Abstract
Previously it was shown that the TNF superfamily member TWEAK (TNFSF12) acts through its receptor, Fn14, to promote proinflammatory responses in kidney cells, including the production of MCP-1, RANTES, IP-10 and KC. In addition, the TWEAK/Fn14 pathway promotes mesangial cell proliferation, vascular cell activation, and renal cell death. To study the relevance of the TWEAK/Fn14 pathway in the pathogenesis of antibody-induced nephritis using the mouse model of nephrotoxic serum nephritis (NTN), we induced NTN by passive transfer of rabbit anti-glomerular antibodies into Fn14 knockout (KO) and wild type (WT) mice. Severe proteinuria as well as renal histopathology were induced in WT but not in Fn14 KO mice. Similarly, a pharmacologic approach of anti-TWEAK mAb administration into WT mice in the NTN model significantly ameliorated proteinuria and improved kidney histology. Anti-TWEAK treatment did not affect the generation of mouse anti-rabbit antibodies; however, within the kidney there was a significant decrease in glomerular immunoglobulin deposition, as well as macrophage infiltrates and tubulointerstitial fibrosis. The mechanism of action is most likely due to reductions in downstream targets of TWEAK/Fn14 signaling, including reduced renal expression of MCP-1, VCAM-1, IP-10, RANTES as well as Fn14 itself, and other molecular pathways associated with fibrosis in anti-TWEAK treated mice. Thus, TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the NTN model, apparently mediating a cascade of pathologic events locally in the kidney rather than by impacting the systemic immune response. Disrupting TWEAK/Fn14 interactions may be an innovative kidney-protective approach for the treatment of lupus nephritis and other antibody-induced renal diseases.
Keywords: Systemic lupus erythematosus (SLE), Nephrotoxic Serum Nephritis, TWEAK, Fn14
1. Introduction
Involvement of the kidney, lupus nephritis (LN), is a major determinant in the prognosis of patients with systemic lupus erythematosus (SLE). Using intensive induction and maintenance regimens, long term outcomes have greatly improved the prognosis of patients with lupus nephritis. However, treatment all too often results in less than a complete response [1]. In addition, the incidence of end stage renal disease may in fact be increasing in certain subpopulations [2-4]. Therefore, while there have been many important advances in our understanding of the pathogenesis of lupus nephritis, these have yet to translate into significant enough gains in the arena of treatment of human disease. Moreover, most of the current therapies that are employed to treat LN are non-specific, and are associated with major side effects. Thus, a kidney-protective modality that would improve the renal prognosis would be of tremendous benefit to LN patients.
Interactions between members of the TNF-ligand superfamily (i.e. CD40, BLyS/BAFF) and their cognate TNF-receptor superfamily members (CD40L, BAFF-R/TACI/BCMA, respectively) are instrumental in the pathogenesis of SLE. Indeed, many studies have shown that inhibition of signaling transduced by these receptor/ligand pairs is beneficial in animal models of lupus featuring nephritis [5-9]. Notably, an anti-BLyS mAb has recently achieved approval for clinical treatment of SLE [10]. Thus, targeting other TNF family members is also of interest for the development of novel therapeutic approaches for treatment of SLE.
TWEAK is a distinct member of the TNF-ligand superfamily with relevance to the pathogenesis of LN. TWEAK, produced primarily as a soluble cytokine by tissue-infiltrating leukocytes, promotes NF-κB and MAPK activation through its sole signaling receptor Fibroblast Growth Factor Inducible 14 (Fn14), a member of the TNF receptor family [11, 12]. Notably, Fn14 is expressed at relatively low levels in normal tissues and is highly induced in injured and diseased tissues, thereby activating the TWEAK/Fn14 pathway locally in those target tissues. Previously, we and others have shown that Fn14 can be expressed by cell types that comprise the kidney, including mesangial cells, podocytes, endothelial cells and tubular cells [13-16]. Interestingly, TWEAK acts on these cell types to induce proinflammatory cytokines and chemokines, including MCP-1 and RANTES which have been implicated in LN [17, 18], as well as vascular activation, including the upregulation of adhesion molecules which are also relevant to LN [19, 20]. In addition to its proinflammatory activity, TWEAK can promote mesangial cell proliferation [14, 15] and tubular cell death [21, 22]. Finally, in the chronic graft versus host (cGVH) model of induced autoimmunity, inhibition of the TWEAK pathway significantly improved glomerulonephritis [23].
Passive transfer into rodents of heterologous sera containing pre-formed antibodies against the glomerular basement membrane (GBM) induces a rapidly progressive, proliferative crescentic glomerulonephritis (GN), modeling human anti-GBM disease (also known as Goodpasture's syndrome). In this rodent experimental model, heterologous antibody-mediated injury primarily involves linear antibody deposition, complement deposits, and acute neutrophil-mediated glomerular injury, followed by an autologous phase involving the generation of a host response to the heterologous antibodies. In a widely used variation of nephrotoxic serum nephritis, herein referred to as nephrotoxic nephritis (NTN), the autologous phase is accelerated by immunization of rodents with heterologous immunoglobulin prior to the passive transfer of the anti-GBM antibodies, thereby promoting the deposition of immune complexes and macrophage-mediated injury [24]. As the NTN model shares these and many other features with human LN, it is a valuable model for exploring LN pathogenesis [19, 25-29]. NTN poses a high hurdle for therapeutic intervention, considering the accelerated kinetics of nephritis as compared to spontaneous disease in susceptible mouse strains. However, considering the mode of induction, it offers the ability to assess the contribution of mechanisms acting locally in the disease target end organ [30].
To explore a possible role for the TWEAK/Fn14 pathway in the pathogenesis of nephritis mediated by pathogenic antibodies and the potential utility of anti-TWEAK antibodies as a kidney-protective approach for the treatment of lupus nephritis, we investigated the effects of inhibiting this pathway on the development and progression of NTN in mice.
2. Materials and Methods
2.1. Mice
Seven to eight week old 129/SvJ (129) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and housed 3-5 mice per cage in the animal facility of the Albert Einstein College of Medicine (Bronx, NY). Fn14 +/+ (WT) and -/- (KO) mice on the 129/Svev (129) background were provided by Biogen Idec investigators. Mice were acclimatized in the facility for 2 weeks prior to experiments. The housing conditions were controlled, with a temperature of 21-23°C and a 12:12 hours light:dark cycle. All animal study protocols were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
2.2. Induction of NTN
Nephrotoxic serum was generated by rabbit immunization with sonicated mouse glomeruli (Lampire Biological Laboratory, Pipersville, PA). Mice on the 129 strain background were primed intraperitoneally with 250 μg of rabbit IgG in complete Freund's adjuvant (Difco Laboratories, Detroit, MI) on day (d) 0. All subsequent time points in this report refer to this initial immunization as baseline, or day 0. On d5, mice received an intravenous injection of either rabbit nephrotoxic serum or phosphate buffered saline (PBS). Blood and urine were obtained at baseline (d0) and at subsequent time points for serological measurements. In this model, mice not succumbing to uremia show spontaneous improvement in renal function starting at two to three weeks after the renal insult. Mice received 300 μg of mAb intraperitoneally, murine anti-TWEAK mAb clone P5G9 [31] or isotype matched control, P1.17 or anti-hen egg lysozyme (HEL), all of which were provided by Biogen Idec, on day 0 and subsequently twice a week until the end of the experiment. Separately, induction of NTN (i.e. the injection of nephrotoxic serum) was performed in Fn14 KO and WT 129 mice using the same schedule. Kidneys from sacrificed mice were used for RNA extraction and for preparation of paraffin sections for histological and immunohistochemical studies as described below.
Levels of proteinuria were determined by Uristix test strips, where gradations in color correspond to categorical urine protein concentrations of 30 mg/dl, 100 mg/dl, 300 mg/dl, and ≥2000 mg/dl (Bayer Corporation, Pittsburgh, PA). Color changes between these categories were assigned the average value. Serum BUN was measured by the Quantichrom DIUR 500 kit (BioAssay Systems, Hayward, CA), following the manufacturer's instructions.
2.3. Assessment of mouse anti-rabbit IgG titers
Microtiter plates were coated with rabbit IgG (5 μg/ml) at 4°C overnight. Plates were washed, blocked with 3% fetal calf serum in PBS, and serum samples at 1:200 were incubated for 2 hours at 37°C. Plates were washed, and the specific secondary (goat anti-mouse IgG-alkaline phosphatase (AP)/goat anti-mouse IgG1-AP/goat anti-mouse IgG2a-AP/goat anti-mouse IgG2b-AP/goat anti-mouse IgG3-AP) (2 μg/ml in block; all from Southern Biotech, Birmingham, AL) was incubated for 1 hour at 37°C, followed by substrate solution.
2.4. Renal histopathology
To examine renal histopathology, paraffin kidney sections (3-5 μm thick) were deparaffinized, hydrated, and stained with hematoxylin & eosin, and periodic acid Schiff (PAS). Kidney histology was evaluated blindly by an experienced nephropathologist, as previously described [32]. Histopathological changes in renal tissue were quantitated as follows. Glomerular proliferation and PAS+ deposits were each scored from 0 to 4 (0, absent; 1, mild; 2, mild-moderate; 3, moderate; 4, severe). The presence of crescents and tubular disease (atrophy, casts, dilatation, inflammatory infiltrates) was each graded on a scale of 0–4, with 0, absent; 1, present in <25% of the section; 2, present in 25–50% of the section; 3, present in 50-75% of the section, and 4, present in >75% of the section. The maximum score for each mouse was 16 (maximum 4 points each for glomerular proliferation, PAS+ deposits, crescent formation, and tubular disease).
2.5. Glomerular immunoglobulin deposition
Glomerular immunoglobulin (Ig) deposition was detected by immunohistochemistry as previously described, with slight modifications [33]. Four μm thick sections from paraffin embedded kidneys were obtained by microtome, deparaffinized, rehydrated, blocked with 2% BSA in PBS in moist chambers, and stained for 2 hr with biotinylated goat anti-mouse IgG at a 1:200 dilution at room temperature (Vector Laboratories, Burlingame, CA). The sections were then incubated for 45 min with streptavidin-alkaline phosphatase ABC reagent (Vectastain ABC kit) and developed with substrate for alkaline phosphatase (5-bromo-4-chloro-3-indoylphosphate p-toludine salt and nitroblue tetrazolium chloride substrate; Roche, Indianapolis, IN) for 5 minutes. Color development was stopped by the addition of distilled water. The sections were mounted on coverslips with toluene solution (Permount, Fisher Scientific, Pittsburgh, PA), sealed, and viewed with a Zeiss microscope. Glomerular Ig deposition was scored blindly on a scale of 0 (absent deposition) to 4 (heavy glomerular staining). In addition, the intensity of glomerular immunoglobulin deposition (staining) in 10 random glomeruli on each slide was blindly quantitated by image J by two independent observers.
2.6. Kidney immunohistochemical staining
Blocks of formalin fixed paraffin embedded kidneys were sectioned at 3 μm and stained for CD3 (rabbit monoclonal, ThermoFisher Scientific), Ki-67 (rabbit monoclonal, Vector Laboratories), or F4/80 (rat monoclonal, AbD Serotec, Raleigh, NC). For trichrome staining, slides were post-fixed in Bouin's fixative solution, and then Gomori's One Step trichrome was used with nuclei stained in Weigert's Hematoxylin.
2.7. RNA isolation, cDNA synthesis and real time PCR
Total RNA was extracted from whole kidney tissue using Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Reverse transcription was performed from 10 μg of total RNA using the SuperScript II system from Invitrogen. Real time PCR was performed in triplicate using the SYBR green stain and the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Warrington, UK), using methods and selected primers previously described [23, 34].
2.8. Statistical methods
Statistical analysis was performed using the STATA 10.0 software package (StataCorp, College Station, Texas, USA). To evaluate bivariate relationships between continuous non-normally distributed variables in Fn14 WT and KO groups, and in isotype control and anti-TWEAK mAb treated groups, we used the non-parametric alternative for the student t-test, the Wilcoxon rank-sum test. We used the chi-square test (or Fisher's exact test when appropriate) to evaluate bivariate relationships between categorical variables in the comparison groups. Two tailed tests were applied except when specified. Continuous variables in each comparison group were reported as median (inter-quartile range) (IQR), and categorical variables were reported as percentages. Differences were considered significant at p≤0.05.
3. Results
3.1. Fn14 KO mice are resistant to renal injury from pathogenic anti-GBM antibodies
To determine if the TWEAK/Fn14 pathway promotes the pathogenesis of immune mediated nephritis, we induced nephrotoxic nephritis by passive transfer of pre-formed rabbit anti-mouse glomerular antibodies into Fn14 WT and KO mice pre-immunized with rabbit IgG. Fn14 WT mice exhibited significantly higher proteinuria than Fn14 KO mice on days 7 (2 days after transfer of nephrotoxic serum), 14, and 21 (Figure 1A). When analyzed by grades of proteinuria, also shown in Figure 1, we found a statistically significant association between proteinuria and genotype. On day 7 in the Fn14 WT group, 17% of mice had proteinuria at ≤100 mg/dl, 33% had >100-<300 mg/dl, and 50% had ≥300 mg/dl. In contrast, in the Fn14 KO group at this time point, 86% had ≤ 100 mg/dl, 14% had >100-<300 mg/dl, and none had ≥300 mg/dl (Figure 1B). Similarly, on day 14, in the Fn14 WT group 17% of mice had proteinuria of ≤100 mg/dl, 33% had 300 mg/dl, and 50% had >300 mg/dl, while in the Fn14 KO group 71% had ≤ 100 mg/dl, 29% had 300 mg/dl, and none had >300 mg/dl (Fisher's exact p-value 0.081). The distribution of proteinuria by categories between Fn14 WT and KO mice remained identical on day 21 (data not shown).
Figure 1. Fn14 KO mice exhibit decreased proteinuria and improved pathology following induction of NTN.
Nephrotoxic serum nephritis was induced as described in the Materials and Methods in Fn14 KO (n=7) and Fn14 WT (n=6) mice, and the kidneys were obtained at sacrifice (day 21). (A) Mean proteinuria was significantly decreased in Fn14 KO mice as compared to Fn14 WT mice on days 7, 14, and 21 (p≤0.02). (B) Proteinuria analyzed as a categorical variable showed a statistically significant association between proteinuria and the genotype at day 7. In the Fn14 WT group, 17% of mice had proteinuria at ≤100 mg/dl, 33% had >100-<300 mg/dl, and 50% had ≥300 mg/dl. In contrast, in the KO group, 86% had ≤ 100 mg/dl, 14% had >100-<300 mg/dl, and none had ≥300 mg/dl, Fisher's exact p-value of 0.029. (C) Total histopathology score (median and IQR are shown). (D),(E) Comparison of Fn14 WT and KO mice in sub-scores for crescents and tubular disease. (F) Representative figures from Fn14 WT (panels A & B) and Fn14 KO mice (panels C & D) with NTN are shown. Panel A (H&E stain, 400x magnification) shows a large proteinaceous tubular cast (arrow) as well as mild interstitial inflammation that was typical of the tubulointerstitial disease seen in the Fn14 WT mice. Also present are 3 glomeruli, one of which shows a crescent (arrowhead). Panel B (PAS, 600x magnification) shows a glomerulus with a large PAS-positive deposit (arrow), as well as a segmental overlying cellular crescent extending from 12 o'clock to 6 o'clock. Panel C (H&E stain, 400x magnification) shows essentially normal tubulointerstitium; only occasional proteinaceous casts and rare foci of interstitial inflammation were seen in the Fn14 KO mice. The glomerulus seen in the bottom right hand corner of this panel shows only mild mesangial expansion and mild mesangial cellular proliferation. Panel D (PAS, 600x magnification) shows a representative glomerulus from a Fn14 KO mouse. While PAS+ deposits are seen in the mesangium (arrow), endocapillary proliferation and crescent formation were rare.
To determine if Fn14 deficiency would abrogate histopathological injury, we analyzed kidney histology in Fn14 WT and KO mice on day 21 following induction of NTN. Total kidney scores in Fn14 WT mice (median (IQR): 3.75 (2.5, 5) were significantly higher than in Fn14 KO mice (median (IQR): 1 (1, 1.5) (p=0.03) (Figure 1C). When pathological features were analyzed separately, we found significant glomerular proliferation and PAS+ deposition in NTN-challenged Fn14 WT mice, with no significant difference between Fn14 WT and KO mice (not shown). However, we found that all (100%) of the Fn14 WT mice had crescent formation with pathological scores of ≥1, as compared to only 14% of Fn14 KO mice (Fisher's exact p-value 0.002) (Figure 1D). In addition, Fn14 deficiency also protected against tubular damage, as 83% of the Fn14 WT mice exhibited tubular disease with pathological scores of ≥1 as compared to only 14% in Fn14 KO mice (Fisher's exact p-value 0.013) (Figure 1E). A representative sample from kidneys of Fn14 WT and KO mice following induction of NTN is showed in Figure 1F. Thus, Fn14 deficiency was protective with respect to both crescent formation and tubular pathology induced by NTN.
In the NTN model, renal injury induced by passive transfer of rabbit anti-mouse GBM antibodies is enhanced by pre-immunization of the mice with rabbit IgG, which results in anti-rabbit antibodies which complex with the passively transferred rabbit anti-mouse antibodies. To determine whether differences in the mouse anti-rabbit response may be contributing to the diminished nephritis observed in Fn14 KO mice, we analyzed the isotype-specific anti-rabbit response at baseline and 14 days after immunization. No differences were observed between Fn14 WT and KO mice in titers of mouse anti-rabbit antibodies of the IgG2a subclass, the most pathogenic of the murine subclasses given its Fc effector functionality [35, 36], nor were there differences in the titers of mouse anti-rabbit IgG1 antibodies (Figure 2A,B). In contrast, Fn14 KO mice had significantly lower titers of mouse IgG anti-rabbit IgG2b and IgG3 antibodies as compared to Fn14 WT mice at the 14 day time point (Figure 2C,D), although there were no significant differences in anti-rabbit IgG2b and IgG3 antibody titers at baseline (data not shown). Glomerular immune complex deposition was also analyzed (Figure 3). We found high murine IgG deposition with scores of ≥2 in 82% of Fn14 WT mice (Figure 3A). By comparison, IgG deposition scores ≥2 were found in only 43% of Fn14 KO mice. The association between glomerular deposition scores and genotype was significant (p<0.05). A similar pattern was observed when total glomerular IgG stain was quantitated by image J, with a trend toward increased IgG deposition in Fn14 WT mice (90.2±7.2 arbitrary units) as compared to Fn14 KO mice (75.4±9.1), p=0.22, Figure 3B). There was no correlation between the titers of mouse anti-rabbit IgG2b and IgG3 antibodies and the intensity of glomerular immunoglobulin deposition or the degree of proteinuria (not shown). Thus, while we cannot rule out that reduced levels of mouse anti-rabbit antibodies contribute to the reduced NTN severity, the data indicate that the beneficial effect of Fn14 deficiency in reducing kidney pathology is not simply mediated by reducing systemic humoral immune responses, and suggests additional mechanisms.
Figure 2. Development of humoral mouse anti-rabbit immune responses in Fn14 WT and KO mice.
Mice were primed intraperitoneally with 250 μg of rabbit IgG in CFA at day 0, followed by intravenous transfer of rabbit anti-glomerular antibodies on day 5. Mice were bled at baseline (pre-immunization) and 14 days later, and the sera analyzed for isotype specific mouse anti-rabbit antibodies. (A)-(D): Data shown are median and IQR, with no differences observed at day 14 between Fn14 WT and KO mice in IgG1 (WT O.D. units 2.5 (2.4, 3.3) vs. KO 2.8 (2.7, 2.9), p=0.48) and IgG2a (WT O.D. units 0.83 (0.77, 1.2) vs. KO 0.85 (0.62, 1.5), p>0.99) mouse anti-rabbit antibody titers, but significant differences in the titers of mouse IgG anti-rabbit antibodies of the IgG2b (WT 1 (0.87, 1.2) vs. KO 0.58 (0.48, 0.8), p=0.02) and IgG3 (WT 0.58 (0.5, 0.73) vs. KO 0.3 (0.29, 0.36), p=0.01) isotypes.
Figure 3. Glomerular Ig deposition and kidney gene expression following induction of NTN in Fn14 WT and KO mice.
Nephrotoxic serum nephritis was induced as described in the Materials and Methods in Fn14 KO (n=7) and Fn14 WT (n=6) mice. Mice were sacrificed on day 21, and kidney paraffin sections were stained for Ig deposition. In addition, one kidney from each mouse was obtained for preparation of RNA, and real time PCR performed as described in the Materials and Methods. (A) Comparison of glomerular immunoglobulin staining intensity between Fn14 WT and KO mice, p<0.05. (B) Glomerular deposition of IgG in each group of mice was quantitated by ImageJ, as described in the Materials and Methods. (C) Data shown are median and IQR kidney mRNA expression levels of the indicated genes. Values are as follows: MCP-1 (WT 7.0 (3.3, 71.7) vs. KO 2.5 (1.4, 3.9), p=0.004) and VCAM-1 (WT 14.7 (12.0, 87.1) vs. KO 9.2 (6.8, 11), p=0.03) were significantly higher in the WT as compared to Fn14 KO mice (marked with asterisks). Differences in median kidney expression levels of IP-10 (WT 21 (12.9, 127.5) vs. KO 12.1 (9.2, 16.3) and TGF-ß (WT 67.7 (56.2, 113.1) vs. KO 51.8 (42.7, 58.3)) showed a similar trend, and reached borderline statistical significance (p=0.06 for both comparisons). The differences in kidney expression of KC, RANTES, and ICAM-1 between Fn14 WT and KO mice were not significant.
We have previously shown [14, 15] that through Fn14 signaling, TWEAK can stimulate resident kidney cells, including mesangial cells, podocytes, and tubular cells, to secrete a variety of cytokines and other inflammatory mediators shown to be involved in the pathogenesis of both nephrotoxic nephritis and lupus nephritis [27]. To further elucidate at the molecular level the mechanism of protection from renal disease observed in Fn14 KO mice following challenge with nephrotoxic antibodies, we compared the kidneys of Fn14 WT and KO mice at day 21 for the expression of genes that mediate inflammation, stimulate vascular activation, and promote fibrosis (Figure 3C). Following induction of nephrotoxic nephritis, median kidney expression levels of MCP-1 (p=0.004) and VCAM-1 (p=0.03) were both significantly higher in the WT as compared to Fn14 KO, while IP-10 and TGF-ß trended in the same direction and approached statistical significance (p=0.06). The differences in kidney expression of RANTES, KC, and ICAM-1 between Fn14 WT and KO mice were not significant (Figure 3C).
3.2. Treatment with an anti-TWEAK mAb decreases proteinuria and renal damage in NTN
To determine whether the protective effect of blocking TWEAK could be achieved with a pharmacologic approach, we induced nephrotoxic nephritis in 129 WT mice and treated them with an anti-TWEAK mAb or an isotype matched control Ig. Notably, the degree of nephritis induced in this experiment was more severe as compared to that described above; mean proteinuria levels peaked at approximately 1000-1500 mg/dl in the therapeutic antibody study (Figure 4A), as compared to approximately 700 mg/dl in the study of Fn14 KO vs. WT mice. Regardless, when the degree of proteinuria was analyzed as a categorical variable, we found a significant association with the treatment group. On day 7 only 14% of anti-TWEAK mAb treated mice had severe proteinuria (≥2000 mg/dl) as compared to 64% in the control treated group (Figure 4B). Moreover, similar to our analysis above in Fn14 WT and KO mice, the percentage of mice with moderate or severe proteinuria (≥300 mg/dl) on days 14 and 21 was higher in mice with induced NTN treated with the isotype matched control antibody (81%), as compared to the group treated with the anti-TWEAK mAb (57%) (not shown). Thus, the results were consistent with those of the genetic deficiency (Fn14 KO) study, in that antibody blockade of TWEAK inhibited the development of renal injury induced by the nephrotoxic antibodies.
Figure 4. Treatment with an anti-TWEAK mAb reduces proteinuria and diminishes renal injury in NTN.
Induction of NTN in Fn14 WT mice was performed as described in the Materials and Methods. In addition, mice received 300 μg of mAb intraperitoneally, murine anti-TWEAK mAb or isotype matched control on day 0, and subsequently twice a week until the end of the experiment. Two independent experiments were conducted; the first experiment included 6 isotype control and 8 anti-TWEAK mAb-treated mice, while the second experiment included 5 isotype control and 5 anti-TWEAK-treated mice. Since similar results were obtained, the combined analysis of anti-TWEAK (n=13) vs. isotype control treated mice (n=11) is shown. A third group of mice (n=12) had intraperitoneal injection of PBS following the same schedule (“PBS”). An additional control group was also immunized with rabbit IgG on day 0, but did not receive nephrotoxic antibody (“Primed only”). (A) Mean proteinuria was significantly decreased by one tailed t-test in anti-TWEAK mAb as compared to isotype control treated mice on day 7 (p=0.026) and day 21 (p=0.047). A similar trend was present at day 14 (p=0.067). The p values for the comparison to no antibody treatment (“PBS”) for days 7, 14, and 21 were 0.09, 0.01, and <0.001 (one tailed t-test), respectively. (B) When proteinuria was analyzed as a categorical variable, the Fisher's exact p-value for day 7 was 0.026, indicating a statistically significant association between proteinuria and treatment. (C) Induction of NTN in Fn14 WT mice was performed as described above. Mice (n=10 per group) were administered 300 μg of anti-TWEAK, isotype control Ig, or PBS intraperitoneally on days 0, 3, and 7, and the mice sacrificed at day 9. The figure shows proteinuria on day 9, with isotype control vs. anti-TWEAK significantly different, p=0.043 by one sided Fisher's exact test. (D) Component histopathology scores. (E) Representative renal histopathology and immunohistochemical staining for F4/80, Ki-67, and trichrome in mice with induced NTN treated with an isotype control or anti-TWEAK mAb. PAS staining (top left panel) shows two glomeruli (600x magnification) with prominent global brightly PAS+ deposits in the glomerular capillaries. This degree of deposition was typical for the isotype control-treated mice. The burden of deposition appears to be less and the distribution is more segmental in anti-TWEAK mAb treated mice (bottom left panel), with relative preservation of the remaining portions of the glomerular tuft. Representative sections show reduced F4/80 and Ki-67 staining in anti-TWEAK treated mice. For the trichrome stained sections, the section from an isotype control treated mouse demonstrates fibrous strands in the interstitium, mild perivascular fibrosis, and slight thickening of the tubular basement membranes, while in the anti-TWEAK treated mouse there is no evidence of interstitial fibrosis or tubular basement membrane thickening. (F) Shown here are median (IQR) scores for staining intensity.
To further evaluate the effect of anti-TWEAK treatment during a nephrotoxic insult, in a separate study we assessed renal histopathology. Since the peak of proteinuria following NTN induction appears between days 7 and 14, we wanted to evaluate kidney tissue at that time. Accordingly, mice (n=10 per group) were administered anti-TWEAK or isotype control antibody at days 0, 3, and 7 (in parallel to the standard immunization with rabbit IgG on day 0, and the transfer of nephrotoxic antibody on day 5, as previously), and sacrificed at day 9. Nephritis in this particular study was even more severe than in prior experiments. Despite this, we observed a significantly decreased incidence of proteinuria ≥2000 on day 9 in the NTN mice that received treatment with the anti-TWEAK mAb (6/10; 60%) as compared to isotype control treated mice which all had urine protein levels of ≥2000 mg/dl; (10/10, 100%)(Figure 4C). Moreover, serum levels of BUN were considerably decreased as well in the anti-TWEAK treatment group, from 76.2±21.2 to 37.2±6.7 mg/dl (p=0.05 one sided t-test; data not shown). Upon histological examination, anti-TWEAK mAb treatment resulted in a decreased incidence of moderate or severe glomerular proliferation (60% vs. 90%, chi-square 0.12), PAS+ immune deposits (60% vs. 90%, chi-square 0.12), and tubular disease (20% vs. 50%, chi-square 0.16) in nephrotoxic nephritis as compared to mice treated with the isotype matched control antibody (Figure 4D). These results are biologically significant, and are consistent with the analysis of Fn14 WT vs. KO mice described above. A representative example of the histopathology in PAS-stained kidneys from mice with NTN treated with an anti-TWEAK or isotype control mAb is shown in Figure 4E.
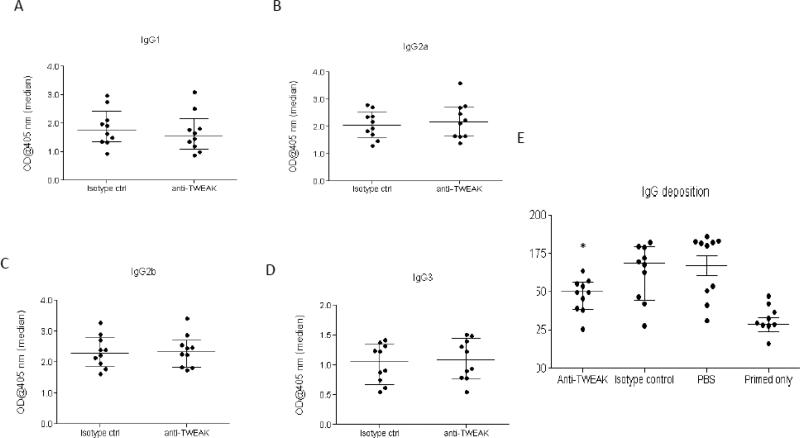
When we evaluated the generation of mouse anti-rabbit IgG subclass antibodies in the NTN model, we found no differences in the levels of mouse anti-rabbit IgG1, IgG2a, IgG2b, and IgG3 antibody levels at day 14 in the mice that received anti-TWEAK mAb as compared to control Ig (Figure 5A-D). Thus, these data are consistent with the hypothesis that targeting TWEAK by an antibody approach is protective by reducing the activity of the TWEAK/Fn14 pathway where it is upregulated, locally in the kidney, rather than by modulating systemic humoral immune responses. Interestingly, however, when glomerular immune complex deposition was quantified (Figure 5E), we found that anti-TWEAK antibody treated mice had significantly decreased glomerular deposition scores as compared to mice treated with the isotype control (163.2±5.8 vs. 148.0±3.5, p<0.04, Figure 5E).
Figure 5. Development of humoral mouse anti-rabbit immune responses and renal IgG deposition in anti-TWEAK antibody treated mice.
(A)-(D) Fn14 WT mice with induced NTN mice (n=10 per group) were administered 300 μg of anti-TWEAK or isotype control intraperitoneally on days 0, 3, and 7, and serum obtained on day 9 analyzed for the isotype-specific anti-rabbit antibody response. Anti-TWEAK antibody treated mice did not have significant differences in the IgG1 (istoype control treated 1.77 (1.35, 2.28) vs. anti-TWEAK treated 1.55 (1.14, 1.99)), IgG2a (2.29 (1.91, 2.75) vs. 2.35 (1.84, 2.63)), IgG2b (2.06 (1.65, 2.47) vs. 2.18 (1.65, 2.71)), and IgG3 (1.12 (0.67, 1.33) vs. 1.17 (0.76, 1.44)) mouse anti-rabbit antibody titers following immunization with rabbit IgG. (E) Glomerular deposition of IgG in each group of mice was quantitated by ImageJ, as described in the Materials and Methods.
To determine the mechanism of protection of anti-TWEAK antibodies in this nephritis model, we stained kidneys of anti-TWEAK and control Ig treated mice with induced NTN for CD3, F4/80, and Ki-67, and assessed the degree of fibrosis by trichrome staining (Figure 4E,F). Using Fisher's exact test to determine an association between the staining scores and antibody treatment with anti-TWEAK or the isotype matched control, we found that kidney infiltration by F4/80+ cells (p=0.013), as well as trichrome staining (p=0.018), were significantly decreased in anti-TWEAK treated mice as compared to the isotype-matched control Ig treated animals (Figure 4E,F). Moreover, there was a statistically significant association between anti-TWEAK treatment and a decrease in Ki-67 staining in both resident glomerular (p<0.05; not shown) and tubulointerstitial cells (p<0.05; Figure 4E,F). There was no significant difference in the number of infiltrating CD3+ cells (Figure 4F).
We explored the mechanism whereby inhibition of the TWEAK/Fn14 pathway reduces NTN severity by investigating the expression of TWEAK and Fn14 in NTN kidneys and the effect of anti-TWEAK treatment. We found that TWEAK mRNA was expressed at relatively similar levels in the normal and NTN kidney (Figure 6A); however, kidney Fn14 mRNA levels were dramatically upregulated after NTN induction, with a 10 fold higher mean value in NTN as compared to naïve kidneys (Figure 6B), supporting local activation of the TWEAK/Fn14 pathway during NTN. In contrast, Fn14 levels in the spleen were low at baseline and did not increase in the course of NTN (data not shown). In addition, we assessed the effect of anti-TWEAK treatment on the upregulation of Fn14 in NTN mice. Interestingly, we found that the increase in Fn14 mRNA levels was significantly ameliorated in kidneys of anti-TWEAK mAb treated NTN mice (Figure 6B).
Figure 6. Gene expression analysis in kidneys of anti-TWEAK and isotype control treated mice following induction of NTN.
Four days after transfer of nephritogenic rabbit sera (day 9) to Fn14 WT mice treated with anti-TWEAK or isotype control Ig (n=10 in each group), one kidney from each mouse was obtained for preparation of RNA, and real time PCR performed as described in the Materials and Methods. Expression of TWEAK (A), Fn14 (B) and selected proinflammatory genes (C) were compared in anti-TWEAK vs. isotype control treated mice with induced NTN. In (C), data shown are median and IQR kidney expression levels; values for median and IQR are as follows: MCP-1 (isotype control 7.6 (3.7, 17.4) vs. anti-TWEAK 2.6 (1.2, 3.9), p=0.008), IP-10 (isotype control 5.7 (2.3, 7.5) vs. anti-TWEAK 2.1 (1.6, 4.1), p=0.02), RANTES (isotype control 7.9 (6.4, 10.7) vs. anti-TWEAK 5.0 (3.3, 5.5), p=0.002), and VCAM-1 (isotype control 2.6 (1.8, 3.5) vs. anti-TWEAK 1.4 (1.2, 1.7), p=0.02) were significantly higher in the isotype control as compared to anti-TWEAK mAb treated mice (marked with asterisks). Differences in median kidney expression levels of KC (isotype control 4.8 (1.9, 8.0) vs. anti-TWEAK 1.6 (0.9, 2.1), p=0.06), ICAM-1 (isotype control 1.5 (1.1, 2.0) vs. anti-TWEAK 1.2 (1.0, 1.5), p=0.09) and TGF-ß (isotype control 1.5 (1.2, 2.0) vs. anti-TWEAK 1.2 (0.9, 1.2), p=0.08) showed a similar trend, and were close to statistical significance. (D) Kidney Affymetrix gene expression profiles are shown for the following genes: COL5A3 (1419703_PM_at), CHAF1B (1423877_PM_at), PRLR (1441102_PM_at), TNFAIP8 (1442753_PM_at) and IGFBP1 (1418918_PM_at). COL5A3 and CHAF1B were significantly upregulated in NTN treated mice (PBS or isotype control), and expression was normalized in anti-TWEAK as compared to istotype control treated mice (p < 0.01). PRLR, TNFAIP8 and IGFBP1 genes were significantly downregulated in NTN treated mice (PBS or isotype control), and expression was normalized in anti-TWEAK as compared to istotype control treated mice (p < 0.01). Data are plotted as normalized intensities, converted from log base 2 into unlogged values. Error bars represent the standard deviation across all sample replicates. The asterisks in the figure are referring to the comparison between anti-TWEAK mAb vs. isotype control treated mice.
Next, to further elucidate the mechanistic underpinnings of TWEAK blockade, we took both focused and unbiased approaches to assess the effects of anti-TWEAK mAb treatment on gene expression in response to a nephrotoxic insult by pathogenic antibodies. Starting with a focused approach, we examined candidate genes by real time PCR to analyze the effects of the different experimental conditions on selected inflammatory mediators relevant to nephritis. Similar to what was observed at the molecular level following induction of NTN in Fn14 KO as compared to WT mice, and consistent with the reduced macrophage infiltration observed in the anti-TWEAK treated mice, we found that the anti-TWEAK mAb attenuated the expression of genes that promote inflammation, vascular activation and fibrosis in response to the nephrotoxic insult. Levels of MCP-1, IP-10, RANTES, and VCAM-1 were significantly decreased in anti-TWEAK as compared to isotype control treated mice (Figure 6C). Moreover, median kidney expression levels of KC, ICAM-1 and TGF-ß were also lower in anti-TWEAK treated mice, approaching statistical significance (Figure 6C).
As an unbiased approach to identify potentially novel genes to inform the mechanism of action of anti-TWEAK mAb treatment, we compared kidney Affymetrix expression profiles in anti-TWEAK vs. isotype control Ig treated mice as described in Supplementary material. As expected, we observed an overlap between the Affymetrix and real time PCR studies (data not shown). In addition, by Affymetrix profiling we identified several novel genes that were significantly increased by NTN treatment and normalized in mice receiving anti-TWEAK but not isotype control mAb treatment (COL5A3, CHAF1B), as well as genes that were significantly down-modulated by NTN treatment and normalized with anti-TWEAK but not isotype control mAb treatment (PRLR, TNFAIP8, IGFBP1). The genes identified includes genes previously associated with cell proliferation/death (TNFAIP8, IGFBP1, CHAF1B), or fibrosis (COL5A3, CHAF1B, PRLR) (Figure 6D).
4. Discussion
Kidney involvement, a relatively common clinical manifestation of SLE, remains a major cause of morbidity and mortality in this disease. In this paper, we show that targeting the TWEAK/Fn14 pathway, by Fn14 deficiency or anti-TWEAK mAb administration in WT mice, results in significantly reduced severity of nephrotoxic injury induced by passive transfer of pathogenic rabbit anti-GBM antibodies in mice preimmunized with rabbit IgG. This is reflected by significantly decreased proteinuria and attenuated renal histopathology, including reduced tubular pathology. Local activation of the TWEAK/Fn14 pathway, as evidenced by upregulated Fn14 expression in kidney tissue, may act directly on renal cell types to effect those changes. Interestingly, we show that anti-TWEAK treatment normalizes kidney Fn14 expression, likely due to blocking the direct stimulatory effect of TWEAK [37] or decreasing the production of TWEAK-induced cytokines and thereby promoting an attenuated local inflammatory milieu. This is one possible mechanism for the salutary effect of anti-TWEAK treatment in nephritis. Furthermore, we elucidate additional mechanisms underlying the beneficial effect of anti-TWEAK in the NTN model, showing that the TWEAK/Fn14 pathway inhibition reduces renal infiltration by macrophages and progression to fibrosis likely through inhibition of downstream targets of TWEAK/Fn14 signaling related to inflammatory pathways, vascular activation and fibrogenesis, including MCP-1, IP-10, RANTES and VCAM-1.
The TWEAK/Fn14 pathway is highly relevant to human lupus nephritis. We previously showed that urinary soluble TWEAK protein levels are significantly elevated in lupus patients with active nephritis and specifically increased during nephritic flares [38, 39]. Moreover, TWEAK and Fn14 mRNA are upregulated locally in both the glomerular and tubular compartments in human LN [40]. Within the kidney, Fn14 can be expressed by various resident cells, including murine and human mesangial cells, podocytes, and tubular cells [14, 15], and potentially on infiltrating macrophages as well [41, 42]. TWEAK is most likely produced by infiltrating leukocytes including macrophages, the latter shown to be a prominent immune cell infiltrating the NTN kidneys in our study and those of others [43], and may also be expressed by renal tubular cells [16]. Significantly, in our NTN mouse model, we found that Fn14 mRNA levels were dramatically upregulated in the kidney. For this and other reasons as follows, the NTN model is highly relevant to clinical LN. Notably, the NTN model features a plethora of pathological features and processes mirrored in LN, and as such is being increasingly employed to discover new pathogenic mechanisms and explore novel therapeutic targets [27, 28]. However, it should also be noted that there are significant differences between experimental anti-GBM nephritis and lupus nephritis. Some important distinctions include the bypassing of pathogenic antibody formation by passive transfer of preformed antibodies and the acute nature of the insult in the NTN model, and the possible contributions of circulating immune complexes in lupus nephritis. In addition, the range of autoantibody specificities is broader in lupus nephritis, resulting in different patterns of glomerular deposition and damage.
The effect of Fn14 deficiency on renal damage as reflected by reduced severity of proteinuria, and similar findings with anti-TWEAK mAb treatment despite induction of very severe nephritis, is notable. These findings are consistent with the benefit of Fn14 deficiency and anti-TWEAK mAb treatment previously observed in cGVHD [23]. However, compared to the cGVHD model, the nephrotoxic nephritis model results in dramatically more severe nephritis and significant histological effects in the kidney. Thus, disruption of the TWEAK/Fn14 pathway in this model is noteworthy in terms of its effect on reducing severe proteinuria as well as in attenuating both glomerular and tubular damage that was seen at the histopathological level. Interestingly, although the NTN model primarily features glomerular disease, we also observed tubular damage and tubulointerstitial fibrosis. Given that TWEAK and Fn14 are expressed in both glomerular and tubular regions [40], the beneficial effect may result from directly reducing TWEAK signaling through Fn14 in glomerular and tubular renal cell types, respectively. Specifically in resident kidney cells, TWEAK promotes the proliferation of renal resident cells, including mesangial cells [14, 15], endothelial cells [44], and tubular cells [15, 16], in vitro and in vivo. Notably, our histopathology data suggest that some of the protective effects that we demonstrated in NTN through blocking the TWEAK pathway, in Fn14 KO and anti-TWEAK mAb treated mice, were mediated by inhibiting glomerular proliferative lesions and crescents - which are among the classic histological features of Class III and IV lupus nephritis.
There is increasing evidence to indicate a role for TWEAK in renal tubulointerstitial injury [21, 45]. Sanz et al reported that TWEAK treatment of a tubular cell line induces Fn14-mediated activation of NF-κB signaling and decreases expression of the kidney-protective factor Klotho [46], together with increased production of MCP-1, RANTES, and IL-6. Moreover, systemic TWEAK administration led to renal interstitial inflammation, and decreased renal Klotho expression [45, 47]. In an experimental model of tubular injury induced by folic acid, TWEAK blockade decreased tubular inflammatory changes and injury [45]. The role of TWEAK in pathways of tubulointerstitial injury may be particularly relevant to lupus nephritis, as progression of tubulointerstitial injury to fibrosis in human lupus nephritis is a major contributor to irreversible injury leading to end stage renal disease. The histology at this terminal stage, class VI, is one of fibrosis rather than active inflammation, and is defined as ≥90% globally sclerosed glomeruli without residual activity. Previous studies have suggested a role for TWEAK/Fn14 in renal tissue fibrogenic responses. Systemic administration of TWEAK via an adenoviral vector induced fibrosis in mouse kidney and other organs (LCB, unpublished data), while Fn14 blockade ameliorated tubulointerstitial fibrosis in an experimental model of ischemia reperfusion injury [48]. In the NTN model, we observed that Fn14 KO mice or mice treated with anti-TWEAK mAb showed decreased trichrome staining, indicative of decreased fibrosis in the kidney, as well as a trend to reduced TGF-ß expression. A recent careful analysis of components of NIH activity and chronicity indexes by Hsieh and colleagues found that tubulointerstitial disease identifies a subset of lupus patients with the highest chance for progression to end stage renal disease [49], confirming previous studies with similar results [50]. Therefore, the protective effect observed with TWEAK inhibition in the NTN model importantly extends to inhibiting tubulointerstitial inflammation and injury.
The passive transfer of pathogenic antibodies in the NTN model provides a focused approach to minimize effects on antibody generation and dissect the effect of an intervention on mechanisms acting downstream of antibody production, locally in kidney. In the NTN model, the titers of mouse anti-rabbit antibodies of the IgG2a and IgG1 subclasses were similar; however, the titers of IgG2b and IgG3 subclasses were reduced in the Fn14 KO as compared to WT mice. This was very surprising in light of prior studies that showed no effect of anti-TWEAK antibody treatment on the generation of systemic humoral immune responses [31, 51], including no effect of anti-TWEAK mAb administration and/or Fn14 deficiency on the generation of autoantibodies in cGVH disease [23], collagen induced arthritis [31] and trinitrobenzene sulfonic acid-induced colitis [52]. Notably, IgG2a antibodies, believed to be highly pathogenic in lupus [36, 53, 54], were not different in the NTN model in Fn14 KO as compared to Fn14 WT mice. Nevertheless, it cannot be ruled out that a diminished anti-rabbit response may have contributed to the attenuated nephritis observed in Fn14 KO mice. Relevant to this discussion, we note that TWEAK KO mice were reported to have several immune abnormalities [55] with potential relevance, including an increased number of natural killer cells and an increase in TH1 cytokine production, including γ-interferon and IL-12 (the latter which may affect IL-17 production [56]). However, similar immune abnormalities are not displayed by independently generated TWEAK KO mice or the Fn14 KO mice used in our current study [[52] and JSM and LCB, data not shown]. Nevertheless, we cannot rule out a developmental phenotype that may result in an altered cytokine environment that influences class switch recombination. Importantly, anti-TWEAK mAb administration in the NTN model did not affect titers of the mouse anti-rabbit antibodies of any IgG isotype as compared to control Ig treatment. Moreover, Fn14 was expressed at very low levels in the spleen and did not increase in NTN mice (data not shown). Accordingly, it is reasonable to conclude that differential responsiveness to rabbit IgG does not play a major role in the improved renal function in anti-TWEAK antibody treated mice. To definitely determine whether the effect of TWEAK/Fn14 pathway blockade is independent of the anti-rabbit response, the anti-TWEAK antibody treatment could be administered following nephrotoxic insult in a future study.
An intriguing potential mechanism to consider underlying the protection observed in NTN is the observation of reduced Ig deposition. This is particularly interesting in the anti-TWEAK treatment study where glomerular Ig deposition was significantly reduced with no corresponding effect on the levels of any of the mouse anti-rabbit IgG subclasses, as previously reported in the cGVHD model of nephritis [23]. Since we found decreased expression of genes associated with inflammation and vascular activation, both which can promote capillary permeability and may thereby enhance Ig deposition, we speculate that targeting TWEAK may have the potential to limit Ig deposition. This mechanism of action should be further explored in other models.
Our studies also shed light on other mechanisms underlying protection of kidneys by Fn14 deficiency or anti-TWEAK in the NTN model. Since TWEAK/Fn14 pathway signaling induces the activation of NF-κB, we investigated the expression of downstream targets that are known to promote the pathological processes of inflammation and vascular activation. We found reduced gene expression of MCP-1, VCAM-1, IP-10, and RANTES. TWEAK has been shown to promote expression of many of the same genes in renal cell types expressing Fn14, and therefore TWEAK likely acts through Fn14 on kidney cells to directly induce expression of these genes. In addition, previous studies demonstrated that TWEAK can promote endothelial cell survival, proliferation, and activation as manifested by upregulated adhesion molecule expression and the production of inflammatory mediators. The reduction in MCP-1 in our study is consistent with the effects of anti-TWEAK antibody treatment previously observed in other autoimmune models [31, 57], and with the reduction in macrophage infiltrates we observed. It is also reminiscent of the TWEAK-induced chemotactic activity preferentially attracting macrophages in vitro [15]. In human SLE, specific genetic polymorphisms in the gene for MCP-1 are associated with the development of LN [58, 59], and urinary MCP-1 levels reflect disease activity and correlate with renal histology and SLEDAI score [60, 61], and predict disease relapse [62]. Urine VCAM-1, an adhesion molecule important in cell recruitment into tissues, is increased in mice and patients with lupus nephritis [19, 63], and correlates with proteinuria, disease activity scores [19], and histological damage [64]. Urinary levels of IP-10, a monocyte secreted chemokine that regulates T cell migration into the kidney, accurately identifies active class IV LN, and predicts response to therapy [65]. Finally, urinary mRNA [66] and protein [67] levels of RANTES were found to be increased in human lupus nephritis, and correlate with disease activity. All of the above point to the involvement of MCP-1, VCAM-1, IP-10, and RANTES in the pathogenesis of nephritis, and suggest that decreased levels of these inflammatory mediators mediated by TWEAK inhibition in NTN would work in concert to attenuate nephritis. Although there were some differences in effects of anti-TWEAK treated and Fn14 KO mice, anti-TWEAK treated mice were sacrificed at the peak of nephritis (day 9), while Fn14 WT and KO mice were sacrificed later, during a more chronic stage (day 21). Therefore, differences in gene expression may be due to the degree of TWEAK inhibition and/or a shift from an inflammatory (early) to more fibrotic (later) milieu, or may reflect the difference in severity of NTN induced in the two studies.
An unbiased approach also revealed a novel set of genes affected by pharmacologic inhibition of TWEAK. Expression of CHAF1B, a gene required for the assembly of histone octamers onto newly-replicated DNA, was increased upon induction of NTN and correspondingly normalized by anti-TWEAK treatment, mirroring the effects on proliferation observed in the study. Conversely, expression of IGFBP1, which can have inhibitory effects on cell proliferation, was decreased in NTN and normalized with anti-TWEAK treatment. Another gene altered in NTN and normalized by anti-TWEAK was TNFAIP8, an NF-κB target known to inhibit apoptosis and promote cell survival [68].
Our studies also potentially elucidate a mechanistic basis for promotion of fibrosis by the TWEAK pathway. Decreased sclerosis of glomeruli in anti-TWEAK treated mice is reflected by the diminished expression of genes such as collagen 5, a fibrillar collagen component of glomerular structure [69]. As a more general mechanism for the reduced fibrosis mediated by inhibition of the TWEAK/Fn14 pathway, expression of TGF-ß tended to be reduced in the NTN model in both Fn14 KO and anti-TWEAK treated mice. Normalization of CHAF1B expression in anti-TWEAK mAb treated mice may be another contributing factor to decreased renal interstitial fibrosis, in light of the association of this gene with Notch signaling [70], a pathway implicated in kidney fibrosis [71]. We also observed normalization of expression of PRLR, the prolactin receptor, in anti-TWEAK treated mice. Notably, a negative association has been reported between PRLR expression and kidney interstitial fibrosis in allograft rejection [72], and kidney expression of PRLR was decreased in MRL/lpr mice, suggesting that the prolactin receptor may function to intrinsically protect the kidney against chronic, irreversible damage ([72, 73] or may simply reflect damage to renal proximal tubules [74]. Thus the TWEAK/Fn14 pathway orchestrates the expression of multiple genes that promote a fibrotic phenotype, and inhibition of the TWEAK pathway likely protects the kidney from fibrosis by its ability to normalize their expression.
5. Conclusions
Our results indicate that the TWEAK/Fn14 pathway plays an important role in the pathogenesis of immune nephritis mediated by pathogenic antibodies. Furthermore, anti-TWEAK mAb, when administered alone, attenuated proteinuria as well as both renal glomerular and tubular damage and tubulointerstitial fibrosis. The mechanism of protection afforded by anti-TWEAK mAb treatment was apparently by normalizing multiple downstream targets locally in the kidney, including genes that promote inflammation, vascular activation and fibrosis, and without apparently impacting systemic humoral immune responses. These studies suggest a novel therapeutic approach to the treatment of proliferative lupus nephritis, and support future efforts to examine the effect of TWEAK inhibition in spontaneous models of lupus associated renal disease.
Supplementary Material
Highlights.
Following pathogenic Ab transfer, nephritis was attenuated in Fn14 deficient mice.
Anti-TWEAK mAb treatment ameliorated proteinuria and improved renal histology.
TWEAK blockade decreased renal inflammation, macrophage infiltration and fibrosis.
Inhibiting TWEAK is a novel approach with a localized mechanism of action.
Acknowledgements
We would like to thank the histopathology facility at Biogen Idec for their assistance in preparation and staining of kidney sections. We acknowledge Suzanne Szak for her assistance with bioinformatics analysis of the Affymetrix data.
Funding
This work was supported by National Institutes of Health grants RO1 AR048692 and RO1 DK 090319, and a research grant from Biogen Idec (to C.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Drs. Wu, Michaelson, and Burkly are full time employees of Biogen Idec. These studies received partial support from a research grant from Biogen Idec (to C. Putterman).
References
- 1.Davidson A, Aranow C. Lupus nephritis: lessons from murine models. Nat. Rev. Rheumatol. 2010;6:13–20. doi: 10.1038/nrrheum.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arth. Care Res. 2010;62:873–80. doi: 10.1002/acr.20116. [DOI] [PubMed] [Google Scholar]
- 3.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996-2004. J. Rheumatol. 2009;36:63–67. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korbet SM, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis: racial differences in presentation and outcome. J. Am. Soc. Nephrol. 2007;18:244–254. doi: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
- 5.Kalled SL, Cutler AH, Datta SK, Thomas DW. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J. Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- 6.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, Vaishnaw A. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 7.Mohan C, Shi Y, Laman JD, Datta SK. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J. Immunol. 1995;154:1470–1480. [PubMed] [Google Scholar]
- 8.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 9.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A tutorial on B cell survival. Ann. Rev. Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 10.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol. Rev. 2011;244:99–114. doi: 10.1111/j.1600-065X.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 12.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat. Rev. Drug. Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR. TWEAK induces angiogenesis and proliferation of endothelial cells. J. Biol. Chem. 1999;274:8455–8459. doi: 10.1074/jbc.274.13.8455. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, et al. Proinflammatory effects of tweak/fn14 interactions in glomerular mesangial cells. J. Immunol. 2006;176:1889–1898. doi: 10.4049/jimmunol.176.3.1889. [DOI] [PubMed] [Google Scholar]
- 15.Gao HX, Campbell SR, Burkly LC, Jakubowski A, Jarchum I, Banas B, et al. TNF-like weak inducer of apoptosis (TWEAK) induces inflammatory and proliferative effects in human kidney cells. Cytokine. 2009;46:24–35. doi: 10.1016/j.cyto.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Justo P, Sanz AB, Sanchez-Nino MD, Winkles JA, Lorz C, Egido J, et al. Cytokine cooperation in renal tubular cell injury: the role of TWEAK. Kidney Int. 2006;70:1750–1758. doi: 10.1038/sj.ki.5001866. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu S, Nakashima H, Masutani K, Inoue Y, Miyake K, Akahoshi M, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates nephritis in MRL/lpr mice. Rheumatology. 2004;43:1121–1128. doi: 10.1093/rheumatology/keh277. [DOI] [PubMed] [Google Scholar]
- 18.Moore KJ, Wada T, Barbee SD, Kelley VR. Gene transfer of RANTES elicits autoimmune renal injury in MRL-Fas (1pr) mice. Kidney Int. 1998;53:1631–1641. doi: 10.1046/j.1523-1755.1998.00911.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J. Immunol. 2007;179:7166–7175. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- 20.Belmont HM, Buyon J, Giorno R, Abramson S. Up-regulation of endothelial cell adhesion molecules characterizes disease activity in systemic lupus erythematosus. The Shwartzman phenomenon revisited. Arthritis Rheum. 1994;37:376–383. doi: 10.1002/art.1780370311. [DOI] [PubMed] [Google Scholar]
- 21.Sanz AB, Sanchez-Nino MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, et al. TWEAK induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J. Cell. Mol. Med. 2009;13:3329–3342. doi: 10.1111/j.1582-4934.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanz AB, Sanchez-Nino MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80:708–718. doi: 10.1038/ki.2011.180. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, et al. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft- versus-host model of systemic lupus erythematosus. J. Immunol. 2007;179:7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- 24.Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J. Amer. Soc. Nephrol. 2002;13:350–358. doi: 10.1681/ASN.V132350. [DOI] [PubMed] [Google Scholar]
- 25.Wu T, Fu Y, Brekken D, Yan M, Zhou XJ, Vanarsa K, et al. Urine proteome scans uncover total urinary protease, prostaglandin D synthase, serum amyloid P, and superoxide dismutase as potential markers of lupus nephritis. J. Immunol. 2010;184:2183–2193. doi: 10.4049/jimmunol.0900292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T, Xie C, Bhaskarabhatla M, Yan M, Leone A, Chen SS, et al. Excreted urinary mediators in an animal model of experimental immune nephritis with potential pathogenic significance. Arthritis Rheum. 2007;56:949–959. doi: 10.1002/art.22556. [DOI] [PubMed] [Google Scholar]
- 27.Du Y, Fu Y, Mohan C. Experimental anti-GBM nephritis as an analytical tool for studying spontaneous lupus nephritis. Arch. Immunol. Ther. Exp. 2008;56:31–40. doi: 10.1007/s00005-008-0007-4. [DOI] [PubMed] [Google Scholar]
- 28.Fu Y, Du Y, Mohan C. Experimental anti-GBM disease as a tool for studying spontaneous lupus nephritis. Clin. Immunol. 2007;124:109–118. doi: 10.1016/j.clim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J. Clin. Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y, Xie C, Chen J, Zhu J, Zhou H, Thomas J, et al. Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-alpha production. J. Immunol. 2006;176:632–639. doi: 10.4049/jimmunol.176.1.632. [DOI] [PubMed] [Google Scholar]
- 31.Perper SJ, Browning B, Burkly LC, Weng S, Gao C, Giza K, et al. TWEAK is a novel arthritogenic mediator. J. Immunol. 2006;177:2610–2620. doi: 10.4049/jimmunol.177.4.2610. [DOI] [PubMed] [Google Scholar]
- 32.Corna D, Morigi M, Facchinetti D, Bertani T, Zoja C, Remuzzi G. Mycophenolate mofetil limits renal damage and prolongs life in murine lupus autoimmune disease. Kidney Int. 1997;51:1583–1589. doi: 10.1038/ki.1997.217. [DOI] [PubMed] [Google Scholar]
- 33.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc. Nat. Acad. Sci. (U.S.A) 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qing X, Zavadil J, Crosby MB, Hogarth MP, Hahn BH, Mohan C, et al. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54:2198–2210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]
- 35.Waldman M, Madaio MP. Pathogenic autoantibodies in lupus nephritis. Lupus. 2005;14:19–24. doi: 10.1191/0961203305lu2054oa. [DOI] [PubMed] [Google Scholar]
- 36.Izui S, Fossati-Jimack L, da Silveira SA, Moll T. Isotype-dependent pathogenicity of autoantibodies: analysis in experimental autoimmune hemolytic anemia. Springer Semin. Immunopathol. 2001;23:433–445. doi: 10.1007/s281-001-8169-1. [DOI] [PubMed] [Google Scholar]
- 37.Tran NL, McDonough WS, Savitch BA, Fortin SP, Winkles JA, Symons M, et al. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–9542. doi: 10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz N, Su L, Burkly LC, Mackay M, Aranow C, Kollaros M, et al. Urinary TWEAK and the activity of lupus nephritis. J. Autoimmun. 2006;27:242–250. doi: 10.1016/j.jaut.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res. Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, et al. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology. 2011;16:426–432. doi: 10.1111/j.1440-1797.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, et al. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ. J. 2004;68:396–399. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- 42.Molano A, Lakhani P, Aran A, Burkly LC, Michaelson JS, Putterman C. TWEAK stimulation of kidney resident cells in the pathogenesis of graft versus host induced lupus nephritis. Immunol. Lett. 2009;125:119–128. doi: 10.1016/j.imlet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Feith GW, Bogman MJ, Assmann KJ, van Gompel AP, Schalkwijk J, van Rooijen N, et al. Decreased PMN accumulation and glomerular damage by clodronate liposome treatment in PMN-dependent anti-GBM nephritis in mice. Exp. Nephrol. 1997;5:301–304. [PubMed] [Google Scholar]
- 44.Donohue PJ, Richards CM, Brown SA, Hanscom HN, Buschman J, Thangada S, et al. TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity, Arterioscler. Thromb.Vasc. Biol. 2003;23:594–600. doi: 10.1161/01.ATV.0000062883.93715.37. [DOI] [PubMed] [Google Scholar]
- 45.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, et al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J. Am. Soc. Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu M-C, Kuro-o M, Moe OW. Klotho and kidney disease. J. Nephrol. 2010;23(Suppl 16):S136–144. [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J. Am. Soc. Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, et al. Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int. 2011;79:179–188. doi: 10.1038/ki.2010.379. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 2011;63:865–874. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu F, Wu LH, Tan Y, Li LH, Wang CL, Wang WK, et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int. 2010;77:820–829. doi: 10.1038/ki.2010.13. [DOI] [PubMed] [Google Scholar]
- 51.Kamata K, Kamijo S, Nakajima A, Koyanagi A, Kurosawa H, Yagita H, et al. Involvement of TNF-like weak inducer of apoptosis in the pathogenesis of collagen-induced arthritis. J. Immunol. 2006;177:6433–6439. doi: 10.4049/jimmunol.177.9.6433. [DOI] [PubMed] [Google Scholar]
- 52.Dohi T, Borodovsky A, Wu P, Shearstone JR, Kawashima R, Runkel L, et al. TWEAK/Fn14 pathway: a nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis. Gastroenterology. 2009;136:912–923. doi: 10.1053/j.gastro.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Ebling F, Hahn BH. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980;23:392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- 54.Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin. Immunopathol. 2006;28:175–184. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 55.Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Gan P-Y, Steinmetz OM, Tan DSY, O'Sullivan KM, Ooi JD, Iwakura Y, et al. Th17 Cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J. Am. Soc. Nephrol. 2010;21:925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desplat-Jego S, Creidy R, Varriale S, Allaire N, Luo Y, Bernard D, et al. Anti-TWEAK monoclonal antibodies reduce immune cell infiltration in the central nervous system and severity of experimental autoimmune encephalomyelitis. Clin. Immunol. 2005;117:15–23. doi: 10.1016/j.clim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Kim HL, Lee DS, Yang SH, Lim CS, Chung JH, Kim S, et al. The polymorphism of monocyte chemoattractant protein-1 is associated with the renal disease of SLE. Am. J. Kid. Dis. 2002;40:1146–1152. doi: 10.1053/ajkd.2002.36858. [DOI] [PubMed] [Google Scholar]
- 59.Tucci M, Barnes EV, Sobel ES, Croker BP, Segal MS, Reeves WH, et al. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004;50:1842–1849. doi: 10.1002/art.20266. [DOI] [PubMed] [Google Scholar]
- 60.Wada T, Yokoyama H, Su SB, Mukaida N, Iwano M, Dohi K, et al. Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int. 1996;49:761–767. doi: 10.1038/ki.1996.105. [DOI] [PubMed] [Google Scholar]
- 61.Chan RW, Lai FM, Li EK, Tam LS, Wong TY, Szeto CY, et al. Expression of chemokine and fibrosing factor messenger RNA in the urinary sediment of patients with lupus nephritis. Arthritis Rheum. 2004;50:2882–2890. doi: 10.1002/art.20471. [DOI] [PubMed] [Google Scholar]
- 62.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. 2005;16:467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 63.Molad Y, Miroshnik E, Sulkes J, Pitlik S, Weinberger A, Monselise Y. Urinary soluble VCAM-1 in systemic lupus erythematosus: a clinical marker for monitoring disease activity and damage. Clin. Exp. Rheumatol. 2002;20:403–406. [PubMed] [Google Scholar]
- 64.Abd-Elkareem MI, Al Tamimy HM, Khamis OA, Abdellatif SS, Hussein MR. Increased urinary levels of the leukocyte adhesion molecules ICAM-1 and VCAM-1 in human lupus nephritis with advanced renal histological changes: preliminary findings. Clin. Exp. Nephrol. 2010;14:548–557. doi: 10.1007/s10157-010-0322-z. [DOI] [PubMed] [Google Scholar]
- 65.Avihingsanon Y, Phumesin P, Benjachat T, Akkasilpa S, Kittikowit V, Praditpornsilpa K, et al. Measurement of urinary chemokine and growth factor messenger RNAs: A noninvasive monitoring in lupus nephritis. Kidney Int. 2006;69:747–753. doi: 10.1038/sj.ki.5000132. [DOI] [PubMed] [Google Scholar]
- 66.Chan RW-Y, Lai FM-M, Li EK-M, Tam L-S, Chow K-M, Li PK-T, et al. Messenger RNA expression of RANTES in the urinary sediment of patients with lupus nephritis. Nephrology. 2006;11:219–225. doi: 10.1111/j.1440-1797.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 67.Tian S, Li J, Wang L, Liu T, Liu H, Cheng G, et al. Urinary levels of RANTES and M-CSF are predictors of lupus nephritis flare. Inflammation Res. 2007;56:304–310. doi: 10.1007/s00011-007-6147-x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang S, Zhang Y, Wei X, Zhen J, Wang Z, Li M, et al. Expression and regulation of a novel identified TNFAIP8 family is associated with diabetic nephropathy. Biochem. Biophys. Acta. 2010;1802:1078–1086. doi: 10.1016/j.bbadis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Houser MT, Scheinman JI, Basgen J, Steffes MW, Michael AF. Preservation of mesangium and immunohistochemically defined antigens in glomerular basement membrane isolated by detergent extraction. J. Clin. Invest. 1982;69:1169–1175. doi: 10.1172/JCI110553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sirin Y, Susztak K. Notch in the kidney: development and disease. J. Pathol. 2012;226:394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eikmans M, Aben JA, Koop K, Baelde HJ, de Heer E, Bruijn JA. Genetic factors in progressive renal disease: the good ones, the bad ones and the ugly ducklings. Nephrol. Dial. Transplant. 2006;21:257–260. doi: 10.1093/ndt/gfi325. [DOI] [PubMed] [Google Scholar]
- 73.Liu J, Karypis G, Hippen KL, Vegoe AL, Ruiz P, Gilkeson GS, Behrens TW. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immunity. 2006;7:156–168. doi: 10.1038/sj.gene.6364286. [DOI] [PubMed] [Google Scholar]
- 74.Ibarra F, Crambert S, Eklof AC, Lundquist A, Hansell P, Holtback U. Prolactin, a natriuretic hormone, interacting with the renal dopamine system. Kidney Int. 2005;68:1700–1707. doi: 10.1111/j.1523-1755.2005.00586.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.