Abstract
The neural processing of auditory information engages pathways that begin initially at the cochlea and that eventually reach forebrain structures. At these higher levels, the computations necessary for extracting auditory source and identity information rely on the neuroanatomical connections between the thalamus and cortex. Here, the general organization of these connections in the medial geniculate body (thalamus) and the auditory cortex is reviewed. In addition, we consider two models organizing the thalamocortical pathways of the non-tonotopic and multimodal auditory nuclei. Overall, the transfer of information to the cortex via the thalamocortical pathways is complemented by the numerous intracortical and corticocortical pathways. Although interrelated, the convergent interactions among thalamocortical, corticocortical, and commissural pathways enable the computations necessary for the emergence of higher auditory perception.
Keywords: thalamus, cortex, medial geniculate body, auditory, thalamocortical, corticocortical, commissural
Introduction
Encoding and processing the rich variety of sounds from the external world requires an equally elaborate and specialized neural processing apparatus. Indeed, the initial sensorineural conversion of sound at the level of the cochlea is a marvel of biophysics (Hudspeth, 1997) and establishes many of the organizing features observed throughout the entirety of the ascending auditory pathway (Trussell, 1999). Multiple brainstem centers, such as the cochlear nuclei (Cant & Benson, 2003) and the superior olivary complex (Moore, 2000; Oliver, 2000; Thompson & Schofield, 2000), parcel the incoming stream into parallel pathways, which eventually converge in the midbrain at the inferior colliculus. From here, ascending tectothalamic pathways project to the auditory thalamus (Wenstrup, 2005), i.e. the medial geniculate body, which in turn projects to the auditory cortex (de la Mothe, Blumell, Kajikawa, & Hackett, 2006b; Lee & Winer, 2008a). These higher auditory forebrain structures, the thalamus and cortex, establish the circuits necessary for the subsequent extraction and decoding of afferent acoustic information.
This advanced forebrain processing of auditory information, and of other sensory modalities, canonically has been viewed as relying on the numerous corticocortical pathways among areas (Felleman & Van Essen, 1991; E. M. Rouiller, Simm, Villa, de Ribaupierre, & de Ribaupierre, 1991). That is, auditory information ascending from the periphery to the medial geniculate body is relayed to a primary auditory cortical area, whereupon after intracortical processing, the transformed information is conveyed to higher cortical areas. From here, higher-level calculations are performed and the process repeats in a hierarchical fashion, until at some stage, putative auditory object, source and higher percepts emerge. Although theoretically attractive, such a wholly corticocentric view ignores the contribution of convergent inputs from thalamic and commissural sources (Lee & Sherman, 2012; Lee & Winer, 2011a), brainstem modulatory inputs (Bao, Chan, & Merzenich, 2001; Miasnikov, Chen, & Weinberger, 2008), and feedback and feedforward corticofugal projections (Sherman & Guillery, 2006; Winer & Prieto, 2001), in particular those to the thalamus. Thus, a more complete account of auditory forebrain processing should encapsulate these pathways into a holistic connectional framework.
As a step towards this goal, the patterns of connectivity in the auditory thalamus and cortex are reviewed here with a consideration of the principles organizing the thalamocortical pathways. In particular, we consider two models of thalamocortical connectivity: the core-matrix model proposed by Jones (2001) and the corticothalamocortical model proposed by Sherman and Guillery (2002). In addition, we examine the corticocortical and commissural connections in relation to their potential roles in auditory information processing.
Thalamocortical pathways
All regions of the neocortex, except those pertaining to olfaction, receive ascending inputs that must first travel through the thalamus (Jones, 2007; Sherman & Guillery, 2006). As such, the role of the thalamus in sensory perception generally is construed primarily in relation to its function in the initial transfer of information to the cortex (Felleman & Van Essen, 1991; Olshausen, Anderson, & Van Essen, 1993; E. M. Rouiller et al., 1991). However, this view of the thalamus as only a relay has evolved into a more multifaceted view, including roles in mediating intracortical communication and in synchronizing widespread cortical activity (Jones, 2003; Lee & Sherman, 2008, 2009; Sherman & Guillery, 2006).
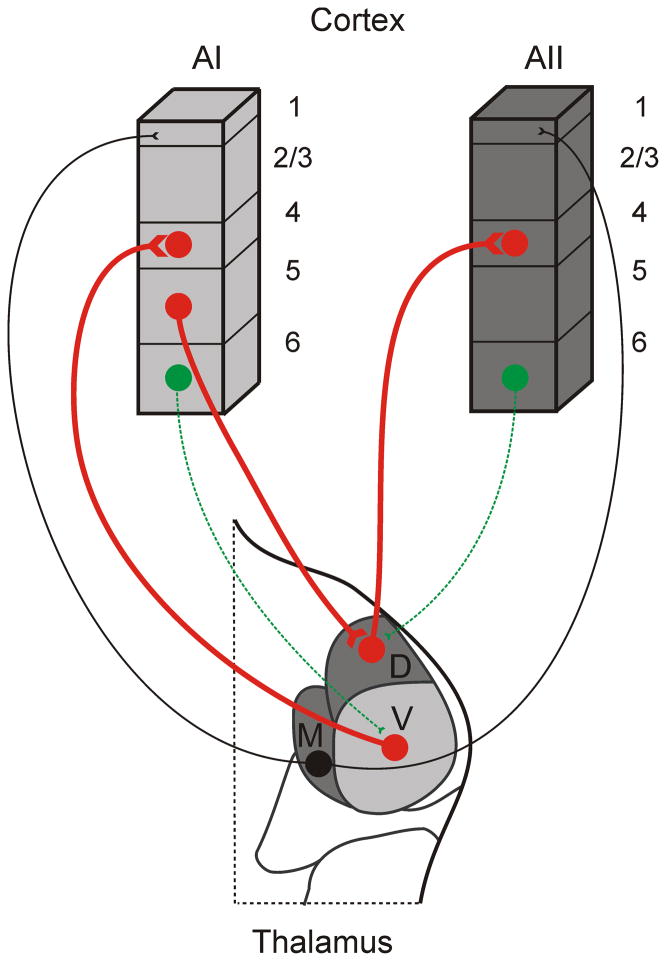
The medial geniculate body (MGB) is the principle nucleus receiving ascending auditory information and is subdivided into three main divisions: the ventral, dorsal, and medial divisions (Fig. 1) (Calford, 1983; Winer, 1984a). The ventral division is the principal nucleus receiving tonotopic inputs from the central nucleus of inferior colliculus (IC) and is further subdivided into the ventral nucleus (MGBv), the medial portion forming the pars ovoidea (Ov), and the rostral pole nucleus (RP); these project to primary auditory cortical areas (de la Mothe et al., 2006b; Morel & Imig, 1987; Winer, 1984a). The dorsal division (MGBd) is composed of several non-tonotopically organized nuclei, which receive inputs from the dorsal cortex of IC (Calford & Aitkin, 1983) and send outputs primarily to secondary non-tonotopic auditory areas (Lee & Winer, 2008a). Finally, the medial division receives non-tonotopic and polymodal inputs from the lateral cortex of the IC (E. M. Rouiller et al., 1989) and sends outputs to all areas of auditory cortex (Lee & Winer, 2008a; Molinari et al., 1995), terminating in non-classical input layers (Fig. 1: black) (Hashikawa, Rausell, Molinari, & Jones, 1991; Huang & Winer, 2000), and to the amygdala (LeDoux, Farb, & Romanski, 1991). Despite the lack of a functional topography in the non-tonotopic thalamic nuclei, each still exhibits highly topographic patterns of thalamocortical connectivity, suggesting that either a metric besides tonotopy organizes these pathways or a general ontogenetic mechanism guides the formation of all auditory thalamocortical connections (J. H. Kaas, 1997; Lee & Winer, 2005; Schreiner & Winer, 2007).
Figure 1.
Thalamocortical connections mediating higher order auditory processing. Primary auditory cortex (AI) receives ascending information from the ventral division of the MGB (V) ending in layer 4, while the secondary auditory cortex (AII) receives information from the dorsal division of the MGB (D) (red thick lines). Feedforward corticothalamic projections from layer 5 (red thick lines) of AI establish a corticothalamocortical route for information transfer between AI and AII. In contrast, feedback corticothalamic projections from layer 6 of AI and AII are not primary conduits of information flow, but rather modulate thalamic activity in MGBv and MGBd, respectively (green dashed lines). Calbindin-expressing neurons in the medial division of the MGB (M) project widely to multiple cortical areas (black thin lines), targeting apical dendrites in layer 1 primarily. These projections have the potential to synchronize activity across broad cortical territories. Light grey shading (tonotopic nuclei and areas), dark grey shading (non-tonotopic nuclei and areas).
Auditory thalamic neurons exhibit a wide range of physiological properties observed at lower stations in the ascending auditory pathway, but vary in their prevalence and distribution. As noted above, frequency tuning differs among the MGB nuclei, with ventral and rostral pole neurons exhibiting the sharpest tuning (Imig & Morel, 1985a; Miller, Escabi, Read, & Schreiner, 2002), while dorsal and medial division neurons have more complex, multipeaked, and sometimes polymodal receptive fields (Imig & Morel, 1985b; Morel, Garraghty, & Kaas, 1993). Intensity coding in the MGB is varied, with a quarter of neurons exhibiting monotonic responses across a range of 60–80 dB and the remaining displaying non-monotonic responses that taper at higher intensities (E. Rouiller, de Ribaupierre, Morel, & de Ribaupierre, 1983). Temporal coding of auditory information in the ventral division is better than in other nuclei, although phase-locked responses, which are robust at the level of the cochlear nucleus, are limited in the MGB (Lennartz & Weinberger, 1992), with only a tenth of neurons exhibiting a maximum following response of 250 Hz (E. Rouiller, de Ribaupierre, Toros-Morel, & de Ribaupierre, 1981). Finally, the binaural responses established in the brainstem are manifested in the ventral nucleus, with about half of the neurons exhibiting binaural excitatory responses (EE), and the remaining neurons split roughly evenly among monaural (EO) and excitatory-inhibitory (EI) responses (Calford, 1983; Cetas et al., 2002). The coding of an auditory space map is uncertain in the MGB, although some neurons are responsive to interaural time (ITD) and intensity (IID) differences (Ivarsson, De Ribaupierre, & De Ribaupierre, 1988).
Neurons in the MGB and in other sensory thalamic nuclei exhibit two distinct firing modes, termed the tonic and burst modes (Deschênes, Roy, & Steriade, 1982; Jahnsen & Llinas, 1984; Ramcharan, Gnadt, & Sherman, 2000; Sherman, 2001). The transition to the burst mode occurs during prolonged periods of hyperpolarization as T-type calcium channels become activated. Stimulation during this hyperpolarized state results in the activation of low-threshold calcium currents, which promote the firing of a rapid burst of action potentials. However, when stimulated from a normal or depolarized state, a regular pattern of action potentials is elicited (Sherman & Guillery, 2002). Generally speaking, the tonic mode is better suited for transferring information to the cortex, while the burst mode signals a state change from quiescence, effectively acting as a ‘wake up’ call to the cortex (Sherman, 2001). Several sources of inhibition can drive auditory thalamic neurons towards a hyperpolarized state, primarily those arising from the inferior colliculus (Peruzzi, Bartlett, Smith, & Oliver, 1997; Winer, Saint Marie, Larue, & Oliver, 1996), the thalamic reticular nucleus (TRN) and local interneurons in humans (Winer & Larue, 1996).
All cortical regions receive a significant fraction (~10%) of their extrinsic input from thalamic sources (Lee & Winer, 2008a, 2011a). And, the termination patterns of both ventral and dorsal divisions of the MGB are anatomically similar (Huang & Winer, 2000; Ryugo & Killackey, 1974), ending in layers 4 and 6 of their cortical targets (see below), but with the synaptic terminations of dorsal division projections to the secondary auditory cortex slightly larger than those of the ventral division projections to the primary auditory cortex (Smith, Uhlrich, Manning, & Banks, 2012). In addition, the synaptic properties of these projections are similar (Cruikshank, Rose, & Metherate, 2002; Lee & Sherman, 2008; Rose & Metherate, 2005). These anatomical and physiological findings suggest that the non-tonotopic thalamocortical projections may serve a similar functional role to that of the tonotopic projections (Guillery & Sherman, 2002; Lee & Sherman, 2011; Sherman & Guillery, 2006). That is, these non-tonotopic thalamocortical connections coupled with the descending feedforward connections from layer 5 of the primary auditory cortex to the dorsal division of the MGB (Bajo et al., 1995; Cappe, Morel, Barone, & Rouiller, 2009; Llano & Sherman, 2008; Ojima, 1994; Winer, Larue, & Huang, 1999) establish a potential alternate route for information flow between cortical areas, via a corticothalamocortical route, as discussed below (Fig. 1: red thick lines).
Two models of thalamocortical processing
The three main subdivisions of the medial geniculate body have unique physiological, anatomical and connectional properties. Classically, these have been subdivided into tonotopic nuclei (ventral and rostral pole divisions), non-tonotopic nuclei (several nuclei in the dorsal division), and multimodal nuclei (medial and suprageniculate nuclei) (Imig & Morel, 1985a, 1985b; Jones, 2007), each of which have unique afferent and efferent connections with midbrain tectal and cerebral cortical structures (Lee & Winer, 2011a; Wenstrup, 2005). Again, the main route for ascending auditory information progresses through the primary lemniscal tonotopic pathway from the central nucleus of the IC to the ventral division of the MGB to the primary auditory cortex (Hackett, 2011; Webster, 1992). After transmission to the cerebral cortex, auditory information is typically depicted as ascending though a series of hierarchical corticocortical connections, e.g. from the primary auditory cortex (AI) to the secondary auditory cortex (AII) (E. M. Rouiller et al., 1991). But, an obvious question arises at this stage. Namely, what are the roles of the non-tonotopic and multimodal thalamocortical projections in auditory processing?
One model, suggested by Sherman and Guillery (2002), is that the higher order thalamic nuclei play an important role in continuing the flow of information between cortical areas. On the basis of several anatomical and physiological criteria (see above and as reviewed in Lee & Sherman, 2011), the non-tonotopic thalamic nuclei are postulated to be ideal conduits for information transfer to higher auditory cortical areas, i.e. from the dorsal division of the MGB to the secondary auditory cortex (AII). However, unlike the ventral division of the MGB, the source of information to the dorsal division does not originate from the inferior colliculus. Instead, layer 5 of AI provides the main information-bearing input. Thus, a potential transthalamic route exists for information transfer between AI and AII via the dorsal division of the MGB. It is important to note here a distinction between the AI feedforward corticothalamic projections originating in layer 5 (Fig. 1: red thick lines) versus the AI feedback corticothalamic projections originating in layer 6 (Fig. 1: green dashed lines) (Llano & Sherman, 2008), which instead target the ventral division and may modulate information ascending through the lemniscal pathway, rather than convey information to the ventral division.
Again, in this model, the non-tonotopic dorsal division of the MGB serves as a key intermediary for communication between the primary and secondary auditory cortical areas, via a corticothalamocortical route (Sherman & Guillery, 2006). It remains unclear how this potential alternate transthalamic route relates and interacts with the direct corticocortical connections between AI and AII (Covic & Sherman, 2011). And, it is important to stress that this speculative role of the non-tonotopic thalamic nuclei is based primarily on anatomical and in vitro physiological findings, which only hint at their potential functional impacts. Their operative roles in vivo could be revealed through inactivation studies, such as those utilizing cooling methods (Carrasco & Lomber, 2009a, 2009b) or an optogenetic approach (Lee, Lam, & Sherman, 2012) to specifically and reversibly silence their activity.
A complementary model of thalamocortical organization proposed by Jones (2001) is based on the observations that MGB nuclei (and other thalamic nuclei) differ in their expression of calcium-binding proteins that correlate with anatomically distinct thalamocortical projection patterns (Hashikawa et al., 1991; Jones, 2003; Lu, Llano, & Sherman, 2009; Molinari et al., 1995). In particular, parvalbumin-expressing neurons are found to concentrate heavily in the ventral division of the MGB and project to the middle layers of a targeted cortical area, e.g. primary auditory cortex. In contrast, calbindin-expressing neurons form a matrix extending throughout the MGB and project in a diffuse manner targeting upper cortical layer 1 (Fig. 1: black lines) (Hashikawa et al., 1991; Jones, 2001; Molinari et al., 1995). These two projection patterns, serve as the basis for Jones’ core-matrix model of thalamic organization, and likely serve different functional purposes, with the ‘core’ parvalbumin neurons transferring information to the auditory cortex in a highly specific and topographic manner, while the ‘matrix’ calbindin neurons project in a widespread fashion across several auditory cortical areas (Jones, 2001). Thus, in this model, the projections of the medial division of the MGB, which exhibit this widespread pattern of thalamocortical projections to upper cortical layer 1 (Huang & Winer, 2000), are theorized to be suited for synchronizing forebrain rhythms to create unified auditory percepts (Hipp, Engel, & Siegel, 2011). However, as with the previous model, the functional roles of these matrix projections are still tentative and await continued refinement through future investigations.
Corticocortical pathways
The bulk of connectivity in the forebrain arises from the numerous corticocortical projections among auditory areas, which comprise nearly 80% of the total extrinsic input to each area (de la Mothe, Blumell, Kajikawa, & Hackett, 2006a; Lee & Winer, 2008b, 2008c). Though the complete boundaries of auditory cortical areas are not fully de ned in humans, it largely appears to reside along the transverse temporal gyrus, Heschl’s gyrus, below the sylvian ssure (Da Costa et al., 2011; Hall, Hart, & Johnsrude, 2003). Primary auditory cortical areas reside inside the sulcal bank, while secondary auditory cortical regions are located along the gyral crest (Warrier et al., 2009; Zatorre, 2007). From homologous studies in non-human primates, based on anatomical connectivity, cytoarchitecture and physiology, auditory areas are grouped into core, belt or parabelt regions (de la Mothe et al., 2006a, 2006b; J.H. Kaas & Hackett, 2000). Those areas within a group, e.g. core areas, share similar connections and physiological properties and are hierarchically arranged, such that core areas receive ascending thalamic information from the ventral division, which is subsequently processed and transferred to the surrounding belt and parabelt regions (Hackett, 2011).
The primary auditory cortical areas, of which there are three in the monkey (Kaas & Hackett, 2000), comprise the core regions, corresponding roughly to Brodmann’s area 41 (Da Costa et al., 2011; Woods et al., 2010), and receive input from the ventral division of the MGB and are tonotopically organized (J.H. Kaas & Hackett, 2000). The secondary belt areas form a ring or ‘belt’ surrounding the core regions. In the monkey, there are at least eight belt areas, which are non-tonotopically organized and receive inputs mainly from the dorsal division nuclei. The lateral belt regions found in the monkey correspond roughly to Brodmann’s area 42 (Hall et al., 2003; Woods et al., 2010). The parabelt areas occupy the lateral edge of the belt areas. These multimodal and association areas, receive inputs from the dorsal nuclei of the MGB as well as multimodal thalamic nuclei, e.g. the medial division, suprageniculate, lateral posterior nuclei, and integrate auditory information with visual, somatosensory and limbic processing streams (de la Mothe et al., 2006a, 2006b; Hackett, 2011; J.H. Kaas & Hackett, 2000).
Tonotopy is the most identifiable organizing feature of the primary auditory cortical areas (Humphries, Liebenthal, & Binder, 2010), while along an isofrequency domain, various parameters, such as bandwidth and binaurality, are arrayed across the extra dimension (Ehret, 1997; Read, Winer, & Schreiner, 2001). Sensitivity to interaural space cues are found in some neurons, but with no distinct mapping (Brugge, Reale, Jenison, & Schnupp, 2001). Temporal following of auditory cortical neurons is generally weak, up to periodicities of 15–30 Hz, but faster to click-train stimuli (Phillips & Hall, 1990). More complex processing of speech is localized outside of the primary auditory cortical core areas, in regions immediately anterior and posterior to Heschel’s gyrus, in close proximity to Wernicke’s area, mainly lateralized to the left hemisphere (Scott SK, 2000; Zatorre, Evans, Meyer, & Gjedde, 1992).
All auditory cortical areas are organized in layers, which are established early in development, such that the more superficial layers, e.g. layer 1, develop last (Kanold & Luhmann, 2010). Of the six distinct layers in the neocortex, layer 1 is remarkable by the general paucity of neurons, instead consisting of apical dendrites of pyramidal neurons from deeper layers and horizontally oriented axons (Winer & Lee, 2007). As described above, layer 1 receives a thalamic input from matrix thalamic neurons, which may synchronize widespread activity in the cortex (Jones, 2001, 2003), although the complete functions of these projections are not fully understood. Layers 2 and 3 are often regarded together, but with differences in their physiological properties (Linden & Schreiner, 2003). Excitatory pyramidal neurons here connect primarily with ipsilateral and contralateral cortical areas (Winer, 1984b, 1985). Layer 4 is the main thalamic recipient layer, projecting to upper cortical layers 2/3 (Huang & Winer, 2000; Linden & Schreiner, 2003). Layer 5 contains large pyramidal neurons that receive inputs from upper cortical layers as well as other cortical areas and send efferent projections that target subcortical sources, such as the higher order auditory thalamic nuclei and the inferior colliculus (Llano & Sherman, 2008; Ojima, 1994; Winer & Prieto, 2001). Layer 6 contains small pyramidal neurons that send feedback connections to the thalamus (Ojima, 1994; Prieto & Winer, 1999), which are largely modulatory in nature (Llano & Sherman, 2008).
These cortical layers are arranged in ‘columns’, with a diameter of about 0.5 mm (Mountcastle, 1997), and whose physiological properties across layers within a column are similar (Linden & Schreiner, 2003). The systematic variation in functional properties between neighboring columns suggest that the cortical column is a fundamental unit for auditory cortical computations (Linden & Schreiner, 2003; Read et al., 2001). Within a column, information is presumed to ascend from the thalamus to layer 4, which then outputs to upper layers 2/3. These in turn project to ‘higher’ cortical areas and to layer 5, which projects corticofugally to the thalamus and the inferior colliculus, as well as layer 6 and other cortical areas (Wallace & He, 2011).
The laminar origins of corticocortical projections establish an anatomical basis for ordering auditory cortical areas into a hierarchical processing network, and underlies many hierarchical models of the auditory, visual, and somatosensory systems in different species (Felleman & Van Essen, 1991; Hackett, 2011; Lee & Winer, 2011a; E. M. Rouiller et al., 1991). In this anatomical framework, ascending projections originate in superficial layers and terminate in layer 4, while descending projections originate in infragranular layers and terminate in all layers except layer 4. Lateral projections originate in both superficial and infragranular layers that terminate across cortical layers (Felleman & Van Essen, 1991; Rockland & Pandya, 1979). Ascending a hierarchical cortical processing network reveals neurally encoded features that increase in general complexity. Thus, in lower cortical areas, such as the primary auditory cortex, relatively simple receptive fields can be mapped with simple tonal stimuli (Miller et al., 2002), while neurons in higher areas in the belt regions, respond to complex and often multimodal inputs (Clarey & Irvine, 1990; Schreiner & Cynader, 1984), reflecting successive convergence or summation of information from lower regions (Hubel & Wiesel, 1962).
An extension of these hierarchical models is the putative separation of cortical streams for processing auditory identity and source information, termed the ‘what’ and ‘where’ pathways, respectively (Lomber & Malhotra, 2008; Rauschecker & Tian, 2000; Romanski et al., 1999). In this formalism, auditory information reaching the core areas are distributed via neuroanatomical connections coursing along a ventral ‘where’ processing pathway or a dorsal ‘what’ processing pathway. Anatomical studies illustrate separate auditory cortical areas targeting either the dorsolateral prefrontal cortex, involved in the ‘what’ pathway, or the ventrolateral prefrontal cortex, involved in the ‘where’ pathway (Romanski et al., 1999). Physiological studies have examined the selectivity of ‘what’ and ‘where’ regions (Lomber & Malhotra, 2008; Poremba et al., 2003; Tian, Reser, Durham, Kustov, & Rauschecker, 2001). Knocking-out cortical processing in each stream via cooling probes in the cat results in a double dissociation of ‘what’ and ‘where’ responses (Lomber & Malhotra, 2008). In humans, focal activation of distinct cortical areas are elicited in response to either auditory pitch or space tasks (De Santis, Clarke, & Murray, 2007). Thus, the dorsal auditory cortical pathway may be involved in the conscious perception of auditory content information, such as speech and language, while the ventral cortical pathway may be engaged to localize the spatial location of the auditory source (Rauschecker & Tian, 2000).
Despite the theoretical simplicity of these hierarchical models, cortical processing may only be roughly hierarchical. In the visual cortex, for example, the average response latencies of cortical areas show significant overlap, with ‘higher’ cortical areas sometimes responding before ‘lower’ cortical areas (Schmolesky et al., 1998). This is perhaps unsurprising given the massively interconnected nature of cortical areas with each other and with convergent thalamic sources (Lee & Winer, 2011a). Indeed, illustrative of this complexity are the auditory cortical inactivation studies that demonstrate differential physiological effects that are area dependent (Carrasco & Lomber, 2009a, 2009b; Kitzes & Hollrigel, 1996). This merging of connections from multiple sources argues that processing of auditory information is distributed across the forebrain, requiring global interactions among cortical areas and thalamic nuclei (Lee & Winer, 2011b; Winer & Lee, 2007). Consequently, those cortical operations concerned with higher auditory processing, such as language, should likewise be distributed, requiring the binding of multiple cortical networks to create identifiable percepts (Burton & Small, 2006; Leech & Saygin, 2011; Mesgarani, David, Fritz, & Shamma, 2009).
Commissural pathways
The commissural connections between auditory cortical hemispheres contribute ~10% of the convergent input to each auditory area (Lee & Winer, 2008b, 2011a) and are the neural substrates supporting the construction of unitary representations of auditory space (Gazzaniga, 2000). The corpus callosum is the fiber bundle linking the two hemispheres of the auditory cortex, principally the homolateral areas on each side, e.g. AI to AI, while heterolateral connections are less prevalent, e.g. AI to AII (Morel et al., 1993; E. M. Rouiller et al., 1991). This is distinct from vision and somatosensation, which instead receive constrained callosal connections along either the vertical meridian (Abel, O’Brien, & Olavarria, 2000; Hubel & Wiesel, 1967) or proximal body representations (Jones & Powell, 1968; E. M. Rouiller et al., 1994). The auditory interhemispheric projections originate primarily from layer 3, but with some areas utilizing layer 5 (Lee & Winer, 2008b). In the primary auditory cortices of several species, callosal connectivity is patchy (Fitzpatrick & Imig, 1980; Luethke, Krubitzer, & Kaas, 1989; Wallace & Harper, 1997) and correlates with the patchy distribution of binaural EE columns in cats (Imig & Brugge, 1978) and bats (Liu & Suga, 1997). Each hemisphere is responsible for contralateral sound perception, but their interactions seems necessary for unifying sound source with perception (Heffner, 1997; Heffner & Heffner, 1989).
In humans, evidence from split-brain studies suggests a hemispheric specialization for acoustic processing in each hemisphere (Gazzaniga, 2000; Springer & Gazzaniga, 1975), with the left hemisphere specialized for language comprehension and production and perhaps the site of conscious integration, while the right hemisphere is specialized for prosody, emotive content, and simple language (Gazzaniga, 2000). The lateralization of auditory cortical processing for speech and language appears unique to humans, yet it remains unclear how concomitant neuroanatomical specializations in the cortex and thalamus underlie these abilities.
Summary
Multiple interrelated thalamic and cortical pathways all contribute to the neural processing of auditory information. The intricate nature of the thalamocortical, corticocortical and commissural connections demonstrates the global and distributed nature of the computations occurring in the auditory forebrain, which all act concertedly to support the emergence of higher auditory percepts. Although a complete understanding of auditory forebrain processing will require linking these varied anatomical and functional properties, only a complete accounting of all the convergent connections to each cortical area will enable an inclusive model of higher auditory forebrain processing.
Highlights.
Auditory cortical areas receive thalamic inputs augmented by the corticocortical pathways
New models of the thalamocortical pathways expand the potential roles of the thalamus
Convergent interactions enable the global processing of acoustic information
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel PL, O’Brien BJ, Olavarria JF. Organization of callosal linkages in visual area V2 of macaque monkey. J Comp Neurol. 2000;428:278–293. doi: 10.1002/1096-9861(20001211)428:2<278::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Rouiller EM, Welker E, Clarke S, Villa AE, de Ribaupierre Y, et al. Morphology and spatial distribution of corticothalamic terminals originating from the cat auditory cortex. Hear Res. 1995;83:161–174. doi: 10.1016/0378-5955(94)00199-z. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Reale RA, Jenison RL, Schnupp J. Auditory cortical spatial receptive fields. Audiol Neurootol. 2001;6:173–177. doi: 10.1159/000046827. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small SL. Functional neuroanatomy of segmenting speech and nonspeech. Cortex. 2006;42:644–651. doi: 10.1016/s0010-9452(08)70400-3. [DOI] [PubMed] [Google Scholar]
- Calford MB. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983;3:2350–2364. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983;3:2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cappe C, Morel A, Barone P, Rouiller EM. The thalamocortical projection systems in primate: an anatomical support for multisensory and sensorimotor interplay. Cereb Cortex. 2009;19:2025–2037. doi: 10.1093/cercor/bhn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco A, Lomber SG. Differential modulatory influences between primary auditory cortex and the anterior auditory field. J Neurosci. 2009a;29:8350–8362. doi: 10.1523/JNEUROSCI.6001-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco A, Lomber SG. Evidence for hierarchical processing in cat auditory cortex: nonreciprocal influence of primary auditory cortex on the posterior auditory field. J Neurosci. 2009b;29:14323–14333. doi: 10.1523/JNEUROSCI.2905-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetas JS, Price RO, Velenovsky DS, Crowe JJ, Sinex DG, McMullen NT. Cell types and response properties of neurons in the ventral division of the medial geniculate body of the rabbit. J Comp Neurol. 2002;445:78–96. doi: 10.1002/cne.10164. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Irvine DRF. The anterior ectosylvian sulcal auditory field in the cat: I. An electrophysiological study of its relation to surrounding auditory cortical fields. J Comp Neurol. 1990;301:289–303. doi: 10.1002/cne.903010211. [DOI] [PubMed] [Google Scholar]
- Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortical areas in mice. Cerebral Cortex. 2011;21:2425–2441. doi: 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Da Costa S, van der Zwaag W, Marques JP, Frackowiak RS, Clarke S, Saenz M. Human Primary Auditory Cortex Follows the Shape of Heschl’s Gyrus. J Neurosci. 2011;31:14067–14075. doi: 10.1523/JNEUROSCI.2000-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Cortical connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006a;496:27–71. doi: 10.1002/cne.20923. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, Hackett TA. Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol. 2006b;496:72–96. doi: 10.1002/cne.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis L, Clarke S, Murray MM. Automatic and intrinsic auditory “what” and “where” processing in humans revealed by electrical neuroimaging. Cereb Cortex. 2007;17:9–17. doi: 10.1093/cercor/bhj119. [DOI] [PubMed] [Google Scholar]
- Deschênes M, Roy JP, Steriade M. Thalamic bursting mechanism: an inward slow current revealed by membrane hyperpolarization. Brain Res. 1982;239:289–293. doi: 10.1016/0006-8993(82)90854-x. [DOI] [PubMed] [Google Scholar]
- Ehret G. The auditory cortex. J Comp Physiol A. 1997;181:547–557. doi: 10.1007/s003590050139. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KA, Imig TJ. Auditory cortico-cortical connections in the owl monkey. J Comp Neurol. 1980;192:589–610. doi: 10.1002/cne.901920314. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relays and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:1–20. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Hackett TA. Information flow in the auditory cortical network. Hear Res. 2011;27:133–146. doi: 10.1016/j.heares.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Hart HC, Johnsrude IS. Relationships between human auditory cortical structure and function. Audiol Neurootol. 2003;8:1–18. doi: 10.1159/000067894. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Rausell E, Molinari M, Jones EG. Parvalbumin- and calbindin-containing neurons in the monkey medial geniculate complex: differential distribution and cortical layer specific projections. Brain Res. 1991;544:335–341. doi: 10.1016/0006-8993(91)90076-8. [DOI] [PubMed] [Google Scholar]
- Heffner HE. The role of macaque auditory cortex in sound localization. Acta Otolaryngol (Stock) Suppl 1. 1997;532:22–27. doi: 10.3109/00016489709126140. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Unilateral auditory cortex ablation in macaques results in a contralateral hearing loss. J Neurophysiol. 1989;62:789–801. doi: 10.1152/jn.1989.62.3.789. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69:387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427:302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol (Lond) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J Neurophysiol. 1967;30:1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19:947–950. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Humphries C, Liebenthal E, Binder JR. Tonotopic organization of human auditory cortex. Neuroimage. 2010;50:1202–1211. doi: 10.1016/j.neuroimage.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig TJ, Brugge JF. Sources and terminations of callosal axons related to binaural and frequency maps in primary auditory cortex of the cat. J Comp Neurol. 1978;182:637–660. doi: 10.1002/cne.901820406. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in lateral part of posterior group of thalamic nuclei in the cat. J Neurophysiol. 1985a;53:836–851. doi: 10.1152/jn.1985.53.3.836. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol. 1985b;53:309–340. doi: 10.1152/jn.1985.53.1.309. [DOI] [PubMed] [Google Scholar]
- Ivarsson C, De Ribaupierre Y, De Ribaupierre F. Influence of auditory localization cues on neuronal activity in the auditory thalamus of the cat. J Neurophysiol. 1988;59:586–606. doi: 10.1152/jn.1988.59.2.586. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: An in vitro study. J Physiol. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Jones EG. Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci. 2003;999:218–233. doi: 10.1196/annals.1284.033. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Vol. 1. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Jones EG, Powell TP. The commissural connexions of the somatic sensory cortex in the cat. J Anat. 1968;103:433–455. [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Topographic maps are fundamental to sensory processing. Brain Res Bull. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Hollrigel GS. Response properties of units in the posterior auditory field deprived of input from the ipsilateral primary auditory cortex. Hear Res. 1996;100:120–130. doi: 10.1016/0378-5955(96)00103-7. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett. 1991;134:139–144. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- Lee CC, Lam YW, Sherman SM. Intracortical convergence of layer 6 neurons. Neuroreport. 2012;23:736–740. doi: 10.1097/WNR.0b013e328356c1aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical intputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009;19:2281–2289. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. On the classification of pathways in the auditory midbrain, thalamus, and cortex. Hear Res. 2011;276:79–87. doi: 10.1016/j.heares.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Principles governing auditory cortex connections. Cereb Cortex. 2005;15:1804–1814. doi: 10.1093/cercor/bhi057. [DOI] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol. 2008a;507:1879–1900. doi: 10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: II. Commissural system. J Comp Neurol. 2008b;507:1901–1919. doi: 10.1002/cne.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol. 2008c;507:1920–1943. doi: 10.1002/cne.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Convergence of thalamic and cortical pathways in cat auditory cortex. Hear Res. 2011a;274:85–94. doi: 10.1016/j.heares.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. A synthesis of auditory cortical connections: thalamocortical, commissural, and corticocortical systems. In: Winer JA, Schreiner CE, editors. The auditory cortex. New York: Springer; 2011b. pp. 147–170. [Google Scholar]
- Lee CC, Sherman SM. Intrinsic modulators of auditory thalamocortical transmission. Hearing Research. 2012;287:43–50. doi: 10.1016/j.heares.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Saygin AP. Distributed processing and cortical specialization for speech and environmental sounds in human temporal cortex. Brain Lang. 2011;116:83–90. doi: 10.1016/j.bandl.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency selectivity is related to temporal processing in parallel thalamocortical auditory pathways. Brain Res. 1992;583:81–92. doi: 10.1016/s0006-8993(10)80011-3. [DOI] [PubMed] [Google Scholar]
- Linden JF, Schreiner CE. Columnar transformations in auditory cortex? A comparison to visual and somatosensory cortices. Cereb Cortex. 2003;13:83–89. doi: 10.1093/cercor/13.1.83. [DOI] [PubMed] [Google Scholar]
- Liu W, Suga N. Binaural and commissural organization of the primary auditory cortex of the mustached bat. J Comp Physiol A. 1997;181:599–605. doi: 10.1007/s003590050143. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for non-reciprocal organization of the mouse auditory thalamocortical-corticothalamic projections systems. J Comp Neurol. 2008;507:1209–1227. doi: 10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nat Neurosci. 2008;11:609–616. doi: 10.1038/nn.2108. [DOI] [PubMed] [Google Scholar]
- Lu E, Llano DA, Sherman SM. Different distributions of calbindin and calretinin immunostaining across the medial and dorsal divisions of the mouse medial geniculate body. Hear Res. 2009;257:16–23. doi: 10.1016/j.heares.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethke LE, Krubitzer LA, Kaas JH. Connections of primary auditory cortex in the New World monkey, Saguinus. J Comp Neurol. 1989;285:487–513. doi: 10.1002/cne.902850406. [DOI] [PubMed] [Google Scholar]
- Mesgarani N, David SV, Fritz JB, Shamma SA. Influence of context and behavior on stimulus reconstruction from neural activity in primary auditory cortex. J Neurophysiol. 2009;102:3329–3339. doi: 10.1152/jn.91128.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008;90:443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Escabi MA, Read HL, Schreiner CE. Spectrotemporal receptive elds in the lemniscal auditory thalamus and cortex. J Neurophysiol. 2002;87:516–527. doi: 10.1152/jn.00395.2001. [DOI] [PubMed] [Google Scholar]
- Molinari M, Dell’Anna ME, Rausell E, Leggio MG, Hashikawa T, Jones EG. Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. 1995. [DOI] [PubMed] [Google Scholar]
- Moore JK. Organization of the human superior olivary complex. Microsc Res Tech. 2000;15:403–412. doi: 10.1002/1097-0029(20001115)51:4<403::AID-JEMT8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335:437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- Morel A, Imig TJ. Thalamic projections to fields A, AI, P, and VP in the cat auditory cortex. J Comp Neurol. 1987;265:119–144. doi: 10.1002/cne.902650109. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Ascending efferent projections of the superior olivary complex. Microsc Res Tech. 2000;15:355–363. doi: 10.1002/1097-0029(20001115)51:4<355::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Anderson CH, Van Essen DC. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J Neurosci. 1993;16:1180–1192. doi: 10.1523/JNEUROSCI.13-11-04700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP, Hall SE. Response timing constraints on the cortical representation of sound time structure. J Acoust Soc Am. 1990;88:1403–1411. doi: 10.1121/1.399718. [DOI] [PubMed] [Google Scholar]
- Poremba A, Saunders RC, Crane AM, Cook M, Sokoloff L, Mishkin M. Functional mapping of the primate auditory system. Science. 2003;299:568–572. doi: 10.1126/science.1078900. [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Winer JA. Layer VI in cat primary auditory cortex: Golgi study and sublaminar origins of projection neurons. J Comp Neurol. 1999;404:332–358. doi: 10.1002/(sici)1096-9861(19990215)404:3<332::aid-cne5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci. 2000;17:55–62. doi: 10.1017/s0952523800171056. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci U S A. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read HL, Winer JA, Schreiner CE. Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci U S A. 2001;98:8042–8047. doi: 10.1073/pnas.131591898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2005;94:2019–2030. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Rouiller E, de Ribaupierre Y, Morel A, de Ribaupierre F. Intensity functions of single unit responses to tone in the medial geniculate body of cat. Hear Res. 1983;11:235–247. doi: 10.1016/0378-5955(83)90081-3. [DOI] [PubMed] [Google Scholar]
- Rouiller E, de Ribaupierre Y, Toros-Morel A, de Ribaupierre F. Neural coding of repetitive clicks in the medial geniculate body of cat. Hear Res. 1981;5:81–100. doi: 10.1016/0378-5955(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Rodrigues-Dagaeff C, Simm G, De Ribaupierre Y, Villa A, De Ribaupierre F. Functional organization of the medial division of the medial geniculate body of the cat: tonotopic organization, spatial distribution of response properties and cortical connections. Hear Res. 1989;39:127–142. doi: 10.1016/0378-5955(89)90086-5. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Simm GM, Villa AE, de Ribaupierre Y, de Ribaupierre F. Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas. Exp Brain Res. 1991;86:483–505. doi: 10.1007/BF00230523. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Killackey HP. Differential telencephalic projections of the medial and ventral divisions of the medial geniculate body of the rat. Brain Res. 1974;82:173–177. doi: 10.1016/0006-8993(74)90903-2. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, et al. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Cynader MS. Basic functional organization of second auditory cortical field (AII) of the cat. J Neurophysiol. 1984;51:1284–1305. doi: 10.1152/jn.1984.51.6.1284. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Winer JA. Auditory cortex mapmaking: principles, projections, and plasticity. Neuron. 2007;56:356–365. doi: 10.1016/j.neuron.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SKBC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Phil Trans R Soc Lond B. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2. London: MIT Press; 2006. [Google Scholar]
- Smith PH, Uhlrich DJ, Manning KA, Banks MI. Thalamocortical projections to rat auditory cortex from the ventral and dorsal divisions of the medial geniculate nucleus. J Comp Neurol. 2012;520:34–51. doi: 10.1002/cne.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SP, Gazzaniga MS. Dichotic testing of partial and complete split brain subjects. Neuropsychologia. 1975;13:341–346. doi: 10.1016/0028-3932(75)90011-1. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Schofield BR. Afferent projections of the superior olivary complex. Microsc Res Tech. 2000;51:330–354. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Neurosci. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Harper MS. Callosal connections of the ferret primary auditory cortex. Exp Brain Res. 1997;116:367–374. doi: 10.1007/pl00005764. [DOI] [PubMed] [Google Scholar]
- Wallace MN, He J. Intrinsic connections of the auditory cortex. In: Winer JA, Schreiner CE, editors. The auditory cortex. New York: Springer; 2011. [Google Scholar]
- Warrier C, Wong P, Penhune V, Zatorre R, Parrish T, Abrams D, et al. Relating structure to function: Heschl’s gyrus and acoustic processing. J Neurosci. 2009;29:61–69. doi: 10.1523/JNEUROSCI.3489-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB. An overview of mammalian auditory pathways with an emphasis on humans. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: neuroanatomy. New York: Springer; 1992. pp. 1–22. [Google Scholar]
- Wenstrup JJ. The tectothalamic system. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 200–230. [Google Scholar]
- Winer JA. The human medial geniculate body. Hear Res. 1984a;15:225–247. doi: 10.1016/0378-5955(84)90031-5. [DOI] [PubMed] [Google Scholar]
- Winer JA. The pyramidal cells in layer III of cat primary auditory cortex. J Comp Neurol. 1984b;229:476–496. doi: 10.1002/cne.902290404. [DOI] [PubMed] [Google Scholar]
- Winer JA. Structure of layer II in cat primary auditory cortex (AI) J Comp Neurol. 1985;238:10–37. doi: 10.1002/cne.902380103. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT. Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc Natl Acad Sci U S A. 1996;93:3083–3087. doi: 10.1073/pnas.93.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Huang CL. Two systems of giant axons terminals in the cat medial geniculate body: convergence of cortical and GABAergic inputs. J Comp Neurol. 1999;413:181–197. [PubMed] [Google Scholar]
- Winer JA, Lee CC. The distributed auditory cortex. Hear Res. 2007;229:3–13. doi: 10.1016/j.heares.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Prieto JJ. Layer V in cat primary auditory cortex (AI): cellular architecture and identification of projection neurons. J Comp Neurol. 2001;434:379–412. doi: 10.1002/cne.1183. [DOI] [PubMed] [Google Scholar]
- Winer JA, Saint Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc Natl Acad Sci U S A. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DL, Herron TJ, Cate AD, Yund EW, Stecker GC, Rinne T, et al. Functional properties of human auditory cortical fields. Front Syst Neurosci. 2010;4:155. doi: 10.3389/fnsys.2010.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ. There’s more to auditory cortex than meets the ear. Hear Res. 2007;229:24–30. doi: 10.1016/j.heares.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]