Abstract
Remodeling of adipose tissue is required to support the expansion of adipose mass. In obesity, an increased death of adipocytes contributes to the accelerated cellular turnover. We have shown that obesity in pregnancy is associated with metabolic and immune alterations in the adipose tissue. In this study we characterized the mechanisms responsible for increased death of adipose cells of pregnant obese women and its functional consequences. We postulated that a higher turnover of dead cells in white adipose tissue of obese women would translate into release of cell free DNA (cfDNA) into their systemic circulation.
Increase in adipose mass of obese compared to lean women results from a lesser number of hypertrophic adipocytes and an accumulation of macrophages in the stromal vascular fraction (SVF). The adipocytes of obese displayed enhanced necrosis with a loss of perilipin staining at the plasma membrane. Apoptosis was prominent in SVF cells with an increased expression of caspase 9 and caspase 3 and a higher rate of TUNEL positive CD68 macrophages in obese vs lean. Whereas circulating fetal cfDNA concentrations were not changed, there was a 2-fold increase in circulating GAPDH cfDNA and adipose tissue GAPDH mRNA in obese women. The maternal systemic GAPDH cfDNA was positively correlated with BMI and gestational weight gain. These data suggest that the active remodeling of adipose tissue of obese pregnant women results in an increased release of cfDNA of maternal origin into the circulation.
Keywords: obesity, adipose tissue, macrophages, apoptosis, necrosis, cell-free DNA, pregnancy
INTRODUCTION
Expansion of the adipose tissue mass involves complex integrated changes in cell structure, endocrine and immune function (1). Increasing evidence suggests that crosstalks between adipose and immune cells play a central role in maintaining adipose tissue homeostasis (2). Obesity-induced remodeling is characterized by the infiltration of macrophages and T cells within the expanded adipose tissue and subsequent recruitment of endogenous immune pathways (3). Subsequent to macrophage activation, a variety of chemokines and cytokines are released both locally and systemically creating a chronic inflammatory environment (4, 5). It has been suggested that adipose tissue macrophages regulate the turnover of adipose tissue by phagocytosis and clearance of dead cell debris (6, 7). Hence, the death of adipose cells may precede or even trigger the macrophage infiltration and activation (8).
The links between turnover of adipocytes, cell death and inflammation are being extensively studied. However, it is still not clear if the clustered macrophages, described as crown like structures surrounding dead adipocytes derive from apoptotic or necrotic activation (6, 9). Additionally, the clearing mechanisms of the cell debris, whether they are all phagocyted by macrophages and/or partly exported outside the tissue, have yet to be described. Likewise, the fate of the activated adipose tissue macrophages is not known.
Recently, cell free DNA has gained attention as a potential biomarker for monitoring of tumor and pathological tissue growth undergoing accelerated apoptosis. Although low levels of cell-free DNA (cfDNA) are detected in the plasma of healthy individuals (10), increased levels have been observed in medical conditions associated with tumor growth (11). Increased release of cfDNA may originate from necrotic and/or apoptotic cell death from rapidly growing or expanding tissues (12,13). Similarities between excessive growth of adipose tissue and that of tumors favors the hypothesis that cfDNA may be generated by remodeling adipose tissue of obese individuals. In support of this concept is the observation that levels of total cfDNA correlate positively with body mass index in normal human pregnancy (14).
We have previously shown that adipose tissue of pregnant women with pre-gravid obesity displays metabolic dysfunction and inflammation associated with macrophage accumulation (5). We postulate that metabolic and immune dysfunction translates into a higher death rate of both adipocytes and macrophages. Therefore, we have investigated the apoptotic characteristics of adipose cells and quantified plasma cfDNA as a potential marker of adipose tissue turnover in pregnancy complicated by obesity.
METHODS AND PROCEDURES
Human subjects
The study was approved by the Institutional Review Board of Metrohealth Medical Center, Case Western Reserve University. Written informed consent was obtained prior to obtaining blood and adipose tissue. Sixteen obese (pre-gravid BMI>30) and 14 lean (pre-gravid BMI<25) women with male fetuses were recruited at term (38–40 weeks) prior to a scheduled cesarean delivery. Women with a multiple gestation, fetal anomalies, IUGR, preeclampsia and placenta previa were excluded. Maternal pre-gravid body mass index (BMI) and metabolic characteristics were obtained from medical records. Maternal peripheral venous blood was collected prior to placement of an intravenous line for hydration, drawn on EDTA and plasma was kept frozen at −20°C. At delivery neonatal male gender was confirmed and anthropometrics were measured (15, Table 1).
Table 1.
Anthropometric parameters of offspring of study subjects at birth (means ± SD)
| Gestational age (weeks) |
Placental weight (g) |
Birth weight (g) |
Neonatal Fat mass (g) |
Neonatal ponderal index |
|
|---|---|---|---|---|---|
| Lean (14) | 38.7±0.6 | 550±128 | 2948±194 | 268±54 | 2.58±0.15 |
| Obese (16) | 38.9±0.7 | 833±212 | 3885±495 | 672±156 | 3.14±0.34 |
| p-value | 0.47 | 0.0004 | 0.0001 | 0.0001 | 0.0001 |
Adipose cell isolation
At the time of incision, 3–5 g of abdominal subcutaneous adipose tissue was obtained and either flash frozen in liquid nitrogen or immediately processed for cell isolation. Adipocytes were isolated by digestion of adipose tissue with 1mg/ml collagenase (Worthington Biochemical, Lakewood, NJ) in Hanks buffered solution for 60 min at 37°C. Freshly isolated adipocytes were counted and their diameter measured using a reticule for microscope Olympus BH-2 (Olympus, Japan). Cells from the stromal vascular fraction (SVF) were pelleted by centrifugation 20 min at 1500 g. The SVF pellet was re-suspended in erythrocyte lysis buffer, centrifuged, re-suspended in RPMI medium and counted. One aliquot of SVF cells was immediately plated at a density of approximate 1.5 − 1.8 × 106 cells/well in 12 well culture plates (precoated with 1% gelatin) containing complete RPMI medium. The remaining of the pellet was subjected to immunoselection with CD14 coated beads and counting of both positive and negative CD14 cells.
Histomorphometry and Immunohistochemistry
Formalin fixed paraffin embedded adipose tissue sections were deparaffinized with xylene, rehydrated in alcohol, boiled for 8 min in 1mM Tris-EDTA with 0.01% Tween and blocked in PBS containing 4% goat normal serum and 2% BSA for 120 min. For immunostaining, the sections were incubated simultaneously with mouse CD68 antibody (1:100 dilution; Abcam, Cambridge, MA) and rabbit perilipin (1:100 dilution, Abcam, Cambridge, MA) overnight at 4°C. Bound antibodies were detected with an Alexa 647-coupled goat anti-mouse antibody for CD68 (1:2500 dilution) and an Alexa 555-coupled goat anti-rabbit in case of perilipin (1:2500 dilution) (Invitrogen, Carlsbad, CA). The tissue sections were then incubated with TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling) reagents for an additional 60 min at 37°C using In Situ Cell Death Detection kit fluorescein (Roche Applied Science). For control, the primary antibodies were omitted and the rest of procedure remained unchanged. Sections were mounted using Vectashield hardset (Vector Laboratories, Inc., Burlingame, CA, USA). 4,6 diamidino-2-phenylindoledihydrochloride (DAPI) was used for nucleus DNA staining. Immunostaining was visualized using a high resolution fluorescent light microscope Leica DM6000 and images were obtained with Leica Volocity software (Leica Microsystems Nederland BV, The Netherlands).
Apoptosis detection assay
Apoptotic cell death was evaluated in cultured stromal vascular cells using In Situ Cell Death Detection kit, fluorescein (Roche Applied Science) for TUNEL detection. Plated stromal vascular cells fixed with 4% paraformaldehyde for 60 min at room temperature. The presence of fluorescein as a cell death marker was visualized using a Leica DM6000 microscope and images were obtained with Leica Volocity software. The number of cells that were TUNEL-positive was calculated and averaged across 5 independent fields from 4– 6 independent cell experiments. The percentage of apoptotic stromal vascular cells was calculated as TUNEL-positive cells/total cells × 100.
Lactate dehydrogenase (LDH) measurement
Isolated adipocytes (3 to 4 × 106 cells/tube) were incubated for 24 hrs in RPMI medium containing 1% penicillin/streptomycin and the supernatant was collected by centrifugation at 500 rpm/5 min). Stromal vascular cells were plated (1.5 to 2 × 106 cells/well) in RPMI medium containing 1% penicillin/streptomycin and the supernatant was collected after 24h culture. Supernatants were assayed for lactate dehydrogenase using Cytotoxicity Detection kit (Roche Applied Science) using an ELISA reader (Gen5 Epoch, Biotek, Vermont, USA). The concentration of LDH present in the supernatants was normalized to the number of cells used and by using an LDH standard curve.
Gene expression analysis
Total RNA was isolated from intact adipose tissue and adipose stromal cells using Trizol reagent (Invitrogen, Carlsbad, CA). Gene expression was monitored by real-time PCR using a Roche thermal cycler (Roche Applied Science, Indianapolis, IN) with Lightcycler Fast-start DNA Sybr Green 1 master mix and primers from Integrated DNA Technologies (Coralville, IA). Specific primers were designed within the 3’ coding region of the genes. IL-6 (NM_000600) forward: 5’-tacccccaggagaagattcc-3’ reverse: 5’-ttttctgccagtgcctcttt-3’; caspase 3 (NM_004346) forward: 5’-tttttcagaggggatcgttg-3’ reverse: 5’-cggcctccactggtatttta-3’; caspase 9 (NM_001229) forward: 5’-ctagtttgcccacacccagt-3’; reverse: 5’-gcattagcgaccctaagcag-3’; β-actin (NM_001101) forward: 5’-ggacttcgagcaagagatgg-3’ reverse: 5’-agcactgtgttggcgtacag3’; GAPDH (NM_002046) forward: 5'-gagtcaacggatttggtcgt; reverse: 3'- ttgattttggagggatctcg; L19 (NM_014763) forward: 5' -agaccccagtgagaccaatg, reverse: 3'-gctgtacccttctgctcacc). Quantitation of relative gene expression normalized for beta-actin was performed by comparative CT method and expressed as fold difference between groups.
cfDNA analysis
DNA was extracted from 400 uL of plasma using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) and eluted in 50 PL of buffer according to the manufacturer’s blood and body fluid protocol. Real time quantitative PCR amplification was performed as previously described (16, 17) to amplify glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the Y chromosome sequence DYS14 as markers of total and fetal DNA, respectively. Analysis was blinded, and a female investigator processed all samples so that there was no risk of contaminating samples with male DNA. The maternal plasma volume from both obese and lean subjects was adjusted for blood volume as previously described (18), as prior experiments in our laboratory suggested an increased blood volume as a function of weight (17).
Statistical analysis
Analysis were performed on blood and adipose tissue of 16 obese and 14 lean women from a metabolically characterized cohort (5). cfDNA measurements were performed in blood samples of 16 obese and 14 lean women. The number of samples analyzed varied among adipose tissue experiments depending on the total amount of tissue obtained at the biopsy site. Morphology studies were done on biopsies from 15 obese and 10 lean women. SVF cells used in molecular ans cellular studies were from 15 obese and 10 lean women with half the samples analyzed for gene expression and the other half analyzed for TUNEL. Differences between dependent variables were examined with one-way or two-way repeated measures analysis of variance (ANOVA). Statistically significant mean differences were identified with a Fisher’s PLSD post-hoc test. Data were analyzed using the Statview II statistical package (Abacus Concepts, Berkeley, CA). Significance was set at p<0.05.
RESULTS
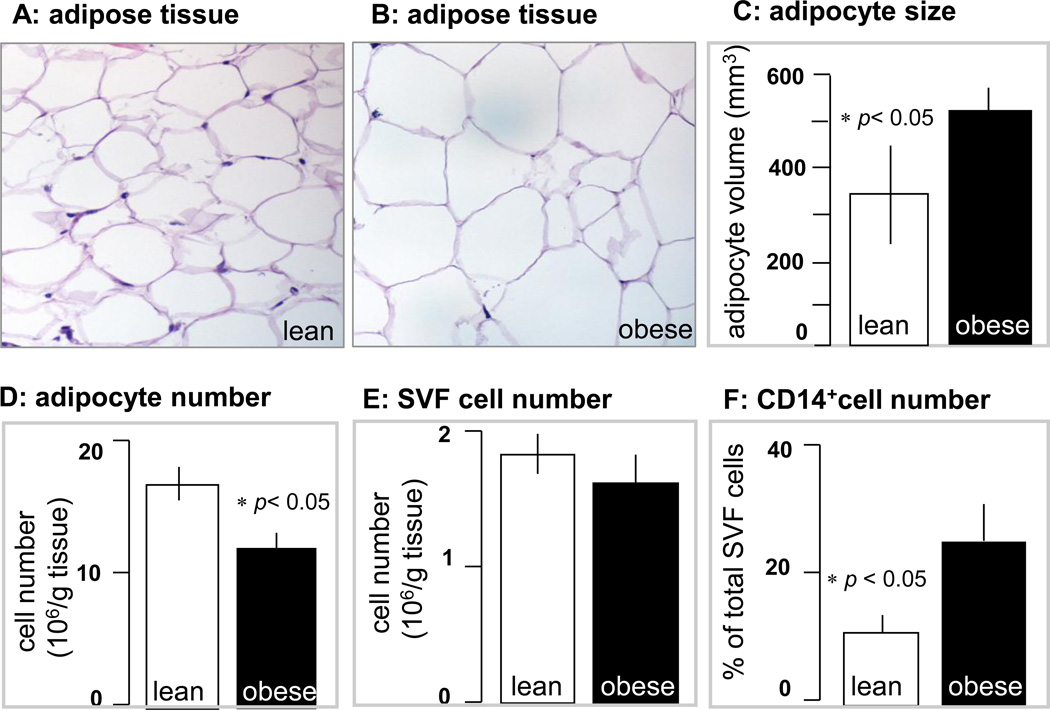
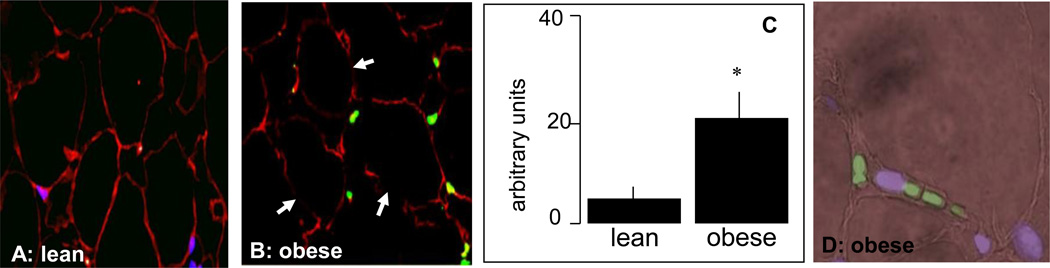
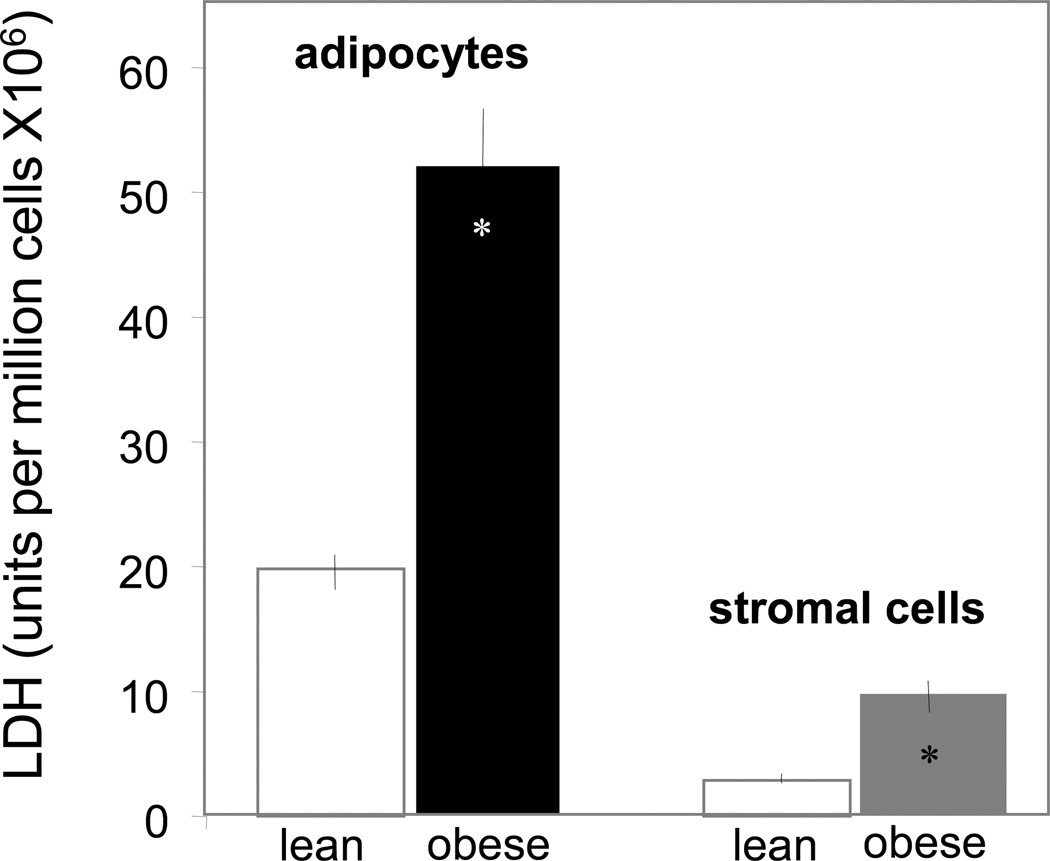
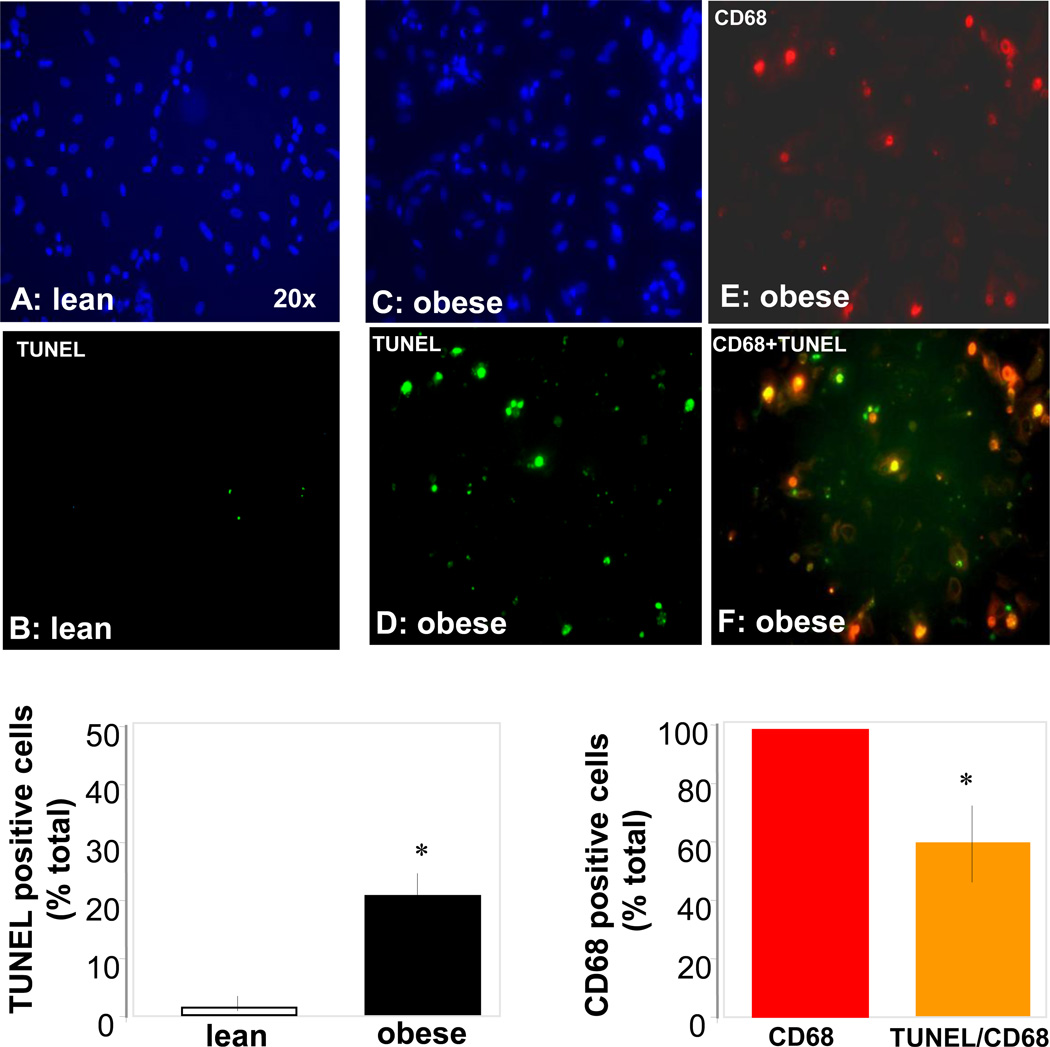
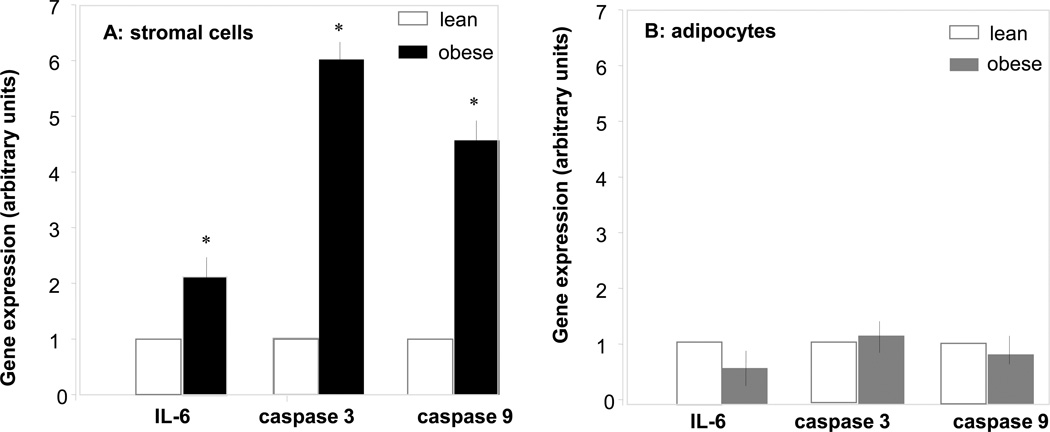
The increased body weight gain in obese pregnant women was associated with hyperleptinemia, hyperinsulinemia and increased plasma IL-6 concentrations, characteristic features of metabolic inflammation (Table 2). The larger adipocyte size (Figure 1A–C) reflected hypertrophy of adipose tissue in obese compared to lean women. Adipose tissue hypertrophy was associated with a lower number of adipocytes but no difference in the total number of cells in the stromal vascular fraction (SVF) (Figure 1 E). However the SVF of obese women was enriched in CD14 positive cells, staining activated macrophages (Figure 1F). The morphological changes were associated with an increased number of necrotic and apoptotic cells in the adipose tissue of obese compared to lean. Enhanced necrosis was documented by decreased perilipin staining of adipocytes, a sign of lesser cell membrane integrity (Figure 2) as well as a 2–3 fold higher release of LDH by adipocytes and stromal cells (Figure 3). Increased apoptosis was indicated by a 5-fold increased in TUNEL immunoreactivity in nuclei surrounding the adipocytes of obese vs lean (Figure 2). TUNEL staining was also increased in SVF cells (Figure 4 A–E). Co-staining of TUNEL with CD68 cells indicated that stromal macrophages represented about 60% of the apoptotic cells (Figure 4 F–H). The higher expression of the caspase 3 and 9 genes (4–6-fold in obese vs lean) confirmed increased apoptosis in the SVF (Figure 5A). In contrast with the SVF cells, neither caspase 3, caspase 9 nor IL-6 expression were modified in the adipocytes of obese (Figure 5B) indicating that apoptosis and inflammation was a primary feature of SVF.
Table 2.
Metabolic characteristics of study subjects (means ± SD)
| Age (years) |
Pre-gravid BMI (kg/m2) |
BMI at delivery (kg/m2) |
Gestational weight gain (kg) |
leptin (ng/ml) |
IL-6 (ng/ml) |
insulin (µU/ml) |
Insulin resistance HOMA index |
smoking status yes/no |
|
|---|---|---|---|---|---|---|---|---|---|
| Lean (14) | 29.5±7.1 | 21.8±2.8 | 27.5±3.0 | 14.7±4.4 | 30±16 | 2.5±1.5 | 11.6±3 | 6.2±4.2 | 3/11 |
| Obese (16) | 28.8±5.5 | 39.2±6.7 | 45.7±7.3 | 17.1±6.2 | 67±23 | 4.5±3.0 | 31.5±2 | 2.3±1.1 | 2/14 |
| p-value | 0.76 | 0.0001 | 0.0001 | 0.05 | 0.0001 | 0.002 | 0.0001 | 0.004 | 0.51 |
Figure 1. Morphological changes in adipose tissue in pregnancy with obesity.
Histochemical analysis of subcutaneous adipose tissue sections from a representative lean woman (pre-gravid BMI: 22.4) and one obese woman (pre-gravid BMI: 35.9). The average adipocyte diameter was 84 ±2 µm in the lean and 95± 3 µm in the obese women (A, B). Magnification 40×. Quantification of adipocyte size (C), number of cells (D) and stromal vascular fraction (E) are mean ± SEM of n = 10 adipose tissue sections from lean (open bars) and 15 obese (solid bars) women. The number of macrophages was estimated as the % of CD14+ cells isolated from the fraction of stromal vascular cells. Significance: *p < 0.05 in obese vs lean.
Figure 2. Histochemical analysis of apoptosis of adipose tissue of obese pregnant women.
Adipose tissue sections of lean (A) and obese (B) women were immunostained for characterizing apoptotic nuclei with dUTP-biotin nick end labeling (TUNEL) and the adipocyte cell membrane marker perilipin. Perilipin positive cells are stained in red and apoptotic nuclei in green. White arrows indicate sites of ruptured plasma membrane with loss of perilipin staining. C: Quantification of TUNEL positive cells from 7 lean and 7 obese. D: higher magnification phase contrast of obese section (B) showing green apoptotic nuclei surrounding an adipocyte. A, B; original magnification × 40. D: original magnification × 100. Significance: *p < 0.05 in obese vs lean.
Figure 3. Biochemical analysis of necrosis in adipose cells of obese pregnant women.
Necrosis was estimated by the amount of lactate dehydrogenase released during a 24 h period in the incubation medium of adipocytes and cells of the stromal vascular fraction. Cells isolations were independently performed from adipose tissue of 10 lean and 15 obese pregnant women. Results expressed as mean ± SD.
Figure 4. Histochemical analysis of apoptosis in adipose stromal vascular cells of obese and lean pregnant women.
Isolated stromal vascular cells were stained with dUTP-biotin nick end labeling (TUNEL) to detect apoptotic nuclei and with the macrophage marker CD68. Positive cells for CD68 were stained in red and nuclei of apoptotic cells were stained in green. Overlay of CD68 and TUNEL staining gives a yellow signal. Dapi was used for counterstaining of nuclei, shown in blue. A and B: cells from a representative lean subject (BMI: 21.2). C, D, E: cells from a representative obese subject (BMI: 33.5). E: quantification of TUNEL positive cells in lean vs obese subjects. F: quantification of CD68-TUNEL positive cells expressed as percent of TUNEL positive cells in obese subjects. Data are from 7 independent cell cultures. 7 fields were counted in each cell sample. Original magnification × 40.
Figure 5. Caspase gene expression in adipocytes and stromal vascular cells.
Total RNA was purified from freshly isolated cells of stromal vascular fraction (A) and adipocytes (B) from 7 obese and 7 lean women. Caspase 3, caspase 9 and IL-6 mRNA levels were measured by quantitative RT-PCR analysis. Real-time Ct values were normalized to β-actin. Statistical significance: * p< 0.05; lean: open bars, obese: solid bars.
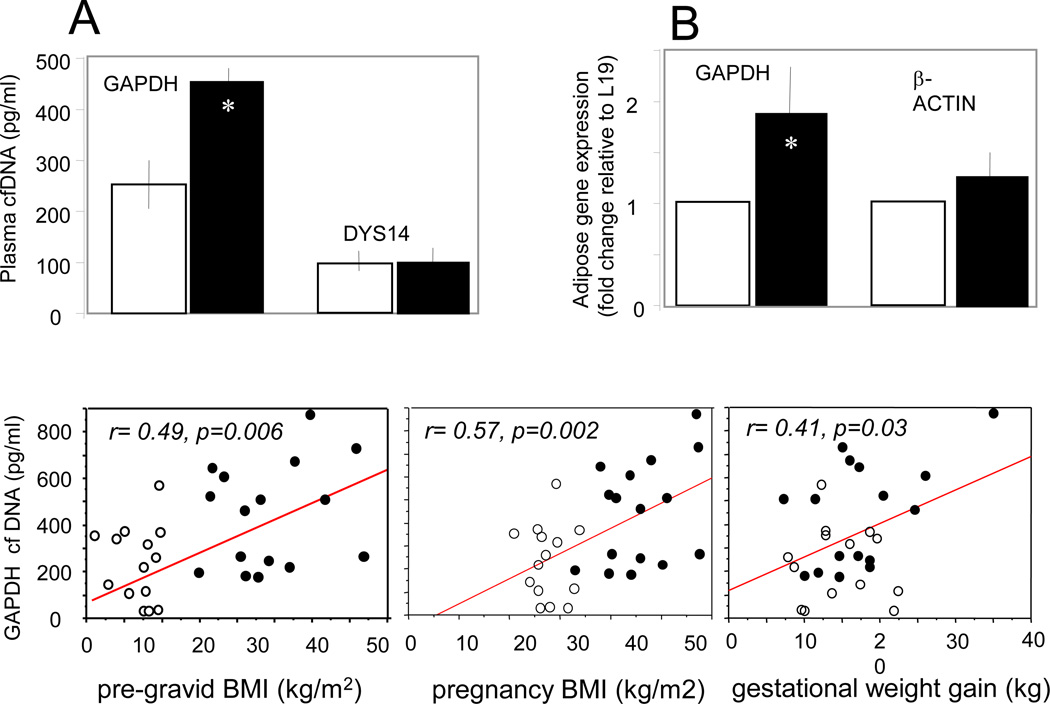
Cell necrosis and apoptosis have been identified as main processes to release free circulating DNA (cfDNA) in plasma. We next quantified cfDNA in plasma of obese vs lean pregnant women using the sequence for the ubiquitous enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). DYS14, a Y-chromosome specific sequence, was used to discriminate between maternal and fetal-placental DNA, since all fetuses were male (14, 19). Circulating GAPDH cfDNA levels were increased by 1.9 fold (p<0.007) in obese vs lean women, whereas there was no difference in levels of DYS14 cfDNA (Figure 6A, upper panel). GAPDH gene expression was increased 1.9 fold in obese vs lean adipose tissue (Figure 6B, upper panel). There was a positive correlation of GAPDH cfDNA with BMI and gestational weight gain, indicating an association between increased fat mass of obese women and total cfDNA released into the maternal circulation (Figure 6, lower panel).
Figure 6. Cell-free DNA concentration in plasma of pregnant women.
Upper panel: GAPDH was used as a marker of total cfDNA (maternal and placental-fetal origin) and DYS14 as a marker of placental-fetal cfDNA (A). GAPDH gene expression in adipose tissue was measured by realtime RT-PCR (B). Black bars represent data from obese women and white bars from lean women. Lower panel: Total cell-free DNA is expressed as copies of glyceraldehyde-3-phosphate (GAPDH) in pg/ml plasma. Values are mean ± SEM after correction for blood volume as detailed in Methods. Filled circles represent data from obese women (n=16) and open circles from lean women (n=14).
DISCUSSION
Adipose tissue mass expansion relies on increasing the amount of stored triglycerides in preexisting adipocytes resulting in cell enlargement (hypertrophy), on generating new small adipocytes (hyperplasia), or on a combination of both mechanisms (20). The primary findings of this study are that circulating levels of cfDNA of maternal origin are increased in obese compared to lean pregnant women. Additionally, adipose tissue of obese undergoes active necrosis and apoptosis compared to lean pregnant women. In addition to the hypertrophy of mature adipocytes, obesity could also have triggered hyperplasia of a preadipocyte subset which have a high turnover rate in obesity (21). This aspect has not been investigated in the current study. Whereas the total number of SVF cells remained constant, an enrichment in macrophages was observed. The morphometric changes in adipose tissue of obese vs lean were coupled with increased IL-6 gene expression in SVF, systemic inflammation and lowered insulin sensitivity (Table 1), two classic traits of metabolic dysfunction in obesity (5, 22, 23).
Contrary to the former dogma that adipose tissue is a quiescent depot, it is now well established that there is a permanent cell turnover in white adipose tissue of adult individuals (24, 25). The enhanced adipose cell death we have observed in obese vs lean indicates an active remodeling of adipose tissue in pregnancy. Necrosis was significantly enhanced in adipocytes and stromal cells of lean vs obese pregnant women. These observations are consistent with enhanced adipocyte death documented in obese rodents and humans (6). Whereas necrosis mostly occurred in the adipocytes, apoptosis was primarily observed in the cells of the SVF with sixty percent of the apoptotic cells being macrophages. Additionally, the remaining apoptotic cells could include vascular cells, a stromal cell type actively remodeling to support adipose angiogenesis (26, 27). A proangiogenic role of adipose tissue macrophages has been characterized in mice (28). It is thus conceivable that crosstalks between macrophages and endothelial cells favor angiogenesis and tissue expansion.
Scavenging of debris and dead cells is a primary function of resident macrophages that is mandatory for cellular maintenance purposes (29). It has been suggested that apoptosis modifies the phenotype of macrophages and helps regulate the turnover of adipose tissue and modifies the phenotype of macrophages (30, 31). In line with their housekeeping role, apoptotic macrophages may serve the need for an increased clearance of dead adipocytes from the adipose tissue of obese pregnant women. We propose that increased apoptosis of stromal macrophages would 1-participate to increase adipocyte turnover and 2- limit excess macrophage accumulation in the SVF. Hence the increased apoptosis of adipose tissue macrophages may serve the goals of maintaining homeostatic balance and limiting locally induced inflammation associated with obesity (5).
Once activated macrophages are engulfed with lipids or cell debris, they need to undergo phagocytosis and be cleared from the tissue. The DNA of apoptotic macrophages can be digested in lysosomes by DNAse 2 prior to egress the cell (32). However, if the rate of cell death is not properly coupled with macrophage digestion and egress capacity, fragmented DNA may be released as circulating DNA (33). Several pathologies such as myocardial infarction, stroke, cancer and preeclamptic pregnancy are associated with elevated cfDNA concentrations in the circulation (34–36). The physiochemical specificities of circulating DNA have led to the suggestion that it originates from internucleosomal cleavage of DNA occurring during the apoptotic process (37). Our study demonstrates that the concentration of total cfDNA but not placental-fetal cfDNA was increased in obese women. Although the placenta represents an important source of nucleic acids in the form of cfDNA (38). our data indicate that circulating cfDNA of obese women originates from maternal rather than feto-placental tissues. The increased tissue mass and GAPDH gene expression points to adipose tissue as a contributor of circulating cfDNA. The positive correlations between GAPDH cfDNA, pre-gestation and late gestation BMI further suggest that obesity is a trigger of adipose cfDNA release. Although it remains to be fully established, adipose released cfDNA would be consistent with the enlargement of maternal adipose mass representing 30–40% of total maternal gestational weight gain (39). An obesity induced release of cfDNA does not preclude that pregnancy specific changes may also generate cfDNA as suggested in non pregnant obese women (14,17).
Nuclear macromolecules such as DNA and dsRNA play an active role in regulating inflammatory mechanisms through recruitment of toll-like innate immune pathways (3) in normal and tumor growth (40, 41). We have previously documented that immune activation of adipose stromal cells contributes to systemic changes and metabolic inflammation in obese pregnant women (5). Taken together, these observations suggest that release of cfDNA into the systemic circulation of obese women may represent additional inflammatory signals associated with metabolic dysfunction.
Acknowledgments
We thank Judi Minium for skillful technical assistance with processing of blood and biological samples. We acknowledge helpful discussion and statistical guidance from Saeid Amini,
This work was supported by grants NIH R01 HD 22965 (PMC/SHM) and NIH T32 HD049341, R01 HD42053-07 (DWB).
Footnotes
Part of the study was presented at the 30th annual Society for Maternal Fetal Medicine meeting, Chicago, IL
Author contributions
Conceived and designed the experiments: SHM. Performed the experiments MH, NV, KJ, SB. Analyzed the data: LP, MH, SHM. Wrote the paper : SHM. Performed critical reading of the manuscript, PMC, DWB.
Contributor Information
Maricela Haghiac, Department of Obstetrics and Gynecology, MetroHealth Medical Center Case Western Reserve University, Cleveland, OH 44109..
Neeta L. Vora, Mother Infant Research Institute at Tufts Medical Center and the Departments of Pediatrics and Obstetrics and Gynecology, Floating Hospital for Children and Tufts Medical Center, Boston, MA 02111.
Subhabrata Basu, Department of Obstetrics and Gynecology, MetroHealth Medical Center Case Western Reserve University, Cleveland, OH 44109..
Kirby L. Johnson, Mother Infant Research Institute at Tufts Medical Center and the Departments of Pediatrics and Obstetrics and Gynecology, Floating Hospital for Children and Tufts Medical Center, Boston, MA 02111.
Larraine Presley, Department of Obstetrics and Gynecology, MetroHealth Medical Center Case Western Reserve University, Cleveland, OH 44109..
Diana W. Bianchi, Mother Infant Research Institute at Tufts Medical Center and the Departments of Pediatrics and Obstetrics and Gynecology, Floating Hospital for Children and Tufts Medical Center, Boston, MA 02111.
Sylvie Hauguel-De Mouzon, Department of Obstetrics and Gynecology, MetroHealth Medical Center Case Western Reserve University, Cleveland, OH 44109..
References
- 1.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum S, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 5.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, Hauguel-de Mouzon S. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity. 2011;19:476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 8.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–3348. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15338. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler C, Barekati Z, Radpour R, Zhong XY. Cell-free DNA in the circulation as a potential cancer biomarker. Anticancer Res. 2011;31:2623–2628. [PubMed] [Google Scholar]
- 11.Chan KC, Lo YM. Circulating tumour-derived nucleic acids in cancer patients: potential applications as tumour markers. Br J Cancer. 2007;96:681–685. doi: 10.1038/sj.bjc.6603625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler A, Angemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev. 2002;28:255–271. doi: 10.1016/s0305-7372(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 13.Kamat AA, Baldwin M, Urbauer D, Dang D, Han LY, Godwin A, Karlan BY, Simpson JL, Gershenson DM, Coleman RL, Bischoff FZ, Sood AK. Plasma Cell-free DNA in Ovarian Cancer: An Independent Prognostic Biomarker. Cancer. 2010;116:1918–1925. doi: 10.1002/cncr.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapaire O, Volgmann T, Grill S, Hösli I, Zanetti-Daellenbach R, Zhong XY, Holzgreve W. Significant correlation between maternal body mass index at delivery and in the second trimester, and second trimester circulating total cell-free DNA levels. Reprod Sci. 2008;16:274–279. doi: 10.1177/1933719108327599. [DOI] [PubMed] [Google Scholar]
- 15.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–1704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KL, Dukes KA, Vidaver J, LeShane ES, Ramirez I, Weber WD, Bischoff FZ, Hahn S, Sharma A, Dang DX, Hire LM, Bianchi DW, Simpson JL, Holzgreve W, Elias S, Klinger KW. Inter-laboratory comparison of fetal male DNA detection from common maternal plasma samples by real-time PCR. Clin Chem. 2004;50:516–521. doi: 10.1373/clinchem.2003.024380. [DOI] [PubMed] [Google Scholar]
- 17.Watanagara T, Peter I, Messerlian GM, Borgatta L, Bianchi DW. Inverse correlation between maternal weight and second trimester circulating cell-free fetal DNA levels. Obstet Gynecol. 2004;104:545–550. doi: 10.1097/01.AOG.0000137352.93110.15. [DOI] [PubMed] [Google Scholar]
- 18.Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16:773–776. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 19.Gorrzelniak K, Janke J, Engeli S, Sharma AM. Validation of endogenous controls for gene expression studies in human adipocytes and preadipocytes. Horm Metab Res. 2001;33:625–627. doi: 10.1055/s-2001-17911. [DOI] [PubMed] [Google Scholar]
- 20.Bjorntorp P, Sjostrom L. Number and size of adipose tissue fat cells in relation to metabolism in human obesity. Metabolism. 1971;20:703–713. doi: 10.1016/0026-0495(71)90084-9. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 22.Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Löfgren P, Rydén M, Thörne A, Wirén M, Palmér M, Thorell A, Toft E, Arner P. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53:2496–2503. doi: 10.1007/s00125-010-1889-3. [DOI] [PubMed] [Google Scholar]
- 24.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 25.Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid cellular turnover in adipose tissue. PLoS One. 2011;6:e17637–e17642. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verseijden F, Jahr H, Posthumus-van Sluijs SJ, Ten Hagen TL, Hovius SE, Seynhaeve AL, van Neck JW, Gerjo JVM, van Osch JV, Hofer S. Angiogenic Capacity of Human Adipose-Derived Stromal Cells During Adipogenic Differentiation: An In Vitro Study. In: Mary Ann Liebert, Inc, editor. Tissue engineering. Part A15. 2009. pp. 445–452. [DOI] [PubMed] [Google Scholar]
- 27.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 28.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab. 2008;295(2):E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas SD, Musson RA. Phagocytic defects-monocytes/macrophages. Clin Immunol Immunopathol. 1986;40:62–68. doi: 10.1016/0090-1229(86)90069-3. [DOI] [PubMed] [Google Scholar]
- 30.Bodles AM, Varma V, Yao-Borengasser A, Phanavanh B, Peterson CA, McGehee RE, Jr, Rasouli N, Wabitsch M, Kern PA. Pioglitazone induces apoptosis of macrophages in human adipose tissue. J Lipid Res. 2006;47:2080–2088. doi: 10.1194/jlr.M600235-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumié A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 32.Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. 2003;10:108–116. doi: 10.1038/sj.cdd.4401161. [DOI] [PubMed] [Google Scholar]
- 33.Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta. 2001;313:139–142. doi: 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 34.Van der varrt M, Pretorius PJ. Circulating DNA its origin and fluctuation. Ann NY Acad. Sci. 2008;1137:18–26. doi: 10.1196/annals.1448.022. [DOI] [PubMed] [Google Scholar]
- 35.Fournie GJ, Martres F, Pourrat JP, Alary C, Rumeau M. Plasma DNA as cell death marker in elderly patients. Gerontology. 1993;39:215–221. doi: 10.1159/000213536. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi DW. Circulating fetal DNA: its origin and diagnostic potential-a review. Placenta. 2004;25(Suppl A):S93–S101. doi: 10.1016/j.placenta.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Kitazumi I, Tsukahara M. Regulation of DNA fragmentation: the role of caspases and phosphorylation. FEBS J. 2011;278:427–441. doi: 10.1111/j.1742-4658.2010.07975.x. [DOI] [PubMed] [Google Scholar]
- 38.Lo YM, Corbetta N, Chamberlain PF, Vik R, DIan LS, Christopher WG, Redman CWG, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 39.Thomson AM, Hytten FE. Body stores in human pregnancy and lactation. Proc Nutr Soc. 1960;19:5–8. doi: 10.1079/pns19600004. [DOI] [PubMed] [Google Scholar]
- 40.Pisetsky DS. The role of nuclear macromolecules in innate immunity. Proc Am Thorac Soc. 2007;4:258–262. doi: 10.1513/pats.200701-027AW. [DOI] [PubMed] [Google Scholar]