Abstract
Hypertrophic growth of the heart occurs in many clinical scenarios, and it confers substantially increased risk of untoward sequelae. Among them, transition to ventricular dilation, wall thinning, contractile dysfunction, and a clinical syndrome of heart failure is paramount. Left ventricular hypertrophy (LVH) is typically diagnosed by either electrocardiography or echocardiography. However, these two means of assessing hypertrophic transformation of the left ventricle can sometimes disagree. At one level, this may not be surprising as the two methodologies are based on entirely divergent signals: electrical potential between two places on the surface of the skin and ultrasound energy reflected from the ventricle itself. Echocardiography is an effective means of assessing ventricular mass, which is a cardinal feature of LVH. Importantly, however, LVH is characterized by a wide range of remodeling events beyond simple increases in muscle mass. Electrocardiographic changes in LVH are reflective of the electrophysiological aspects of hypertrophic transformation. Here, I present an overview of the complex biology of left ventricular hypertrophy with an eye toward enhancing our understanding of its ECG manifestations.
Keywords: cardiac hypertrophy, remodeling, signal transduction, ECG
Introduction
Hypertrophic growth of ventricular myocytes is a hallmark feature of disease-related pathological cardiac remodeling. This hypertrophic process is complex, involving a vast array of transcriptional, signaling, structural, electrophysiological, and functional events within the growing cell. In addition, other cellular elements within the ventricle – vascular smooth muscle cells, endothelium, fibroblasts – manifest intricate stress responses, resulting in fibrosis, inflammatory cell infiltration, and vascular stiffness. Current thinking holds that these events – the heart’s reaction to a host of pathological stresses – confer short-term benefit. However, if hypertrophy-inducing stress remains unchecked, these remodeling events become maladaptive and predispose to cardiovascular morbidity and mortality.
Hypertrophic growth of the heart occurs in many clinical scenarios, conferring substantially increased risk for untoward sequelae, including transition to ventricular dilation, wall thinning, contractile dysfunction, and a clinical syndrome of heart failure. Left ventricular hypertrophy (LVH) is typically diagnosed by either electrocardiography or echocardiography. However, these two means of assessing hypertrophic transformation of the left ventricle do not always agree. A moment’s contemplation reveals that the two approaches, which are based on entirely distinct signals – electrical potential at the body surface versus ultrasound waves reflected from the ventricle itself – query different aspects of hypertrophic biology. Echocardiography is an effective means of assessing ventricular mass, which is a cardinal feature of LVH. Electrocardiography, by contrast, measures a voltage gradient between two places on the patient’s skin, which is in the case of LVH is influenced by a complex amalgamation of changes in the cardiomyocyte action potential, cell-cell electrical conduction, contractile events, and growth-related changes. These events, in turn, derive from multifaceted cascades of transcriptional, signaling, metabolic, structural, electrophysiological, and functional changes.
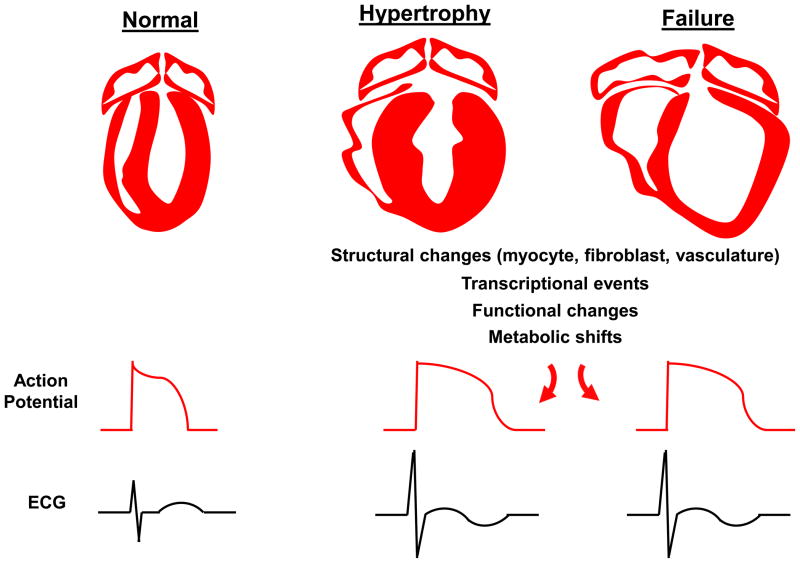
As hypertrophic transformation of the heart is an elaborate, multifaceted process, its manifestations on the ECG are complex (Figure). The aim of this review is to provide an overview of the complex biology of left ventricular hypertrophy with an eye toward enhancing our understanding of its ECG manifestations.
Figure. Hypertrophic transformation of the ventricle and its electrophysiological and electrocardiographic manifestations.
In the setting of disease-related stress, the heart responds with a comprehensive array of structural, transcriptional, signaling, and metabolic events taking place in each of the cellular elements within the tissue. These events culminate in alterations in cardiac-derived electrical potentials at the surface of the skin which can be recorded on the ECG.
Hypertrophic growth of the cardiac myocyte
Whereas recent work has revealed that a small fraction of cells within the ventricle are capable of re-entering the cell cycle, the vast majority of cardiac myocytes are post-mitotic and hence do not retain the ability to divide. Rather, they respond to stress by growing, shrinking, or dying. In the context of many disease-related stresses, cardiac myocytes undergo hypertrophic transformation culminating in significant cellular growth. As part of this, a wide range of transcriptional and post-translational events occurs, including activation of a pattern of gene expression reminiscent of that observed during fetal development (“fetal gene program”).
Increases in mechanical stress stimulate proliferation and recruitment of cardiomyocyte sarcomeres laid down in parallel with consequent increases in wall thickness. This, according to Grossman’s stress-adaptation hypothesis, is an adaptive response ; based on Laplace’s law, ventricular wall stress is proportional to both ventricular pressure and cavity radius and inversely proportional to ventricular wall thickness. Thus, increases in wall thickness tend to lessen wall stress and thereby diminish oxygen demand. Following myocardial infarction, surviving cardiac myocytes in border and remote zones of tissue can increase in size in response to increases in hemodynamic demand that arise secondary to loss of ventricular tissue. When the pressure overload state is persistent, however, the concentric hypertrophic phenotype of the myocardium inexorably progresses to a state of decompensation and clinical heart failure. Mechanisms governing this transition from adaptive hypertrophy to maladaptive failure remain poorly understood.
Myocyte growth is determined by a delicate balance between protein synthesis and protein degradation. In the setting of elevated afterload, protein synthesis predominates, culminating in a hypertrophic phenotype. However, it is important to recognize that hypertrophic remodeling is not a simple process of addition of new sarcomeres. Rather, this highly dynamic cellular remodeling response involves intricate coordination of de novo protein synthesis and organelle biogenesis, protein degradation, organelle breakdown, transcriptional reprogramming, metabolic shifts, and much more.
Cardiomyocyte hypertrophy: Comprehensive reprogramming of the cell
Hypertrophic transformation of the cardiac myocyte involves much more than simple cell growth. Rather, it entails a near-comprehensive reworking of multiple aspects of the cellular machinery and architecture. One way of looking at the process is to view the disease-stressed myocyte as undergoing a dedifferentiation process, reactivating numerous transcriptional, signaling, electrical, and metabolic events which characterized that same cell during development. In some ways, the “fetal gene program” which was extinguished shortly after birth re-ignites the day that stress is imposed. For a 60-year old experiencing a heart attack, surviving cardiac myocytes reactivate genes that had been dormant for the past 60 years!
As part of this, signaling and transcriptional programs emerge wherein a wide range of genes are reactivated. Some evidence suggests that cell size is regulated by shared signaling pathways, but cell shape and sarcomeric organization are regulated by distinct pathways. Current understanding does not allow us to parse the effects of all those genes and pathways yet, but it is thought that some confer benefit whereas others are maladaptive. Time, and further study, will be required to sort out these critical questions.
There is substantial evidence that alterations in transmembrane Ca2+ fluxes – another central feature of pathological remodeling – contribute to the pathogenesis of hypertrophy and failure, both by perturbing excitation-contraction coupling and by abnormally activating Ca2+-responsive signaling pathways.
Cardiac myocyte death
Death of cardiac myocytes is characteristic of a number of cardiac diseases, and it can occur to some extent in cardiac hypertrophy. The major types of cardiomyocyte death are necrosis and apoptosis, with the former being seen more frequently and to greater extents. [An emerging body of research has demonstrated that necrosis is a series programmed events, rather than a simple catastrophic dismantling of the cell. Indeed, programmed necrosis and apoptosis share certain features and may represent different aspects of a common biology termed necroptosis.]
Dying cells often manifest evidence of up-regulated autophagy, an evolutionarily ancient process of ordered recycling of intracellular contents. However, whether activation of the autophagic cascade reflects a cellular response to stress, serving to promote cell survival, or is a process which, itself, contributes to cell death and disease progression, is context-dependent. Whether autophagy is capable of actually killing a cell is debated.
Fibrosis
Another hallmark feature of pathological hypertrophic remodeling is accumulation and deposition of excessive extracellular matrix (ECM). This surplus ECM, which constitutes tissue “scar” or fibrosis, promotes dysfunction of both ventricular contraction and relaxation, perturbations of electrical conduction, and rhythm disturbances. As a result, cardiac fibrosis contributes to morbidity and mortality of cardiac hypertrophy. Indeed, the amount of fibrotic scar in the myocardium correlates directly with increased incidence of arrhythmias and sudden cardiac death.
ECM deposition and fibrosis formation occur through the action of cardiac fibroblasts. These cells, the most abundant cell type in the myocardium, proliferate in response to pathological stress and differentiate into myofibroblasts, thereby gaining the capacity to contract and secrete collagen I, collagen III, and fibronectin. In the setting of hypertension-induced pressure overload, fibrosis is predominantly reactive (perivascular or interstitial), as opposed to replacement fibrosis occurring at the site of an eliminated myocyte. Myofibroblasts derive from activated, resident fibroblasts, but also may be derived from adult epicardial cells and circulating, collagen-secreting, bone marrow-derived cells. Both individual myofibroblasts and collagenous septa within the left ventricle facilitate and propagate the arrhythmic phenotype of the hypertrophied heart.
Cardiac fibrosis is an independent and predictive risk factor for heart failure development in the setting of ischemic or non-ischemic cardiomyopathy. Interestingly, recent work has demonstrated that cardiac fibrosis, long held to be irreversible, may regress under certain conditions.
Electrophysiology
Patients with left ventricular hypertrophy are at significantly increased risk of developing malignant arrhythmia, which contributes significantly to morbidity and mortality associated with this disorder. Indeed, arrhythmia, especially ventricular tachyarrhythmia, is a major cause of death in patients with cardiac hypertrophy or failure. Underlying mechanisms, collectively termed “electrical remodeling”, encompass alterations in multiple electrogenic transport processes within the cardiac myocyte. Whereas numerous insights have emerged in elucidating the molecular pathogenesis of cardiac hypertrophy, our understanding of mechanisms underlying the myriad facets of electrical remodeling is limited. As a result, clinical means of treating hypertrophy-associated arrhythmias continue to disappoint, and device-based therapy has emerged as a widely used surrogate.
The action potential phenotype of ventricular hypertrophy is characterized by delayed repolarization leading to prolongation of action potential duration (APD). This derives, at least in part, from disordered transmembrane electrical currents. Delayed recovery of excitability, in turns, predisposes to early and late after-depolarizations. Hypertrophy is also associated with myocardial fibrosis (see above), altered electrotonic coupling between cells, slowed conduction, and dispersion of refractoriness, which together promote re-entrant arrhythmias. Collectively, these interlacing responses manifest as changes in the QRST morphology of the ECG and underlie the propensity to arrhythmia, syncope, and sudden death.
Lengthening of the ventricular cardiomyocyte APD is characteristic of both cardiac hypertrophy and failure, a finding which contrasts with APD shortening observed in the stressed (fibrillating) atrium. In the setting of excessive afterload, such as in severe transverse aortic constriction-induced heart failure, APD was prolonged more in subepicardial ventricular myocytes than in subendocardial myocytes. Further evidence for heterogeneity of APD prolongation has been reported in a model of pacing-induced heart failure in dogs where APD prolongation in mid-myocardial cells was substantially greater than in subepicardial cells. Together, prolongation of APD, combined with its spatial heterogeneity, culminates in a complex series of changes in the surface ECG, and together underlies a cellular substrate that promotes emergence of ventricular arrhythmia.
In ventricular hypertrophy, up-regulated inward Ca2+ current contributes to APD prolongation, particularly in models of modest hypertrophy. For example, L-type Ca2+ current (ICa,L) is a major mechanism of Ca2+ influx in cardiac myocytes, and some evidence suggests that ICa,L density correlates inversely with disease progression; in models of mild-to-moderate hypertrophy, ICa,L is often increased, whereas in severe hypertrophy and failure, ICa,L can manifest significant declines. Importantly, as membrane impedance in many species is relatively high during phase 2 of the action potential, small changes in ICa,L can have significant effects on action potential morphology and duration. Finally, entry of small amounts of extracellular Ca2+ triggers release of much larger amounts of Ca2+ from intracellular stores, so modest changes in inward Ca2+ flux are amplified within the cell.
A wide range of alterations in myocyte ion channels and electrogenic ion transporters contribute to APD prolongation (reviewed elsewhere ). Briefly, increases in late inward Na+ current can occur, arising from loss of voltage-gated sodium channel inactivation. Further, down-regulation of outward K+ currents, up-regulation of inward Ca2+ currents, and perturbations in Ca2+ current inactivation all contribute to APD prolongation. Indeed, diminished outward, repolarizing current secondary to down-regulated K+ channel levels (particularly Ito) is a common feature often observed in cardiac hypertrophy and failure. In fact, reduced Ito density is among the most consistent electrophysiological changes observed in heart failure. Also, alterations in the function of the Na+-Ca2+ exchanger (NCX), a major mechanism of Ca2+ elimination during diastole, contribute to late after-depolarizations and triggered ventricular activity.
Electrical activity within the myocardium hinges critically on electrotonic cell-cell coupling, such that depolarization in one cell is transmitted seamlessly to its neighboring cells. This coupling is mediated through gap junctions, such as connexin 43, which can become disorganized in the hypertrophied or failing heart leading to disruption of normal impulse conduction.
It is worth noting that the atria are also touched by remodeling events in cardiac hypertrophy and failure. Reduced contractility, development of fibrosis, and chamber enlargement can each be seen leading to heterogeneity of conduction velocity and propensity to atrial fibrillation. This arrhythmia, in turn, promotes a reduction in atrial effective refractory period and shortened APD, which together promote sustained atrial fibrillation.
Metabolism
The metabolic demands of the myocardium are exceptionally high; never-ending cycles of ventricular contraction and relaxation consume enormous quantities of ATP derived largely from fatty acid oxidation. That being said, the heart is a metabolic omnivore which can flexibly burn fuel derived from a wide range of sources.
One of the most dramatic changes occurring with cardiac hypertrophy is a shift in energy substrate utilization, a process termed metabolic remodeling. Numerous studies have shown that upon hypertrophic transformation of the myocardium, glucose uptake and glycolysis are significantly up-regulated, while β-oxidation of fatty acid is reduced. Ballpark numbers are that glycolysis accounts for roughly 10% of ATP production in the normal heart and 20% in the hypertrophied heart. Conversely, fatty acid metabolism drops from accounting for 90% of ATP production to 80%. These shifts are consistent with the overarching phenomenon of cellular dedifferentiation in the pathologically stressed myocardium; hypertrophied heart manifests a shift in metabolism which mimics the metabolic program in the fetal myocardium.
Whereas the metabolic changes characteristic of cardiac hypertrophy have long been appreciated, underlying mechanisms remain elusive. Among those mechanisms, autophagy is induced in heart by increases in afterload. Accumulating evidence suggests that activation of autophagy may play a critical role in both hypertrophic cellular growth and the associated metabolic changes.
Hypertrophic growth of cardiomyocytes involves synthesis of new macromolecules and organelles. To accomplish this, exogenous nutrients, such as glucose, cannot be metabolized exclusively for ATP production. Rather, metabolic intermediates must be channeled to support anabolic pathways. Again, interplay between the plasticity of metabolic pathways and activation of autophagy is critical to providing key intermediate metabolites that feed into the TCA cycle for ATP production as well as serving to promote macromolecule synthesis.
Inflammation
Activation of the immune system plays a significant role in ventricular remodeling, contributing to long-term cardiac injury in certain contexts. Better characterized in heart failure, where a variety of inflammatory molecules and pathways are activated, it is likely that these mechanisms pertain at least to some extent in cardiac hypertrophy, as well. For example, in pressure-overload models, macrophages infiltrate the ventricle leading to myocardial expression of NF-κB and inflammatory cytokines. Recently, a novel connection between autophagy, pressure overload and inflammation was uncovered where lack of autophagy-mediated removal of mitochondrial DNA promoted depressed cardiac function from increases in a cardiac inflammatory response.
Vascular Remodeling
During the course of pathological hypertrophic growth, the ventricular vasculature remodels. In the setting of hypertension, vascular smooth muscle proliferates and hypertrophies, culminating in vascular wall thickening. In some settings, flow reserve is compromised.
Alterations in angiogenesis occur, as well. Indeed, one model holds that a hallmark feature of pathological hypertrophy, as opposed to the physiological variety, is that capillary growth does not keep up with myocyte growth leading to decreased oxygen diffusion capacity. Also, it has also been postulated that the transition from compensatory hypertrophy to decompensated heart failure is due to an imbalance in the capillary to cardiac myocyte ratio. Consistent with this notion, enhancing angiogenesis in a pressure overload model can be protective.
Summary and perspective
In recent years, significant strides have been achieved in our understanding, and therapeutic targeting of, pathological hypertrophic remodeling. Looking to the future, the ECG will continue to serve a vital role in the evaluation of the hypertrophied ventricle. That said, it is critical to recognize that hypertrophic transformation of the ventricle is just that – a transformation involving cellular dedifferentiation and comprehensive reprogramming of the cardiac myocyte and other cellular elements within the ventricle. Whereas increases in myocyte size, and consequent increases in ventricular mass, are hallmark features, a wide range of additional events occurs in these stressed cells. With time, critical information with clinical relevance regarding this biology may emerge from continued careful analysis of the electrocardiographic features of LVH.
Acknowledgments
This work was supported by grants from the NIH (HL-075173; HL-080144; HL-090842), AHA (0640084N, JAH), and the AHA-Jon Holden DeHaan Foundation (0970518N).
Footnotes
Conflict of interest
The author has declared that no conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. The New England journal of medicine. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN, editors. Muscle: Fundamental Biology and Mechanisms of Disease. Amsterdam: Elsevier/Academic Press; 2012. [Google Scholar]
- 3.Bacharova L, Estes H, Bang L, Rowlandson I, Schillaci G, et al. The first statement of the Working Group on Electrocardiographic Diagnosis of Left Ventricular Hypertrophy. Journal of electrocardiology. 2010;43:197–9. doi: 10.1016/j.jelectrocard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bacharova L, Estes EH, Bang LE, Hill JA, Macfarlane PW, et al. Second statement of the working group on electrocardiographic diagnosis of left ventricular hypertrophy. Journal of electrocardiology. 2011;44:568–70. doi: 10.1016/j.jelectrocard.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO molecular medicine. 2011;3:701–12. doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burch GE, Ray CT, Cronvich JA. Certain mechanical peculiarities of the human cardiac pump in normal and diseased states. Circulation. 1952;5:504–13. doi: 10.1161/01.cir.5.4.504. [DOI] [PubMed] [Google Scholar]
- 10.Bass GT, Ryall KA, Katikapalli A, Taylor BE, Dang ST, et al. Automated image analysis identifies signaling pathways regulating distinct signatures of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2012;52:923–30. doi: 10.1016/j.yjmcc.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature reviews Molecular cell biology. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 12.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annual review of physiology. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nature cell biology. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annual Review of Pharmacology and Toxicology. 2005;45:657–87. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 15.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–9. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 16.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 19.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. Journal of Clinical Investigation. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. International Journal of Biochemistry & Cell Biology. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 23.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–10. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 24.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–8. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 25.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–84. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 26.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–8. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 27.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 28.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, et al. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res. 2011;109:407–17. doi: 10.1161/CIRCRESAHA.110.228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–35. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill JA. Electrical remodeling in cardiac hypertrophy. Trends in cardiovascular medicine. 2003;13:316–22. doi: 10.1016/j.tcm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Hill JA. Electrophysiological remodeling in heart failure. Journal of molecular and cellular cardiology. 2010;48:619–32. doi: 10.1016/j.yjmcc.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure. Circulation. 2006;113:1849–56. doi: 10.1161/CIRCULATIONAHA.106.615682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res. 2003;93:638–45. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 34.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaab S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, et al. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–73. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 36.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Tandan S, Cheng J, Yang C, Nguyen L, et al. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J Biol Chem. 2008;283:25524–32. doi: 10.1074/jbc.M803043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–21. [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 40.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–8. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 41.Pang H, Ronderos R, Perez-Riera AR, Femenia F, Baranchuk A. Reverse atrial electrical remodeling: a systematic review. Cardiology journal. 2011;18:625–31. doi: 10.5603/cj.2011.0025. [DOI] [PubMed] [Google Scholar]
- 42.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–88. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 43.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–73. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 44.Allard MF. Energy substrate metabolism in cardiac hypertrophy. Curr Hypertens Rep. 2004;6:430–5. doi: 10.1007/s11906-004-0036-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang ZV, Ferdous A, Hill JA. Cardiomyocyte Autophagy: Metabolic Profit and Loss. Heart failure reviews. 2012 doi: 10.1007/s10741-012-9350-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang ZV, Rothermel BA, Hill JA. Autophagy in hypertensive heart disease. The Journal of biological chemistry. 2010;285:8509–14. doi: 10.1074/jbc.R109.025023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–8. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 49.Aukrust P, Gullestad L, Lappegard KT, Ueland T, Aass H, et al. Complement activation in patients with congestive heart failure: effect of high-dose intravenous immunoglobulin treatment. Circulation. 2001;104:1494–500. doi: 10.1161/hc3801.096353. [DOI] [PubMed] [Google Scholar]
- 50.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–82. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 51.Soejima H, Irie A, Fukunaga T, Oe Y, Kojima S, et al. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ J. 2007;71:1879–84. doi: 10.1253/circj.71.1879. [DOI] [PubMed] [Google Scholar]
- 52.Caforio AL, Mahon NG, Baig MK, Tona F, Murphy RT, et al. Prospective familial assessment in dilated cardiomyopathy: cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation. 2007;115:76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]
- 53.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins JT, Boyle JJ, McKay IC, Richens D, McPhaden AR, Lindop GB. Vascular remodelling in intramyocardial resistance vessels in hypertensive human cardiac transplant recipients. Heart. 1997;77:353–6. doi: 10.1136/hrt.77.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karam R, Healy BP, Wicker P. Coronary reserve is depressed in postmyocardial infarction reactive cardiac hypertrophy. Circulation. 1990;81:238–46. doi: 10.1161/01.cir.81.1.238. [DOI] [PubMed] [Google Scholar]
- 56.Jugdutt BI, Menon V, Kumar D, Idikio H. Vascular remodeling during healing after myocardial infarction in the dog model: effects of reperfusion, amlodipine and enalapril. J Am Coll Cardiol. 2002;39:1538–45. doi: 10.1016/s0735-1097(02)01805-3. [DOI] [PubMed] [Google Scholar]
- 57.Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, et al. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–10. doi: 10.1016/j.athoracsur.2003.11.003. discussion 11. [DOI] [PubMed] [Google Scholar]