Abstract
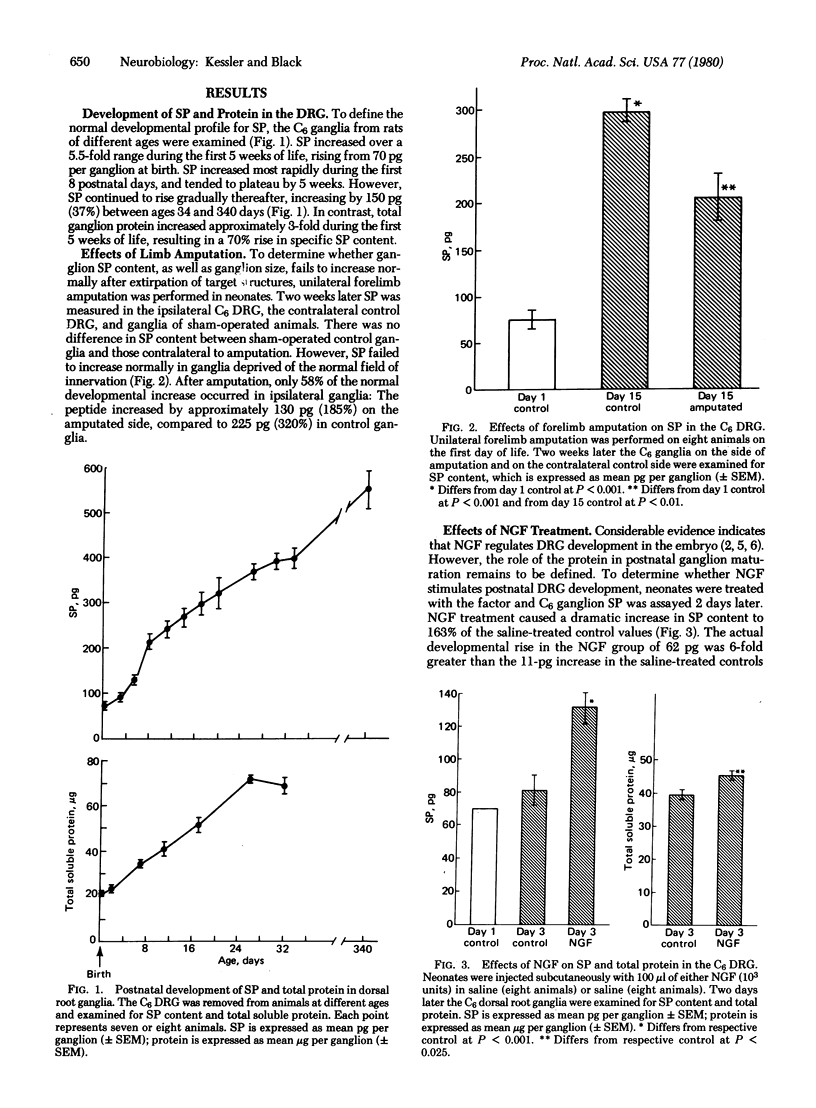
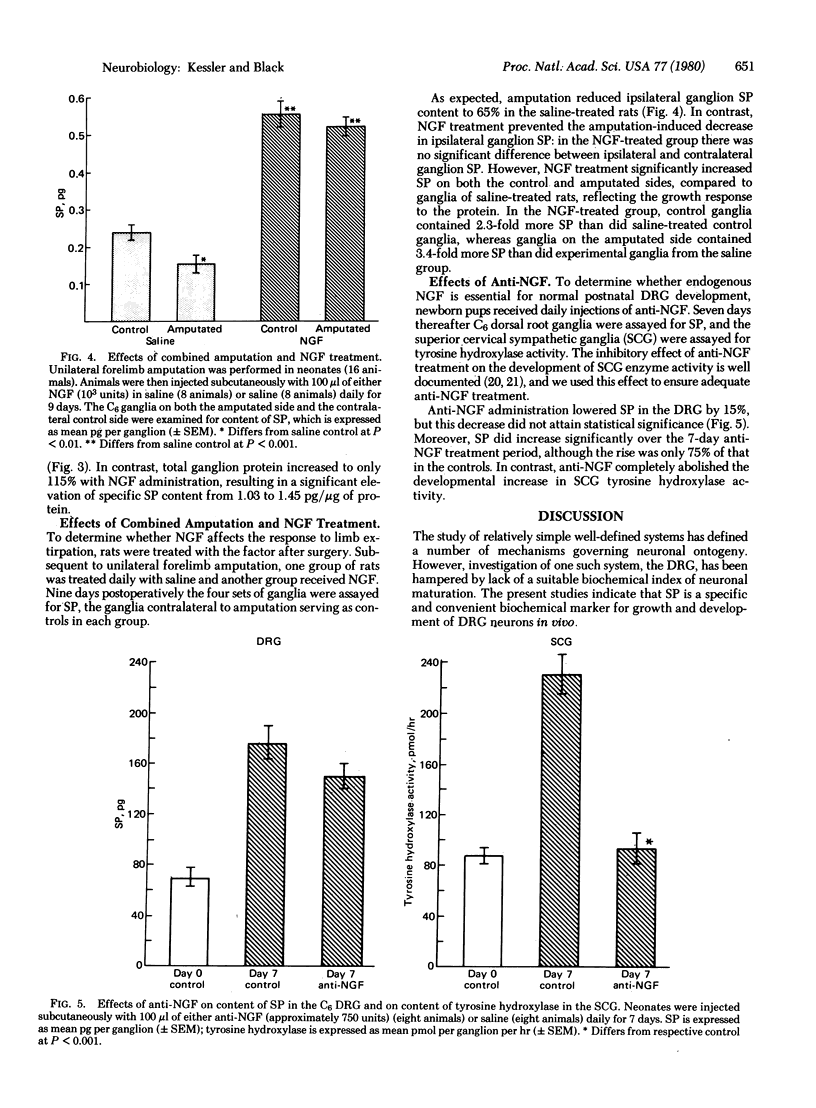
The development of the putative neurotransmitter substance P (SP) in rat dorsal root ganglion (DRG) was defined in vivo. The sixth cervical DRG of newborn rats contained 70 pg of SP, and the ganglionic content increased 5.5-fold during the first 5 weeks of life. Forelimb amputation partially prevented the normal developmental increase of SP in the sixth cervical DRG destined to innervate that limb. Conversely, treatment with nerve growth factor (NGF) increased both ganglionic SP and total ganglion protein. Moreover, NGF administration prevented the failure of SP development that followed amputation, suggesting that NGF may mediate the limb-DRG interaction. However, treatment with antiserum to NGF failed to significantly inhibit development of ganglion SP. Consequently, neonatal ganglia may remain responsive to NGF, without requiring the protein for survival. SP appears to be an excellent index of the maturation of neurons in dorsal root ganglia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti P. U., Levi-Montalcini R., Kettler R., Thoenen H. Comparative studies on the effect of the nerve growth factor on sympathetic ganglia and adrenal medulla in newborn rats. Brain Res. 1972 Sep 15;44(1):197–206. doi: 10.1016/0006-8993(72)90375-7. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Effects of surgical decentralization and nerve growth factor on the maturation of adrenergic neurons in a mouse sympathetic ganglion. J Neurochem. 1972 May;19(5):1367–1377. doi: 10.1111/j.1471-4159.1972.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I., Iversen L. L. Differences in the regulation of tyrosine hydroxylase and dopa decarboxylase in sympathetic ganglia and adrenals. Nat New Biol. 1971 May 5;231(18):27–29. [PubMed] [Google Scholar]
- Brunso-Bechtold J. K., Hamburger V. Retrograde transport of nerve growth factor in chicken embryo. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1494–1496. doi: 10.1073/pnas.76.3.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham P., Raiborn C., Varon S. Replacement of nerve-growth factor by ganglionic non-neuronal cells for the survival in vitro of dissociated ganglionic neurons. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3556–3560. doi: 10.1073/pnas.69.12.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970 Sep 25;245(18):4784–4790. [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBURGER V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958 May;102(3):365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Iversen L. L. Effect of nerve growth factor and its antiserum on tyrosine hydroxylase activity in mouse superior cervical sympathetic ganglion. Brain Res. 1971 Jun 4;29(1):159–162. doi: 10.1016/0006-8993(71)90429-x. [DOI] [PubMed] [Google Scholar]
- Herrup K., Shooter E. M. Properties of the beta-nerve growth factor receptor in development. J Cell Biol. 1975 Oct;67(1):118–125. doi: 10.1083/jcb.67.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Meyerson B., Nilsson G., Pernow B., Sachs C. Immunohistochemical evidence for substance P-containing nerve endings in the human cortex. Brain Res. 1976 Mar 5;104(1):181–186. doi: 10.1016/0006-8993(76)90662-4. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Jessell T. Post mortem changes and regional distribution of substance P in the rat and mouse nervous system. Brain Res. 1976 Nov 26;117(2):362–367. doi: 10.1016/0006-8993(76)90748-4. [DOI] [PubMed] [Google Scholar]
- Konishi S., Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974 Dec 20;252(5485):734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., MEYER H., HAMBURGER V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 1954 Jan;14(1):49–57. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J Physiol. 1974 Sep;241(3):715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. EXCESSIVE GROWTH OF THE SYMPATHETIC GANGLIA EVOKED BY A PROTEIN ISOLATED FROM MOUSE SALIVARY GLANDS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):373–384. doi: 10.1073/pnas.46.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley W. C., Schenker A., Shooter E. M. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry. 1976 Dec 14;15(25):5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Substance P and excitatory transmitter of primary sensory neurons. Cold Spring Harb Symp Quant Biol. 1976;40:135–143. doi: 10.1101/sqb.1976.040.01.015. [DOI] [PubMed] [Google Scholar]
- Powell D., Leeman S., Tregear G. W., Niall H. D., Potts J. T., Jr Radioimmunoassay for substance P. Nat New Biol. 1973 Feb 21;241(112):252–254. doi: 10.1038/newbio241252a0. [DOI] [PubMed] [Google Scholar]
- Prestige M. C. The control of cell number in the lumbar ventral horns during the development of Xenopus laevis tadpoles. J Embryol Exp Morphol. 1967 Dec;18(3):359–387. [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975 May 16;89(1):1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Otsuka M. Regional distribution of substance P in the spinal cord and nerve roots of the cat and the effect of dorsal root section. Brain Res. 1975 Apr 4;87(1):1–11. doi: 10.1016/0006-8993(75)90774-x. [DOI] [PubMed] [Google Scholar]
- Varon S., Raiborn C., Tyszka E. In vitro studies of dissociated cells from newborn mouse dorsal root ganglia. Brain Res. 1973 May 17;54:51–63. doi: 10.1016/0006-8993(73)90033-4. [DOI] [PubMed] [Google Scholar]


