Abstract
Objective
The risk of clinically significant depressive symptoms increases during the perimenopause. With highly active antiretroviral treatment (HAART), more HIV-infected women survive to transition through the menopause. In a cross-sectional analysis, we evaluated the association of menopausal stage and vasomotor symptoms with depressive symptoms in an ethnically diverse, cohort of women with a high prevalence of HIV.
Methods
Participants included 835 HIV-infected women and 335 HIV-uninfected controls from the Women’s Interagency HIV Study (WIHS; 63% African-American). The Center for Epidemiological Studies Depression (CES-D) scale was used to screen for elevated depressive symptoms. Menopausal stages were defined according to standard definitions. Logistic regression analysis was used to identify predictors of elevated depressive symptoms.
Results
Compared to premenopausal women, early perimenopausal (OR 1.74, 95%CI 1.17–2.60), but not late perimenopausal or postmenopausal women were more likely to show elevated depressive symptoms in adjusted analyses. The odds were similar in HIV-infected and HIV-uninfected women. Persistent vasomotor symptoms also predicted elevated depressive symptoms in HIV-infected and uninfected women (OR 1.45, 95%CI 1.02–2.06). In HIV-infected women, menopausal stage interacted with antiretroviral use (p=0.02); the likelihood of elevated depressive symptoms in early perimenopause compared with premenopause was especially high in HAART-untreated women (OR 3.87, 95%CI 1.57–9.55).
Conclusions
In HIV+ and HIV− women, the odds of elevated depressive symptoms were significantly higher during the early perimenopause. Elevated depressive symptoms were associated with nonadherence to HAART, underscoring the importance of screening and treating depressive symptoms in HIV+ women who have experienced a change in the regularity of their menstrual cycles.
Keywords: HIV, Depression, Menopause, Perimenopause, African American, Vasomotor
Although mood complaints at midlife have long been attributed to menopause, there has been substantial debate regarding the impact of menopause on mood and cognition.1 The 2005 NIH State-of-the-Science Conference Statement on Management of Menopause-Related Symptoms concluded that there is strong evidence from both longitudinal and cross-sectional observational studies that the menopausal transition causes vasomotor symptoms and vaginal dryness.1 By contrast, the evidence that the menopausal transition leads to mood changes was considered “mixed.” Although large gaps in knowledge remain, a number of recent studies have informed our understanding of mood symptoms at midlife. The scientific literature indicates that most women experience the menopausal transition without mood difficulties. However, there are six studies that characterize the perimenopausal stage as a period of increased risk for elevated depressive symptoms and/or depressive disorders, including a study of women from Eindhoven Netherlands,2 the Study of Women’s Health Across the Nation (SWAN),3–6 the National Institutes of Mental Health,7 the Penn Ovarian Aging (POA) study,8, 9 the Harvard Study of Moods and Cycles,10 and the Seattle Midlife Women’s Health Study11. The magnitude of this risk is sizeable, with studies using diagnostic clinical interviews finding a two- to four-fold increased risk of depression during the transition in women with no previous history of mood disorder.5, 7, 10
One limitation of our understanding of perimenopausal-related depression is that research on this topic has largely been confined to cohorts of healthy women of middle-class or higher socioeconomic status. Two exceptions are the ethnically diverse cohort of women studied in the SWAN, a sample that included 28% African American women and 8% Latina,3 and the Penn Ovarian Aging Study, a sample that included 50% African American women.8, 9 One significant, and as-yet unstudied question concerning perimenopausal depression is whether women of lower socioeconomic status may be at increased risk for perimenopausal depression because of other exacerbating factors including low income, low educational attainment, unemployment, interpersonal violence, and drug and alcohol use.12 Similarly, since previous studies were based on cohorts of healthy women, little is known about the differential risk of depression among healthy women versus those with serious medical illnesses. Understanding factors that are associated with depression in HIV-infected women is especially important is especially important because depression is associated with nonadherence to HAART as well as increased mortality.13, 14 We therefore investigated the potential interactive effects of menopausal stage and HIV status on elevated depressive symptoms in the Women’s Interagency HIV Study (WIHS). The WIHS is the largest study of the natural and treated history of HIV in women. Here we report data from 835 HIV-infected women and 335 HIV-uninfected controls. One substantial benefit of examining this question in the WIHS is that the controls are similar to the HIV-infected women in risk factors for depressive symptoms and HIV. We hypothesized that there would be an increased risk for elevated depressive symptoms in the perimenopausal period and that this risk would be higher in HIV-infected women. We further hypothesized that women treated with HAART would show lower depressive symptoms in the perimenopausal period compared to women who have not received HAART because of evidence that initiation or maintenance of HAART decreases depressive symptoms in patients with HIV.15, 16
METHODS
Study Population
The Women’s Interagency HIV Study (WIHS) is a longitudinal, multi-center study of the natural and treated history of HIV infection in women and includes six clinical sites including: Bronx/Manhattan, NY; Brooklyn, NY; Chicago, IL; Washington, DC; San Francisco and Los Angeles, CA.17, 18 Study methodology, training, and quality assurance procedures as well as the cohort’s baseline characteristics have been reported previously.17, 18 In brief, there were two waves of enrollment in the WIHS, the first in October 1994 and the second in 2001–2002 (N=3,766). At each enrollment, HIV-infected and uninfected women were similar in age, race/ethnicity, level of education, recruitment site, and risk factors including history of injection drug use, and number of sexual partners.17, 18 Eligibility criteria at enrollment into the WIHS included age 13 years or older, provision of informed consent, and ability to complete an interview in either English or Spanish. WIHS participants complete “core” visits every 6 months and at each of these visits they undergo clinical examination, extensive medical interview, questionnaires (including the Center for Epidemiologic Studies Depression Scale (CES-D)), and a blood draw. This analysis was restricted to data collected as part of a cross-sectional study of menopause, cognition, and mood that was incorporated into the WIHS core visits in April 2007 to April 2008. A total of 1901 WIHS participants were assessed during that time frame, and 1458 of those women met the entry criteria of: a) age 30 to 65 (n = 203 excluded); b) completion of the CES-D (n = 21 excluded); and c) completion of detailed questions about menstrual cycle characteristics used to determine menopausal status (n = 219). Of the 1458 participants, we included 1170 (80% of the cohort) after excluding participants meeting one or more of the following exclusion criteria: a) current pregnant or breastfeeding (n=9); b) hysterectomy or bilateral oophorectomy (n=180); and/or use of hormone therapy or oral contraceptives within the past 6 months (n=124). In the Results section we compare the characteristics of women who were included in this analysis (n=1170; 80% of overall sample) and those who were excluded (n=731).
Measures
Primary outcome
Depressive Symptoms
The primary outcome was clinically indicated depression as assessed by the CES-D,19 a 20-item self-report measure of depressive symptoms. The CES-D has excellent reliability, validity, and factor structure19 and is commonly used in studies of HIV-positive cohorts,20 including studies of HIV-infected women.21 The instrument has been studied extensively in the WIHS, including in studies of perinatal depression.22 Importantly, a validation study in the WIHS demonstrated that the standard cutoff of 16 produced virtually identical associations with antiretroviral therapy use and with the woman’s demographic characteristics when compared with alternative cut-offs, including a more stringent cutoff of 23, and an interval-level version of the subscale that excluded somatic items similar to HIV symptoms.13 Thus, this study used the standard cutoff score of 16. The CES-D sensitivity for the diagnosis of major depression is excellent, in the range of 80–90%, with a somewhat lower specificity, in the range of 70–80%.12, 16, 23
Menopausal Stage Classification
Menopausal stage was explicitly assessed in the medical interview and classified according to definitions used in the SWAN. This allowed us to compare our findings with those from the SWAN, which is the largest study of the menopausal transition in the United States and which has produced a series of important findings regarding depression and the menopausal transition3–6. Importantly, the SWAN criteria are very similar to criteria set forth in the Stages of Reproductive Aging Workshop (STRAW) consensus statement.24 The definitions were as follows: premenopausal (menses in the past 3 months with no changes in regularity); early perimenopausal (menses in the past 3 months with change in regularity); late perimenopausal (no menses within the past 3 months but some menstrual bleeding within the past 12 months); and postmenopausal (no menses within the past 12 months).
Covariates
The primary factors of interest were menopausal stage, HIV serostatus, and the interaction of the two variables. In addition, to age, race/ethnicity, level of education (< high school education versus ≥ high school education), and site (Bronx, Brooklyn, Washingon, DC, Los Angeles, San Francisco, Chicago), a number of potential covariates and predictors of risk factors for elevated depressive symptoms were examined based on previous literature in the WIHS and other studies.12 Socio-demographic covariates included marital status (married versus living with a partner versus all other participants), employment status, average household income (≤$12,000 versus >$12,000), and insurance status. Other covariates included current smoking status, recent alcohol use (abstainer versus moderate ≤ 7 drinks/week versus heavy/ > 7 drinks/week), past heavy alcohol use during WIHS (> 7 drinks/week), recent (last six months) marijuana/hash use, use of crack/cocaine/or heroin via any means of administration (never, former, current), and current number of sexual partners (< 2 versus ≥ 2). We also included medical factors: self-reported use of antidepressant medication (never, former, current), prior CES-D ≥16 during WIHS, and Hepatitis C virus antibody. We also examined covariates related to reproductive health including past use of menopausal hormone therapy, parity, and body mass index as well as vasomotor symptoms. Vasomotor symptoms were ascertained using a questionnaire from the SWAN and were categorized as high when women reported experiencing more than half of the individual vasomotor items (hot flashes or night sweats) on ≥6 days in the past 2 weeks3. In analyses restricted to HIV-infected women we also assessed HAART use (no HAART versus on HAART and <95% compliant with prescribed dosing versus on HAART and ≥95% compliant), duration on HAART (years), CD4 count (a measure of immunologic function in HIV infection), and viral load (number of copies of HIV RNA per ml of plasma which is an indicator of the rate of viral replication at the current time).
Statistical Analyses
Differences between HIV-infected and uninfected women in demographic characteristics were examined using independent t-tests for continuous variables and Chi-square (Χ2) tests for categorical variables. Next, Χ2 tests were computed to examine the prevalence of elevated depressive symptoms (≥ 16 on the CES-D) as a function of menopausal stage), HIV serostatus (infected versus uninfected), and menopausal stage × serostatus. Subsequently, unadjusted logistic regression models were used to assess the association of serostatus, menopausal stage, and other potential risk factors (e.g., risky health behaviors, socio-demographic factors, and reproductive health factors as described above) with elevated depressive symptoms. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using maximum likelihood estimates from the logistic regression models. Based on simple regression techniques, predictors that were found to be significant were examined in subsequent multivariable logistic regression analyses examining the impact of menopausal stage, serostatus, and their interaction on elevated depressive symptoms. Age, race/ethnicity, site, and education were included in all analyses. Forward and backward selection procedures (done manually) were used to determine the best fitting model, with menopausal stage and serostatus forced in the models. All p values are two-sided, and the statistical significance level was set at p<0.05. All analyses were performed using SAS (version 9.2, SAS Institute Inc, Cary, NC).
RESULTS
Population Characteristics
Table 1 shows demographic and clinical information for the 835 HIV-infected women, the 335 HIV-uninfected women, and the combined sample of 1170 women. Participants ranged in age from 30 to 65 years (M = 44.19, SD = 7.42). Minority representation was high, with 63% African-American and 20% Latina participants. The average income was low with 51% of the samples having annual incomes below $12,000 annually. Lifetime use of illicit substances (i.e., crack/heroin/and/or powder cocaine) was common (64%). Only 13% reported use of illicit substances since the previous study visit 6 months earlier, with more HIV-uninfected (16%) than infected (12%) women reporting current use (p<.05). Recent use of marijuana, cigarettes, and alcohol, were also higher among uninfected versus infected women (p<.001). Among HIV-infected women, 16% had a current CD4 + lymphocyte count less than 200 cells/µl. Plasma HIV RNA was not detected (lower limit of detection was 80 copies per ml) in 50% indicating optimal treatment response. Viral load was greater than the lower limit of quantitation (LLQ) but < 10,000 copies/mL for 32% and > 10,000 copies/mL for 18% of HIV-infected women. Sixty-three percent had been prescribed HAART therapy, and 75% reported adherence to >95% of prescribed doses.
Table 1.
Demographic characteristics of HIV-infected, HIV-uninfected, and the Two Groups Combined.
| HIV Status | |||
|---|---|---|---|
| Background Characteristics (n, %) | Infected n = 835 |
Uninfected n = 335 |
Overall N = 1170 |
| Menopausal Status** | |||
| Pre | 440 (53) | 205 (61) | 645 (55) |
| Early Peri | 116 (14) | 58 (17) | 174 (15) |
| Late Peri | 45 (5) | 12 (4) | 57 (5) |
| Post | 234 (28) | 60 (18) | 294 (25) |
| Age (M, SD)** | 44.7 (7.3) | 42.9 (7.5) | 44.2 (7.4) |
| Race/Ethnicity | |||
| African American, non-Hispanic | 526 (63) | 209 (62) | 735 (63) |
| White, non-Hispanic | 123 (14) | 35 (10) | 158 (13) |
| Hispanic | 155 (19) | 76 (23) | 231 (20) |
| Other | 31 (4) | 15 (5) | 46 (4) |
| Site** | |||
| Bronx | 168 (20) | 89 (27) | 257 (22) |
| Brooklyn | 197 (24) | 53 (16) | 250 (21) |
| Washington DC | 138 (16) | 48 (14) | 186 (16) |
| Los Angeles | 85 (10) | 51 (15) | 136 (12) |
| San Francisco | 124 (15) | 61 (18) | 185 (16) |
| Chicago | 123 (15) | 33 (10) | 156 (13) |
| At least high school graduate or equivalent | 548 (66) | 217 (65) | 765 (65) |
| Average household income ≤ 12,000/yr | 424 (51) | 167 (50) | 591 (51) |
| Currently married or with a partner* | 217 (26) | 116 (35) | 333 (29) |
| Currently employed* | 321 (38) | 165 (49) | 486 (42) |
| Carrying insurance** | 781 (94) | 258 (77) | 1039 (89) |
| Parous | 770 (92) | 311 (93) | 1081 (92) |
| Body Mass Index (BMI) (M, SD)** | 28.6 (8.0) | 31.5 (8.6) | 29.4 (8.3) |
| Current number of sex partners (≥ 2)* | 62 (7) | 46 (14) | 108 (9) |
| Recent Use1 | |||
| Crack, cocaine, and/or heroin* | 100 (12) | 55 (16) | 155 (13) |
| Marijuana** | 135 (16) | 83 (25) | 218 (19) |
| Smoking** | 380 (46) | 196 (59) | 576 (49) |
| Alcohol** | |||
| Abstainer | 466 (56) | 163 (49) | 629 (54) |
| Moderate (≤ 7 drinks/week) | 319 (38) | 128 (38) | 447 (38) |
| Heavy (> 7 drinks/week) | 50 (6) | 44 (13) | 94 (8) |
| Antidepressant medication* | 134 (16) | 36 (11) | 170 (15) |
| Past Use2 | |||
| Crack, cocaine, and/or heroin | 531 (64) | 230 (69) | 761 (65) |
| Heavy Alcohol (> 7 drinks/week)** | 267 (32) | 152 (45) | 419 (36) |
| Hormone Therapy | 83 (10) | 30 (9) | 113 (10) |
| Antidepressant medication** | 309 (37) | 91 (27%) | 400 (34) |
| History of CES-D ≥16 | 709 (85) | 275 (82) | 984 (84) |
| Frequent Vasomotor Symptoms (%)3 | 151 (18) | 56 (17) | 207 (18) |
| Disease | |||
| CD4 Count (cells/µl) | |||
| > 500 | 350 (42) | - | - |
| ≥ 200 and < 500 | 351 (42) | - | - |
| < 200 | 134 (16) | - | - |
| Viral Load (HIV RNA (cp/ml)) | |||
| Undetectable | 419 (50) | - | - |
| < 10,000 | 268 (32) | - | - |
| ≥ 10,000 | 148 (18) | - | - |
| Medication Use | - | - | |
| HAART | 526 (63) | - | - |
| Non-HAART (mono or combination therapy) | 25 (3) | - | - |
| No therapy | 284 (34) | - | - |
| Medication Compliance (≥ 95%) | 412 (75) | - | - |
| ART duration (years)(M, SD)† | 9.3 (3.0) | - | - |
| HAART duration (years)(M, SD)‡ | 7.6 (2.6) | - | - |
| HCV** | 288 (35) | 80 (24) | 368 (32) |
Note.
p < 0.01,
p < 0.05;
“Recent Use” refers to within 6 months of the most recent WIHS visit.
“Past Use” refers to any previous use.
Frequent vasomotor symptoms (hot flash or night sweats) occurred at least 6 days in the past 2 weeks.
Reflects the mean for 745 of the HIV-infected women (89%) who started ART prior to menopause data collection.
Reflects the mean for 719 HIV-infected women (86%) who started HAART prior to menopause data collection.
HCV = Hepatitis C virus antibody.
Compared to the 731 women excluded from this analysis (see Participants section above), the 1170 women included in the analysis were more likely to be older (44.2 vs. 41.0, p<.001), to be Black non-Hispanic (63% vs. 49%, p<.001), to have at least a high school education (65% vs. 56%, p<.001), to be living alone (72% vs. 64%, p=.001), to smoke (49% vs. 34%, p<.001), to have engaged in hazardous drinking in the past (36% vs. 27%, p<.001), to have used crack, cocaine, and/or heroin recently or in the past (65% vs. 42%), to have recently used marijuana (19% vs. 14%, p=.02), and to have more depressive symptoms (38% vs. 32%, p=.01).
Prevalence of Depressive Symptoms
In unadjusted analyses, 38% of the women had CES-D scores ≥ 16. The prevalence of elevated depressive symptoms was the same among HIV-infected and uninfected women, at 38% each. Notably, the prevalence of elevated depressive symptoms differed by menopausal stage, with 33% among premenopausal women, 47% among early perimenopausal women, 42% among late perimenopausal women, and 43% among postmenopausal women, χ2 (1, n = 1170) = 15.95 p<0.001. Within these four strata, the prevalence of elevated depressive symptoms was similar for HIV-infected and uninfected women, p’s>.18.
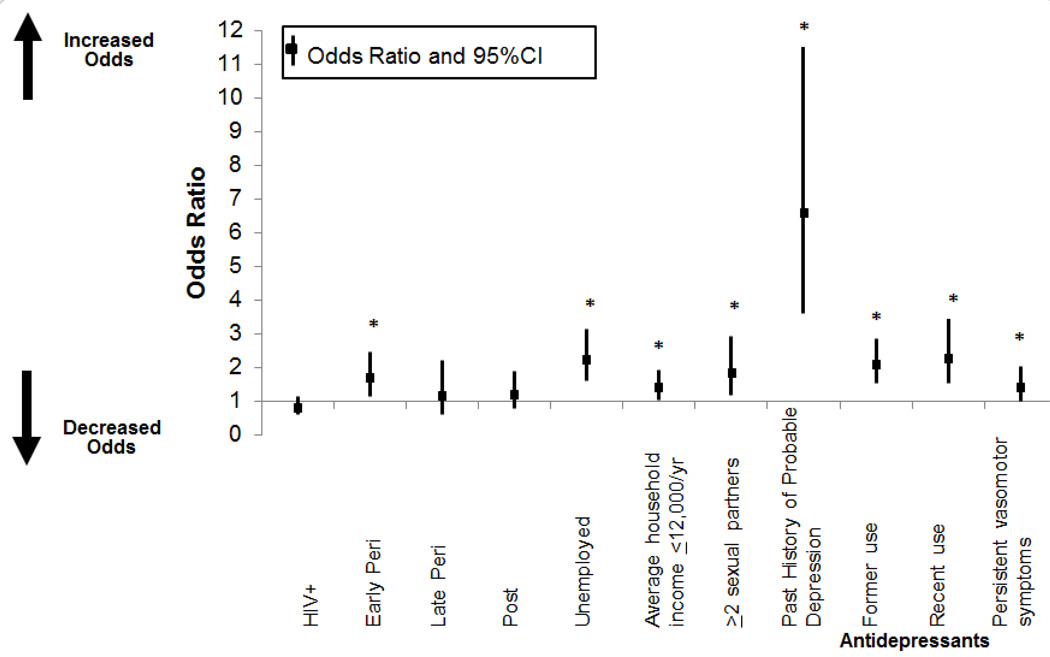
Table 2 shows results from the unadjusted and adjusted regression analyses. Variables found to be significant in the unadjusted analyses included unemployment, low social support, low household income, higher number of current sexual partners, current smoking, past and recent heavy alcohol use, former and recent illicit use of crack, cocaine, and/or heroin, HCV infection, recent use of antidepressants, and persistent vasomotor symptoms. In the total population, early perimenopausal stage was associated with a greater likelihood of elevated depressive symptoms, both in unadjusted (OR 1.77, 95%CI 1.26–2.48, p=0.001) and adjusted analyses (OR 1.74, 95%CI 1.17–2.60, p=0.007; Figure 1). Similarly, in analyses restricted to HIV-infected women, those in the early perimenopausal stage had significantly higher depressive symptom severity than those in the premenopausal stage, both in unadjusted (OR 1.97, 95%CI 1.29–2.99, p=0.002) and adjusted analyses (OR 1.92, 95%CI 1.16–3.19, p=0.01). Contrary to our hypotheses, there was no interaction between HIV infection status and menopausal stage, indicating that HIV-infected women were not more likely to report elevated depressive symptoms in the early perimenopausal stage when compared with HIV-uninfected women. Among HIV-infected women, a CD4 count < 200 cells/µl was also associated with a higher rate of depression (OR = 2.14, 95%CI 1.29–3.56, p <= .003), and HAART adherence was associated with a lower rate of depression (OR = 0.67, 95%CI = 0.46–0.96, p = .03).
Table 2.
Unadjusted and Adjusted Logistic Regression Analyses Examining Predictors of Depressive Symptoms in the Entire Sample and in HIV-Infected Women Separately.
| Overall sample | HIV-infected women | |||
|---|---|---|---|---|
| Predictors | Unadjusteda ORb (95% CIc) |
Adjustedd ORb (95% CIc) |
Unadjusteda ORb (95% CIc) |
Adjustedd ORb (95% CIc) |
| HIV-infected (vs uninfected) | 1.01 (0.78–1.31) | 0.84 (0.62–1.14) | - | - |
| Menopausal stage (vs pre) | ||||
| Early Peri | 1.77 (1.26–2.48)* | 1.74 (1.17–2.60)* | 1.97 (1.29–2.99)* | 1.92 (1.16–3.19)* |
| Late Peri | 1.48 (0.85–2.56) | 1.18 (0.63–2.22) | 1.85 (0.99–3.43) | 1.61 (0.77–3.36) |
| Post | 1.54 (1.16–2.05)* | 1.23 (0.80–1.89) | 1.57 (1.13–2.19)* | 1.55 (0.92–2.63) |
| Socio-demographic Factors | ||||
| Age (per year) | 1.01 (0.99–1.03) | 0.98 (0.96–1.01) | 1.00 (0.98–1.02) | 0.96 (0.93–0.99)* |
| Race (vs White non-Hispanic) | ||||
| Black non-Hispanic | 0.97 (0.68–1.39) | 1.01 (0.67–1.54) | 1.15 (0.76–1.74) | 1.27 (0.77–2.10) |
| Hispanic | 1.24 (0.82–1.87) | 1.22 (0.74–2.00) | 1.68 (1.03–2.76)* | 1.65 (0.89–3.01) |
| Other | 1.08 (0.55–2.12) | 1.15 (0.53–2.52) | 1.43 (0.64–3.20) | 2.07 (0.78–5.54) |
| Site (vs Brooklyn) | ||||
| Bronx | 1.49 (1.03–2.15)* | 0.92 (0.60–1.41) | 1.57 (1.01–2.44)* | 1.10 (0.65–1.87) |
| Washington DC | 1.14 (0.76–1.71) | 1.38 (0.86–2.21) | 1.06 (0.66–1.72) | 1.40 (0.80–2.46) |
| Los Angeles | 1.74 (1.12–2.68)* | 1.30 (0.79–2.14) | 1.76 (1.03–3.01)* | 1.59 (0.85–2.99) |
| San Francisco | 2.03 (1.36–3.01)* | 1.29 (0.81–2.05) | 2.01 (1.25–3.21)* | 1.45 (0.83–2.54) |
| Chicago | 1.67 (1.10–2.53)* | 1.30 (0.81–2.09) | 1.96 (1.22–3.16)* | 1.71 (0.98–2.96) |
| Less than high school education (vs At least high school graduate or equivalent) |
1.77 (1.38–2.26)* | 1.28 (0.97–1.71) | 1.96 (1.46–2.64)* | 1.27 (0.89–1.80) |
| Currently unemployed (vs employed) | 3.46 (2.66–4.50)* | 2.27 (1.63–3.16)* | 3.75 (2.71–5.20)* | 2.85 (1.95–4.17)* |
| Not carrying insurance (vs yes) | 0.94 (0.64–1.36) | 1.07 (0.61–1.89) | ||
| No social support (vs married/partner) | 1.49 (1.14–1.94)* | 1.57 (1.13–2.19)* | ||
| Average household income ≤12,000/yr (vs > 12,000) | 2.40 (1.88–3.06)* | 1.43 (1.05–1.95)* | 2.38 (1.78–3.18)* | |
| Risk Behaviors | ||||
| Current number of sexual partners ≥2 (vs <2) | 1.94 (1.30–2.89)* | 1.86 (1.18–2.94)* | 1.46 (0.86–2.48) | |
| Currently smoking (vs no) | 1.93 (1.52–2.45)* | 1.78 (1.34–2.37)* | ||
| Recent alcohol use (vs abstainer) | ||||
| Moderate (1–7 drinks/week) | 0.80 (0.62–1.03) | 0.75 (0.55–1.01) | ||
| Heavy (>7 drinks/week) | 1.71 (1.10–2.64)* | 1.42 (0.78–2.60) | ||
| Past heavy alcohol (>7 drinks/week)(vs <7) | 1.67 (1.30–2.13)* | 1.51 (1.11–2.03)* | ||
| Crack/cocaine and/or heroin use (vs never) | 2.35 (1.67–3.31)* | |||
| Former | 1.59 (1.21–2.08)* | 1.35 (0.99–1.86) | ||
| Current | 3.12 (2.13–4.58)* | 2.92 (1.82–4.69)* | ||
| Recent marijuana use (vs no) | 1.75 (1.30–2.36)* | 1.72 (1.18–2.51)* | ||
| Reproductive Health | ||||
| Persistent vasomotor symptoms | 1.98 (1.46–2.68)* | 1.45 (1.02–2.06)* | 2.08 (1.45–2.98)* | 1.49 (0.97–2.29)* |
| Parous (vs not) | 1.45 (0.91–2.31) | 1.94 (1.08–3.48)* | ||
| Body mass index | 0.99 (0.97–1.003) | 0.98 (0.96–0.99)* | ||
| Never used hormone therapy (vs yes) | 1.24 (0.82–1.86) | 1.46 (0.89–2.42) | 1.68 (0.93–3.02) | |
| Medical Factors | ||||
| Past antidepressant medication use (vs no) | 3.03 (2.32–3.97)* | 2.11 (1.55–2.87)* | 2.80 (2.02–3.86)* | 1.96 (1.34–2.87)* |
| Current antidepressant medication use (vs no) | 3.51 (2.46–5.00)* | 2.31 (1.55–3.46)* | 2.93 (1.93–4.43)* | 2.10 (1.28–3.42)* |
| Past CES-D ≥16 | 10.4 (5.85–18.5)* | 6.62 (3.62–12.1)* | 13.1 (6.04–28.6)* | 9.10 (4.03–20.5)* |
| HCV Antibody + (vs negative) | 1.67 (1.30–2.15)* | 1.67 (1.25–2.25)* | ||
| HIV-related factors | ||||
| CD4 (vs >500) | - | - | ||
| ≥200 and <500 | 1.17 (0.85–1.60) | 1.03 (0.72–1.47) | ||
| <200 | 2.73 (1.79–4.16)* | 2.14 (1.29–3.56)* | ||
| Viral load (vs undetectable) | - | - | ||
| <10,000 | 1.79 (1.30–2.48)* | |||
| ≥10,000 | 2.12 (1.44–3.12)* | |||
| Medication use (vs no HAART) | - | - | ||
| HAART and <95% adherent | 0.99 (0.65–1.51) | 0.90 (0.55–1.45) | ||
| HAART and ≥95% adherent | 0.64 (0.47–0.88)* | 0.67 (0.46–0.96)* | ||
| Duration on HAART (years) | - | - | 0.99 (0.95–1.03) | |
Note.
p < 0.05.
Unadjusted analysis refers to a simple logistic regression equation with the dependent variable (CES-D 16 cutoff) and only one independent variable.
OR=odds ratio.
CI=confidence interval.
Adjusted analysis refers to the covariates that were able to best predict CES-D using forward and backward selection procedures to determine the best fitting multivariable model.
Figure 1.
Association of Menopausal Stage with Depressive Symptoms
To examine the influence of HAART treatment on perimenopausal depression, a final exploratory multivariable regression was conducted in HIV-infected women only. Medication use (no HAART, <95% compliant and on HAART, ≥95% compliant and on HAART), menopausal stage, and their interaction were forced in the model along with relevant covariates from the analysis of the larger sample. There was a significant interaction of menopausal stage (i.e., early perimenopausal versus premenopausal) and HAART use (p=0.02). Specifically, among HIV-infected women who reported not taking HAART, those in the early perimenopausal stage were over 3.9 times as likely to report elevated depressive symptoms as those in the premenopausal stage (OR 3.87, 95%CI 1.57–9.55, p=0.003).
DISCUSSION
The aim of this investigation was to examine the likelihood of elevated depressive symptoms in relation to menopausal stage in an ethnically diverse cohort of low-income HIV-infected and HIV-uninfected women. The finding that depressive symptoms are elevated during the perimenopausal stage in healthy cohorts has been established in a number of studies2–11. The present study demonstrates that the link between the perimenopausal stage and elevated depressive symptoms extends to women with a high prevalence of poverty (i.e., 51% had household incomes below the established poverty line) and ethnic diversity (i.e., 66% African American, 20% Latina). Notably, the majority of women in this study were HIV-infected (i.e., 69%), but HIV-infected women were not more likely to show elevated depressive symptoms during the perimenopausal stage compared to HIV-uninfected women. Overall, the likelihood of elevated depressive symptoms was 74% higher in the early perimenopausal stage compared to the premenopausal stage, after controlling for significant covariates. Elevated risk was also evident in analyses restricted to HIV-infected women. Women with persistent vasomotor symptoms were 45% more likely to show elevated depressive symptoms. Other factors associated with depressive symptoms in this cohort have been reported in previous studies and include history of probable depression, unemployment, low income, higher number of sexual partners, and current and former antidepressant medication use.5, 9 In HIV-infected women, adherence with HAART was protective, and CD4 lymphocyte counts less than 200 cells/ml (indicating more advanced HIV immune injury) were associated with elevated depressive symptoms.
It is useful to compare findings from the present study with those from SWAN and the POA Study. Like the present study, the SWAN and the POA used similar definitions of menopausal stage and elevated depressive symptoms (i.e., CES-D > 16), and drew on ethnically diverse (albeit healthier and more economically advantaged) cohorts. Importantly, although all three studies used premenopausal stage as a reference for risk in later reproductive stages, the SWAN and POA studies assessed risk longitudinally,4, 9 allowing within-person estimates of risk. In contrast, the WIHS estimates are based on a cross-sectional design. The likelihood of elevated depressive symptoms in the early perimenopausal versus premenopausal stage was significant in all three studies: 74% higher in the WIHS, 30% higher in the SWAN,4 and 55% higher in the POA Study.9 Given our study’s low representation of women in the late perimenopausal stage (only 57 out of 1170 participants), it may be that our analysis was underpowered to detect differences for late perimenopausal stage which were observed in both SWAN and POA. The risk in the postmenopausal period was not significant in the present study, was 57% higher in the SWAN, and was not significant in the POA which was underpowered to detect that particular effect. Ongoing data collection in WIHS will allow for comparisons with SWAN and the POA based on a longitudinal design.
It is notable that an association between menopausal stage and depressive symptom severity is observed despite the high prevalence of unemployment, poverty, and other risk factors that contribute to depressive symptoms in this cohort. In our cohort, 58% of women were unemployed, and unemployment was associated with a 2.3-fold increased likelihood of elevated depressive symptoms. In SWAN, 8% of the cohort reported that paying for basics was “very” hard, and the likelihood of elevated depressive symptoms was 2 times higher among those women.4 In the POA study, 18% of participants were unemployed, and unemployment was associated with a 97% increased likelihood of elevated depressive symptoms.
Although HIV-infected women showed significantly higher depressive symptom severity during the early perimenopausal period than the premenopausal period, the magnitude of severity was not significantly different from that observed among the HIV-uninfected women. Similarly, we recently reported that HIV-infected women were more at risk for depression during the perinatal period than in the antenatal period, but their risk was not higher than that of controls.25 Together, these two findings suggest that HIV-infected WIHS participants were not more likely than HIV-uninfected participants to show elevated depressive symptoms during reproductive transitions. It remains to be seen whether these results generalize to other cohorts of HIV-infected women, including those who do not receive HAART treatment and who are not involved in studies that can provide social support and improved medical monitoring. Contrary to other HIV-infected women, HIV-infected women who were adherent to HAART did not show an elevated risk of depression during the perimenopausal period. Based on that finding, we might expect to find higher rates of perimenopausal depression among cohorts of HIV-infected women who are less adherent to HAART. As noted earlier, elevated depressive symptoms are linked to non-adherence to HAART and greater mortality.13, 14
The factors contributing to higher depressive symptom severity in the perimenopausal period compared with the premenopausal period are unclear. Consistent with other investigations, we found that moderate to severe vasomotor symptoms were associated with increased odds for elevated depressive symptoms.4, 5, 11 Risk for depression has been associated with high levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) as well as increased variability in estradiol, FSH, and LH as measured over two consecutive months,8, 9 although other studies find no association between annual hormone measurements and risk of depression.5 Other studies report an association between a history of postpartum depression and increased risk of depression in the perimenopausal stage.9, 26
Our results suggest that HIV care providers should be alert to the possibility of increased depressive symptoms in HIV-infected women who report new onset of menstrual cycle irregularity. Longitudinal analyses of depressive symptoms and adherence in WIHS participants demonstrate an association between depressive symptoms and non-adherence to HAART.13 In that light, treatment of perimenopausal depressive symptoms may enhance adherence to antiretroviral therapy and thereby improve health outcomes in HIV-infected women. Related to this is our exploratory cross-sectional finding that women in the present study not taking HAART showed a 3.9-fold increased odds of elevated depressive symptoms during the early perimenopause. Longitudinal follow-up of these women is ongoing and will provide important information about patterns of depression and HAART use over time. Information about efficacy of hormonal and non-hormonal interventions in the treatment of mood disorders in the perimenopausal and postmenopausal stages comes from clinical trials in healthy women, but these therapies have not been tested in HIV-infected women with perimenopausal depression. For example, transdermal estrogen therapy, escitalopram, and desvenlafaxine have been shown to be an effective therapy for perimenopausal mood disorders in HIV-uninfected women.27–29 Both SSRIs are used in treating depression in individuals with HIV, and a recent review provides useful information about diagnosing and treating depression in women with HIV.30 Use of estrogen therapies requires further investigation since HIV infection is associated with increased risk of thrombotic events.31, 32
There were several limitations to the study. Primary among these is the cross-sectional design. Second, elevated depressive symptoms were measured using the CES-D rather than a diagnostic interview. Third, compared to WIHS participants who were excluded from this study (e.g., based on age, pregnancy, hormone use, etc.), those included in this study showed higher depressive symptoms and more risk factors for depressive symptoms (e.g., past drug use), so the generalizability of these findings is to be determined. We do not have information on important mental health variables that might alter the findings including history of depression diagnosis, indications for antidepressant use (women with HIV are prescribed antidepressants for neuropathy among other reasons), and years of HIV infection. Longitudinal studies are underway in the WIHS to evaluate the risk of elevated depressive symptoms and clinical depression based on diagnostic interview as women transition through the menopause and to further assess the impact of vasomotor symptoms and HAART adherence in perimenopausal depression.
CONCLUSION
Our results extend previous studies of perimenopausal depression in healthy, high-SES cohorts to a large sample of women with HIV. Our finding that depressive symptoms were most evident in women who were nonadherent to HAART support a need to further understand the relationship between medication adherence and symptoms of depression in perimenopausal women with HIV.
Acknowledgements
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). L.Rubin’s participation was funded by grant number K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women's Health (ORWH).The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures/conflicts of interest: PM has served as a consultant for Noven Pharmaceuticals.
Preliminary data presented: North American Menopause Society, San Diego, CA, Oct 1–3, 2009.
References
- National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–1013. [PubMed] [Google Scholar]
- 2.Maartens LW, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas. 2002;42(3):195–200. doi: 10.1016/s0378-5122(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 3.Bromberger JT, Assmann SF, Avis NE, Schocken M, Kravitz HM, Cordal A. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158(4):347–356. doi: 10.1093/aje/kwg155. [DOI] [PubMed] [Google Scholar]
- 4.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN) Psychol Med. 2011;41(9):1879–1888. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161(12):2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Archives of General Psychiatry. 2004;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 10.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 11.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2008;15(2):223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 12.Richardson J, Barkan S, Cohen M, et al. Experience and covariates of depressive symptoms among a cohort of HIV infected women. Soc Work Health Care. 2001;32(4):93–111. doi: 10.1300/J010v32n04_05. [DOI] [PubMed] [Google Scholar]
- 13.Cook JA, Cohen MH, Burke J, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30(4):401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Cook JA, Grey D, Burke J, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan KS, Orlando M, Joyce G, et al. Combination antiretroviral therapy and improvements in mental health: results from a nationally representative sample of persons undergoing care for HIV in the United States. J Acquir Immune Defic Syndr. 2003;33(1):104–111. doi: 10.1097/00126334-200305010-00015. [DOI] [PubMed] [Google Scholar]
- 16.Low-Beer S, Chan K, Yip B, et al. Depressive symptoms decline among persons on HIV protease inhibitors. J Acquir Immune Defic Syndr. 2000;23(4):295–301. doi: 10.1097/00126334-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 18.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 20.Moore J, Schuman P, Schoenbaum E, Boland B, Solomon L, Smith D. Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. Aids. 1999;13(17):2459–2468. doi: 10.1097/00002030-199912030-00018. [DOI] [PubMed] [Google Scholar]
- 21.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LH, Cook JA, Grey DD, et al. Perinatal Depressive Symptoms in HIV-Infected Versus HIV-Uninfected Women: A Prospective Study from Preconception to Postpartum. J Womens Health (Larchmt) 2011 doi: 10.1089/jwh.2010.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breslau N. Depressive symptoms, major depression, and generalized anxiety: a comparison of self-reports on CES-D and results from diagnostic interviews. Psychiatry Res. 1985;15(3):219–229. doi: 10.1016/0165-1781(85)90079-4. [DOI] [PubMed] [Google Scholar]
- 24.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8(6):402–407. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rubin LH, Cook JA, Grey DD, et al. Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. J Womens Health (Larchmt) 2011;20(9):1287–1295. doi: 10.1089/jwh.2010.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods NF, Mariella A, Mitchell ES. Patterns of depressed mood across the menopausal transition: approaches to studying patterns in longitudinal data. Acta Obstetricia et Gynecologia Scandinavica. 2002;81:623–632. doi: 10.1034/j.1600-0412.2002.810708.x. [DOI] [PubMed] [Google Scholar]
- 27.Soares CN, Almeida OP, Joffe H, LS C. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry. 2001;58(6):529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 28.Kornstein SG, Jiang Q, Reddy S, Musgnung JJ, Guico-Pabia CJ. Short-term efficacy and safety of desvenlafaxine in a randomized, placebo-controlled study of perimenopausal and postmenopausal women with major depressive disorder. Journal of Clinical Psychiatry. 2010;71(8):8. doi: 10.4088/JCP.10m06018blu. [DOI] [PubMed] [Google Scholar]
- 29.Soares CN, Thase ME, Clayton A, et al. Open-label treatment with desvenlafaxine in postmenopausal women with major depressive disorder not responding to acute treatment with desvenlafaxine or escitalopram. CNS Drugs. 2011;25(3):9. doi: 10.2165/11586460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Watkins CC, Pieper AA, Treisman GJ. Safety considerations in drug treatment of depression in HIV-positive patients: an updated review. Drug Safety. 2011;34(8):16. doi: 10.2165/11592070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Crum-Cianflone NF, Weekes J, Bavaro M. Thromboses among HIV-Infected Patients during the Highly Active Antiretroviral Therapy Era. AIDS Patient Care and STDs. 2008;22(10):7. doi: 10.1089/apc.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiser KL, Badowski ME. Risk factors for venous thromboembolism in patients with human immunodeficiency virus infection. Pharmacotherapy. 2010;30(12):10. doi: 10.1592/phco.30.12.1292. [DOI] [PubMed] [Google Scholar]