Abstract
Very few studies have addressed the phylogenetic diversity of fungi from Northeast India under the Eastern Himalayan range. In the present study, an attempt has been made to study the phylogenetic diversity of culturable soil fungi along the altitudinal gradients of eastern Himalayas. Soil samples from 24 m above sea level to 2,000 m above sea level altitudes of North-East India were collected to investigate soil micro-fungal community structure and diversity. Molecular characterization of the isolates was done by PCR amplification of 18S rDNA using universal primers. Phylogenetic analysis using BLAST revealed variation in the distribution and richness of different fungal biodiversity over a wide range of altitudes. A total of 107 isolates were characterized belonging to the phyla Ascomycota and Zygomycota, corresponding to seven orders (Eurotiales, Hypocreales, Calosphaeriales, Capnodiales, Pleosporales, Mucorales, and Mortierellales) and Incertae sedis. The characterized isolates were analysed for richness, evenness and diversity indices. Fungal diversity had significant correlation with soil physico-chemical parameters and the altitude. Eurotiales and Hypocreales were most diverse and abundant group of fungi along the entire altitudinal stretch. Species of Penicillium (D = 1.44) and Aspergillus (D = 1.288) were found to have highest diversity index followed by Talaromyces (D = 1.26) and Fusarium (D = 1.26). Fungal distribution showed negative correlation with altitude and soil moisture content. Soil temperature, pH, humidity and ambient temperature showed positive correlation with fungal distribution.
Keywords: Altitude, Diversity indices, Microfungal diversity, Richness, 18S rDNA
Introduction
The relationship between biodiversity of soil fungi and ecosystem function is an issue of paramount importance, particularly in the face of global climate change and human alteration of ecosystem processes. Soil micro-flora performs a variety of ecosystem functions that are crucial to maintaining ecosystem stability [1-3]. Fungi are an important component of the soil micro-biota typically constituting more of the soil biomass than bacteria, depending on soil depth and nutrient conditions. The saprobic fungi represent the largest proportion of fungal species in soil and they perform a crucial role in the decomposition of plant structural polymers, such as, cellulose, hemicellulose and lignin, thus contributing to the maintenance of global carbon cycle [4]. Researches on distribution of fungi in soil have focused in agricultural soils and less is known about the occurrence of fungi under natural soil conditions [5, 6]. Fungal flora of the soil is attributed to native soils [7]. Some fungi are widely distributed in soil while others are limited to certain habitats. The distribution of fungi is influenced by the abundance and nature of the organic content of the soil, as well as by other soil and climatic conditions, surface vegetation and soil texture. Soil micro-fungal diversity has important implications in ecosystem stability and productivity. They are a well recognized source for variety of chemicals, several of which are valuable pharmaceuticals, agrochemicals and industrial products [8].
Fungi have historically been identified and classified using morphological characteristics such as sexual structure. However, fungi, especially microscopic ones, often have few useful morphological features and show pronounced morphological variability [9]. Many fungi are anamorphic without sexual production, yet possess a surprisingly high level of genetic variation [10]. Furthermore, an increasing number of morphologically indistinguishable (cryptic) species have recently been described [11]. Accordingly, the use of morphology for fungal identification and classification can be severely biased. In the past decade, the ability to identify species at the molecular level has changed our understanding of the species concept for different groups of fungi. Particularly, molecular phylogenetic approaches avoid subjectivity in determining the limits of a species by relying on the concordance of more than one gene genealogy and have been approved to well suit teleomorphic and anamorphic fungi [12].
Northeast (NE) India is regarded as one of the biodiversity hotspots of the globe [13] and blessed with a wide range of physiography and ecoclimatic conditions. It forms one of the major regions of tropical forests in India, especially the species-rich tropical rain forests [14]. The tropical semi-evergreen and moist deciduous forests in the lowlands of this region extend south and west into the subcontinent, and east into Southern China and Southeast Asia. The subtropical forests of the region follow the foothills of the Himalaya to the west and extend into Southeast China in the east. Himalayan temperate and subalpine zone forests extend from northern Pakistan and adjacent Afghanistan through NE India to Southwest China. Northeast region of India sprawling over 262,379 sq.km, 22~30° N and 89~97° E comprising of the states of Arunachal Pradesh, Assam, Meghalaya, Manipur, Tripura, Mizoram, Nagaland and Sikkim can be physiographically categorized into the Eastern Himalayas, Northeast hills (Patkai-Naga Hills and Lushai Hills) and the Brahmaputra and Barak Valley plains. NE India represents the transition zone between the Indian, Indo-Malayan and Indo-Chinese biogeographic regions and a meeting place of the Himalayan mountains and peninsular India. At the confluence of the Indo-Malayan, Indo-Chinese and Indian biogeographical realms, this region is unique in providing a profusion of habitats, which features diverse biota with a high level of endemism. Highly undulating topography leads to marked variation in edapho-climatic conditions, and has resulted in great variation in altitude, irrespective of distance due to which it is rich in diverse groups of flora and fauna which have been documented but the microbial groups have not attracted any attention as yet. In the present study, an attempt has been made to investigate soil microfungal community structure and phylogenetic diversity from soil from different altitudes of NE India under Eastern Himalayan range and to assess if altitude has any impact on the diversity of fungi in the range.
Materials and Methods
Study area
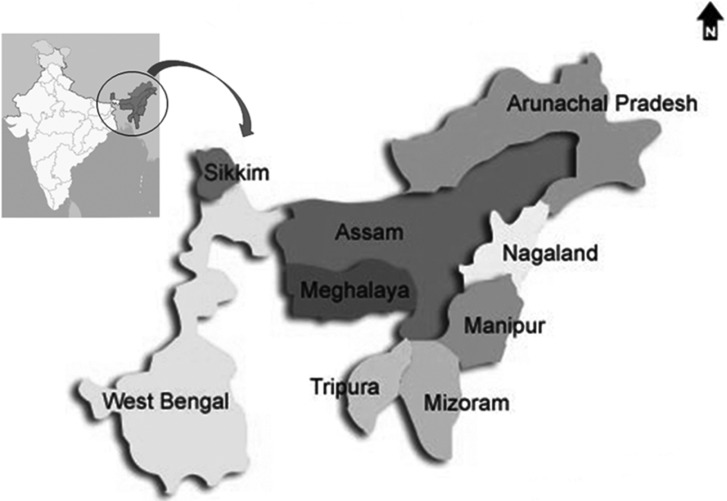
The study area comprises of an area sprawling over 262,379 sq.km in NE India spread from 22~30° N and 89~97° E at altitude from 24 m above sea level to 2,000 m above sea level (Fig. 1).
Fig. 1.
Map showing sampling area under Eastern Himalayan range.
Collection of soil samples
Soil samples were collected from various vegetational and climatic zones spread over different altitudes of NE India under Eastern Himalayan range. Surface and sub-surface soil samples were collected aseptically from different microhabitats. Every sample was a mixture of soils from 5 to 10 holes at a depth from 0 to 30 cm. The soil samples were transported in sterile containers to the laboratory where it was stored at 4℃ until processing.
Physico-chemical characteristics of soil
Different physical parameters of the sampling site were recorded. The ambient temperature and humidity was recorded using a Hygrometer. The altitudes of the sampling sites were determined by using GPS (Garmin 76 CSX, USA). Soil pH was determined using a soil-water mixture (1 : 5, w/v) with a pH meter (Chemiline, India).
Isolation and enumeration of fungi
The colony forming units (CFU) of the fungi were determined by serial dilution method. 10 g of the soil sample was suspended in 90 mL of sterile 0.86% NaCl solution and shaken vigorously on a magnetic stirrer for 30 min to obtain uniform suspension. A serial dilution up to 10-5 was made and 0.1 mL aliquot from each dilution was inoculated on potato dextrose agar (PDA; HiMedia, India) plates supplemented with Streptomycin (20 ppm) and incubated at 25℃ for 48~72 hr. All fungal colonies which appeared on plates were counted. Morphologically different strains were isolated and purified. Purified fungal isolates were maintained on PDA slant (stored at 4℃) and also in sterile distilled water (stored at -80℃).
Characterization of fungal isolates
Fungal isolates were examined and identified to genus level using light microscopy. Classical morphological features of the fungal isolates were used for these preliminary identifications of the fungal isolates. Selected fungal isolates were characterized using molecular approach. Genomic DNA of the isolates was extracted using genomic DNA miniprep purification spin kit (Qiagen, Germany). The universal 18S rRNA gene primers; forward nu-SSU-0817-5, 5'-TTAGCATGGAATAATRRAATA-3' and reverse nu-SSU-1536-3, 5'-ATTGCAATGCYCTATCCCCA-3' [15] were used for amplification of the 18S rRNA gene. The PCR reaction mixture comprised of 10 µL fungal DNA, 5 µL 10 × PCR buffer, 1.5 µL of 50 mM MgCl2, 1 µL of 10 mM dNTP, 0.25 µL Taq polymerase, 40 pM each of the forward and the reverse primers in a total reaction volume of 50 µL [16]. Amplification of DNA was carried out in 9700 Gold thermal cycler (Applied Biosystems, Warrington, UK) under the following conditions: initial denaturation at 94℃ for 2 min; 35 cycles of denaturation at 94℃ for 1 min, annealing at 56℃ for 10 sec, extension at 72℃ for 30 sec, and a final extension at 72℃ for 2 min. Approximately 740 nucleotides were amplified. Amplified PCR products were purified using QIA quick gel extraction kit (Qiagen) and were bi-directionally sequenced (BigDye terminator; Applied Biosystems).
Sequence similarity searches were performed for each of the representative fungal sequences against the non-redundant database maintained by the National Center for Biotechnology Information (NCBI) using the BLAST algorithm (http://www.ncbi.nlm.nih.gov).
Nucleotide sequence accession number
The 18S rDNA nucleotide partial sequences were submitted to GenBank and accessions were obtained.
Statistical analysis
The diversity of the culturable fungal diversity in our study was estimated using the Shannon index (H') [17]. However the Shannon index is not itself a measure of diversity. Conversion of this value to effective number of species, or true Diversity (D), is the key to a unified and intuitive interpretation of diversity [18]. Simpson's index of diversity was also estimated [19]. The reciprocal form of Simpson's index (1/D) usually is presented, ensuring that the index increases with increasing diversity. Evenness (J) of species was evaluated using the formula as given by Pielou [20]. The number of species divided by the square root of the number of individuals results in species richness, S.
The correlation between the different environmental factors, soil physico-chemical parameters and fungal counts was determined by calculating Pearson product moment correlation coefficients [21]. The correlations were considered significant if p < 0.05. Karl Pearson correlation coefficient was done using SPSS ver. 20 (IBM, NY, USA).
Results and Discussion
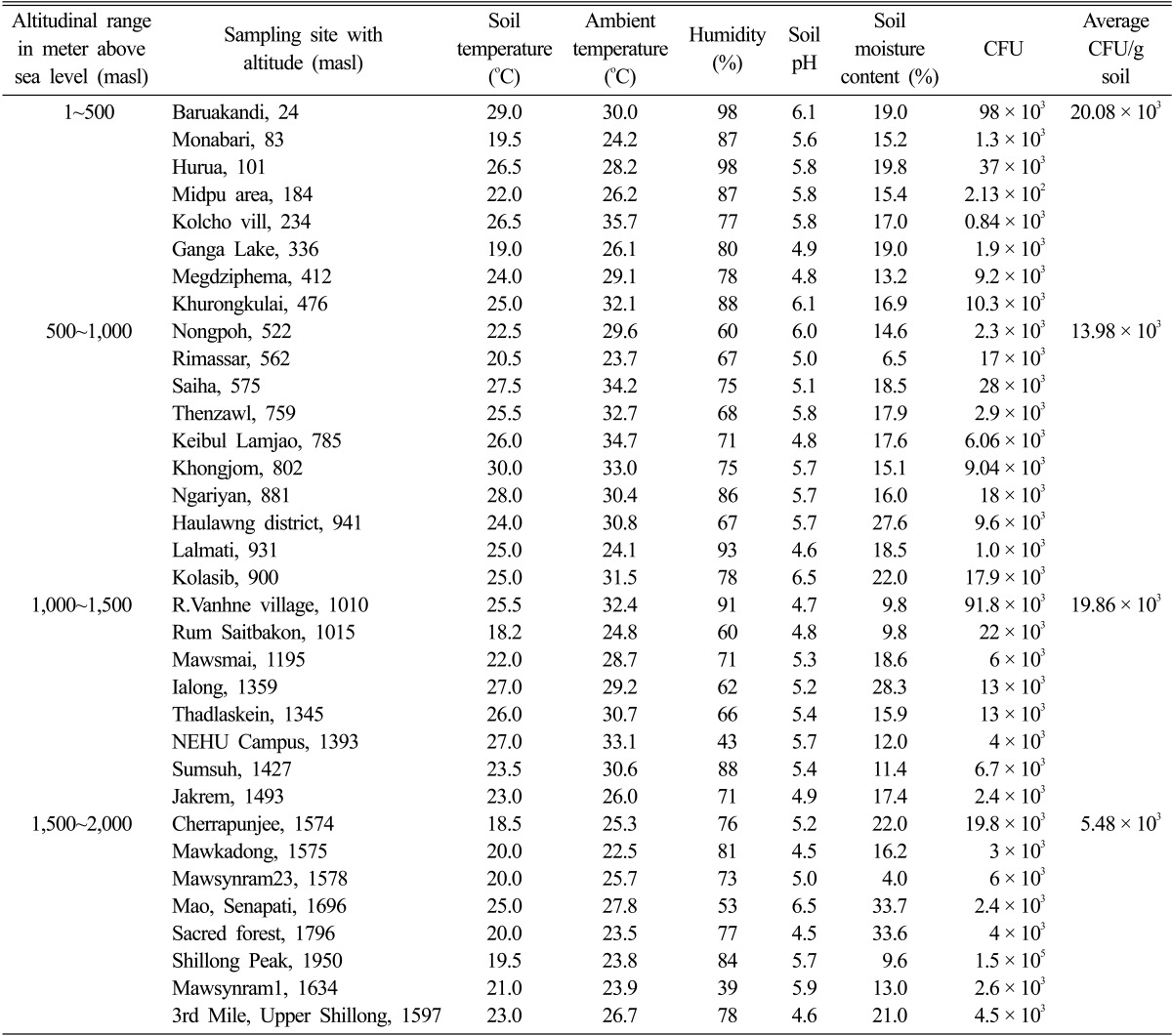
There was significant variation in the soil physico-chemical parameters and fungal CFU counts along the altitudinal gradient (Table 1).
Table 1.
Soil physico-chemical parameters and fungal colony forming unit (CFU) counts along the altitudinal gradient
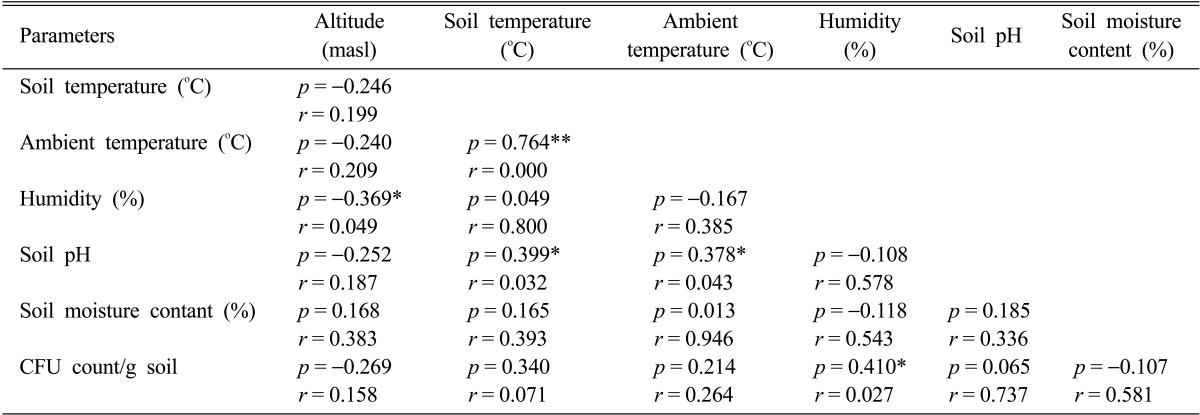
Soil temperature, pH and moisture are some of the major factors affecting the fungal population and diversity [22]. Altitude is also considered important factor acting on fungal diversity. Generally, an increase in altitude is accompanied by a decrease in temperature, affecting negatively fungal diversity. In our study, the range of soil pH along the altitudinal stretch did not show much variation. The results obtained in our study clearly indicate that there is a marked decrease in the number of isolates with increasing altitude which is in agreement with other studies [23, 24]. The CFU counts of fungi decreased with increasing altitude, as highest CFU counts were recorded at lower altitudes and lowest CFU counts were recorded at higher altitudes (Table 1). However, there was a sharp increase in CFU count at 1,000~1,500 altitudinal range which can be attributed to the tropical semi-evergreen to sub-tropical evergreen forest prevalent in the range. Bissett and Parkinson [25] have demonstrated that moisture, soil pH and temperature were the most important abiotic variables influencing the distribution and community composition of alpine soil fungi. Microbial community structure and diversity are sensitive to any small change in their microhabitat especially due to anthropogenic activities [26-28]. Soil physico-chemical factors, ambient temperature and humidity were found to influence soil fungal distribution and population density at various level of significance (p < 0.05, p < 0.01). Fungal distribution showed negative but insignificant correlation with altitude (r = -0.269) and soil moisture content (r = -0.107). Soil parameters like pH (r = 0.065), soil temperature (r = 0.340), ambient temperature (r = 0.214) showed positive but insignificant correlation whereas humidity (r = 0.410) showed positive significant correlation with the fungal distribution (Table 2).
Table 2.
Pearson correlation coefficients analysis of different parameters
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
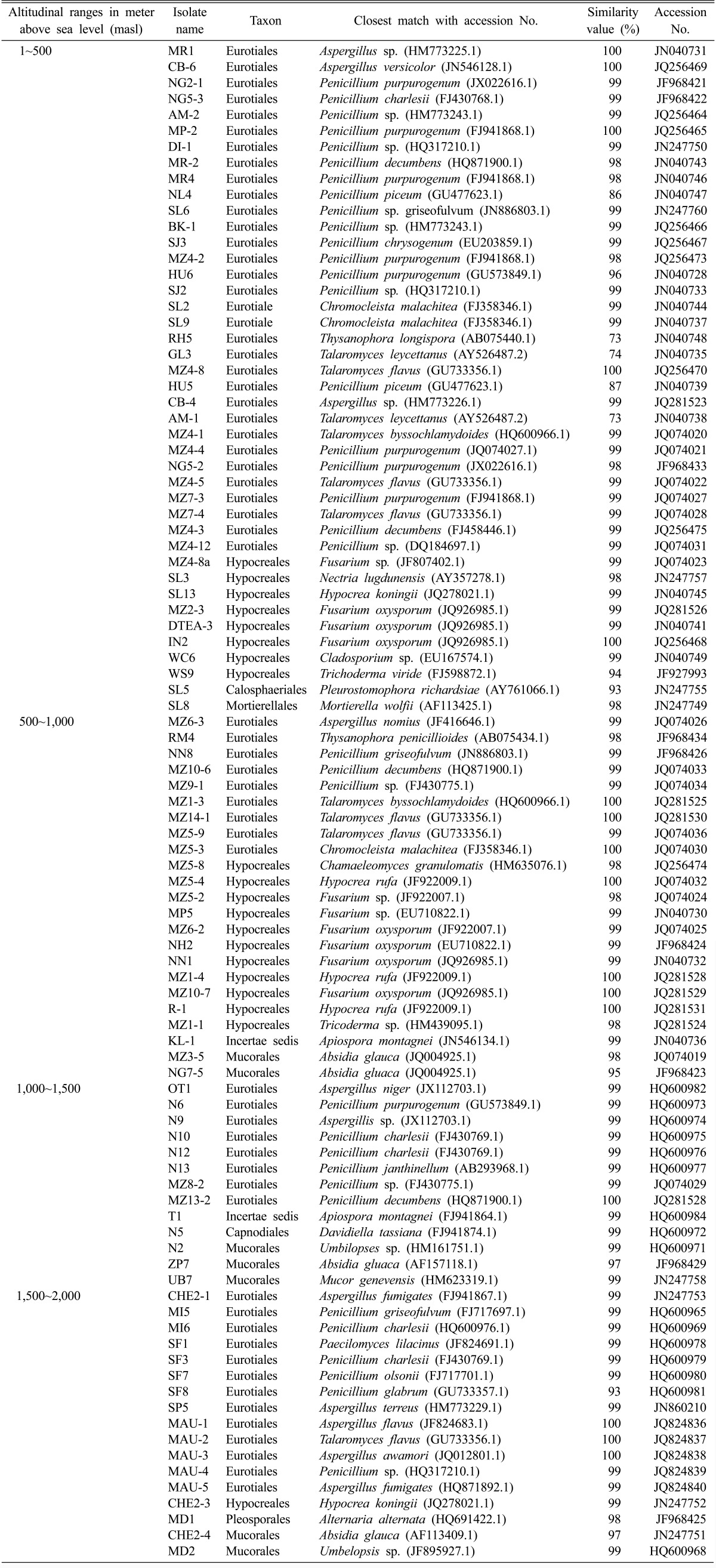
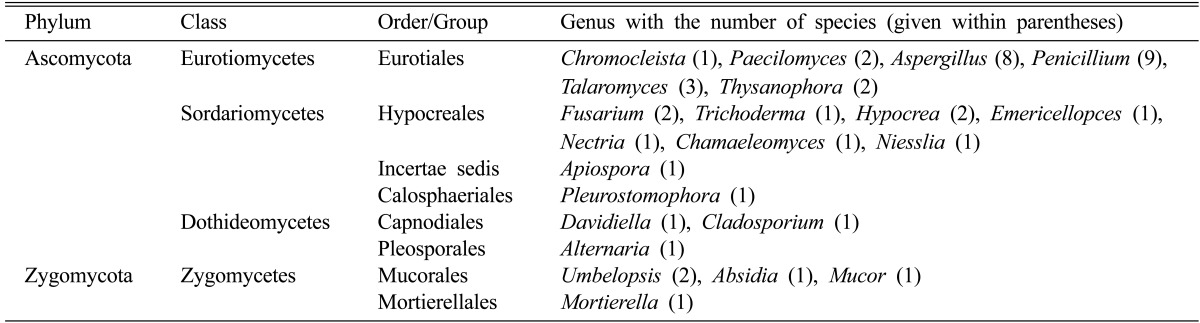
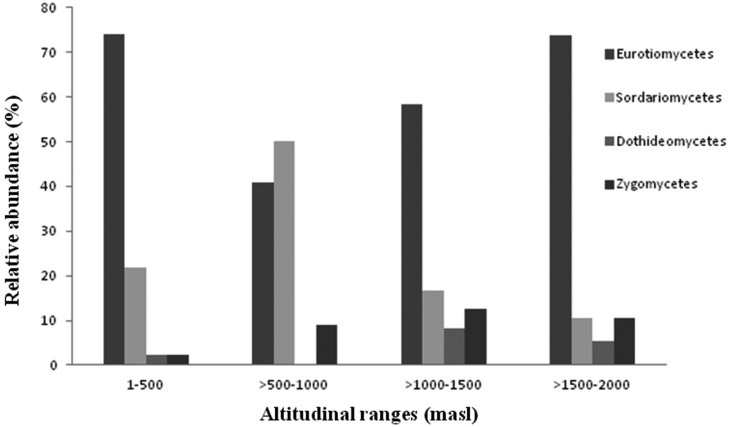
Cultivation of fungi from the four different altitudinal ranges yielded a total of 107 isolates (Table 3). Sequence and phylogenetic analysis revealed the soils to be rich in fungal diversity belonging to the phyla Ascomycota and Zygomycota, corresponding to seven orders (Eurotiales, Hypocreales, Calosphaeriales, Capnodiales, Pleosporales, Mucorales, and Mortierellales) and Incertae sedis which were distributed in 22 genera and 44 species (Table 4). Ascomycota was the dominant phyla in all the four altitudinal ranges. Eurotiomycetes presented the highest relative abundance in the three altitudinal ranges (1~500, 1,000~1,500, and 1,500~2,000) followed by Sordariomycetes whereas in the altitudinal range 500~1,000 Sordariomycetes had the highest relative abundance followed by Eurotiomycetes (Fig. 2). Dothideomycetes was not characterized from the altitudinal range 500~1,000 which could be attributed to the vegetation types. In the present study, under Euritoiales, Penicillium and Aspergillus were observed to be the most diverse genus and were evenly distributed in the entire stretch. Among Ascomycota, diversity index of genus Penicillium (1.44) was found to be highest followed by Aspergillus (1.29) and Talaromyces (1.26). Fusarium and Hypocrea belonging to the class Hypocreales were dominant genus among the phyla Ascomycota with diversity index of 1.16 and 1.15 respectively.
Table 3.
Phylogenetic affiliations of fungi isolated from soils on the basis of 18S rRNA genes sequences showing closest match and their NCBI accession No.
Table 4.
Taxonomic grouping of fungi isolated from different altitudes
Fig. 2.
Relative abundance (%) of fungal order along the altitudinal gradient of the studied Himalayan range.
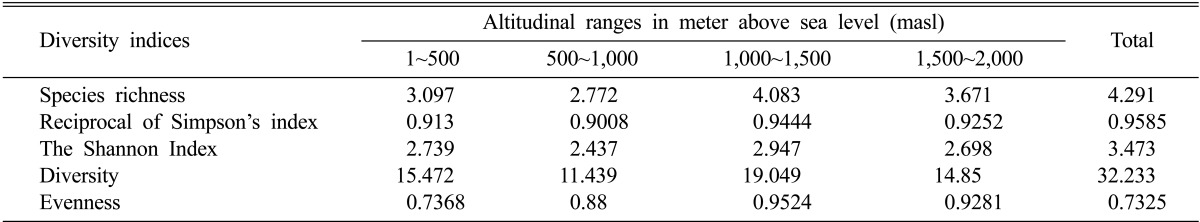
Shannon-Wiener's index, Diversity index, reciprocal of Simpson's index, species richness and evenness index for the studied range was found to be 3.473, 32.233, 0.9585, 4.291, and 0.732 respectively (Table 5). The Shannon-Wiener's index (H') for the entire range varied from 2.99 to 3.35. The highest value for most of the diversity indices as well as species richness was recorded for the altitudinal range 1,000~1,500 (H' = 3.473, D = 32.233, 1/D = 0.9585, S = 4.291).
Table 5.
Statistical analysis of fungal diversity at different altitudinal ranges
These findings provide important insights that aid our understanding of the diversity of fungi in natural ecosystems since fungi comprise important component of microbial diversity in high altitudes and are considered key organisms in inland ecosystems. Information on fungal diversity and functions might provide scope for bioprospecting of new source of drugs and other industrially important biomolecules and enzymes. New technologies, particularly in nucleic acid analysis, bioinformatics, analytical chemistry, and habitat sampling and characterization place the study of microbial diversity on the cutting edge of science. Bacillus from high altitudes of eastern Himalayan range has been reported to produce thermostable enzyme [29]. Previous study also demonstrated that the fungi isolated from soil systems of high altitudinal cold climatic zones in eastern Himalayan range were capable of synthesizing stable silver nanoparticles [30]. Despite the acknowledged value of microorganisms especially fungi, our knowledge of their diversity in NE India under Eastern Himalayan range is still very scarce. This calls for continued research to inventorize and protect the unexplored resources not only for the conservation of natural ecosystems but also for the future benefits through bioprospection.
Acknowledgements
Authors acknowledge the financial support received from Department of Information technology (MC & IT), Government of India to carry out the present study.
References
- 1.Zak JC, Visser S. An appraisal of soil fungal biodiversity: the crossroads between taxonomic and functional biodiversity. Biodivers Conserv. 1996;5:169–183. [Google Scholar]
- 2.Schlesinger WH. Biogeochemistry: an analysis of global change. 2nd ed. San Diego: Academic Press; 1997. pp. 3–15. [Google Scholar]
- 3.Zhang Q, Zak JC. Potential physiological activities of fungi and bacteria in relation to plant litter decomposition along a gap size gradient in a natural subtropical forest. Microb Ecol. 1998;35:172–179. doi: 10.1007/s002489900071. [DOI] [PubMed] [Google Scholar]
- 4.Hobbie EA, Macko SA, Shugart HH. Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia. 1999;118:353–360. doi: 10.1007/s004420050736. [DOI] [PubMed] [Google Scholar]
- 5.Trevors JT. Bacterial biodiversity in soil with an emphasis on chemically-contaminated soils. Water Air Soil Pollut. 1998;10:45–67. [Google Scholar]
- 6.Gleason FH, Letcher PM, McGee PA. Some Chytridiomycota in soil recover from drying and high temperatures. Mycol Res. 2004;108:583–589. doi: 10.1017/s0953756204009736. [DOI] [PubMed] [Google Scholar]
- 7.Manoharachary C, Sridhar K, Singh R, Adholeya A, Suryanarayanan TS, Rawat S, Johri BN. Fungal biodiversity: distribution, conservation and prospecting of fungi from India. Curr Sci. 2005;89:58–71. [Google Scholar]
- 8.Petersen RH, Hughes KW. Species and speciation in mushrooms: development of a species concept poses difficulties. BioScience. 1999;49:440–452. [Google Scholar]
- 9.Burnett J. Fungal populations and species. Oxford: Oxford University Press; 2003. [Google Scholar]
- 10.Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- 11.Myers N, Mittermeier RA, Mittermeier CG, de Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 12.Ramakantha V, Gupta AK, Kumar A. Biodiversity of Northeast India: an overview. Envis Bull. 2003;4:1–24. [Google Scholar]
- 13.Rao RR. Biodiversity. In: Singh B, Singh MP, editors. India (floristic aspects) India: 1994. p. 315. [Google Scholar]
- 14.Chatterjee S, Saikia A, Dutta P, Ghosh D, Pangging G, Goswami AK. Background paper on biodiversity significance of North East India for the study on natural resources, water and environment nexus for development and growth in North Eastern India; Forest Conservation Programme, 2006 WWF-India; 2006 Jun 30; New Delhi, India. [Google Scholar]
- 15.Borneman J, Hartin RJ. PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol. 2000;66:4356–4360. doi: 10.1128/aem.66.10.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaolis DK. Method for direct detection of fungal pathogens [Internet] United States Patent; 2007. [cited 2010 Nov 2]. Available from: http://www.freepatentsonline.com/7291465.html. [Google Scholar]
- 17.Shannon CE, Weaver W. The mathematical theory of communication. Urbana: University of Illinois Press; 1949. [Google Scholar]
- 18.Jost L. Entropy and diversity. Oikos. 2006;113:363–375. [Google Scholar]
- 19.Simpson EH. Measurement of diversity. Nature. 1949;163:688. [Google Scholar]
- 20.Pielou EC. The measurement of diversity in different types of biological collections. J Theor Biol. 1966;13:131–144. [Google Scholar]
- 21.Zar JH. Biostatistical analysis. Upper Saddle River: Prentice Hall; 1984. [Google Scholar]
- 22.Song FQ, Tian XJ, Li ZQ, Yang CL, Chen B, Hao JJ, Zhu J. Diversity of filamentous fungi in organic layers of two forests in Zijin Mountain. J For Res. 2004;15:273–279. [Google Scholar]
- 23.Bissett J, Parkinson D. Fungal community structure in some alpine soils. Can J Bot. 1979;57:1630–1641. [Google Scholar]
- 24.Osono T, Hirose D. Altitudinal distribution of microfungi associated with Betula ermanii leaf litter on Mt. Rishiri, northern Japan. Can J Microbiol. 2009;55:783–789. doi: 10.1139/w09-030. [DOI] [PubMed] [Google Scholar]
- 25.Bissett J, Parkinson D. Functional relationships between soil fungi and environment in alpine tundra. Can J Bot. 1979;57:1642–1659. [Google Scholar]
- 26.Joshi SR, Chauhan M, Sharma GD, Mishra RR. Effect of deforestation on microbes, VAM fungi and their enzymatic activity in Eastern Himalaya. In: Rajwas GS, editor. Studies in Himalayan ecobiology. New Delhi: Today and Tomorrows Publication; 1991. pp. 141–152. [Google Scholar]
- 27.Joshi SR, Kumar R, Saikia P, Bhagobaty RK, Thokchom S. Impact of roadside pollution on microbial activities in subtropical forest soil of North East India. Res J Environ Sci. 2010;4:280–287. [Google Scholar]
- 28.Revenga C, Kura Y. Status and trends of biodiversity of inland water ecosystems. Technical series no. 11. Montreal: Secretariat of the Convention on Biological Diversity; 2003. [Google Scholar]
- 29.Devi SL, Khaund P, Joshi SR. Thermostable α-amylase from natural variants of Bacillus spp. prevalent in eastern Himalayan range. Afr J Microbiol Res. 2010;4:2534–2542. [Google Scholar]
- 30.Devi LS, Joshi SR. Antimicrobial and synergistic effects of silver nanoparticles synthesized using soil fungi of high altitudes of eastern Himalaya. Mycobiology. 2012;40:27–34. doi: 10.5941/MYCO.2012.40.1.027. [DOI] [PMC free article] [PubMed] [Google Scholar]