Abstract
Background
Abnormal immune function is a key factor in predisposition to non-Hodgkin lymphoma (NHL). We evaluated the association of 30 cytokines individually and as a profile with diffuse large B-cell (DLBCL) and follicular (FL) lymphomas.
Methods
We used a multiplexed assay to measure 30 cytokine concentrations in pre-treatment serum in a case-control study of 234 FL, 188 DLBCL, and 400 control participants. Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CI) adjusted for age and sex, and polytomous regression was used to evaluate heterogeneity between FL and DLBCL. Principal components analysis (PCA) was used to assess cytokine profiles associated with FL and DLBCL.
Results
In single cytokine modeling, we found that 12 of the 30 circulating serum cytokines were significantly (P<0.05) associated with FL and/or DLBCL after accounting for multiple testing (q<0.05). Soluble IL-2R (sIL-2R) had the strongest association with both FL (OR=6.0 for highest versus lowest tertile, 95% CI 3.8–9.5; p-trend=1.8 × 10−21) and DLBCL (OR=7.6, 95% CI 4.5–13.1; p-trend=7.2 × 10−20). IL1RA and IL-12p40 also showed similar associations for DLBCL and FL. In contrast, HGF, MIG, and MIP-1α had a stronger association with DLBCL compared to FL, and IL-6, IL-8, IL-10, IFN-γ, IP-10, and VEGF were only statistically significantly associated with DLBCL after accounting for multiple testing. However, in PCA modeling, a cytokine profile based on sIL-2R, IL-1RA, MIG, IP-10, IL-8, and IL-12p40 explained most of the variability between controls and both FL and DLBCL.
Conclusions
We identified some single cytokines unique to DLBCL, but overall cytokine associations were more similar than distinct for DLBCL and FL. While these data are limited by concerns of reverse causality, they do suggest cytokines and cytokine profiles that can be prioritized in future studies.
Keywords: non-Hodgkin lymphoma, biomarkers, cytokines, case-control
1. Introduction
There were approximately 66,360 newly diagnosed cases of non-Hodgkin lymphoma (NHL) in the US in 2011 [1]. Among NHL cases, around 8–85% are B-cell lymphomas, which consist of both aggressive and indolent subtypes [2]. Diffuse large B cell lymphoma (DLBCL) is the most common aggressive B cell subtype [3] and follicular lymphoma (FL) is another common but indolent B-cell lymphoma [4] Altered immune function has consistently been linked with NHL risk in a number of studies [5]. For example, several autoimmune conditions [6] as well as certain viral and bacterial infections appear to increase risk of developing NHL [5,7]. These associations suggest that chronic inflammatory conditions may initiate or promote tumor formation and progression. Additionally, a considerable number of immune related gene polymorphisms have been found to influence the risk of NHL [8–12]. Several case-control studies nested in cohorts with pre-disease banked serum have also linked levels of circulating cytokines and other immune markers to the development of NHL [13–19].
These nested case-control studies provide evidence that serum cytokine levels may be related to lymphoma risk. However, the study populations so far have generally been limited in numbers, combined multiple biologically different subtypes, analyzed only a few cytokines or analyzed cytokines individually, rather than assessing cytokine profiles. Only one prior study of 63 HIV associated NHL cases of mixed subtypes and serums collected 0.1–2.0 years prior to NHL diagnosis has evaluated and simultaneously assessed 30 cytokines in association with NHL [19]. In prior work, we measured 30 cytokines using a multiplex assay from pretreated serum samples collected at diagnosis from FL or DLBCL patients [20]. Due to the availability of a control group as part of our ongoing etiology work [21], the same cytokines were measured in a set of matched controls. This study design measures pre-treatment, but not pre-disease (e.g., as in a nested case-control design), cytokine levels, and associations must be considered in the context of reverse causality (cytokine differences due to the lymphoma rather than causing the lymphoma). Nevertheless, the large sample size of the two most common NHL subtypes accompanied by a broader set of cytokines allowed a preliminary assessment to inform future work. This also allowed us to assess the differences in magnitude and type of cytokines associated with FL compared to DLBCL and to determine if there was a general cytokine profile associated with either of these subtypes.
2. Materials and Methods
2.1. Study Population
Participants in the studies provided written informed consent. The studies were reviewed and approved by the Human Subjects Institutional Review Board at the Mayo Clinic and the University of Iowa. FL and DLBCL case patients were from the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER). Briefly, newly diagnosed lymphoma patients were prospectively enrolled into the MER and the diagnosis was confirmed by the study hematopathologist (WRM) in all cases [22]. Patients selected for this analysis were diagnosed with FL or DLBCL from 2002–2008 and had a pre-treatment serum sample available. Controls were obtained from an ongoing case-control study of lymphoma [21]. Controls were selected from patients visiting the Mayo Clinic Department of Medicine for a pre-scheduled general medical examination, and eligibility requirements were age 18 years or older and a resident of Minnesota, Iowa or Wisconsin, and no prior history of lymphoma, leukemia or HIV infection. Controls were frequency matched to the Mayo cases by 5-year age group, gender, and distance from Rochester, Minnesota. All controls were enrolled over the same timeframe as the cases.
2.2. Serum cytokine assay
Serum cytokines were measured from pre-treatment blood draws using a multiplex ELISA (Invitrogen, Camarillo, CA) as previously described [20]. Thirty cytokines, including proinflammatory, Th1 and Th2 associated cytokines, were analyzed using the Luminex-100 system Version 1.7 (Luminex, Austin, TX). The following cytokines were tested – EGF (epidermal growth factor), eotaxin, FGF-2 (basic fibroblast growth factor), GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony stimulating factor), HGF (hepatocyte growth factor), IFNα (interferon alpha), IFNγ (interferon gamma), IL-2 (interleukin-2), IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-15, IL-17, IL-12p40, sIL-2R (interleukin-2 receptor), IL-1RN (interleukin-1 receptor antagonist), IL-1β (interleukin-1 beta), MCP-1 (monocyte chemotactic protein 1), IP-10 (inducible protein 10/CXCL10), MIG (monokine induced by interferonγ/CXCL9), MIP-1α (CCL3), MIP-1b (CCL4), RANTES (regulated on activation, normal T-cell expressed and secreted/CCL5), TNFα (tumor necrosis factor alpha) and VEGF (vascular endothelial growth factor). Data were acquired using STar Station software (Applied Cytometry, Dinnington, Sheffield, UK) and analysis was performed using the MasterPlex QT 1.0 system (MiraiBio, Alameda, CA). Samples were randomly assigned to plates, stratified by case (DLBCL and FL) and control status, so that each plate was mixture of cases and controls. Each cytokine was examined graphically for batch effects by plate; control distributions were consistent across plates for cytokines with distributions above the limit of detection, therefore no batch adjustment was made during the analyses. Inter-assay variation was assessed by inclusion of an internal control serum on all assay plates; the CV for the internal control log2 cytokine values across plates ranged from 0.3% (RANTES) to 12.2% (IL-6).
2.3. Statistical Analysis
We used unconditional logistic regression to calculate the odds ratios (OR) and 95% confidence intervals (95% CI) for the association of serum cytokine concentrations with risk of FL or DLBCL, adjusting for age and sex. Serums with undetectable cytokines were assigned a value of the limit of detection divided by (LOD/2). Cytokines levels were modeled as ordinal variables corresponding to the tertile cut points among the controls, with the lowest group serving as the reference. However, if fewer than 2/3 of the control levels were above the LOD, then samples falling below LOD were the referent group and the median cut point was used for the remaining samples with detectable levels. We calculated a one-degree of freedom trend test, using the original scoring of the cytokine grouping, and statistical significance was declared for p<0.05. We used polytomous logistic regression to assess heterogeneity between FL and DLBCL, and calculated a one-degree of freedom Wald test to assess heterogeneity in the trend tests between DLBCL and FL; statistical significance was declared for q-values <0.05 to account for multiple testing. All analyses were performed using SAS v9.2 (SAS Institute, Cary, NC).
To assess cytokines simultaneously, we used principal components analysis (PCA). PCA was performed on the combined FL and control samples and repeated on the combined DLBCL and control samples, agnostic to case or control status for both datasets. The top principal components were then examined graphically to explore patterns between cases and controls. We additionally calculated q-values to account for multiple testing. JMP v9.0.1 (SAS Institute, Cary, NC) was used to create correlation graphs.
3. Results
3.1. Patient Characteristics
The FL and DLBCL cases were reasonably similar to controls with respect to age and sex (Table 1).
Table 1.
Characteristics of case and control subjects
| Characteristic | Controls (N=400) |
Follicular (N=234) |
DLBCL (N=188) |
|---|---|---|---|
| Age, Median (range), years | 63.0 (21.0–87.0) | 60.0 (23.0–93.0) | 63.0 (20.0–92.0) |
| Age in years, distribution, N (%) | |||
| <40 | 41 (10.2) | 16 (6.8) | 16 (8.5) |
| 40–49 | 51 (12.8) | 46 (19.7) | 16 (8.5) |
| 50–59 | 77 (19.2) | 54 (23.1) | 41 (21.8) |
| 60–69 | 113 (28.2) | 65 (27.8) | 42 (21.3) |
| 70+ | 118 (29.5) | 53 (22.6) | 73 (38.8) |
| Sex, N (%) | |||
| Male | 213 (53.2) | 128 (54.7) | 98 (52.1) |
| Female | 187 (46.8) | 106 (45.3) | 90 (47.9) |
| Recruitment Site, N (%) | |||
| Iowa | NA | 90 (38.5) | 87(46.3) |
| Mayo | 400 (100) | 144 (61.5) | 101 (53.7) |
| BMI, mean (SD), kg/m2 | 27.3 (4.9) | 27.9 (5.3) | 27.4 (4.7) |
| BMIa,WHO categories, N (%) | |||
| Low/Normal (<25 kg/m2) | 119 (34.7) | 38 (32.2) | 20 (30.8) |
| Overweight (25 to <30 kg/m2) | 139 (40.5) | 43 (36.4) | 26 (40.0) |
| Obese (≥30 kg/m2) | 85 (24.8) | 37 (31.4) | 19 (29.2) |
Percents do not include missing values
3.2. Serum cytokine concentrations in cases and controls
In the control group, a majority of the cytokines were above the limit of detection, while IFN-γ, IL-5, IL-6, IL-7, IL-15, IL-1β, and VEGF had 20–50% of values above the detection limit and TNF-α and IL-17 had less than 20% of values above the detection limit (Table 2). Median serum levels were significantly higher in both FL and DLBCL compared to controls for HGF, IL-10, IL-12p40, sIL-2R, IL-1RA, MIG, and MIP-1α. In FL only, median FGF-2 levels were significantly lower compared to controls, and in DLBCL only, median IFN-γ, IL-6, IL-8, IL-13, IP-10, MIP-1β, TNF-α and VEGF were significantly elevated compared to controls. Among the controls, age, sex and BMI were not highly correlated with any of the 30 cytokines tested (data not shown).
Table 2.
Cytokine median expression level and percent above limit of detection
| Controls (N=400) |
Follicular Lymphoma (N=234) |
DLBCL (N=188) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | N Above LOD (%) |
Median (pg/ml) |
Interquartile Range | N Above LOD (%) |
Median (pg/ml) |
Interquartile Range | Pa | N Above LOD (%) |
Median (pg/ml) |
Interquartile Range | Pa |
| EGF | 380 (95.0) | 92.9 | (52.1–145.8) | 223 (95.3) | 92.7 | (56.2–142.8) | 0.89 | 175 (93.1) | 100.6 | (54.2–149.4) | 0.30 |
| Eotaxin | 400 (100) | 126.1 | (96.9–160.8) | 233 (99.6) | 121.8 | (96.1–153.9) | 0.57 | 188 (100) | 123.4 | (91.7–159.1) | 0.66 |
| FGF-2 | 239 (59.8) | 25.9 | (11.0–41.4) | 116 (49.6) | 22.0 | (11.0–35.4) | 0.041 | 95 (50.5) | 22.5 | (11.0–37) | 0.26 |
| G-CSF | 223 (55.8) | 32.0 | (20–93.2) | 150 (64.1) | 34.8 | (20.0–104.5) | 0.19 | 117 (62.2) | 38.2 | (20.0–116) | 0.19 |
| GM-CSF | 200 (50.0) | 5.2 | (5.0–50.0) | 100 (42.7) | 5.0 | (5.0–30.6) | 0.062 | 95 (50.5) | 5.4 | (5.0–53.4) | 0.78 |
| HGF | 388 (97.0) | 361.3 | (240.0–534.3) | 230 (98.3) | 437.4 | (304.1–623.9) | 0.001 | 186 (98.9) | 566.1 | (364.4–879.0) | <.0001 |
| IFN-α | 341 (85.2) | 53.0 | (25.6–102.3) | 190 (81.2) | 52.8 | (19.8–100.3) | 0.62 | 167 (88.8) | 61.2 | (26.9–114.4) | 0.23 |
| IFN-γ | 119 (29.8) | 5.0 | (2.5–5.7) | 73 (31.2) | 5.0 | (2.5–6.4) | 0.31 | 72 (38.3) | 5.0 | (2.5–8.2) | 0.026 |
| IL-1RA | 396 (99.0) | 403.9 | (272.8–614.2) | 233 (99.6) | 618.9 | (417.8–999.8) | <0.0001 | 187 (99.5) | 639.0 | (397.9–999.4) | <0.0001 |
| IL-1β | 92 (23.0) | 15.0 | (15.0–15.0) | 54 (23.1) | 15.0 | (15.0–15.0) | 0.83 | 50 (26.6) | 15.0 | (15.0–20.3) | 0.30 |
| IL-2 | 231 (57.8) | 9.2 | (6.0–15.5) | 138 (59.0) | 9.0 | (6.0–15.2) | 0.96 | 121 (64.4) | 10.2 | (6.0–15.4) | 0.50 |
| IL-4 | 278 (69.5) | 27.4 | (5.0–45.0) | 162 (69.2) | 27.4 | (5.0–40.5) | 0.63 | 137 (72.9) | 24.9 | (5.0–41.4) | 0.71 |
| IL-5 | 143 (35.8) | 3.0 | (3.0–7.3) | 90 (38.5) | 3.0 | (3.0–8.1) | 0.26 | 81 (43.1) | 3.0 | (3.0–8.1) | 0.066 |
| IL-6 | 166 (41.5) | 3.0 | (3.0–8.3) | 106 (45.3) | 3.0 | (3.0–11.5) | 0.30 | 120 (63.8) | 8.5 | (3.0–34.3) | <0.0001 |
| IL-7 | 122 (30.5) | 10.0 | (10.0–20.0) | 66 (28.2) | 10.0 | (10.0–14.6) | 0.64 | 57 (30.3) | 10.0 | (10.0–18.3) | 0.79 |
| IL-8 | 380 (95.0) | 29.3 | (19.5–43.1) | 224 (95.7) | 32.3 | (20.5–51.9) | 0.059 | 179 (95.2) | 49.7 | (28.7–89.2) | <0.0001 |
| IL-10 | 337 (84.2) | 16.4 | (8.8–26.8) | 200 (85.5) | 19.9 | (11.3–30.8) | 0.010 | 161 (85.6) | 27.5 | (14.8–53.4) | <0.0001 |
| IL-12p40 | 400 (100.0) | 200.3 | (162.6–258.6) | 233 (99.6) | 276.8 | (199.2–432.5) | <0.0001 | 187 (99.5) | 264.0 | (182.9–398.7) | <0.0001 |
| IL-13 | 280 (70.0) | 26.8 | (5.0–43.5) | 170 (72.6) | 29.1 | (5.0–44.7) | 0.54 | 145 (77.1) | 31.0 | (8.3–45.2) | 0.048 |
| IL-15 | 117 (29.2) | 10.0 | (10.0–14.3) | 66 (28.2) | 10.0 | (10.0–13.2) | 0.91 | 68 (36.2) | 10.0 | (10.0–19.9) | 0.60 |
| IL-7 | 26 (6.5) | 16.0 | (16.0–16.0) | 24 (10.3) | 16.0 | (16.0–16.0) | 0.64 | 13 (6.9) | 16.0 | (16.0–16.0) | 0.68 |
| sIL-2R | 399 (99.8) | 390.5 | (302.5–517.0) | 232 (99.2) | 892.6 | (462.0–1690) | <0.0001 | 184 (97.9) | 1100.0 | (538.1–2516.2) | <0.0001 |
| IP-10 | 400 (100) | 39.7 | (30.0–56.2) | 234 (100) | 42.4 | (29.1–63.0) | 0.30 | 186 (98.9) | 70.1 | (38.1–129.9) | <0.0001 |
| MCP-1 | 400 (100) | 758.9 | (565.4–949.0) | 234 (100) | 765.8 | (573.6–948.4) | 0.74 | 188 (100) | 783.4 | (602.9–1041.9) | 0.14 |
| MIG | 393 (98.2) | 63.8 | (41.6–95.7) | 231 (98.7) | 96.8 | (57.4–207.8) | <0.0001 | 177 (94.2) | 193.3 | (89.8–502.4) | <0.0001 |
| MIP-1α | 283 (70.8) | 43.0 | (10.0–59.8) | 168 (71.8) | 48.6 | (10.0–66.0) | 0.036 | 154 (81.9) | 49.4 | (29.1–76.9) | <0.0001 |
| MIP-1β | 389 (97.2) | 86.4 | (58.6–126.5) | 232 (99.2) | 90.4 | (63.2–132.7) | 0.20 | 187 (99.5) | 92.5 | (68.3–147.8) | 0.003 |
| RANTES | 400 (100) | 7440 | (6740–8202) | 234 (100) | 7737 | (6759–8359) | 0.068 | 188 (100) | 7491.5 | (6706.–8317) | 0.89 |
| TNF-α | 65 (16.2) | 5.0 | (5.0–5.0) | 49 (20.9) | 5.0 | (5.0–5.0) | 0.22 | 48 (25.5) | 5.0 | (5.0–5.4) | 0.004 |
| VEGF | 89 (22.2) | 2.5 | (2.5–5.0) | 73 (31.2) | 2.5 | (2.5–5.7) | 0.17 | 84 (44.7) | 5.0 | (2.5–8.4) | <0.0001 |
Kruskal-Wallis test statistic comparing cytokine concentrations in controls to those in lymphoma patients
3.3. Single cytokine associations with FL and DLBCL
The associations of cytokine levels with FL or DLBCL were evaluated in logistic regression models, adjusted for age and sex (Table 3). Six cytokines, IL-1RA, sIL-2R, IL-12p40, HGF, MIG and MIP-1α, were statistically significantly associated with both FL and DLBCL after accounting for multiple testing (q<0.05). Of these six cytokines, the associations for HGF, MIG, and MIP-1α were significantly stronger for DLBCL compared to FL (p-heterogeneity<0.05). In addition, six cytokines – IL-6, IL-8, IL-10, IFN-γ, IP-10, and VEGF – were specifically associated with DLBCL risk.
Table 3.
Association of cytokine level with risk of follicular and DLBCL
| Control | Follicular | DLBCL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | pg/ml | N | N | ORa | 95% CI | P Trend | q-value | N | ORa | 95% CI | P Trend | q-value | Pb |
| EGF | 10.0–65.0 | 132 | 68 | 1.0 | reference | 52 | 1.0 | reference | |||||
| 65.0–127.7 | 136 | 95 | 1.3 | 0.9–2.0 | 70 | 1.3 | 0.9–2.1 | ||||||
| 127.8–9295.0 | 132 | 71 | 1.1 | 0.7–1.6 | 0.28 | 0.31 | 66 | 1.3 | 0.8–2.0 | 0.37 | 0.20 | 0.63 | |
| Eotaxin | 5.0–106.5 | 132 | 78 | 1.0 | reference | 63 | 1.0 | reference | |||||
| 106.5–146.8 | 136 | 84 | 1.1 | 0.7–1.6 | 62 | 0.9 | 0.6–1.4 | ||||||
| 146.8–645.7 | 132 | 72 | 1.0 | 0.6–1.4 | 0.88 | 0.54 | 63 | 0.9 | 0.6–1.4 | 0.93 | 0.39 | 0.86 | |
| FGF-2 | ≤LOD | 161 | 118 | 1.0 | reference | 93 | 1.0 | reference | |||||
| 22.1–37.9 | 120 | 62 | 0.7 | 0.5–1.0 | 50 | 0.7 | 0.5–1.1 | ||||||
| 38.0–4408.9 | 119 | 54 | 0.6 | 0.4–0.9 | 0.034 | 0.056 | 45 | 0.7 | 0.4–1.0 | 0.14 | 0.093 | 0.94 | |
| G-CSF | ≤LOD | 177 | 84 | 1.0 | reference | 71 | 1.0 | reference | |||||
| 20.1–92.0 | 109 | 85 | 1.6 | 1.1–2.4 | 60 | 1.4 | 0.9–2.2 | ||||||
| 92.1–12478.3 | 114 | 65 | 1.2 | 0.8–1.8 | 0.046 | 0.060 | 57 | 1.3 | 0.8–1.9 | 0.25 | 0.11 | 0.67 | |
| GM-CSF | ≤LOD | 200 | 134 | 1.0 | reference | 93 | 1.0 | reference | |||||
| 5.1–49.8 | 99 | 60 | 0.9 | 0.6–1.3 | 46 | 1.1 | 0.7–1.6 | ||||||
| 49.8–2786.8 | 101 | 40 | 0.6 | 0.4–0.9 | 0.033 | 0.056 | 49 | 1.1 | 0.7–1.7 | 0.86 | 0.37 | 0.027 | |
| HGF | 15.0–275.1 | 132 | 50 | 1.0 | reference | 27 | 1.0 | reference | |||||
| 275.2–480.5 | 136 | 81 | 1.6 | 1.0–2.4 | 41 | 1.5 | 0.9–2.5 | ||||||
| 480.5–26822.3 | 132 | 103 | 2.1 | 1.4–3.2 | 2.10E-03 | 7.00E-03 | 120 | 4.4 | 2.7–7.1 | 7.58E-11 | 2.84E-10 | 6.00E-04 | |
| IFN-α | 5.0–32.6 | 132 | 81 | 1.0 | reference | 57 | 1.0 | reference | |||||
| 32.6–82.0 | 136 | 79 | 0.9 | 0.6–1.4 | 63 | 1.1 | 0.7–1.7 | ||||||
| 82.0–17351.0 | 132 | 74 | 0.9 | 0.6–1.3 | 0.87 | 0.54 | 68 | 1.2 | 0.8–1.9 | 0.69 | 0.32 | 0.46 | |
| IFN-γ | ≤LOD | 281 | 161 | 1.0 | reference | 116 | 1.0 | reference | |||||
| 5.1–9.0 | 56 | 29 | 0.9 | 0.5–1.4 | 27 | 1.2 | 0.7–2.1 | ||||||
| 9.0–221.9 | 63 | 44 | 1.2 | 0.8–1.8 | 0.57 | 0.48 | 45 | 1.8 | 1.2–2.8 | 0.031 | 0.029 | 0.14 | |
| IL-1β | ≤LOD | 308 | 180 | 1.0 | reference | 138 | 1.0 | reference | |||||
| 15.1–45.1 | 46 | 24 | 0.8 | 0.5–1.4 | 22 | 1.1 | 0.7–2.0 | ||||||
| 45.1–15723.0 | 46 | 30 | 1.1 | 0.7–1.8 | 0.74 | 0.51 | 28 | 1.4 | 0.8–2.3 | 0.43 | 0.22 | 0.56 | |
| IL-1RA | 15.0–319.8 | 132 | 37 | 1.0 | reference | 28 | 1.0 | reference | |||||
| 319.8–522.5 | 136 | 52 | 1.4 | 0.8–2.2 | 42 | 1.5 | 0.9–2.5 | ||||||
| 522.6–100323.2 | 132 | 145 | 3.9 | 2.5–6.1 | 1.64E-11 | 1.37E-10 | 118 | 4.2 | 2.6–6.8 | 1.71E-10 | 4.81E-10 | 0.97 | |
| IL-2 | ≤LOD | 169 | 96 | 1.0 | reference | 67 | 1.0 | reference | |||||
| 6.1–14.4 | 115 | 73 | 1.1 | 0.8–1.6 | 68 | 1.5 | 1.0–2.3 | ||||||
| 14.4–1489.4 | 116 | 65 | 1.0 | 0.7–1.4 | 0.82 | 0.54 | 53 | 1.2 | 0.8–1.9 | 0.13 | 0.093 | 0.35 | |
| sIL-2R | 12.0–342.4 | 132 | 30 | 1.0 | reference | 19 | 1.0 | reference | |||||
| 342.4–473.2 | 136 | 30 | 0.9 | 0.5–1.6 | 23 | 1.2 | 0.6–2.3 | ||||||
| 473.2–15285.3 | 132 | 174 | 6.0 | 3.8–9.5 | 1.84E-21 | 3.07E-20 | 146 | 7.6 | 4.5–13.1 | 7.18E-20 | 8.08E-19 | 0.75 | |
| IL-4 | ≤LOD | 122 | 72 | 1.0 | reference | 51 | 1.0 | reference | |||||
| 5.1–37.9 | 137 | 83 | 1.0 | 0.7–1.5 | 76 | 1.4 | 0.9–2.1 | ||||||
| 37.9–11906.4 | 141 | 79 | 0.9 | 0.6–1.4 | 0.92 | 0.55 | 61 | 1.0 | 0.7–1.6 | 0.31 | 0.17 | 0.57 | |
| IL-5 | ≤LOD | 257 | 144 | 1.0 | reference | 107 | 1.0 | reference | |||||
| 3.1–8.6 | 70 | 36 | 0.9 | 0.6–1.5 | 36 | 1.2 | 0.8–2.0 | ||||||
| 8.65–424.6 | 73 | 54 | 1.3 | 0.9–2.0 | 0.35 | 0.36 | 45 | 1.5 | 1.0–2.3 | 0.18 | 0.11 | 0.50 | |
| IL-6 | ≤LOD | 234 | 128 | 1.0 | reference | 68 | 1.0 | reference | |||||
| 3.1–12.5 | 83 | 52 | 1.2 | 0.8–1.7 | 39 | 1.6 | 1.0–2.6 | ||||||
| 12.5–1167 | 83 | 54 | 1.2 | 0.8–1.8 | 0.62 | 0.49 | 81 | 3.4 | 2.2–5.1 | 4.53E-08 | 5.10E-08 | 5.21E-05 | |
| IL-7 | ≤LOD | 278 | 168 | 1.0 | reference | 131 | 1.0 | reference | |||||
| 10.1–43.6 | 61 | 39 | 1.0 | 0.7–1.6 | 28 | 1.0 | 0.6–1.6 | ||||||
| 43.6–1131.1 | 61 | 27 | 0.7 | 0.4–1.2 | 0.44 | 0.41 | 29 | 1.0 | 0.6–1.7 | 0.99 | 0.40 | 0.50 | |
| IL-8 | 1.5–23.1 | 133 | 67 | 1.0 | reference | 36 | 1.0 | reference | |||||
| 23.2–37.7 | 136 | 69 | 1.1 | 0.7–1.6 | 35 | 0.9 | 0.6–1.6 | ||||||
| 37.7–21299.7 | 131 | 98 | 1.6 | 1.1–2.5 | 0.032 | 0.056 | 117 | 3.2 | 2.0–5.1 | 1.62E-09 | 3.65E-09 | 1.20E-03 | |
| IL-10 | 2.5–11.4 | 132 | 61 | 1.0 | reference | 33 | 1.0 | reference | |||||
| 11.4–22.8 | 135 | 75 | 1.2 | 0.8–1.8 | 44 | 1.3 | 0.8–2.2 | ||||||
| 22.9–12806.1 | 133 | 98 | 1.6 | 1.1–2.4 | 0.069 | 0.088 | 111 | 3.4 | 2.1–5.3 | 3.05E-08 | 3.81E-08 | 2.30E-03 | |
| IL-12p40 | 5.0–172.8 | 132 | 40 | 1.0 | reference | 38 | 1.0 | reference | |||||
| 172.8–235.7 | 136 | 52 | 1.3 | 0.8–2.1 | 40 | 1.0 | 0.6–1.7 | ||||||
| 235.7–6743.1 | 132 | 142 | 3.6 | 2.4–5.5 | 7.35E-11 | 4.08E-10 | 110 | 2.8 | 1.8–4.4 | 2.40E-08 | 3.38E-08 | 0.64 | |
| IL-13 | 2.5–9.1 | 133 | 71 | 1.0 | reference | 50 | 1.0 | reference | |||||
| 9.1–37.9 | 130 | 76 | 1.1 | 0.7–1.6 | 58 | 1.2 | 0.7–1.8 | ||||||
| 37.9–1267.1 | 137 | 87 | 1.2 | 0.8–1.8 | 0.68 | 0.51 | 80 | 1.5 | 1.0–2.3 | 0.14 | 0.093 | 0.54 | |
| IL-15 | ≤LOD | 283 | 168 | 1.0 | reference | 120 | 1.0 | reference | |||||
| 10.1–48.2 | 58 | 40 | 1.1 | 0.7–1.8 | 39 | 1.7 | 1.0–2.6 | ||||||
| 48.2–6265.2 | 59 | 26 | 0.7 | 0.4–1.2 | 0.38 | 0.37 | 29 | 1.2 | 0.7–1.9 | 0.096 | 0.077 | 0.15 | |
| IP-10 | 5.0–32.5 | 132 | 77 | 1.0 | reference | 37 | 1.0 | reference | |||||
| 32.6–49.9 | 135 | 71 | 0.9 | 0.6–1.4 | 34 | 0.9 | 0.5–1.5 | ||||||
| 50.0–712.9 | 133 | 86 | 1.2 | 0.8–1.8 | 0.48 | 0.42 | 117 | 3.1 | 1.9–4.9 | 3.55E-09 | 6.66E-09 | 1.66E-05 | |
| MCP-1 | 99.8–640.8 | 132 | 86 | 1.0 | reference | 59 | 1.0 | reference | |||||
| 640.8–889.5 | 136 | 74 | 0.9 | 0.6–1.3 | 57 | 0.9 | 0.6–1.4 | ||||||
| 889.5–11226.4 | 132 | 74 | 0.9 | 0.6–1.3 | 0.72 | 0.51 | 72 | 1.2 | 0.8–1.8 | 0.50 | 0.24 | 0.52 | |
| MIG | 3.0–49.9 | 132 | 43 | 1.0 | reference | 24 | 1.0 | reference | |||||
| 49.9–83.6 | 135 | 52 | 1.2 | 0.8–2.0 | 20 | 0.8 | 0.4–1.6 | ||||||
| 83.6–5412.8 | 133 | 139 | 3.6 | 2.4–5.6 | 2.07E-10 | 8.63E-10 | 144 | 6.1 | 3.6–10.1 | 8.23E-19 | 4.63E-18 | 2.80E-03 | |
| MIP-1α | ≤LOD | 117 | 66 | 1.0 | reference | 34 | 1.0 | reference | |||||
| 10.1–51.9 | 139 | 56 | 0.7 | 0.5–1.1 | 66 | 1.6 | 1.0–2.6 | ||||||
| 51.9–7235.9 | 144 | 112 | 1.4 | 0.9–2.0 | 5.30E-03 | 0.015 | 88 | 2.1 | 1.3–3.4 | 6.50E-03 | 6.65E-03 | 0.014 | |
| MIP-1β | 5.0–67.4 | 132 | 67 | 1.0 | reference | 46 | 1.0 | reference | |||||
| 67.4–109.6 | 136 | 76 | 1.1 | 0.7–1.7 | 65 | 1.4 | 0.9–2.1 | ||||||
| 109.6–3971.0 | 132 | 91 | 1.4 | 0.9–2.1 | 0.26 | 0.31 | 77 | 1.6 | 1.0–2.5 | 0.095 | 0.077 | 0.74 | |
| RANTES | 1085.6–7044.6 | 132 | 72 | 1.0 | reference | 65 | 1.0 | reference | |||||
| 7044.6–7914.5 | 135 | 61 | 0.8 | 0.5–1.3 | 58 | 0.9 | 0.6–1.3 | ||||||
| 7914.6–11813.7 | 133 | 101 | 1.4 | 0.9–2.0 | 0.037 | 0.056 | 65 | 1.0 | 0.7–1.5 | 0.74 | 0.33 | 0.23 | |
| VEGF | ≤LOD | 311 | 161 | 1.0 | reference | 104 | 1.0 | reference | |||||
| 5.1–7.2 | 45 | 40 | 1.7 | 1.1–2.8 | 27 | 1.8 | 1.1–3.1 | ||||||
| 7.2–252.8 | 44 | 33 | 1.5 | 0.9–2.4 | 0.035 | 0.056 | 57 | 3.9 | 2.5–6.1 | 2.34E-08 | 3.38E-08 | 5.00E-04 | |
OR adjusted for gender and age at diagnosis or first contact for controls.
P-heterogeneity across subtypes
3.4. Cytokine profiles associated with FL and DLBCL
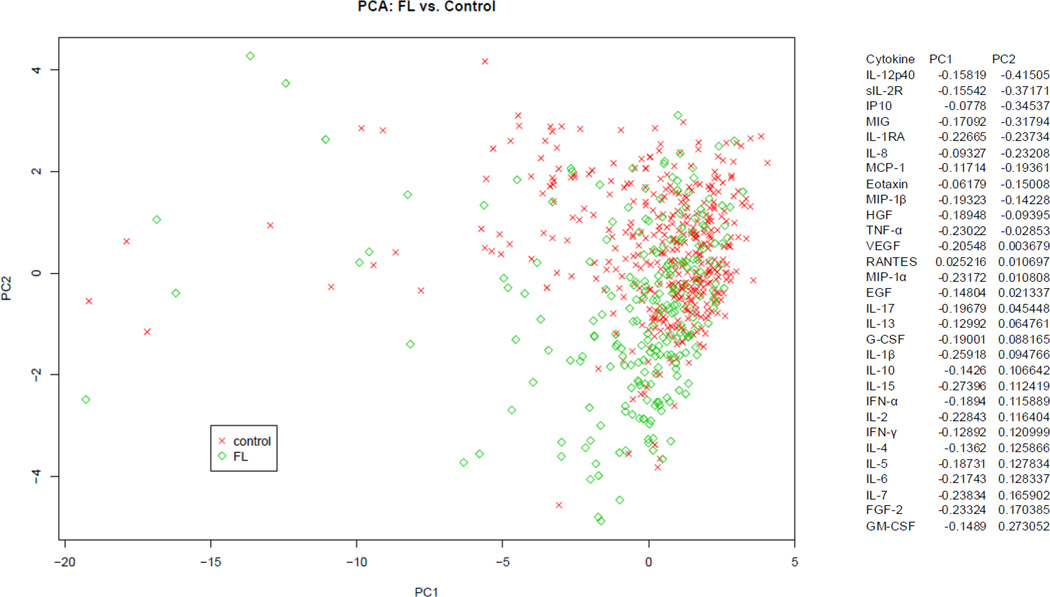
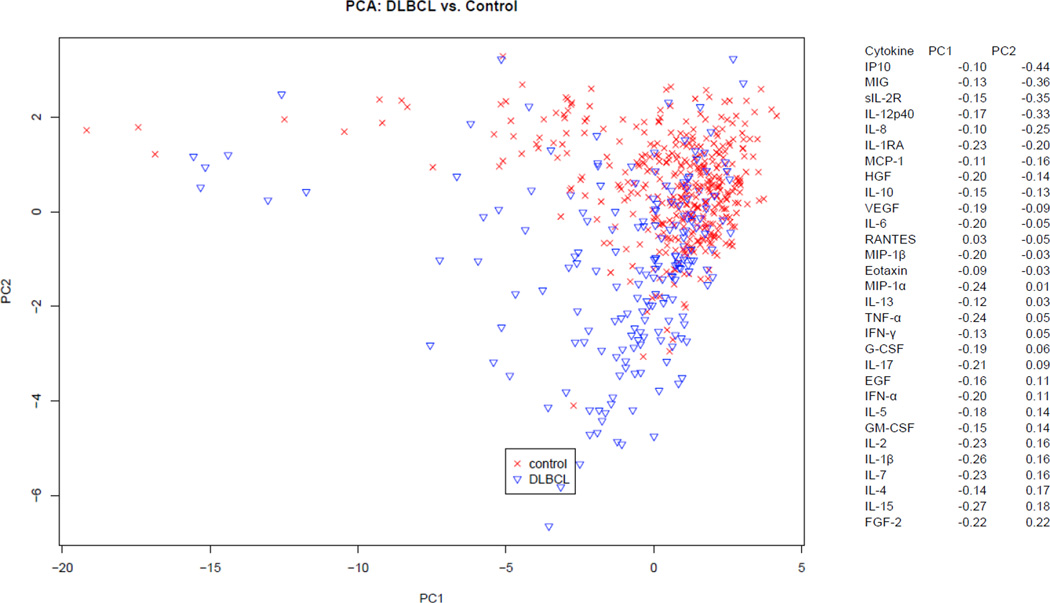
Given the large number of statistically significant associations and the known biologic correlation among cytokines, we next conducted a multivariable analysis for each subtype using principal components analysis (PCA). This analysis allowed us to examine the influence of all 30 cytokines simultaneously in detecting patterns of cytokine variation between controls and each subtype. The top 3 principal components (PC1-PC3) accounted for most of the total variation in the DLBCL (45%) and FL (43%) data. When assessing the different components, PC2 appeared to best separate the controls compared to each subtype (Figure 1 and Figure 2). For both FL and DLBCL, the top 6 loading cytokines for PC2 included sIL-2R, IL1RA, MIG, IP-10, IL-8 and IL-12p40. Although the ranking of these cytokines differed between the two subtypes, these six cytokines were clearly the primary cytokines with differential levels between controls and the two subtypes, suggesting a similar biologic process occurring in the two lymphoma subtypes that was distinct from a control population. Most of the top loading cytokines were highly correlated with each other in controls as well as in FL or DLBCL cases (Supplemental Figure 1).
Figure 1.
Multivariate analysis of FL and Controls: Graphic representation of the variability of circulating cytokines between FL patients (green) and controls (red) using PCA. Shown PC2 versus PC1, demonstrating the greatest variability between cases and controls is explained by PC2.
Figure 2.
Multivariate analysis of DLBCL and Controls: Graphic representation of the variability of circulating cytokines between DLBCL patients (blue) and controls (red) using PCA. Shown PC2 versus PC1, demonstrating the greatest variability between cases and controls is explained by PC2.
4. Discussion
In single cytokine logistic regression modeling, 12 of the 30 circulating serum cytokines were associated with FL and/or DLBCL after accounting for multiple testing (q<0.05). Both DLBCL and FL were most strongly associated with sIL-2R. When formally assessing single cytokine differences between FL and DLBCL, serum cytokines HGF, MIG, Mip-1α, IL-8, VEGF, IL-6, IL-10, IP-10, and GM-CSF were all significantly different. Positive significant associations were found for both FL and DLBCL for those first three cytokines after accounting for multiple testing, but the effect sizes for the DLBCL associations were stronger. Using a principal components analysis approach, IL-12p40, sIL-2R, IP-10, MIG, IL-1RA, and IL-8 best explained the variability between controls and DLBCL and FL cases, regardless of subtype. It should be noted that there is a fairly high correlation between these cytokines in the controls, and therefore it is difficult to determine in the setting of a case-control study if one cytokine is driving the others, if they are synergistic, or if they are acting independently.
There are several strengths of this study. Incident cases of FL and DLBCL were enrolled with pre-treatment serum, and there was a relatively large control group that was representative of the underlying source population that generated the cases [21]. A high quality and reproducible Luminex-based assay was utilized to simultaneously evaluate the largest number of cytokines of any study published to date. Our study also included a large number of FL and DLBCL cases, as well as controls, compared to previously published studies, which allowed us to formally assess etiologic heterogeneity between FL and DLBCL. We also accounted for multiple testing and found robust results. Finally, we are the first to assess such a large panel of cytokines simultaneously in association with NHL.
The major limitation of this study was that serum samples were collected after a lymphoma diagnosis, rather than before diagnosis using banked samples. This raises the concern of reverse causality, making it difficult to distinguish cytokine changes that predispose to lymphoma versus changes that are produced by malignant cells following the development of lymphoma or by other immune or microenvironment cells as a response to the lymphoma. The microenvironment is important in DLBCL and FL [23]. In addition, there were a large number of case and control serums falling below the limit of detection for IL-17 and TNF-α with the Luminex-based assay. A more sensitive assay or plasma may be necessary to more accurately assess these specific cytokines in peripheral blood of lymphoma patients versus controls. Although it is also possible that these cytokines are not relevant for FL and DLBCL, three other studies found at least a marginal association between TNF-α and DLBCL [16–18]. Our study adjusted for age, and sex but did not have covariate data on other lifestyle or environmental factors on all of the cases. Finally, the study was conducted in a predominantly white, upper Midwest population which may impact generalizability to other racial and ethnic groups.
In the present study, sIL-2R expression had a strong positive association with both FL and DLBCL. This cytokine was also one of the top loading components of PC2, which explained most of the variability between lymphomas and controls. Elevated serum concentrations of this soluble receptor have been previously shown in a number of cancers, particularly those of hematopoietic origin [24]. Additionally, elevated sIL-2R expression has been found in autoimmune and infectious conditions, which could predispose lymphocytes to malignant transformation [25]. This soluble receptor was previously reported to be elevated in NHL, however, only one nested case-control study with prospectively collected serum examined this relationship, and found elevated levels of this soluble receptor in serum collected 2 years prior to NHL diagnosis compared to controls [16]. In the present study, elevated serum levels of the IL-2R ligand, IL-2, an important cytokine for lymphocyte proliferation [26], was not significantly associated with either FL or DLBCL in single cytokine analysis. One of two NHL studies with prospectively collected serum/plasma that examined levels of IL-2 found an association with NHL and this cytokine [18], while the other did not find an association [16]. Recent studies have found sIL-2R expression in serum increases with increasing IPI or Ann Arbor stage, supporting that this expression may be reflective (in part or whole) of increased tumor burden [27,28]. It is difficult to assess whether elevated sIL-2R levels promote tumor growth through immunosuppression or other mechanisms, whether these levels are simply a byproduct of constitutively activated immune cells, or both. Of note, sIL-2R has been previously shown to bind IL-2 and promote its signaling in lymphoma, aiding in regulatory T-cell formation and inhibiting CD8 T-cells [29].
IL-1R antagonist (IL-1RA), which inhibits signaling by IL-1α and IL-1β [30,31], has been implicated by mouse models in protection against autoimmune arthritis [32]. In our study, this cytokine antagonist was also found to be strongly and positively associated with both FL and DLBCL in single cytokine analysis, and was a top loading component in our PCA model. However, in two prior nested case-control studies examining IL-1RA in banked pre-disease serum/plasma, no association was found between this cytokine receptor antagonist and NHL [16,19], raising concerns that the elevated levels we observed were due to the tumor. One prior study of IL-1RA found significantly elevated serum levels in FL patients (n=60) compared to controls (n=20), although serums were collected from patients at relapse and during remission in addition to primary diagnosis [33].
Serum concentration of IL-12p40, a subunit of IL-12, which is an important cytokine for Th1 development [34], was positively associated with both FL and DLBCL in single cytokine and PCA analyses. This cytokine was also examined in three of the prior nested case-control studies of all NHL and no associations were found with NHL risk [16,18,19], raising the possibility that IL-12p40 may be elevated as the result of lymphoma, either by lymphoma cells themselves or other cells in the tumor microenvironment. Probably due to its ability to enhance innate and cellular immunity, this cytokine has been demonstrated to be a useful mediator of anti-tumor response [35]. However, a recent study found that IL-12 induced T-cell exhaustion and decreased immune function in follicular lymphoma [36], which might explain the elevated levels of this cytokine with lymphoma in the present study. It is important to note that this protein is also a subunit of the cytokine, IL-23, which acts to promote proliferation in memory T-cells [37].
In single cytokine analyses, serum chemokines IL-8 (CXCL8) and MIG (CXCL9) were associated with both FL (borderline for IL-8) and DLBCL, although the associations appeared to be stronger for DLBCL. Both were also top loading cytokines in the PC2 component of PCA models for both FL and DLBCL. MIG is an IFN-γ inducible chemokine involved in T lymphocyte trafficking [38], and to our knowledge has not been previously associated with FL and DLBCL. IL-8, which plays a crucial role in neutrophil recruitment and angiogenesis [39], has been assessed in a few studies of NHL. In an Italian nested case-control study, IL-8 was examined but not found to be associated with lymphoma, although FL and DLBCL made up less than half of their 86 cases [18]. Another study with 46 DLBCL cases and 40 controls found elevated IL-8 in patients versus controls, however, as in our study, the serum was collected at the time of diagnosis [40]. This chemokine may therefore be expressed as a result of the lymphoma rather than predisposing patients to the disease.
IP-10 is another chemokine inducible by IFN-γ that acts as a chemoattractant for effector T cells [41], and appears to play a role in autoimmune diseases like colitis [42]. Interestingly, although single cytokine analysis only detected a significant association between IP-10 and DLBCL, it was one of the top loading cytokines in PC2 for both FL and DLBCL, suggesting its effect may be more pronounced in the setting of other cytokines. This chemokine has been assessed for association with NHL risk in two prior prospective studies [18,19], one in an HIV+ population, where a positive significant association was found with the HIV-associated lymphoma [19]. Because IL-12 induces IFN-γ expression, which in turn increases expression of both IP-10 and MIG [43], it is likely that these cytokines really represent activation of the same pathway [43,44]. Additionally, in this NHL population, our group recently found and reported an association between this chemokine and prognosis in DLBCL [20].
In at least one of three previous nested case-control studies of NHL in HIV negative study subjects, significant associations were found for IL-10, TNF-α, sIL-2R, IL-13 (inverse), IL-2, IFN-γ, ICAM, sTNFR1, and sCD27 [16–18]. We did not assess the ICAM, sTNFR1, and sCD27 in our study, and our assay may not have been sensitive enough to detect low level differences in TNF-α, given the high percentage of patients and controls with serum concentrations below the limit of detection. Consistent with these studies, we did find that sIL-2R was significantly associated with both FL and DLBCL in the single cytokine analysis and sIL-2R explained much of the variability seen in our multivariate model. Additionally, IFN-γ and IL-10 were significantly associated with DLBCL in the single cytokine analysis. However, IL-13 and IL-2 did not trend significantly with either lymphoma subtype in our study. We also found a statistically significant positive association with IL-6 and VEGF and DLBCL. IL-6 [14,15,19] and VEGF [19] have also been found to be positively associated with HIV-associated NHL, although three studies in HIV− populations assessed IL-6 and found no association [16–18]. Finally, in the only other study to date to assess 30 cytokines simultaneously, IL-1α, IL-4, IL-5, IL-13, and GM-CSF were the top loading cytokines in the factor found to be significantly associated with combined subtypes of NHL in an HIV+ cohort [19]. The top loading cytokines for our most significant principal component did not match any of these, although their panel did not include MIG and sIL-2R, two of our top loading components. These differences are not unexpected, as our findings reflect those of patients with a presumably higher functioning immune system, as opposed to the severely impaired immune system in HIV infection [45]. The discrepancies in our findings and those of prior studies may also be due to differences in cytokine levels in serum versus plasma, different multiplex systems, or these differences might begin to explain cytokines that predispose to lymphoma versus those that are the result of lymphoma. It is also important to note that most other studies pooled subtypes and some included T-cell lymphomas.
Although our study lacks collection of serum samples before onset of lymphomas, we were able to simultaneously assess a large panel of serum cytokines both FL and DLBCL patients compared to controls. In most studies of cytokines and NHL risk, all subtypes were combined. Our study raised the question of whether combined analysis is appropriate, hypothesizing that cytokine patterns might differ between the two subtypes, due to differing aggressiveness and influence of microenvironment. Although we did observe some differences in the single cytokine analyses of serum cytokine concentrations in association with the two subtypes of NHL assessed in this study, these differences mostly reflect higher magnitude of association/ higher cytokine concentrations with DLBCL, rather than completely different patterns of cytokine abnormalities. As mentioned before, this could likely result from the more aggressive nature of this disease. On the other hand, in the PCA model, which allows us to assess all 30 cytokines simultaneously, the same 6 cytokines appear to most strongly influence differences between each subtype and controls. Generally speaking, there was fairly close agreement between most of the results in the two models, but it should be noted that differences could result from the fact that single cytokine analysis was based on tertiles of cytokine concentrations, while the PCA analysis modeled cytokines continuously. Most prior studies of this type utilized the former method.
5. Conclusions
The results of this study support limited previous studies that found associations of serum or plasma concentrations of sIL-2R (single cytokine and PCA analyses with both subtypes), IL-6, IL-10, IFN-γ (single-cytokine analysis with DLBCL) and VEGF (single-cytokine analysis with DLBCL and borderline with FL) with lymphoma, while suggesting potential subtype specific differences. Prior nested case-control studies that examined IL-1RA, IP-10, IL-8, and IL-12p40 did not find significant single cytokine associations with NHL, suggesting our findings for these cytokines may reflect reverse causality. The association of MIG with DLBCL and FL is novel and warrants follow-up in future studies. Regarding heterogeneity of cytokine associations between DLBCL and FL subtypes, we found in single cytokine analyses that DLBCL was specifically associated with six cytokines (IL-6, IL-8, IL-10, IFN-γ, IP-10, and VEGF), while three cytokines (HGF, MIG, and Mip-1α) were associated with both subtypes, but with a stronger association for DLBCL, which may be reflective of the more aggressive nature of this lymphoma. However, when all cytokines were assessed simultaneously with PCA modeling, the same 6 cytokines, sIL-2R, IL-1RA, MIG, IP-10, IL-8, and IL-12p40 explained most of the variability between controls and DLBCL and FL. Overall, we conclude that cytokines were more similar than distinct for these two subtypes, but that specific cytokines may vary in their strength of association.
Supplementary Material
Highlights.
Of 30 cytokines analyzed, sIL-2R was most strongly associated with FL and DLBCL.
IL1RA and IL-12 were also strongly associated with both subtypes.
HGF, MIG, and MIP-1α had stronger associations with DLBCL than with FL.
Associations with IL-6, IL-8, IL-10, IFN-γ, IP-10 and VEGF were specific to DLBCL.
IL-1RA, sIL-2R, MIG, IP-10, IL-8 and IL-12 best explained case-control differences.
Acknowledgements
This work was supported in part by grants CA92104, CA92153, CA97274, and CA25224 from the National Institutes of Health and by the Predolin Foundation; Dr. Charbonneau was supported by R25 CA92049.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest: none.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Byrd JC, Czuczman MS, Fayad L, Forero A, Glenn MJ, Gockerman JP, et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2010;8:288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 3.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman A. Follicular lymphoma: 2011 update on diagnosis and management. Am J Hematol. 2011;86:768–775. doi: 10.1002/ajh.22099. [DOI] [PubMed] [Google Scholar]
- 5.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene. 2004;23:6524–6534. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 6.Ekstrom Smedby K, Vajdic CM, Falster M, Engels EA, Martinez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori Costantini A, Bracci PM, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:401–404. doi: 10.1158/1055-9965.EPI-06-1056. [DOI] [PubMed] [Google Scholar]
- 8.Purdue MP, Lan Q, Kricker A, Grulich AE, Vajdic CM, Turner J, Whitby D, Chanock S, Rothman N, Armstrong BK. Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: findings from the New South Wales non-Hodgkin Lymphoma Study. Carcinogenesis. 2007;28:704–712. doi: 10.1093/carcin/bgl200. [DOI] [PubMed] [Google Scholar]
- 9.Wang SS, Cozen W, Cerhan JR, Colt JS, Morton LM, Engels EA, Davis S, Severson RK, Rothman N, Chanock SJ, et al. Immune mechanisms in non-Hodgkin lymphoma: joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors. Cancer Res. 2007;67:5042–5054. doi: 10.1158/0008-5472.CAN-06-4752. [DOI] [PubMed] [Google Scholar]
- 10.Cerhan JR, Ansell SM, Fredericksen ZS, Kay NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares W, et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007;110:4455–4463. doi: 10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest MS, Skibola CF, Lightfoot TJ, Bracci PM, Willett EV, Smith MT, Holly EA, Roman E. Polymorphisms in innate immunity genes and risk of non-Hodgkin lymphoma. Br J Haematol. 2006;134:180–183. doi: 10.1111/j.1365-2141.2006.06141.x. [DOI] [PubMed] [Google Scholar]
- 12.Nieters A, Beckmann L, Deeg E, Becker N. Gene polymorphisms in Toll-like receptors, interleukin-10, and interleukin-10 receptor alpha and lymphoma risk. Genes Immun. 2006;7:615–624. doi: 10.1038/sj.gene.6364337. [DOI] [PubMed] [Google Scholar]
- 13.Breen EC, Boscardin WJ, Detels R, Jacobson LP, Smith MW, O'Brien SJ, Chmiel JS, Rinaldo CR, Lai S, Martinez-Maza O. Non-Hodgkin's B cell lymphoma in persons with acquired immunodeficiency syndrome is associated with increased serum levels of IL10, or the IL10 promoter -592 C/C genotype. Clin Immunol. 2003;109:119–129. doi: 10.1016/s1521-6616(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 14.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, Kaslow RA, Variakojis D, Bream JH, Rinaldo CR, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen EC, van der Meijden M, Cumberland W, Kishimoto T, Detels R, Martinez-Maza O. The development of AIDS-associated Burkitt's/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6. Clin Immunol. 1999;92:293–299. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y, Shore RE, Arslan AA, Koenig KL, Liu M, Ibrahim S, Lokshin AE, Zeleniuch-Jacquotte A. Circulating cytokines and risk of B-cell non-Hodgkin lymphoma: a prospective study. Cancer Causes Control. 2010;21:1323–1333. doi: 10.1007/s10552-010-9560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purdue MP, Lan Q, Bagni R, Hocking WG, Baris D, Reding DJ, Rothman N. Prediagnostic serum levels of cytokines and other immune markers and risk of non-hodgkin lymphoma. Cancer Res. 2011;71:4898–4907. doi: 10.1158/0008-5472.CAN-11-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saberi Hosnijeh F, Krop EJ, Scoccianti C, Krogh V, Palli D, Panico S, Tumino R, Sacredote C, Nawroly N, Portengen L, et al. Plasma cytokines and future risk of non-Hodgkin lymphoma (NHL): a case-control study nested in the Italian European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2010;19:1577–1584. doi: 10.1158/1055-9965.EPI-09-1237. [DOI] [PubMed] [Google Scholar]
- 19.Rabkin CS, Engels EA, Landgren O, Schuurman R, Constanza Camargo M, Ruth P, Goedert JJ. Circulating cytokine levels, Epstein-Barr viremia, and risk of acquired immunodeficiency syndrome-related non-Hodgkin lymphoma. Am J Hematol. 2011;86:875–878. doi: 10.1002/ajh.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansell SM, Maurer MJ, Ziesmer SC, Slager SL, Habermann TM, Link BK, Witzig TE, Macon WR, Dogan A, Cerhan JR, et al. Elevated pretreatment serum levels of interferon-inducible protein-10 (CXCL10) predict disease relapse and prognosis in diffuse large B-cell lymphoma patients. Am J Hematol. 2012 doi: 10.1002/ajh.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerhan JR, Fredericksen ZS, Wang AH, Habermann TM, Kay NE, Macon WR, Cunningham JM, Shanafelt TD, Ansell SM, Call TG, et al. Design and validity of a clinic-based case-control study on the molecular epidemiology of lymphoma. Int J Mol Epidemiol Genet. 2011;2:95–113. [PMC free article] [PubMed] [Google Scholar]
- 22.Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, Kelly JL, Macon WR, Nowakowski GS, Inwards DJ, et al. Vitamin D insufficiency and prognosis in non-Hodgkin's lymphoma. J Clin Oncol. 2010;28:4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coupland SE. The challenge of the microenvironment in B-cell lymphomas. Histopathology. 2011;58:69–80. doi: 10.1111/j.1365-2559.2010.03706.x. [DOI] [PubMed] [Google Scholar]
- 24.Bien E, Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers. 2008;13:1–26. doi: 10.1080/13547500701674063. [DOI] [PubMed] [Google Scholar]
- 25.Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm. 2005;2005:121–130. doi: 10.1155/MI.2005.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittington R, Faulds D. Interleukin-2. A review of its pharmacological properties and therapeutic use in patients with cancer. Drugs. 1993;46:446–514. doi: 10.2165/00003495-199346030-00009. [DOI] [PubMed] [Google Scholar]
- 27.Jo SA, Hwang SH, Chang CL, Kim SY, Shin HJ, Chung JS, Sol MY, Lee EY. Clinical relevance of elevated levels of serum soluble interleukin-2 receptor alpha (sIL-2Ralpha) in patients with non-Hodgkin's lymphoma. Korean J Lab Med. 2010;30:600–605. doi: 10.3343/kjlm.2010.30.6.600. [DOI] [PubMed] [Google Scholar]
- 28.Morito T, Fujihara M, Asaoku H, Tari A, Sato Y, Ichimura K, Tanaka T, Takata K, Tamura M, Yoshino T. Serum soluble interleukin-2 receptor level and immunophenotype are prognostic factors for patients with diffuse large B-cell lymphoma. Cancer Sci. 2009;100:1255–1260. doi: 10.1111/j.1349-7006.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang ZZ, Grote DM, Ziesmer SC, Manske MK, Witzig TE, Novak AJ, Ansell SM. Soluble IL-2Ralpha facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood. 2011;118:2809–2820. doi: 10.1182/blood-2011-03-340885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seckinger P, Williamson K, Balavoine JF, Mach B, Mazzei G, Shaw A, Dayer JM. A urine inhibitor of interleukin 1 activity affects both interleukin 1 alpha and 1 beta but not tumor necrosis factor alpha. J Immunol. 1987;139:1541–1545. [PubMed] [Google Scholar]
- 31.Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 32.Horai R, Nakajima A, Habiro K, Kotani M, Nakae S, Matsuki T, Nambu A, Saijo S, Kotaki H, Sudo K, et al. TNF-alpha is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J Clin Invest. 2004;114:1603–1611. doi: 10.1172/JCI20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labidi SI, Menetrier-Caux C, Chabaud S, Chassagne C, Sebban C, Gargi T, Biron P, Blay JY, Ghesquieres H. Serum cytokines in follicular lymphoma. Correlation of TGF-beta and VEGF with survival. Ann Hematol. 2009 doi: 10.1007/s00277-009-0777-8. [DOI] [PubMed] [Google Scholar]
- 34.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 35.Younes A, Pro B, Robertson MJ, Flinn IW, Romaguera JE, Hagemeister F, Dang NH, Fiumara P, Loyer EM, Cabanillas FF, et al. Phase II clinical trial of interleukin-12 in patients with relapsed and refractory non-Hodgkin's lymphoma and Hodgkin's disease. Clin Cancer Res. 2004;10:5432–5438. doi: 10.1158/1078-0432.CCR-04-0540. [DOI] [PubMed] [Google Scholar]
- 36.Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 38.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 40.Nacinovic-Duletic A, Stifter S, Dvornik S, Skunca Z, Jonjic N. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol. 2008;30:230–239. doi: 10.1111/j.1751-553X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 41.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 42.Singh UP, Singh S, Taub DD, Lillard JW., Jr. Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol. 2003;171:1401–1406. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 43.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 44.Tannenbaum CS, Wicker N, Armstrong D, Tubbs R, Finke J, Bukowski RM, Hamilton TA. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. J Immunol. 1996;156:693–699. [PubMed] [Google Scholar]
- 45.Miedema F, Petit AJ, Terpstra FG, Schattenkerk JK, de Wolf F, Al BJ, Roos M, Lange JM, Danner SA, Goudsmit J, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.