and bronchial blood flow in the airways [60].
5. Smaller animals
The McDevitt and Nies study [2] demonstrates some of the problems associated with application of the method to small animals. These investigators do not provide data on blood flow in heart and brain. The authors explained the absence of heart perfusion data by their reluctance to inject microspheres directly into the left ventricular or left atrial cavities and because the potential error in estimates of myocardial flows might be higher than for other organs.
McDevitt and Nies used Tween 80 in Dextran 10% to suspend the microspheres. Later studies showed that both agents, used to prevent aggregation of microspheres, can cause transient hypotension [61,62]. Instead of dextran, other macromolecular solutions, such as Ficoll/Isopaque or plasma replacement solutions, can be used [13,61]. Measurement of absolute, rather than relative, flows, and measurement of cardiac output requires withdrawing an arterial reference sample, which itself can be hypotensive, and again more so in small animals. Combined withdrawal of reference blood and infusion with blood substitute has been applied to prevent/minimize these hemodynamic effects [61].
McDevitt and Nies used microspheres in rats, animals relatively small compared with the species generally used at that time, but not smaller than fetal sheep [1]. Nowadays, there are strong incentives to use even smaller species. Transgenic mice are now often used to determine the physiological function of certain genes in vivo. The use of mice, whose size is only 10% of that of rats, amplifies the problems mentioned above and may create more problems than those encountered in rats. For example, anesthesia and cannulation, either acute or chronic, is technically demanding.
In a first paper on the use of microspheres in mice, Wetterlin et al. [63] reported unreliable and non-reproducible results. Later studies reported better results, using a left ventricular injection catheter with a diameter of 0.4 mm and injection of about 150,000 spheres in 100 µl [64]. Similar numbers have been used in the chick embryo, but application in this model was facilitated by the possibility to inject the spheres in a peripheral vein, due to the right–left shunt in the fetal circulation [65]. Even after more than 20 years only a few investigators used the microsphere method including the reference sample approach in mice. Dowell et al. [66] used LV injection in anesthetized mice, while others [64] limited their studies to measurement of the distribution of cardiac output, which does not require a reference sample. Studies on the distribution of blood flow in abdominal organs used injection into the ascending aorta [29,30], apparently successfully. The withdrawal of arterial reference samples can be avoided when the animals are instrumented with a flowprobe to measure absolute blood flow in a certain part of the circulation. This reduces the problem of management of the amount of fluid withdrawn from these small animals.
6. Conclusion
The microsphere method is an important technique in cardiovascular research. Although the method has few artifacts, the particulate nature of the spheres likely causes a slight overestimation of inhomogeneities in flow. McDevitt and Nies [2] demonstrated the utility of the method in what was then considered a small animal, the rat, and now, with modernization, it is being refined for use in mice. Non-radioactive microspheres increase the applicability of the technique outside isotope laboratories and even offer new possibilities. Extension of the microsphere technique to studies in transgenic animals appears just a matter of time.
References
- 1.Rudolph M, Heymann MA. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967;21:163–185. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- 2.McDevitt DG, Nies AS. Simultaneous measurement of cardiac output and its distribution with microspheres in the rat. Cardiovasc Res. 1976;10:494–498. doi: 10.1093/cvr/10.4.494. [DOI] [PubMed] [Google Scholar]
- 3.Mendell PL, Hollenberg NK. Cardiac output distribution in the rat: comparison of rubidium and microsphere methods. Am J Physiol. 1971;221:1617–1620. doi: 10.1152/ajplegacy.1971.221.6.1617. [DOI] [PubMed] [Google Scholar]
- 4.Rakusan K, Blahitka J. Cardiac output distribution in rats measured by injection of radioactive microspheres via cardiac puncture. Can J Physiol Pharmacol. 1974;52:230–235. doi: 10.1139/y74-031. [DOI] [PubMed] [Google Scholar]
- 5.Daemen MJAP, Thijssen HHW, Van Essen H, et al. Liver blood flow measurement in the rat: the electromagnetic vs. the microsphere and the clearance methods. J Pharmacol Methods. 1989:287–297. doi: 10.1016/0160-5402(89)90066-1. [DOI] [PubMed] [Google Scholar]
- 6.Buckberg GD, Luck JC, Payne DB, et al. Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol. 1971;31:598–604. doi: 10.1152/jappl.1971.31.4.598. [DOI] [PubMed] [Google Scholar]
- 7.Dole WP, Jackson DL, Rosenblatt JI, Thompson WL. Relative error and variability in blood flow measurements with radiolabeled microspheres. Am J Physiol. 1982;243:H371–H378. doi: 10.1152/ajpheart.1982.243.3.H371. [DOI] [PubMed] [Google Scholar]
- 8.Nose Y, Nakamura T, Nakamura M. The microsphere method facilitates statistical assessment of regional blood flow. Basic Res Cardiol. 1985;80:417–429. doi: 10.1007/BF01908186. [DOI] [PubMed] [Google Scholar]
- 9.Reneman RS, Jageneau AHM, Van Gerven W, Dony J, Beirnaert P. The radioactive microsphere method for the assessment of regional myocardial blood flow after coronary artery occlusion. Pflug Arch. 1975;353:337–347. doi: 10.1007/BF00587030. [DOI] [PubMed] [Google Scholar]
- 10.Flameng F, Winkler B, Wuesten B, Schaper W. Minimum requirements for the measurement of regional myocardial flow using tracer microspheres. Bibl Anat. 1977;15:24–29. [PubMed] [Google Scholar]
- 11.Prinzen FW, Glenny RW. Developments in non-radioactive microsphere techniques for blood flow measurement. Cardiovasc Res. 1994;28:1467–1475. doi: 10.1093/cvr/28.10.1467. [DOI] [PubMed] [Google Scholar]
- 12.Stanek KA, Smith TL, Murphy WR, Coleman TG. Hemodynamic disturbances in the rat as a function of the number of microspheres injected. Am J Physiol. 1983;245:H920–H923. doi: 10.1152/ajpheart.1983.245.6.H920. [DOI] [PubMed] [Google Scholar]
- 13.Van Oosterhout MFM, Willigers HMM, Reneman RS, Prinzen FW. Validation of fluorescent microsphere technique for measurement of organ perfusion with improved sample processing method. Am J Physiol. 1995;269:H725–H733. doi: 10.1152/ajpheart.1995.269.2.H725. [DOI] [PubMed] [Google Scholar]
- 14.Bassingthwaighte JB, Malone MA, Moffett TC, et al. Molecular and particulate depositions for regional myocardial flows in sheep. Circ Res. 1990;66:1328–1344. doi: 10.1161/01.res.66.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richmond DR, Tauxe WN, Bassingthwaighte JB. Albumin macroaggregates and measurements of regional blood flow: validity and application of particle sizing by Coulter counter. J Lab Clin Med. 1970;75:336–346. [PMC free article] [PubMed] [Google Scholar]
- 16.Heymann MA, Payne BD, Hoffman JIE, Rudolph AM. Blood flow measurements with radionuclide-labeled microspheres. Prog Cardiovasc Dis. 1977;20:55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- 17.Austin RE, Hauck WW, Aldea GS, et al. Quantitating error in blood flow measurements with radioactive microspheres. Am J Physiol. 1989;257:H280–H288. doi: 10.1152/ajpheart.1989.257.1.H280. [DOI] [PubMed] [Google Scholar]
- 18.Love WD, Burch GE. Differences in the rate of Rb86 uptake by several regions of the myocardium of control dogs and dogs receiving l-norepinephrine or pitressin. J Clin Invest. 1957;36:479–484. doi: 10.1172/JCI103445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tancredi RG, Yipintsoi T, Bassingthwaighte JB. Capillary and cell wall permeability to potassium in isolated hearts. Am J Physiol. 1975;229:537–544. doi: 10.1152/ajplegacy.1975.229.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renkin EM. Exchangeability of tissue potassium in skeletal muscle. Am J Physiol. 1959;197:1211–1215. doi: 10.1152/ajplegacy.1959.197.6.1211. [DOI] [PubMed] [Google Scholar]
- 21.Ueda H, Kaihara S, Ueda K, et al. Regional myocardial blood flow measured by I-131 labelled macroaggregated albumin (I-131-MAA) Jpn Heart J. 1965;6:534–542. doi: 10.1536/ihj.6.534. [DOI] [PubMed] [Google Scholar]
- 22.Taplin GV, Johnson DE, Dore EK, et al., editors. Organ visualization by photoscanning using micro- and macroaggregates of radioalbumin. Vienna: IAEA; 1964. pp. 3–31. [Google Scholar]
- 23.Richmond DR, Yipintsoi T, Coulam CM, Titus JL, Bassingthwaighte JB. Macroaggregated albumin studies of the coronary circulation in the dog. J Nucl Med. 1973;14:129–134. [PMC free article] [PubMed] [Google Scholar]
- 24.Bassingthwaighte JB, Dobbs WA, Yipintsoi T. In: Heterogeneity of myocardial blood flow. Maseri A, editor. 1972. pp. 197–205. [Google Scholar]
- 25.Domenech RJ, Hoffman JIE, Noble MIM, et al. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res. 1969;25:581–596. doi: 10.1161/01.res.25.5.581. [DOI] [PubMed] [Google Scholar]
- 26.Yipintsoi T, Dobbs WA, Scanlon PD, Knopp TJ, Bassinghtwaighte JB. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res. 1973;33:573–587. doi: 10.1161/01.res.33.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy MN, De Olivera JM. Regional distribution of myocardial blood flow in the dog as determined with Rb86. Circ Res. 1961;9:96–98. doi: 10.1161/01.res.9.1.96. [DOI] [PubMed] [Google Scholar]
- 28.King RB, Bassingthwaighte JB, Hales JRS, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ Res. 1985;57:285–295. doi: 10.1161/01.res.57.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarin SK, Groszmann RJ. Splanchnic and systemic hemodynamics in mice using a radioactive microsphere technique. Am J Physiol. 1990;258:G365–G369. doi: 10.1152/ajpgi.1990.258.3.G365. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson P-O, Andersson A, Jansson L. Pancreatic islet blood flow in normal and obese-hyperglycemic (ob/ob) mice. Am J Physiol. 1996;271:E990–E995. doi: 10.1152/ajpendo.1996.271.6.E990. [DOI] [PubMed] [Google Scholar]
- 31.Hales JRS, Cliff WJ, editors. Direct observations of the behaviour of microspheres in microvasculature. Basel: Karger, S; 1977. pp. 87–91. [PubMed] [Google Scholar]
- 32.Reeves WJ, Rakusan K. Myocardial capillary flow pattern as determined by the method of coloured microspheres. Adv Exp Med Biol. 1988;222:447–453. doi: 10.1007/978-1-4615-9510-6_53. [DOI] [PubMed] [Google Scholar]
- 33.Cicutti N, Rakusan K, Downey HF. Colored microspheres reveal interarterial microvascular anastomoses in canine myocardium. Basic Res Cardiol. 1992;87:400–409. doi: 10.1007/BF00796525. [DOI] [PubMed] [Google Scholar]
- 34.Consigny PM, Verrier ED, Payne BD, et al. Acute and chronic microsphere loss from canine ventricular myocardium. Am J Physiol. 1982;242:H392–H404. doi: 10.1152/ajpheart.1982.242.3.H392. [DOI] [PubMed] [Google Scholar]
- 35.Utley J, Carlson EL, Hoffman JIE, Martinez HM, Buckberg GD. Total and regional myocardial blood flow measurements with 25 µ, 15 µ, 9 µ and filtered 1–10 µ diameter microspheres and antipyrine in dogs and sheep. Circ Res. 1974;34:391–405. doi: 10.1161/01.res.34.3.391. [DOI] [PubMed] [Google Scholar]
- 36.Ofjord ES, Clausen G, Aukland K. Skimming of microspheres in vitro: implications for measurement of intrarenal blood flow. Am J Physiol. 1981;241:H342–H347. doi: 10.1152/ajpheart.1981.241.3.H342. [DOI] [PubMed] [Google Scholar]
- 37.Yen RT, Fung YC. Effect of velocity distribution on red cell distribution in capillary blood vessels. Am J Physiol. 1978;235:H251–H257. doi: 10.1152/ajpheart.1978.235.2.H251. [DOI] [PubMed] [Google Scholar]
- 38.Bassingthwaighte JB, Malone MA, Moffett TC, et al. Validity of microsphere depositions for regional myocardial flows. Am J Physiol. 1987;253:H184–H193. doi: 10.1152/ajpheart.1987.253.1.H184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowallik P, Schulz R, Guth BD, et al. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation. 1991;83:974–982. doi: 10.1161/01.cir.83.3.974. [DOI] [PubMed] [Google Scholar]
- 40.Abel FL, Cooper RH, Beck RR. Use of fluorescent latex microspheres to measure coronary blood flow distribution. Circ Shock. 1993;41:156–161. [PubMed] [Google Scholar]
- 41.Reed JH, Wood EH. Effect of body position on vertical distribution of pulmonary flow. J Appl Physiol. 1970;28:303–311. doi: 10.1152/jappl.1970.28.3.303. [DOI] [PubMed] [Google Scholar]
- 42.Medvedev OS, Martynova ER, Akchurin RS, Khalatov VY. Experimental estimation of chronic microsphere loss from the rat myocardium. Biull Eksp Biol Med. 1987;103:8–10. [PubMed] [Google Scholar]
- 43.Hales JRS, Bennett JW, Fawcett AA. Effects of acute cold exposure on the distribution of cardiac output in the sheep. Pflug Arch. 1976;366:153–157. doi: 10.1007/BF00585871. [DOI] [PubMed] [Google Scholar]
- 44.Hales JRS, King RB, Fawcett AA. Observations on the validity of using NEN-Trac microspheres for measuring organ blood flow. Pflug Arch. 1979;379:295–296. doi: 10.1007/BF00581435. [DOI] [PubMed] [Google Scholar]
- 45.Van Oosterhout MFM, Prinzen FW, Sakurada S, Glenny RW, Hales JRS. Fluorescent microspheres are superior to radioactive microspheres in chronic blood flow measurements. Am J Physiol. 1998;275:H110–H115. doi: 10.1152/ajpheart.1998.275.1.H110. [DOI] [PubMed] [Google Scholar]
- 46.Hale SL, Vivaldi MT, Kloner RA. Fluorescent microspheres: a new tool for visualization of ischemic myocardium in rats. Am J Physiol. 1986;251:H863–H868. doi: 10.1152/ajpheart.1986.251.4.H863. [DOI] [PubMed] [Google Scholar]
- 47.Wieland W, Wouters PF, Van Aken H, Flameng W. Computers in cardiology. Los Alamitos, USA: IEEE Society Press; 1993. Measurement of organ blood flow with coloured microspheres: a first time-saving improvement using automated spectrophotometry; pp. 691–694. [Google Scholar]
- 48.Hakkinen JP, Miller MW, Smith AH, Knight DR. Measurement of organ blood flow with coloured microspheres in the rat. Cardiovasc Res. 1995;29:74–79. [PubMed] [Google Scholar]
- 49.Hodeige D, de Pauw M, Eechaute W, Weyne J, Heyndrickx GR. On the validity of blood flow measurement using colored microspheres. Am J Physiol. 1999;276:H1150–H1158. doi: 10.1152/ajpheart.1999.276.4.H1150. [DOI] [PubMed] [Google Scholar]
- 50.Glenny RW, Bernard S, Brinkley M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. J Appl Physiol. 1993;74:2585–2597. doi: 10.1152/jappl.1993.74.5.2585. [DOI] [PubMed] [Google Scholar]
- 51.Mazoit JX, Le Guen R, Decaux A, Albaladejo P, Samii K. Application of HPLC to counting of colored microspheres in determination of regional blood flow. Am J Physiol. 1998;274:H1041–H1047. doi: 10.1152/ajpheart.1998.274.3.H1041. [DOI] [PubMed] [Google Scholar]
- 52.Austin GE, Martino-Salzman D, Justicz AG, et al. Determination of regional myocardial blood flow using fluorescent microspheres. Am J Cardiovasc Pathol. 1993;4:352–357. [PubMed] [Google Scholar]
- 53.Raab S, Thein E, Harris AG, Messmer K. A new sample-processing unit for the fluorescent microsphere method. Am J Physiol. 1999;276:H1801–H1806. doi: 10.1152/ajpheart.1999.276.5.H1801. [DOI] [PubMed] [Google Scholar]
- 54.Barlow C, Ewen J, Glenny RW, Kelly J. Fluorescent imaging for measurement of regional blood flow. FASEB J. 1996;10:A324. [Google Scholar]
- 55.Gundersen HJG. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- 56.Stereo DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 57.Luchtel DL, Boykin JC, Bernard SL, Glenny RW. Histological methods to determine blood flow distribution with fluorescent microspheres. Biotech Histochem. 1998;73:291–309. doi: 10.3109/10520299809141123. [DOI] [PubMed] [Google Scholar]
- 58.Stapleton DD, Moffett TC, Baskin DG, Bassingthwaighte JB. Autoradiographic assessment of blood flow heterogeneity in the hamster heart. Microcirculation. 1995;2:277–282. doi: 10.3109/10739689509146773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jasper MS, McDermott P, Gann DS, Engeland WC. Measurement of blood flow to the adrenal capsule, cortex and medulla in dogs after hemorrhage by fluorescent microspheres. J Auton Nerv Syst. 1990;30:159–168. doi: 10.1016/0165-1838(90)90140-e. [DOI] [PubMed] [Google Scholar]
- 60.Bernard SL, Glenny RW, Polissar NL, Luchtel DL, Lakshminarayan S. Distribution of pulmonary and bronchial blood supply to airways measured by fluorescent microspheres. J Appl Physiol. 1996;80:430–436. doi: 10.1152/jappl.1996.80.2.430. [DOI] [PubMed] [Google Scholar]
- 61.Stanek KA, Coleman TG, Smith TL, Murphy WR. Two hemodynamic problems commonly associated with the microsphere technique for measuring regional blood flow in rats. J Pharmacol Methods. 1985;13:117–124. doi: 10.1016/0160-5402(85)90055-5. [DOI] [PubMed] [Google Scholar]
- 62.Grund F, Sommerschild HT, Kirkeboen KA, Ilebekk A. Cardiovascular effects of the microsphere suspending agent, Tween 80, in pigs. Acta Physiol Scand. 1995;155:331–332. doi: 10.1111/j.1748-1716.1995.tb09982.x. [DOI] [PubMed] [Google Scholar]
- 63.Wetterlin S, Aronson KF, Byorkman I, Ahlgren I. Studies on methods for determination of the distribution of cardiac output in the mouse. Scand J Clin Lab Invest. 1977;37:451–454. [PubMed] [Google Scholar]
- 64.Quintana A, Raczka E, Bonaccorsi A. Cardiac output distribution measured with radioactive microspheres in the mouse. Pharmacol Res Commun. 1979;11:245–252. doi: 10.1016/s0031-6989(79)80086-7. [DOI] [PubMed] [Google Scholar]
- 65.Mulder ALM, Van Golde J, Prinzen FW, Blanco C. Cardiac output distribution in the chicken embryo from stage 36 to 45. Cardiovasc Res. 1997;34:525–528. doi: 10.1016/s0008-6363(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 66.Dowell RT, Gairola CG, Diana JN. Reproductive organ blood flow measured using radioactive microspheres in diestrous and estrous mice. Am J Physiol. 1992;262:R666–R670. doi: 10.1152/ajpregu.1992.262.4.R666. [DOI] [PubMed] [Google Scholar]
- 67.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res. 1989;65:578–590. doi: 10.1161/01.res.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]