Abstract
Cocaine-induced alterations in synaptic glutamate function in nucleus accumbens are thought to mediate drug-related behaviors such as psychomotor sensitization. However, previous studies examined global alterations in randomly selected accumbens neurons regardless of their activation state during cocaine-induced behavior. We recently found that a minority of strongly activated Fos-expressing accumbens neurons are necessary for cocaine-induced psychomotor sensitization while the majority of accumbens neurons are less directly involved. Here, we assessed synaptic alterations in these strongly activated accumbens neurons in c-fos-GFP mice that express a fusion protein of Fos and green fluorescent protein (GFP) in strongly activated neurons and compared these alterations with those in surrounding non-activated neurons. Cocaine sensitization produced higher levels of ‘silent synapses’ that contained functional NMDA receptors and non-functional AMPA receptors in only GFP-positive neurons, 6–11 days after sensitization. Thus unique synaptic alterations are induced in the most strongly activated accumbens neurons that mediate psychomotor sensitization.
Keywords: cocaine sensitization, nucleus accumbens, neuronal ensemble, silent synapse, c-fos-GFP transgenic mice
Introduction
Cocaine produces short and long-lasting neuroadaptations at excitatory glutamatergic synapses within the ventral tegmental area and nucleus accumbens1–4, two brain areas that mediate drug reward5,6. These neuroadaptations include alterations of synaptic strength assessed by AMPAR/NMDAR ratios7–12, AMPAR subunit composition7,12–14, and changes in the expression of synaptic plasticity7,10–17 that are hypothesized to underlie long-lasting alterations in drug-associated behaviors1–4.
It is important to note that all these alterations were assessed in randomly selected neurons in ex vivo brain slices regardless of their previous activation state during behavior, with the implicit assumption that most or all of these neurons undergo similar alterations and play similar roles in cocaine-induced behaviors. However, we recently found that cocaine-induced locomotor sensitization in a novel environment is mediated primarily by a distinct minority of sparsely distributed accumbens neurons that are more strongly activated than the surrounding majority of neurons, as indicated by Fos expression18. Cocaine sensitization in a novel environment is context-specific where cocaine induces sensitized locomotion only in the environment paired with repeated cocaine injections during sensitization, and not in a non-drug-paired environment. Cocaine administration to these sensitized rats correspondingly induces the activation marker Fos in only 2–3% of accumbens neurons that were selected by stimuli in the drug-paired environment18. Selective inhibition of these neurons activated in the drug-paired environment disrupted sensitized cocaine-induced locomotion, while inhibition of other neurons activated in a non-drug-paired environment had no effect18. Thus, these selectively activated neurons undergo different alterations and play different roles in behavior than the majority of less activated neurons following cocaine sensitization in a novel environment.
We hypothesize that repeated strong activation of these selectively activated neurons during sensitization induces unique synaptic alterations relative to the surrounding majority of neurons that are not as strongly activated. In the present study, we used c-fos-GFP transgenic mice with a transgene containing a c-fos promoter that drives expression of a gene encoding a fusion protein of Fos and green fluorescent protein (GFP) in strongly activated neurons19,20. We compared glutamatergic synaptic properties in the strongly activated neurons that express GFP with those in the surrounding majority of less activated accumbens neurons that do not express GFP following cocaine-induced locomotion in previously cocaine-sensitized and naïve mice
Results
Cocaine-induced locomotor activity and GFP expression
We have previously shown that cocaine sensitization in a novel environment produces enhanced or ‘sensitized’ cocaine-induced activation of nucleus accumbens neurons as measured by Fos immunohistochemistry, that corresponds with sensitized cocaine-induced locomotion18,21,22. In the current study, cocaine-induced locomotion and neuronal activation in accumbens could also be sensitized in c-fos-GFP mice. Mice in the Repeated cocaine group received 5 once daily injections of 15 mg/kg cocaine, while the Acute cocaine group received 5 once daily injections of saline. 6–11d after these repeated injections, mice in both groups were given test injections of 20 mg/kg cocaine. Prior repeated cocaine injections enhanced cocaine-induced locomotion on test day relative to cocaine-induced locomotion for the Acute cocaine group (Repeated: 449±38m, Acute: 254±36m; n=7,7 mice, t(12)=3.71, p<0.01).
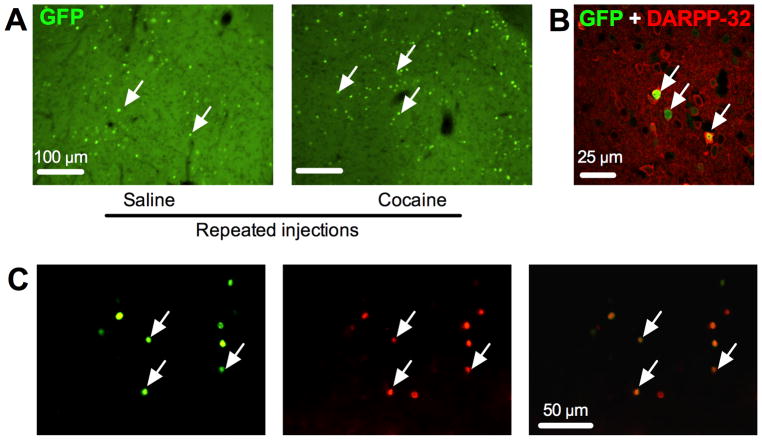
Neuronal activation was assessed in the same mice by counting GFP-expressing (GFP+) neurons in the nucleus accumbens shell (Fig. 1A). Cocaine test injections induced significantly higher levels of GFP expression in the Repeated cocaine group relative to levels in the Acute cocaine group (Repeated: 136±10 cells/mm2, Acute: 97±14 cells/mm2; n=7,7 mice, t(12)=2.29, p<0.05). In the accumbens shell region, 136 out of 167 GFP+ neurons (81.4%; n=3 mice) were also Fos+ (Fig. 1C). Lack of co-expression of Fos in a minority of GFP+ neurons could be due to different time courses for induction and degradation of the two proteins after neuronal activation, or different antibody sensitivities and thresholds chosen for each protein when determining whether a nucleus was immunoreactive or not.
Figure 1.
Cocaine-induced GFP expression in nucleus accumbens. (A) Representative images of cocaine-induced GFP expression in nucleus accumbens. White arrows indicate GFP-expressing cells as measured by native GFP fluorescence in c-fos-GFP transgenic mice. (B) Double-labeling immunofluorescence histochemistry of GFP (green) and the medium spiny neuron marker DARPP-32 (red) from a cocaine-sensitized mouse. White arrows indicate cells positive for both GFP and DARPP-32. (C) Co-labeling of GFP (green) and Fos (red) using immunofluorescence histochemistry from a cocaine-sensitized mouse. White arrows indicate cells positive for both GFP and Fos.
We assessed the phenotype of GFP+ neurons using DARPP-32 as a marker of GABAergic medium spiny projection neurons23 (Fig. 1B). In the Repeated cocaine group (n=6 mice), 92 out of 95 GFP+ neurons (96.8%) were DARPP-32+, while only 3 GFP+ neurons were DARPP-32−. In the Acute cocaine group (n=5 mice), 53 out of 55 GFP+ neurons (96.4%) were DARPP-32+, while only 2 GFP+ neurons were DARPP-32−. Thus the vast majority of cocaine-activated GFP+ neurons in both groups were medium spiny neurons. The high-degree of co-labeling in GFP+ neurons for both DARPP-32 and Fos ensured that whole-cell recordings from the majority of GFP+ neurons in this study were from strongly activated medium spiny neurons.
Synaptic strength
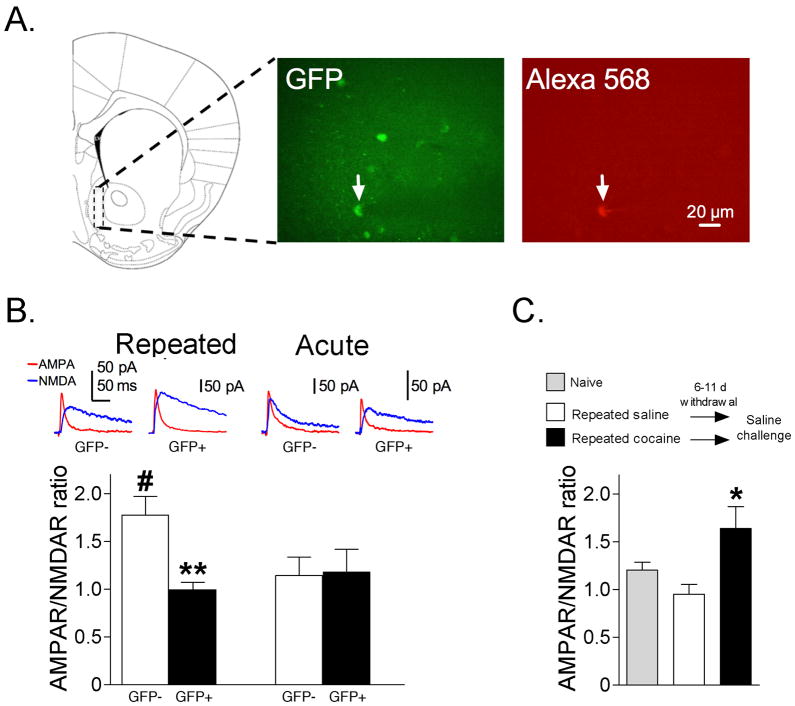
Glutamatergic synaptic transmission was assessed in accumbens shell neurons using the ratio of peak AMPAR- to peak NMDAR-mediated evoked synaptic currents8,10. We observed a significant interaction of GFP and repeated cocaine exposure (F(1,49)=5.08; p<0.05). Post-hoc tests revealed significantly lower AMPAR/NMDAR ratios in GFP+ neurons compared to GFP- neurons following cocaine test injections in the Repeated cocaine group (p<0.01), but not in the Acute cocaine group (p>0.05) (Fig. 2B). Thus, glutamatergic synaptic strength was lower in cocaine-activated GFP+ neurons compared to GFP− neurons only after repeated cocaine injections. For non-activated (GFP−) neurons, post-hoc tests revealed significantly increased AMPAR/NMDAR ratios in the Repeated cocaine group compared to GFP− neurons in the Acute cocaine group (#p<0.05) (Fig. 2B), indicating that glutamatergic synaptic strength increased in the general neuronal population. For comparison with previous studies, we measured AMPAR/NMDAR ratios from randomly selected neurons following acute saline challenge in mice withdrawn 6–11 d from either repeated cocaine or repeated saline. We observed significantly higher AMPAR/NMDAR ratios following repeated cocaine injections compared to that following repeated saline (Fig. 2C; F(2,26)=5.22, p<0.05), similar to results from Kourrich et al.8 Overall, activated GFP+ neurons in Repeated cocaine mice exhibited relatively low synaptic strength at a time when synaptic strength increased globally in the accumbens.
Figure 2.
(A) Representative area of medial accumbens shell where whole-cell recordings were performed (dashed line rectangle) and where GFP-expressing (GFP+) nuclei were counted. A GFP+ neuron in the accumbens shell is indicated by a white arrow in the GFP panel. The same neuron was filled with Alexa 568 fluorophore included in the patch pipette and indicated with a white arrow in the Alexa 568 panel. (B) AMPAR/NMDAR ratios are significantly lower in GFP+ versus GFP− neurons following cocaine test injections in the Repeated cocaine group (GFP+ neurons: n=16/16, GFP− neurons: n=17/12; **p<0.01), but not in the Acute cocaine group (GFP+ neurons: n=8/6, GFP− neurons: n=12/7). AMPAR/NMDAR ratios are significantly higher in GFP− neurons in the Repeated cocaine group compared to GFP− neurons in the Acute cocaine group (#p<0.01). (C) AMPAR/NMDAR ratios obtained from randomly selected accumbens neurons from mice challenged with saline on test day, 6–11d after repeated cocaine or repeated saline injections. AMPAR/NMDAR ratios are significantly higher in the Repeated cocaine group compared to the Repeated saline group (Repeated cocaine: n=10/4, Repeated saline: n=12/4, Naïve: n=7/2; *p<0.05). Data are expressed as mean±SEM.
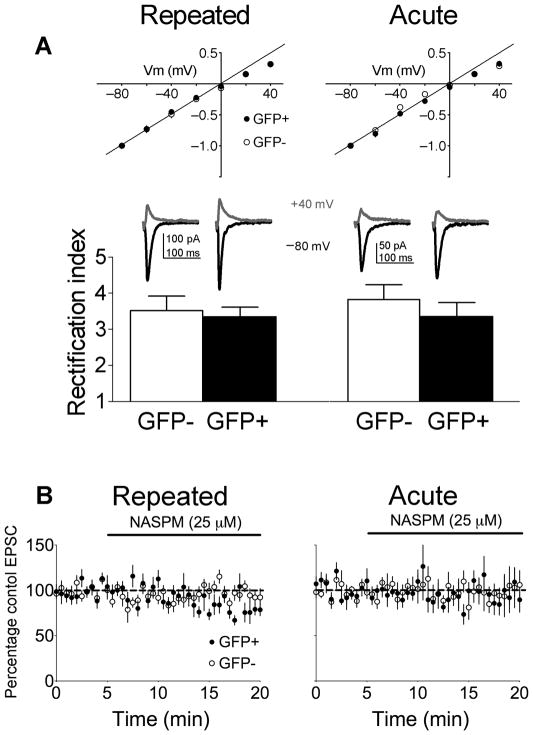
Reductions in AMPAR/NMDAR ratios may be due to higher proportions of GluR2-lacking AMPARs since the loss of this subunit would increase inward rectification24. We measured rectification of glutamatergic EPSCs by dividing the EPSC amplitude at −80mV by the amplitude at +40mV25. If GluR2-lacking AMPARs increased following cocaine sensitization, then EPSCs should show greater inward rectification at depolarized membrane potentials. However, we did not observe a significant interaction of GFP and repeated cocaine exposure on AMPA EPSC rectification (F(1,27)=0.15; p=0.70) (Fig. 3A). Also, NASPM, a selective blocker of GluR2-lacking AMPARs, did not alter the amplitude of evoked EPSCs between GFP+ and GFP− neurons in both Repeated (F(32,416)=1.26; p=0.157) or Acute cocaine groups (F(32,288)=0.82; p=0.752; Fig. 3B). Thus reduced AMPAR/NMDAR ratios in GFP+ neurons following Repeated cocaine were not due to significant changes in AMPAR subunit composition, and single cocaine injections do not result in incorporation of GluR2 lacking AMPARs in GFP+ neurons in Acute cocaine mice.
Figure 3.
AMPAR subunit composition does not differ between GFP+ or GFP− neurons following cocaine test injections in Repeated or Acute cocaine mice. (A) AMPAR rectification indices do not differ between GFP+ and GFP− neurons from both Repeated cocaine mice (GFP+ n=8/6, GFP− n=9/6) and Acute cocaine mice (GFP+ neurons: n=6/4, GFP− neurons: n=8/4). (B) NASPM had no effect on the amplitude of AMPAR-mediated EPSCs in Repeated cocaine mice (GFP+ n= 8/7, GFP− n=7/6) and Acute cocaine mice (GFP+ neurons: n=6/6, GFP− neurons: n=6/5). Data are expressed as mean±SEM.
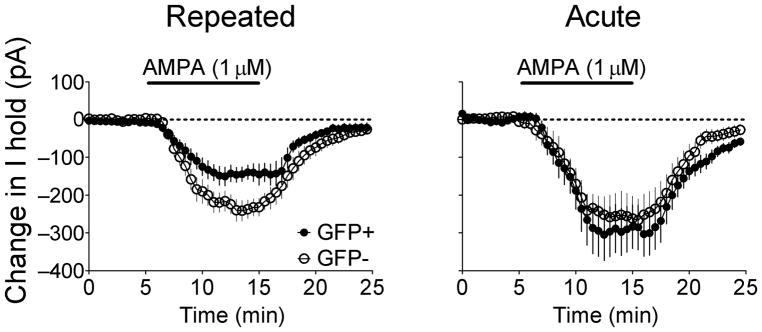
To further assess whether smaller AMPAR/NMDAR ratios resulted from decreased AMPAR function, the effects of bath-applied AMPA were determined. AMPA initiated inward currents in all neurons (Fig. 4). However, this current was significantly smaller in GFP+ neurons compared to GFP− neurons in the Repeated cocaine group (F(34,816)=3.37; p<0.0001). In contrast, no differences of AMPA-mediated inward currents were observed between GFP+ and GFP− neurons in the Acute cocaine group (F(34,442)=0.47; p=0.995). Thus lower AMPAR/NMDAR in GFP+ neurons, compared to GFP− neurons, in Repeated cocaine mice was due in part to decreased AMPAR function.
Figure 4.
Effects of bath-applied AMPA on GFP+ and GFP− accumbens neurons following cocaine test injections in Repeated cocaine and Acute cocaine groups. In the Repeated cocaine group, bath-applied AMPA generated a smaller inward current in GFP+ neurons compared to GFP− neurons (GFP+ neurons: n=14/12, GFP− neurons: n=12/9; p<0.0001). In the Acute cocaine group, no differences were observed between GFP+ and GFP− neurons (GFP+ neurons: n=7/5, GFP− neurons: n=8/4). Data are expressed as mean±SEM.
Spontaneous EPSC properties
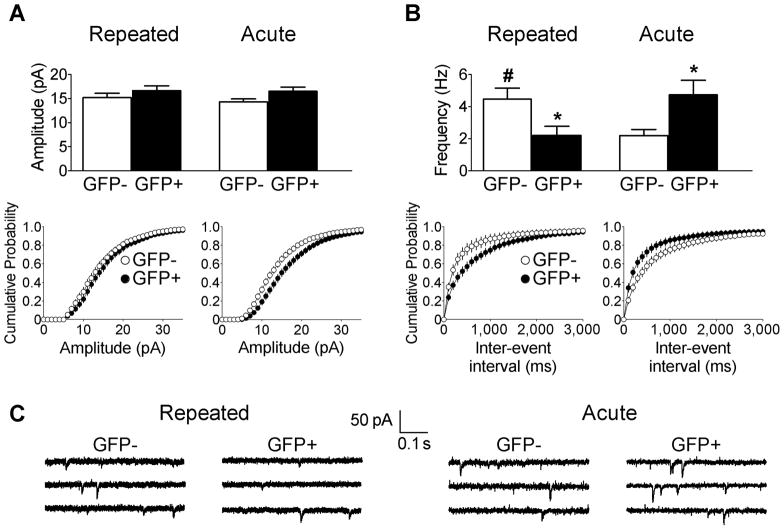
We recorded spontaneous AMPAR-mediated EPSCs (sEPSCs) in GFP+ and GFP− neurons. These sEPSCs likely represent action potential-independent quantal release events since tetrodotoxin did not affect sEPSC frequency or amplitude in GFP+ or GFP− neurons from Repeated cocaine mice (frequency: F(1,22)=0.05, p=0.824; amplitude (F(1,22)=0.04, p=0.834; GFP+ n=6/5, GFP− n=7/3, cells/mice, data not shown). Spontaneous EPSC amplitudes did not significantly differ between GFP+ and GFP− neurons in either the Repeated or Acute cocaine groups (F(1,88)=0.21; p=0.645) (Fig. 5A). However, for spontaneous EPSC frequency, we observed a significant interaction of GFP and repeated cocaine exposure (F(1,88)=13.1, p<0.01). Post-hoc tests revealed significantly lower spontaneous EPSC frequency in GFP+ neurons than in GFP− neurons from the Repeated cocaine group (p<0.05). In contrast, sEPSC frequency was significantly greater in GFP+ neurons than in GFP− neurons from the Acute cocaine group (p<0.05). Also, post-hoc tests revealed significantly higher sEPSC frequency in the GFP− neurons of the Repeated cocaine group compared to GFP− neurons from the Acute cocaine group (#p<0.05) (Fig. 5B). Altered sEPSC frequency in the absence of amplitude changes suggest presynaptic alterations in glutamate release. However, measurement of paired-pulse facilitation of evoked EPSCs, thought to reveal changes in the probability of neurotransmitter release (pr), did not differ between GFP+ and GFP− neurons from Repeated and Acute cocaine groups (Repeated: F(6,90)=0.16; p=0.986; GFP+ n=8/6, GFP− n=9/7 (cells/mice); Acute: F(6,78)=0.67; p=0.677; GFP+ n=7/5, GFP− n=8/4 (cells/mice); Supplementary Figure). This lack of difference argues against presynaptic changes in pr. Therefore, our data indicate a reduction in the number of functional AMPA receptors in the absence of a change of AMPAR properties in neurons from the Repeated cocaine group.
Figure 5.
Quantal spontaneous EPSC (sEPSC) properties in GFP+ and GFP− accumbens neurons following cocaine test injections in Repeated cocaine (GFP+ neurons: n=21/15, GFP− neurons: n=20/16), and Acute cocaine mice (GFP+ n=24/15, GFP− n=27/11). (A) sEPSC amplitudes do not differ between GFP+ and GFP− neurons in Repeated cocaine and Acute cocaine mice. (B) sEPSC frequencies were significantly lower in GFP+ compared to GFP− neurons in the Repeated cocaine group, whereas sEPSC frequencies were significantly higher in GFP+ neurons compared to GFP− neurons in the Acute cocaine group (*p<0.05). Also, sEPSC frequencies were significantly higher in GFP− neurons of Repeated cocaine mice compared to GFP− neurons of Acute cocaine mice (#p<0.05). (C) Sample traces of sEPSCs from GFP+ and GFP− neurons. Data are expressed as mean±SEM.
Silent synapses
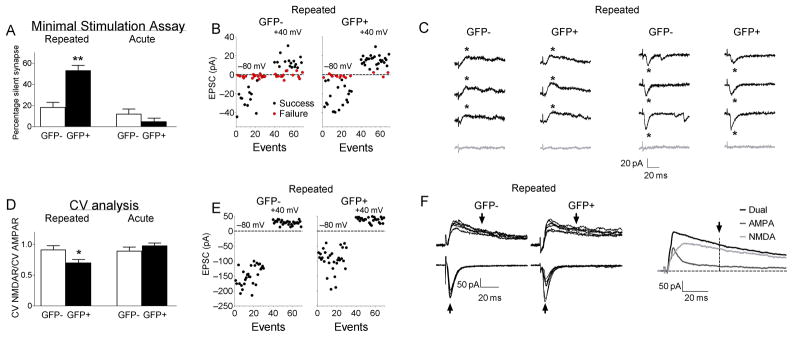
What might explain the lower AMPAR/NMDAR ratios, lower AMPA-induced inward currents with bath application, lower sEPSC frequency, and no changes in mean sEPSC amplitude or paired-pulse facilitation in GFP+ neurons from cocaine-sensitized mice? One possibility is that a larger subset of glutamate synapses on GFP+ neurons are silent, in that they contain functional NMDARs, but not AMPARs. Silent synapses26–32 can be detected by measuring evoked EPSCs at −80mV where AMPARs, but not NMDARs, are activated, and at +40mV where NMDARs contribute strongly to EPSCs. Minimal stimulus intensity to evoke EPSCs was set by adjusting stimulator output to elicit AMPAR-mediated currents in approximately 30–70% of trials at −80mV. Once set, stimulus intensity was unchanged during assessment of synaptic failures at +40mV. There was a significant interaction of GFP and Repeated cocaine exposure for the difference in the failure rates between −80 and +40mV holding potentials (F(1,51)=12.1; p<0.01). Post-hoc tests revealed that this difference was significantly different in GFP+ neurons (at −80mV, 46.9±3.2%; at +40mV, 19.8±4.2%; p<0.001), but not significantly different in GFP− neurons (at −80mV, 33.6±3.8%; at +40mV, 28.8±4.4%; p>0.05) (Fig.6A–C). In contrast, failure rates in neurons from Acute cocaine mice did not differ between −80 and +40 mV holding potentials in GFP+ neurons (at −80mV, 51.4±7.5%; at +40mV, 54.8±7.7%, p>0.05) or in GFP− neurons (at −80mV, 48.0±7.7%; at +40mV, 45.1±6.4%; p>0.05).
Figure 6.
Higher levels of silent synapses were found on GFP+ neurons compared to GFP− neurons following cocaine test injections in Repeated cocaine, but not in Acute cocaine mice. (A–C) Minimal stimulation assays: (A) GFP+ accumbens neurons contain more silent synapses than GFP− neurons in Repeated cocaine (GFP+ neurons: n=17/9, GFP− neurons: n=22/11; **p<0.001), but do not differ in Acute cocaine mice (GFP+ neurons: n=9/4, GFP− neurons: n=7/5). Data are expressed as mean±SEM. (B) Representative plots of peak minimally evoked-EPSC amplitudes from 30 consecutive responses at −80mV and +40mV from individual GFP+ and GFP− neurons from Repeated cocaine mice. (C) Representative traces of successes (black) and failures (gray) from individual GFP+ and GFP− neurons at −80mV and +40mV from Repeated cocaine mice. (D–F) Coefficient of Variation (CV) analysis: (D) GFP+ neurons showed lower CV-NMDAR/CV-AMPAR ratios compared to GFP- neurons in Repeated cocaine (GFP+ neurons: n=8/7, GFP− neurons: n=10/6; *p<0.05), but not in Acute cocaine mice (GFP+ neurons: n=5/3, GFP− neurons: n=5/4). Data are expressed as mean±SEM. (E) Representative event plots from 30 consecutive EPSCs at −80mV and +40mV from CV analysis from individual GFP+ and GFP− neurons from Repeated cocaine mice. (F) Representative traces at −80mV (AMPAR EPSCs) and +40mV (NMDAR EPSCs) from individual GFP+ and GFP− neurons from Repeated cocaine mice. Arrows indicate where peak measurements were obtained for AMPAR and NMDAR components of the EPSCs.
These failure rates obtained at −80mV and +40mV were used to estimate the proportion of silent synapses using the equation: 1−Ln(Failure rate at 80mV)/Ln(Failure rate at +40mV)27,32. We observed a significant interaction of GFP and Repeated cocaine exposure (F(1,51)=12.9; p<0.01). Post-hoc tests revealed a significantly greater proportion of silent synapses in GFP+ neurons than in GFP− neurons (p<0.001) (Fig. 6A–C), while the proportion of silent synapses was not different between GFP+ and GFP− neurons in the Acute group (p>0.05).
Silent synapses were also assessed using the coefficient of variation (CV) for AMPAR EPSCs at −80mV and NMDAR EPSCs at +40mV. When silent synapse levels are increased, the CV of AMPAR EPSCs is higher compared to when silent synapses are absent resulting in a lower CV-NMDAR/CV-AMPAR ratio. We observed a significant interaction of GFP and Repeated cocaine exposure (F(1,24)=4.79, p<0.05). Post-hoc tests revealed in the Repeated cocaine group that the CV-NMDAR/CV-AMPAR ratio was significantly lower (p<0.05) in GFP+ neurons (CV-AMPAR=0.40±0.03, CV-NMDAR=0.27±0.02, ratio=0.70±0.05) compared to GFP− neurons (CV-AMPAR=0.28±0.03, CV-NMDAR=0.25±0.02, ratio=0.91±0.07) (Fig. 6D–F). In the Acute cocaine group, the CV-NMDAR/CV-AMPAR ratio did not differ (p>0.05) in GFP+ neurons (CV-AMPAR=0.39±0.03, CV-NMDAR=0.38±0.02, ratio=0.97±0.05) compared to GFP− neurons (CV-AMPAR=0.33±0.04, CV-NMDAR=0.29±0.03, ratio=0.89±0.07). Taken together, the minimal stimulation and CV analyses indicate that cocaine sensitization was associated with a greater proportion of silent synapses in GFP+ neurons compared to GFP− neurons.
A previous study identified significantly elevated levels of silent synapses in medium spiny neurons 1–2d, but not 7–14d following 3–5d of repeated cocaine injections in rats30. For comparison, we used the minimal stimulation assay to measure silent synapses from randomly selected neurons in our mice 1–2 d and 6–11d following repeated cocaine or repeated saline injections. Although there was no significant interaction of Repeated cocaine and Withdrawal duration for the difference in the failure rates between −80 and +40mV holding potentials (F(1,54)=2.9), there was a main effect of Repeated cocaine (F(1,54)=6.8). Post-hoc tests revealed significantly different failure rates for neurons in the 1–2d withdrawal group for the repeated cocaine group (at −80mV, 55.8±4.2%; at +40mV, 39.8±5.6%; p<0.05), but not for neurons from the repeated saline group (at −80mV, 48.8±4.9%; at +40mV, 47.6±5.2%; p>0.05). In the 6–11d withdrawal groups, failure rates did not differ between neurons from the repeated cocaine group (at −80mV, 57.3±4.2%; at +40mV, 54.1±4.4%; p>0.05) and neurons from the repeated saline group (at −80mV, 53.9±7.6%; at +40mV, 53.8±7.0%; p>0.05).
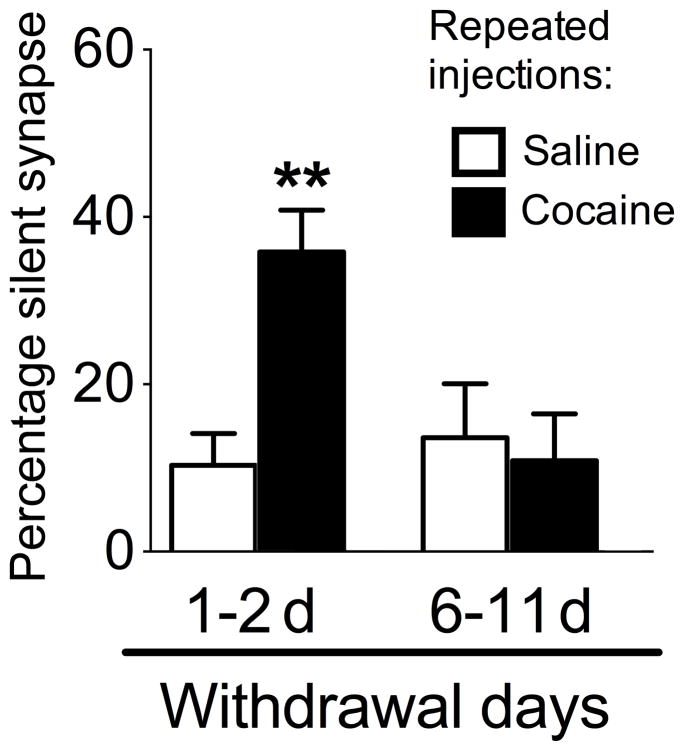
When the proportion of silent synapses were calculated for each group, we observed a significant interaction of Repeated cocaine exposure and Withdrawal duration (F(1,54)=7.2, p<0.01). In the 1–2 d withdrawal groups, post-hoc tests revealed a significantly higher proportion of silent synapses in neurons from the repeated cocaine group than in the repeated saline group (p<0.01) (Fig. 7). However, the proportion of silent synapses was not significantly different between repeated cocaine and repeated saline groups following 6–11d withdrawal (p>0.05). Thus, in randomly selected (presumably GFP−) neurons, the increase in silent synapses after repeated cocaine was observed only at 1–2d, and not 6–11d, after cocaine withdrawal.
Figure 7.
The proportion of silent synapses from a randomly selected population of accumbens neurons, following 1–2d or 6–11d withdrawal from 3–5 days of repeated cocaine injections. Repeated cocaine increased the proportion of silent synapses in the general population of nucleus accumbens neurons following 1–2d withdrawal (Repeated cocaine 1–2d: n=19/6, Repeated saline 1–2d: n=16/5, **p<0.01), but not following 6–11d withdrawal (Repeated cocaine 6–11d: n=13/4, Repeated saline 6–11d: n=10/5). Data are expressed as mean±SEM.
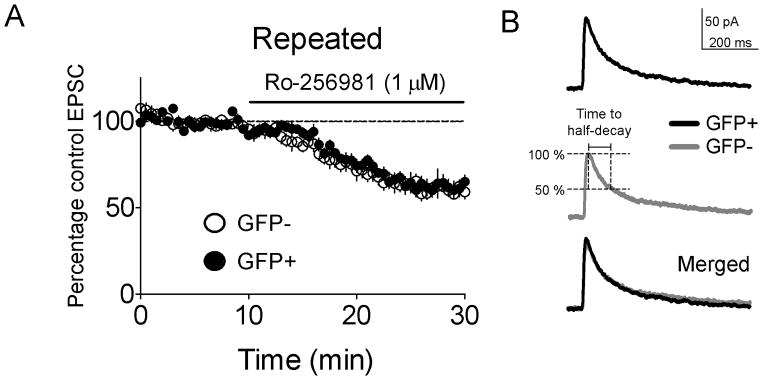
Huang et al. 30 demonstrated that silent synapses in the general population of neurons (presumably GFP−) 1–2d after repeated cocaine had increased levels of NR2B-containing NMDARs. We assessed whether the silent synapses we observed in GFP+ neurons 6–11d following repeated cocaine (Repeated cocaine group) also contained NR2B NMDARs. The selective antagonist of NR2B-containing NMDA receptors Ro-25698133 did not differentially alter evoked NMDAR EPSC amplitudes in GFP+ or GFP− neurons (F(53,918)=0.63, p=0.98) (Fig. 8A). NR2B containing NMDARs also exhibit slower decay kinetics than NR2A containing counterparts30,36. Therefore, we measured the half-decay times of NMDAR EPSCs34,35 6–11d following repeated cocaine. NMDAR EPSC half-decay times did not differ between GFP+ and GFP− neurons (72.3±7.7ms, 68.6±4.3ms, respectively; t(25)=0.42, p>0.05) (Fig. 8B). Overall our data indicate that increased silent synapses in GFP+ neurons from cocaine-sensitized mice are not associated with altered NR2B subunit expression, and that these silent synapses are different from those found on randomly-selected neurons 1–2d following withdrawal from cocaine.
Figure 8.
Silent synapses in GFP+ neurons from Repeated cocaine mice (i.e. mice challenged with cocaine following 6–11 d of withdrawal from repeated cocaine) were not associated with alterations in NR2B expression. (A) Ro-256981 application did not produce significantly different reductions in the amplitudes of NMDAR-mediated EPSCs (GFP+ neurons: n=9/4, GFP− neurons: n=10/6; p>0.05) and (B) The time to half-decay was not significantly different between GFP+ and GFP− neurons (GFP+ neurons: n=13/5, GFP− neurons: n=14/7). Data are expressed as mean±SEM.
Discussion
Repeated cocaine administration to rats and mice in a novel environment induces context-specific sensitization of cocaine-induced locomotion, where the sensitized response is expressed only in the drug-paired environment, and not in a non-paired environment18,37,38. In the present study, sensitization of mice in a novel environment enhanced cocaine-induced activation of a small number of sparsely distributed accumbens neurons that express GFP as an indicator of strong activation. The high degree of DARPP-32 and GFP co-expression (96%) indicates that nearly all GFP-expressing neurons observed in this study are medium spiny neurons, and previous work suggests that cocaine-induced Fos expression occurs primarily in neurons expressing dopamine D1 receptors39. GFP-expressing neurons had higher levels of silent synapses following cocaine test injections in cocaine-sensitized mice (Repeated cocaine group) and exhibited down-regulated synaptic AMPAR function compared to the majority of neurons that did not express GFP. These properties did not generalize to GFP-expressing neurons activated by cocaine test injections in naïve mice (Acute cocaine group). We previously demonstrated that these strongly activated neurons are selected by stimuli in the drug-paired environment and represent a neuronal ensemble that encodes the learned association between drug and environment that is necessary for context-specific sensitization18,38. Based on the present results, we hypothesize that the GFP-expressing neurons forming this ensemble undergo unique synaptic alterations that likely play a role in the learned associations underlying context-specific sensitization.
The synaptic alterations found in these strongly activated GFP-expressing neurons are distinct from those described in previous studies that examined randomly selected accumbens neurons regardless of their activation state following cocaine exposure8,10,13,16,17,29,30. Neurons in these studies likely correspond to the less-activated majority of neurons that did not express GFP in our study, and we confirmed these prior observations by showing that AMPAR/NMDAR ratios and sEPSCs were increased in GFP-negative neurons from cocaine-sensitized mice8. Whereas these global alterations have been hypothesized to play a direct role in sensitization, our previous study demonstrated that alterations within these non-activated neurons likely play a lesser role than the selectively activated GFP-expressing neurons in context-specific cocaine sensitization18.
Silent synapses contain functional NMDARs but lack functional AMPARs, resulting in an absence of synaptic transmission near the resting membrane potential where NMDA channels are inactive26–32. Silent synapses are observed at high levels in juvenile animals40,41 and are hypothesized to represent sites of future synapse maturation28. However Huang et al.30 found that silent synapses can also be induced in adult animals and are likely involved in synaptic remodeling during cocaine exposure. Repeated cocaine injections (3–5d, once daily) to rats in their home cage transiently induced silent synapses in randomly selected accumbens neurons 1–2d, but not 7d, following repeated cocaine30. Our results confirm those of Huang et al. in that silent synapses were observed in randomly selected neurons1–2d, but not 6–11d, following repeated cocaine. However, since sensitized cocaine-induced locomotion is normally still present 7d and 6–11d following repeated cocaine18,21,22, silent synapses in GFP-negative neurons may play a transient role in sensitization, but are likely not necessary for maintenance of sensitization of cocaine-induced locomotion.
In contrast to these results, we observed silent synapses in strongly activated GFP-expressing neurons up to 11 days following repeated cocaine. Thus, silent synapse expression in the strongly-activated GFP-expressing neurons is detected after prolonged withdrawal from repeated cocaine, in contrast to that found in randomly selected GFP-negative neurons. One explanation for silent synapse expression after extended withdrawal is that it occurs following more prolonged or stronger activation of these neurons during each repeated cocaine injection, perhaps due to cocaine exposure in a novel environment. In support of this, we and others have shown that Fos is induced more strongly in accumbens when cocaine is given in a novel environment, rather than in the less-arousing home cage 21,43. We have also shown that the same set of accumbens neurons is activated by each cocaine injection in a novel environment, which implies repeated activation of the same set of synapses38. Thus stronger activation of GFP-expressing neurons with each repeated cocaine injection in a novel environment may induce a more persistent increase of the same silent synapses relative to that in the surrounding population of non-activated neurons.
The silent synapses we observed in GFP-expressing neurons differed from Huang et al30 in that NMDARs in silent synapses from their study of randomly selected accumbens neurons contained increased levels of NR2B subunits, while those observed in GFP+ neurons in the present study did not contain increased levels of NR2B. These data suggest that silent synapses on GFP-expressing neurons, that are implicated in long-lasting cocaine sensitization in a novel environment are distinct from those reported previously.
It is also possible that higher levels of silent synapses in GFP-expressing neurons represent an acute short-lasting alteration due to enhanced activation of these neurons following cocaine test injections, and are not due to a persistent increase following cocaine sensitization. Previously observed cocaine-induced glutamatergic synaptic alterations including AMPAR surface expression44,45, AMPAR/NMDAR ratios8,10, and silent synapses found in randomly selected accumbens neurons29,30 are thought to represent homeostatic responses to increased excitation during repeated cocaine injections and/or by cocaine test injections3,42. This homeostatic response is also referred to as ‘synaptic scaling’ where prolonged increases in synaptic activity result in decreased excitatory synaptic transmission via AMPAR removal, and prolonged decreases in synaptic activity result in increased excitatory transmission via AMPAR insertion46. In this regard, GFP-expressing neurons in our study may receive higher levels of excitation shortly after the cocaine test injection that activates the c-fos promoter. But continuing high levels of excitation in these neurons, enabled by prior repeated cocaine-induced synaptic alterations, may produce a greater degree of synaptic scaling than in the surrounding majority of less strongly activated neurons. This scaling could be due to the removal of AMPARs from existing synapses, leaving only NMDARs at these sites. Alternatively, the silent synapses observed in the GFP-expressing neurons may be generated via acute activity-dependent synaptogenesis. Thus, silent synapses in GFP-expressing neurons may be a consequence rather than a cause of enhanced cocaine-induced activity in these neurons. A determination of the causal role of silent synapses in cocaine sensitization awaits methodology that permits manipulation of glutamate receptor expression specifically within these critical neurons.
Since we cannot identify GFP-expressing neurons prior to cocaine test injections, we cannot distinguish whether higher levels of silent synapses are induced during cocaine sensitization or following the last acute cocaine injection. Also, we do not know if this putative form of synaptic scaling is short or long-lasting since electrophysiological recordings from these neurons were constrained by the transient expression of GFP from approximately 2 h to 6–8 h after cocaine injections. This requirement for GFP expression also precludes examining alterations induced within neurons that are selectively inhibited during cocaine-induced locomotion but are still contributing to behavior.
In summary, we find that repeated cocaine injections in a novel environment increased silent synapses on neurons that are strongly implicated in mediating learned associations between cocaine and the drug-paired environment in context-specific cocaine sensitization18. Silent synapses on strongly-activated GFP-expressing neurons appear distinct because they do not exhibit altered NR2B expression as found in previously described silent synapses in randomly selected accumbens neurons following cocaine sensitization29,30. We hypothesize that GFP-expressing neurons comprise a neuronal ensemble that is recruited during cocaine sensitization in a novel environment, and that increased silent synapses on these neurons represents either an acute adaptive response to increased glutamatergic excitation or is more directly involved in the development of sensitization behavior.
Sensitization is a useful model for examining drug and environment interactions, but it does not model many components of addiction. In future studies, it will be important to determine whether silent synapses are similarly induced in accumbens neurons that are selectively activated during cue- and context-induced reinstatement, which are common animal models of drug relapse. Although beyond the scope of the present study, it is also possible that similar silent synapses may be found in neuronal ensembles that encode other reward-context associations, such as those between food reward and the feeding context. However, ensembles encoding food reward-context associations are likely distinct from those involved in context-specific cocaine sensitization, either intermingling with each other in the same brain region or located in other brain regions. Finally, our study demonstrates the need to distinguish between synaptic alterations induced specifically within neurons activated during behavior and global synaptic alterations induced within the general neuronal population.
Materials and Methods
Animals
Animals protocols were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA) Intramural Research Program and were carried out according to National institutes of Health guidelines. c-fos-GFP mice previously bred onto a C57Bl/6 background were generously donated by Dr. Allison Barth (Carnegie Mellon University). Heterozygous males continued to be bred in our facilities with wild-type C57Bl/6 females obtained from Charles Rivers. All experimental mice were males heterozygous for the c-fos-GFP transgene. Mice were single housed in a temperature and humidity controlled environment under a 12-h light:12-h dark cycle, and experiments took place during the light phase. Water and food were available ad libitum. Mice were habituated to the colony room for at least 5–7 days before experimentation.
Cocaine treatment and behavioral experiments
For behavioral experiments, two groups of mice (mean age 8.1 ± 0.2 weeks old at time of testing) were used: the Repeated cocaine group received 5 once daily injections of 15 mg/kg of cocaine (intraperitoneal; NIDA). The Acute cocaine group received 5 saline injections. After each injection, mice were placed in 46×25×19 cm clear plastic cages in light and sound-attenuated Optovarimax activity monitors (Columbus Instruments, USA) for 1 h. On test day, 6–11 days after the last repeated injection, mice were habituated to the test environment for 90 min, then injected with saline and placed in the cage for an additional hour, and then injected again with cocaine (20mg/kg) and placed back in the cage for an additional hour. Locomotion was assessed as distance traveled for one hour following each injection.
Two more groups of mice were utilized for the electrophysiology experiments. Mice in the Repeated cocaine group (mean age 7.9 ± 0.2 weeks at time of testing) received 5 once daily injections of cocaine (15mg/kg) and placed in locomotor activity chambers for 1h each time. On test day, 6–11 days after the last injection, mice were given a test injection of cocaine (20mg/kg) and placed back in activity chambers for 1h, then placed in their home cages for an additional 30 min until decapitation. Mice in the age-matched Acute cocaine group (mean age 7.8±0.2 weeks) did not receive prior repeated cocaine injections, but were injected on test day with 20mg/kg cocaine and handled similar to mice in the Repeated cocaine group.
To assess AMPAR/NMDAR ratios in randomly selected neurons, mice received repeated cocaine or saline injections once daily for 5 days in activity chambers. On test day, 6–11 days later, each mouse received a saline test injection in the activity chamber and was sacrificed 90 min later. To assess silent synapses in randomly selected neurons, mice received repeated cocaine or saline injections once daily for 3–5d in activity chambers and sacrificed 1–2 d or 6–11 d later without test injections.
Histochemical procedures
Ninety min after cocaine test injections, mice from behavioral experiments were transcardially perfused with 4% paraformaldehyde and their brains frozen. Thirty μm thick sections containing the nucleus accumbens were cut as previously described18.
To assess cocaine-induced GFP expression, free-floating sections from the behavioral experiment (n=7 per group) were washed in Tris-buffered saline (TBS; 0.025 M Tris-HCl, 0.5 M NaCl, pH 7.5), mounted on chrom-alum/gelatin coated slides, air-dried, and coverslipped with Aqua-Mount (#TA-030-FM, Thermo Scientific). Fluorescence images of native GFP from left and right hemispheres of accumbens shell from 2 coronal sections per animal were captured using an Exi Aqua camera (Qimaging) attached to a Zeiss Axioskop 2 microscope (Zeiss). The number of GFP+ nuclei was assessed using iVision (version 4.0.15, Biovision Technologies).
Another series of sections were double-labeled for GFP and DARPP-32, a marker for striatal medium-sized spiny neurons23. Sections from 11 mice from the behavioral experiments (n=5,6) were immunolabeled for GFP and DARPP-32 using chicken anti-GFP (1:3000 dilution; #GFP-1020, Aves Lab) and mouse anti-DARPP-32 (1:3000 dilution, kindly donated by the Paul Greengard laboratory) primary antibodies and rabbit anti-chicken CF488 (#20079, Biotium) and Alexa 568-labeled goat anti-mouse (#A11044, Invitrogen) secondary antibodies. Fluorescence images of GFP and DARPP-32-immunoreactivity (IR) from left and right hemispheres of accumbens shell from 1 coronal section were captured with a Zeiss LSM 710 microscope system and ZEN 2009 software (Carl Zeiss Microimaging). In order to prevent antibody penetration problems from affecting the determination of the proportion of DARPP-32-labeled neurons, we limited the analysis to the upper 6–7μm focal plane of the tissue48. GFP-expressing and GFP+DARPP-32 co-expressing cells were manually counted.
We assessed co-expression of GFP and Fos in accumbens sections from 3 mice from the Repeated cocaine group using rabbit anti-c-Fos primary antibody (1:500 dilution; #c-Fos sc-52, Santa Cruz Biotechnology) and chicken anti-GFP primary antibody (1:500 dilution; #GFP-1020, Aves Labs) primary antibodies and Streptavidin Alexa 350 (1:200 dilution; #S11249, Invitrogen) and biotinylated donkey anti-chicken IgG (1:200; #703-065-155, Jackson Immunoresearch), and Alexa 568 conjugated goat anti-rabbit IgG secondary antibodies (#A11011, Invitrogen). Fluorescence images of Fos-IR and GFP-IR were captured from left and right hemispheres of accumbens shell from 1–2 coronal sections per mouse. GFP-expressing and GFP+Fos co-expressing cells were manually counted.
Brain slice preparation
Ninety minutes following test injection of cocaine, when GFP expression is near maximal19, mice were decapitated, their brains rapidly removed and immersed into near-freezing oxygenated high-sucrose artificial cerebrospinal fluid (aCSF) with the following composition (mM): 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 D-glucose, saturated with 95% O2/5% CO2. To determine AMPAR/NMDAR ratios and the proportion of silent synapses in randomly selected neurons following repeated cocaine or saline, mice were decapitated 90 min after saline injections (AMPAR/NMDAR) or without test injections (silent synapses). Coronal slices (280μm; ~Bregma +0.98–1.54mm, Paxinos and Franklin atlas49) containing the nucleus accumbens shell (Fig. 2A), were prepared using a vibrating tissue slicer (VT1000S; Leica Instruments). Hemisectioned brain slices were stored in a holding chamber containing normal aCSF containing (mM) 126 NaCl, 4.5 KCl, 2.5 CaCl2, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 11 D-glucose for approximately 20 min at 35oC before imaging and recording. A brain slice was transferred to the recording chamber and superfused with normal aCSF (2mL/min) at 30–32°C. Drugs were added to superfusion using a syringe pump (Razel Scientific Instruments).
Electrophysiological recordings
Neurons were viewed with an Olympus (Tokyo, Japan) BX51WI microscope equipped with a spinning disk laser confocal system (Perkin-Elmer), near-infrared differential interference (DIC) optics and a 40× water-immersion objective. Images were acquired with a high-resolution CCD camera (Hamamatsu).
Confocal imaging of nucleus accumbens GFP-positive neurons was conducted with 488nm laser light and images acquired using the Ultraview RS software (Perkin-Elmer). GFP signals were then digitally superimposed on to DIC images of the same neuron. To confirm that recordings were from GFP-positive cells they were loaded with Alexa 568 (Cat # A10437, Invitrogen) via whole-cell patch pipette. Co-localizaton of GFP and Alexa 568 fluorescence was confirmed within minutes of break-in (Fig. 2A). Cells not exhibiting GFP fluorescence were considered GFP-negative. From each mouse, data from 1–3 GFP-positive and negative cells were obtained. All recordings were obtained from the accumbens shell region within 5–6h after decapitation.
Whole-cell recordings of evoked EPSCs in accumbens shell region were obtained with an Axopatch 200B amplifier (Axon Instruments) and electrodes pulled from borosilicate capillary glass (1.5mm OD, 0.86mm ID). Currents were measured in whole-cell mode using pipettes filled with (mM): 120 CsMeSO3, 5 NaCl, 10 TEA-Cl, 10 HEPES, 4QX-314, 1.1 EGTA, 4 Mg-ATP, and 0.3 Na-GTP, 0.1 spermine, 0.1 μM Alexa 568, pH = 7.2. Signals were amplified, filtered at 3–5khz, digitized at > 10khz, and stored on a PC hard drive using WinWCP (courtesy of Dr. John Dempster, University of Strathclyde) via an A/D board (PCI 6024E, National Instruments). Responses were evoked by stimulating within the medial accumbens shell (Fig. 2A), 200–400μm away from a recorded neuron with single pulses (0.1ms) at 0.033Hz, from a Formvar insulated Ni-chromium wire (180μm diameter) bipolar electrode. Series resistance was monitored using −10mV voltage steps (200ms) and only cells maintaining stable access (<10% change) were included in the analyses. EPSCs were measured in aCSF containing picrotoxin (100μM, Sigma) to block GABAA receptor-mediated IPSCs. Neurons were voltage-clamped at −70mV except where noted. Spontaneous EPSC currents were acquired using WinEDR (courtesy of Dr. John Dempster, University of Strathclyde, UK) and analyzed using miniAnalysis (Synaptosoft).
AMPAR/NMDAR ratios were measured from the averages of 10 EPSCs evoked at +40mV with and without 50μM D-AP-5 (Ascent, UK). Average responses recorded during D-AP-5 application (AMPAR-only EPSC) were subtracted from responses recorded without D-AP-5 (AMPAR+NMDAR) to determine the NMDAR EPSC. The peak AMPAR EPSC was divided by the peak NMDAR EPSC to yield the AMPAR/NMDAR ratio. To determine whether spontaneous EPSCs were action potential independent, 1μM tetrodotoxin (TTX) (Sigma) was applied for 10 minutes and mean spontaneous EPSC frequency and amplitude were compared to a pre-TTX control period.
In some experiments AMPA (1μM, Tocris Bioscience) was applied for 10 minutes and holding current (Vhold = −70 mV) was measured every 30 seconds. AMPAR rectification indices were calculated by obtaining the ratio of mean evoked peak AMPAR EPSCs from 3 responses at −80 mV divided by mean peak AMPAR EPSCs at +40mV25. To assess GluR2 lacking AMPA receptors, 25μM 1-naphthyl acetyl-spermine (NASPM; Sigma) was applied for 15 minutes while measuring evoked EPSCs at −80mV before and during NASPM application.
Minimal stimulation assay experiments were performed as previously described30,31 from epochs of 30 consecutive trials at −80mV and +40mV. The percentage of silent synapses was calculated using the equation 1-ln(%Failures at −80mv)/(% Failures at +40mV)27,32.
The coefficient of variation (CV) analysis was performed as previously described30,31,50. We calculated the CVs from epochs of 30 consecutive trials at −80mV for AMPAR-mediated currents and at +40mV for NMDAR-mediated currents. The peak responses of NMDAR-EPSCs were measured approximately 35ms after the onset of the AMPAR-EPSC at −80mV. The CV was calculated for AMPAR and NMDAR-mediated currents using the equation: , where SV=sample variance. The observer was blind to the cell type when determining successes or failures used to calculate the percentage of silent synapses.
To assess NR2B containing NMDA receptors, a selective antagonist (Ro-25698133, 1μM; Sigma) was applied for 20 minutes and evoked EPSCs obtained at +40mV before and during application were compared. The time to half-decay for NMDAR-mediated EPSCs was obtained while holding the cell at +40mV in ACSF containing 100μM picrotoxin (Sigma) and 20μM DNQX (Sigma).
Statistics
Group data are presented as mean±SEM. The number of cells and animals used for each experiment are denoted as cells/mice, and were established based upon the magnitude of the effect and the robustness of the statistical model. Paired Student’s t-tests were used for cocaine-induced locomotor activity, cocaine-induced GFP expression, and time to half-decay measurements. A two-factor ANOVA with GFP expression and repeated or acute cocaine as factors, followed by Bonferroni’s post-hoc test was used for spontaneous EPSC parameters, AMPAR/NMDAR ratios, AMPAR rectification, difference in failure rates at −80 mV and +40mV , proportion of silent synapses, and CV-NMDAR/CV-AMPAR experiments. For difference in failure rates between −80 mV and +40mV, and percentage of silent synapses from neurons in the Repeated cocaine and saline groups withdrawn at 1d and 6–11d, data were analyzed using a two-factor ANOVA with Repeated cocaine and Withdrawal duration as factors, followed by Bonferroni’s post-hoc test. For the TTX experiment, data were analyzed using a two-factorial ANOVA with GFP and TTX as factors. For AMPAR/NMDAR ratios in randomly selected neurons following repeated cocaine and saline injections, data were analyzed using a one-way ANOVA. Two-factor ANOVAs with repeated measures were used for AMPA, NASPM, Ro-256981 bath application and paired-pulse ratio experiments. For AMPAR/NMDAR ratios and spontaneous EPSC experiments, cells that exhibited frequencies two standard deviations from the mean were excluded from analysis. Only 2-tailed p values < 5% were considered significant for all parametric statistical tests used in these studies.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Intramural Research Program. We thank Dr. Alison Barth for providing the first line of c-fos-GFP mice and Kriss Knestaut and Jennifer Hug for breeding the c-fos-GFP mice and Evan Goldart and Robert Ator for genotyping assistance. We thank Drs. Scott Hall and Toni Shippenberg for use of their locomotor activity chambers. We thank Dr. Said Kourrich for many fruitful discussions regarding synaptic plasticity in the nucleus accumbens.
Footnotes
Author contributions: E.K., F.C.C. and B.T.H. designed the behavioral experiments. E.K. performed these experiments with F.C.C., R.A., S.A.G. and B.T.H. E.K., A.F.H., and C.R.L. designed the electrophysiology experiments. C.R.L. designed and built the spinning disc confocal and electrophysiology apparatuses. E.K. conducted and analyzed the data from the behavior, immunohistochemistry, and electrophysiology experiments. E.K., B.T.H., and C.R.L. wrote the paper.
References
- 1.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang YH, Schluter OM, Dong Y. Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 6.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 7.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 8.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 10.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 11.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 12.Mameli M, Bellone C, Brown MT, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- 13.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 14.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 15.Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fourgeaud L, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CC, et al. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koya E, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu LJ, Kim SS, Li X, Zhang F, Zhuo M. Sexual attraction enhances glutamate transmission in mammalian anterior cingulate cortex. Mol Brain. 2009;2:9. doi: 10.1186/1756-6606-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- 22.Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 23.Matamales M, et al. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Good CH, Lupica CR. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J Neurosci. 2010;30:7900–7909. doi: 10.1523/JNEUROSCI.1507-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 28.Rumpel S, Hatt H, Gottmann K. Silent synapses in the developing rat visual cortex: evidence for postsynaptic expression of synaptic plasticity. J Neurosci. 1998;18:8863–8874. doi: 10.1523/JNEUROSCI.18-21-08863.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown TE, et al. A Silent Synapse-Based Mechanism for Cocaine-Induced Locomotor Sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Liao D, Malinow R. Deficiency in induction but not expression of LTP in hippocampal slices from young rats. Learn Mem. 1996;3:138–149. doi: 10.1101/lm.3.2-3.138. [DOI] [PubMed] [Google Scholar]
- 33.Fischer G, et al. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 34.Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 35.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 37.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 38.Mattson BJ, et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- 39.Bertran-Gonzalez J, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sametsky EA, Disterhoft JF, Geinisman Y, Nicholson DA. Synaptic strength and postsynaptically silent synapses through advanced aging in rat hippocampal CA1 pyramidal neurons. Neurobiol Aging. 2010;31:813–825. doi: 10.1016/j.neurobiolaging.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: Silent synapse and beyond. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uslaner J, et al. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 44.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 47.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 48.Reiner A, Perera M, Paullus R, Medina L. Immunohistochemical localization of DARPP32 in striatal projection neurons and striatal interneurons in pigeons. J Chem Neuroanat. 1998;16:17–33. doi: 10.1016/s0891-0618(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 49.Paxinos G, KBJ F. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 2001. [Google Scholar]
- 50.Kullmann DM. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.