Abstract
We previously reported that ranolazine improved exercise myocardial perfusion. Ranolazine ameliorates myocardial ischemia by augmenting myocardial blood flow; likely due to a reduction in extra-vascular compression of small vessels. We hypothesized that ranolazine could improve left ventricular (LV) dyssynchrony as assessed by phase analysis of gated SPECT myocardial perfusion imaging (MPI). Patients (n=32) with known or suspected coronary artery disease and reversible perfusion defects on a clinically indicated stress MPI were re-studied 4 weeks after ranolazine (500–1000 mg orally twice daily) was added to their conventional treatment in an open-label trial (data previously reported). The LV systolic and diastolic dyssynchrony indices were obtained using automated phase analysis before and after ranolazine. There were no significant changes in heart rate and blood pressure (at rest or during stress) after treatment. The perfusion pattern improved in 13 of 18 patients who had exercise testing but in only 3 of 14 patients who had vasodilator stress testing. There were no significant changes in LV ejection fraction or volumes after treatment. The systolic and diastolic LV dyssynchrony improved after ranolazine therapy; there was a significant decrease in systolic phase standard deviation (SD) (21±17 vs. 18±13, P=0.04), systolic bandwidth (BW) (69±60 vs. 53±38, P=0.03), diastolic SD (29±18 vs. 24±15, P=0.047) and diastolic BW (91±61 vs. 72±45, P=0.02). In conclusion, this is the first study to show improvement in diastolic and systolic LV synchrony with ranolazine as measured by automated phase analysis of gated SPECT MPI.
Keywords: Ranolazine, SPECT, Dyssynchrony, Phase analysis
Introduction
Ranolazine, a prototype late I- Na inhibitor, reduces myocardial ischemia by a novel mechanism that involves inhibition of late sodium current which decreases sodium dependent intracellular calcium overload of cardiomyocytes (1–2). This effect of ranolazine results in a decrease in left ventricular (LV) stiffness, a decrease in extra-vascular compressive forces on coronary microcirculation and thereby improves myocardial blood flow (MBF) (1–6). In a previous study, we showed that ranolazine improved myocardial perfusion during exercise (7). We hypothesized that ranolazine might improve LV synchrony, especially during diastole; such an effect could improve MBF by decreasing LV stiffness and by increasing early diastolic flow. We used automated analysis of gated SPECT MPI to measure LV systolic and diastolic dyssynchrony indices.
Methods
This was an open label study conducted at the University of Alabama at Birmingham Medical Center and was approved by the institutional review board and all participants signed an informed consent form. The patient population, baseline clinical and demographic data and myocardial perfusion imaging (MPI) results have been reported (7, 8).
All images were acquired and processed according to the ASNC guidelines using either single-day or 2-day (depending on body weight) stress/rest –Tc- 99m sestamibi (9). Identical doses of the tracer were used for the baseline and follow-up studies. All raw data obtained from the gated SPECT images (2 sets/patient) were reconstructed using standard filtered backprojection and identical low-pass filtering (Butterworth filter with a critical frequency of 0.4 cycles/second, order 5). Motion correction was applied prior to reconstruction of images if >1 pixel of x or y axis deviation was observed over the 180-degree acquisition. No attenuation correction was used. An automated program was used to derive summed stress score (SSS), summed rest score (SRS) and summed difference score (SDS) based on the conventional 17-segment model. The program assigned a score of 0–4 to each segment based on activity level, whereby 0=normal and 4=absent.
The phase analysis method has been described in detail elsewhere and was derived from the post-stress studies (10, 11). In brief, a three-dimensional count distribution was extracted from each of the LV short-axis datasets and subjected to one-harmonic Fourier analysis for systolic dyssynchrony and three-harmonic Fourier analysis for diastolic dyssynchrony. The Fourier analyses generated a systolic phase distribution and a diastolic phase distribution, both ranging between 0–360° and spanning the entire R-R interval. The systolic and diastolic phase distributions were displayed on polar maps and histograms. Two indices were extracted from each phase distribution: 1) histogram bandwidth (BW), which marked the range of degrees of the cardiac cycle during which 95% of the myocardium-initiated contraction and 2) standard deviation (SD), which represented the SD of the phase distribution. The effective temporal resolution of the technique correlated to the 1/64th of the cardiac cycle, without any significant impact on the phase SD and BW whether 8 or 16 frames per cycle were applied.
Statistical Analysis
Data are expressed as percentages for discrete variables and mean ± standard deviation for continuous variables. Descriptive statistics were computed for phase variables and were compared between baseline and follow-up tests with paired t-tests. All statistical analyses were performed using SAS version 9.1 (SAS Inc, Cary, NC). All tests were 2-tailed and a P <0.05 was used as the level of significance. Paired t-tests were used to compare continuous pre- and post-ranolazine variables.
Results
Of the 38 patients (exercise=20, vasodilator=18), phase analysis was not possible in 6 patients (exercise=2, vasodilator=4) due to gating errors; the analysis was performed in the remaining 32 patients. The baseline clinical variables are summarized in Table 1. No changes were made in other medications between the baseline and follow-up MPI. The perfusion pattern improved in 13 of 18 patients who had exercise MPI and 3 of 14 patients who had vasodilator MPI.
Table 1.
Clinical and Laboratory variable distribution.
| Variable | N=32 |
|---|---|
| Age (years) | 65 ± 10 |
| Exercise stress | 18(56%) |
| Women | 9 (28%) |
| Diabetes mellitus | 17 (53%) |
| Hypertension (on anti-hypertensive therapy) | 26 (81%) |
| Smokers | 12 (38%) |
| Prior coronary revascularization | 27 (84%) |
| Angiotensin converting enzyme-Inhibitors | 21 (66%) |
| Beta blockers | 29 (91%) |
| Statins | 28 (88%) |
| Glomerular filtration rate (ml/min per 1.73m2) | 71 ± 15 |
There were no significant differences in heart rate, blood pressure, LV ejection fraction, LV end-diastolic and LV end-systolic volumes with ranolazine therapy (Table 2).
Table 2.
Comparison of hemodynamic variables between baseline and follow-up stress tests.
| Variable | Pre-Ranolazine* (n=32) |
Post- Ranolazine* (n=32) |
|---|---|---|
| Resting HR (bpm) | 68 ± 10 | 66 ± 9 |
| Resting systolic BP (mm Hg) | 129 ± 27 | 125 ± 17 |
| Resting diastolic BP (mm Hg) | 72 ± 14 | 72 ± 8 |
| Resting QT (ms) | 418 ± 29 | 423 ± 31 |
| Ejection Fraction | ||
| LV end-diastolic volume (ml) | 58 ± 15 | 59 ± 15 |
| 111 ± 49 | 111 ± 50 | |
| LV end-systolic volume (ml) | 54 ± 44 | 52 ± 43 |
BP=Blood pressure, HR= Heart rate; LV= Left Ventricle
Figures represent Mean ± SD
Paired-t-test
The post-stress systolic and diastolic phase SD and BW decreased significantly after ranolazine therapy (Figure 1). The prevalence of abnormal phase variables (based on previously reported normal values) decreased after ranolazine therapy (11) (Figure 2). The percentage changes in systolic and diastolic phase variables are shown in Figure 3. A representative example showing improvement in systolic and diastolic indices is shown in Figure 4.
Figure 1.
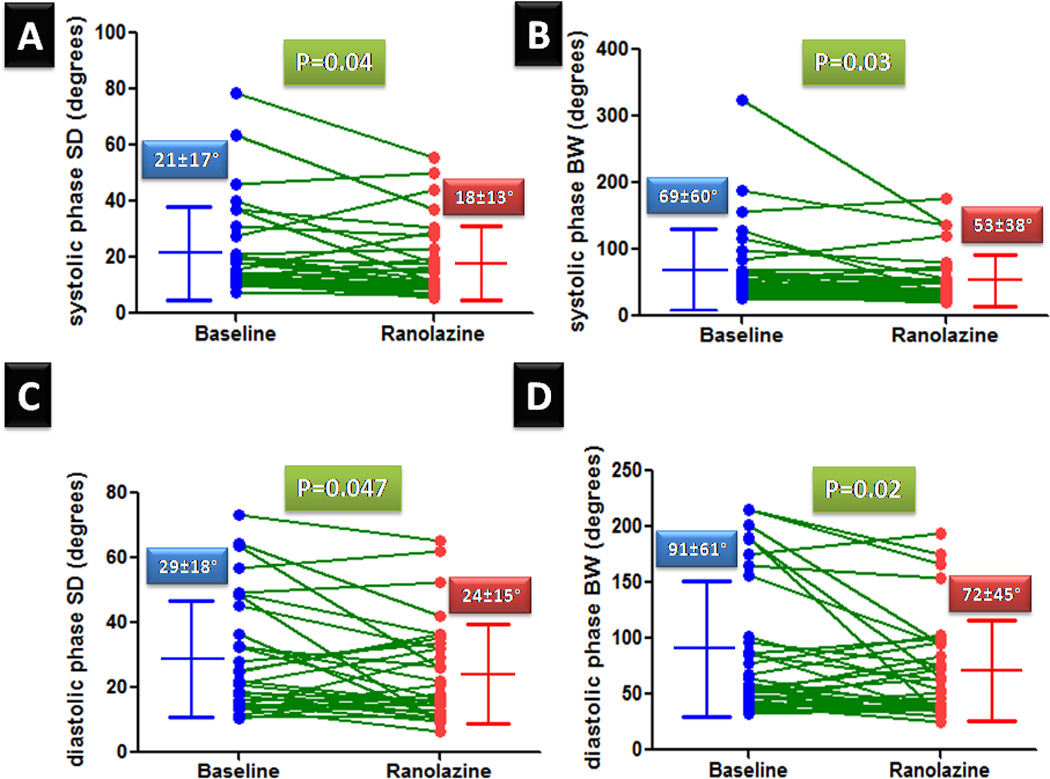
Systolic and diastolic phase variables at baseline and follow-up after ranolazine therapy. (SD-Phase standard deviation, BW-phase bandwidth)
Figure 2.
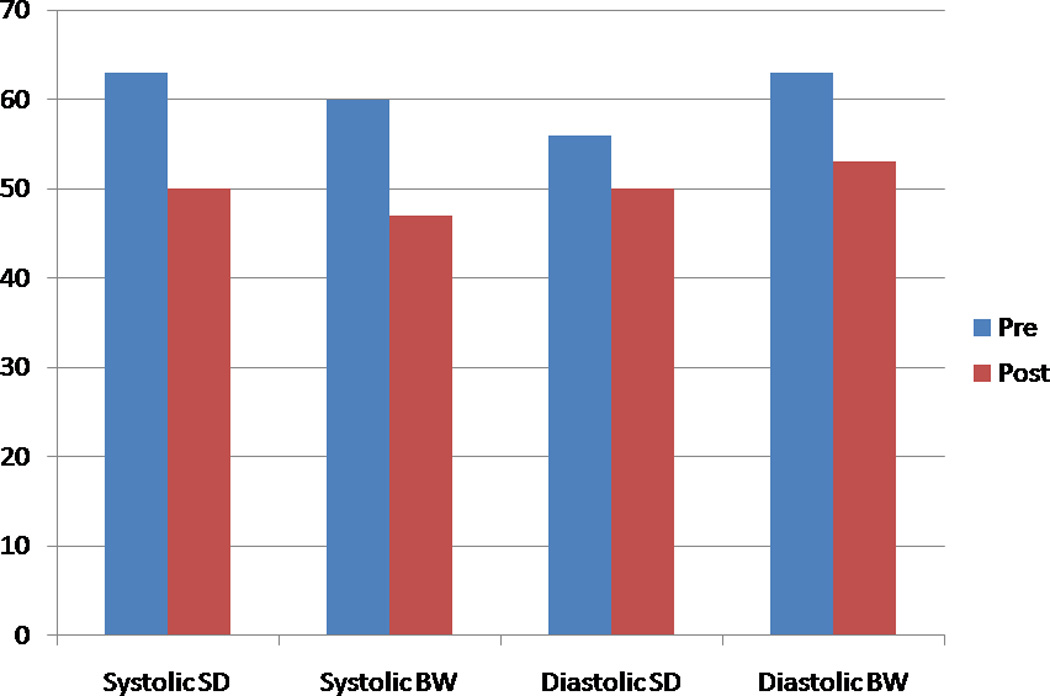
The prevalence of those with abnormal phase variables, before and after ranolazine therapy. (SD-Phase standard deviation, BW-phase bandwidth)
Figure 3.
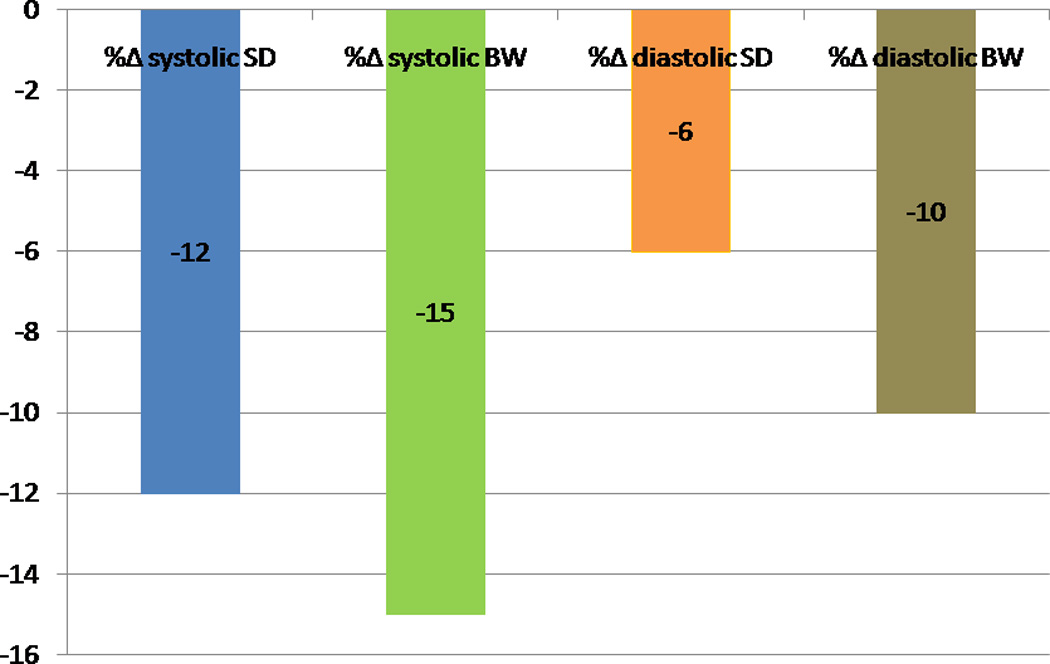
Percentage change in systolic and diastolic phase variables in the entire cohort after ranolazine therapy. (SD-Phase standard deviation, BW-phase bandwidth)
Figure 4.
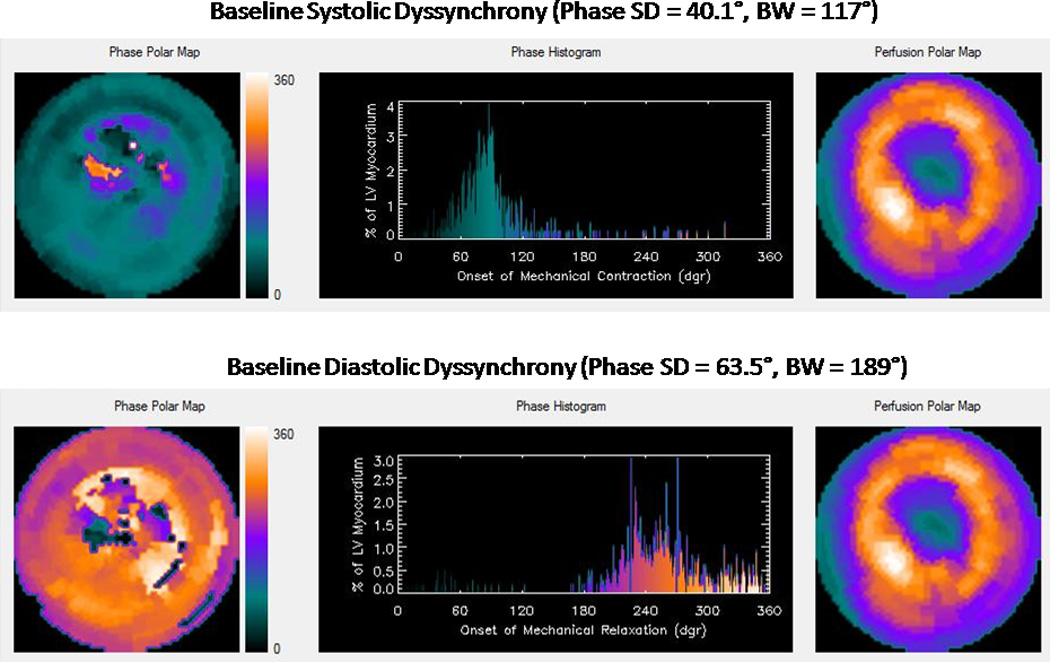
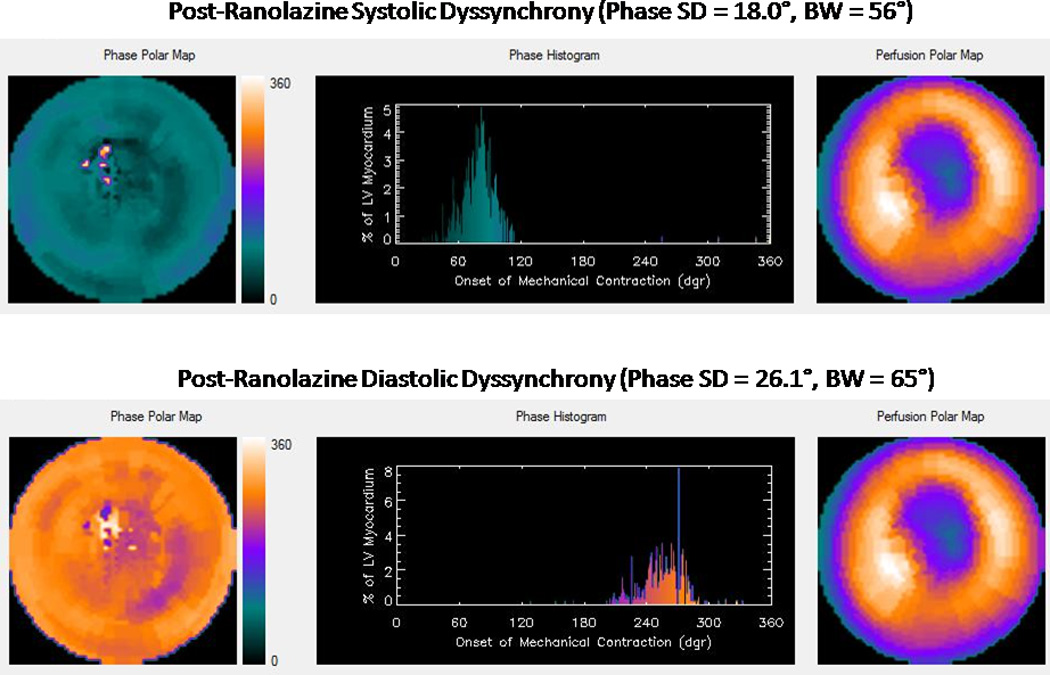
A: Baseline systolic and diastolic dyssynchrony
B: Post-ranolazine, improved systolic and diastolic dyssynchrony
The percentage change in systolic and diastolic phase variables were not significantly different between those with improved perfusion and those with no change in perfusion (Table 3). There was no effect of heart rate or type of stress on the change in systolic and diastolic phase variables. There was no correlation between baseline and percentage change in systolic and diastolic phase variables. There was a strong and positive correlation between both percentage change in systolic SD and percentage change in diastolic SD and the percentage change in systolic BW and percentage change in diastolic BW (Figures 5 and 6).
Table 3.
Change in phase variables in those with improved perfusion and those without improvement in perfusion.
| Perfusion | ||
|---|---|---|
| Variable | Improved (n=16) |
No change (n=16) |
| % change in systolic SD | −6 ± 37 | −17 ± 35 |
| % change in systolic BW | −12 ± 28 | −18 ± 30 |
| % change in diastolic SD | 2 ± 45 | −15 ± 41 |
| % change in diastolic BW | −2 ± 38 | −18 ± 38 |
SD=phase standard deviation, BW= Bandwidth
Figures represent Mean ± SD
Independent sample t-test
Figure 5.
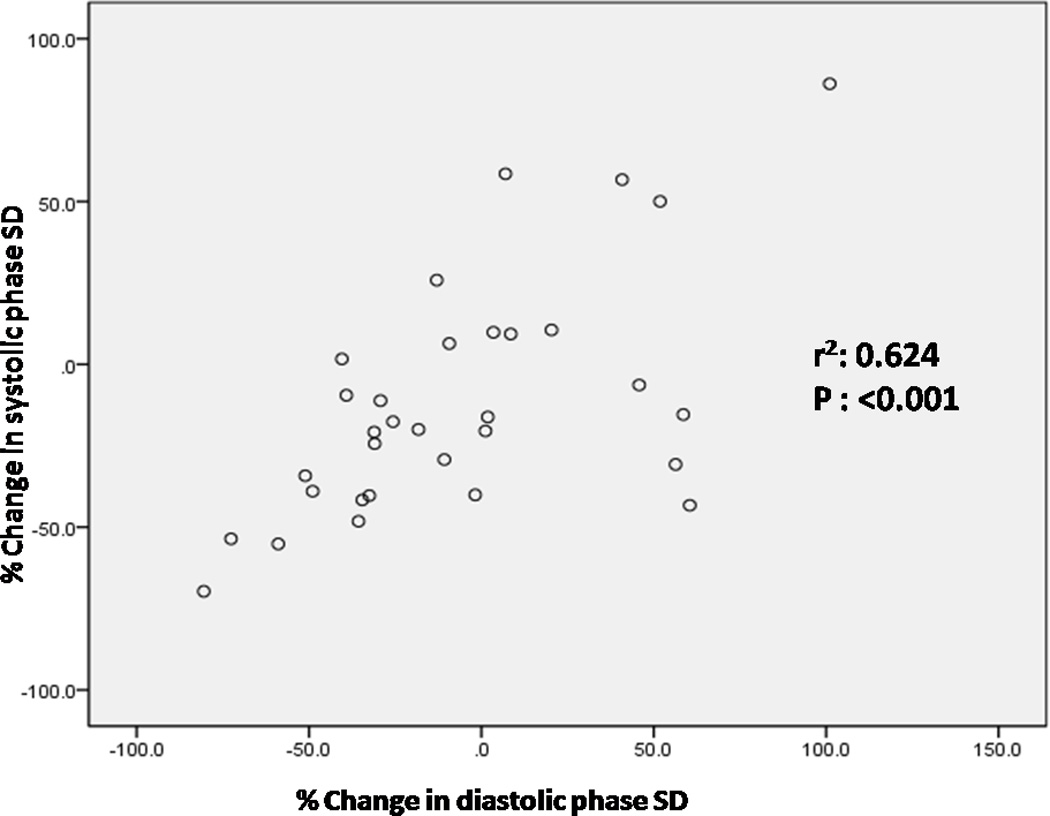
Correlation between percentage change in systolic SD and percentage change in diastolic SD (SD-Phase standard deviation, r2: Pearson’s correlation coefficient)
Figure 6.
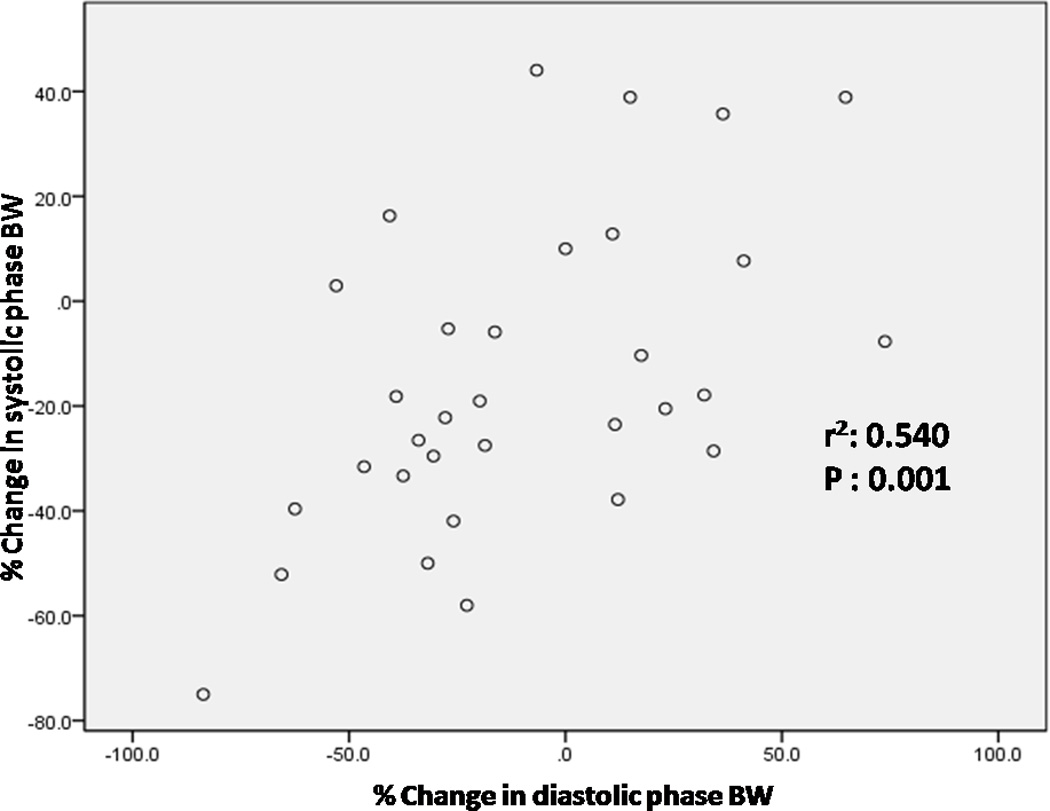
Correlation between percentage change in systolic BW and percentage change in diastolic BW (BW-phase bandwidth, r2: Pearson’s correlation coefficient)
Discussion
The main finding of this study is that systolic and diastolic indices of LV dyssynchrony, measured by automated phase analysis of gated SPECT MPI, improved after short term therapy with ranolazine in patients with coronary artery disease. It should be noted that these changes were observed even though none of the patients had left bundle branch block and the mean LV ejection fraction was normal. Ranolazine inhibits the late sodium current and thus decreases the diastolic calcium overload in ischemic myocytes (1–2). Myocardial ischemia is known to cause late sodium current dependent intracellular calcium overload. The cellular calcium overload increases diastolic tension which decreases perfusion due to increase in extravascular compression, which then becomes a vicious cycle as ischemia begets more ischemia. Thus, a decrease in diastolic calcium overload should lead to an increase in coronary blood flow by improving diastolic relaxation of the ventricular myocardium (1–2). Improved synchrony and decreased stiffness might contribute to the improvement in MBF. It is unlikely that improvement in MBF lead to improvement in dyssynchrony variables as the resting MBF is often normal despite coronary stenosis. Also, “the stress images” were obtained 30–60 minutes after the stress.
Heart failure with preserved LV ejection fraction is a major cause of morbidity and mortality (12). Diastolic dysfunction is characterized by impaired LV relaxation, leading to decreased LV filling and reduced stroke volume. Enhanced late sodium current and increase in diastolic calcium overload have been shown to impair relaxation of ventricular myocytes, hence there has been considerable interest in the effects of ranolazine in diastolic dysfunction (13). A study in isolated human ventricular myocytes from end-stage failing hearts showed that ranolazine reduced the frequency dependent increase in diastolic tension without having negative inotropic effects on contractility (14). Ranolazine also significantly reduced the regional peak filling rate in patients with previous transmural infarction (15). Interestingly, ranolazine improved diastolic relaxation of noninfarcted myocardium under chronic ischemic conditions. A recent analysis of the MERLIN-TIMI 36 trial suggested that ranolazine may have enhanced efficacy in high-risk patients with acute coronary syndrome with increased B-type natriuretic peptide (16). Patients with increased B-type natriuretic peptide likely have increased wall stress and it is reasonable to speculate that the effect of ranolazine in these patients may be related to improvement in LV synchrony, since ranolazine does not affect cardiac contractility per se. A recent small clinical study indeed demonstrated improvement in diastolic echocardiographic indices of LV dyssynchrony with ranolazine therapy (17). Two large double blinded cross-over trials are currently underway to rigorously study the effect of ranolazine on diastolic function as assessed by cardiac catheterization and Doppler echocardiography (18,19).
Echocardiography is the method of diastolic function assessment of the on-going trials with ranolazine (18–19). Phase analysis by SPECT MPI has been shown to correlate well with echocardiography for assessment of both systolic and diastolic dyssynchrony (20–21). Unlike echocardiography, dyssynchrony assessment by phase analysis with SPECT MPI is not user dependent and can be derived from the same study done for assessment of perfusion.
Our study has limitations and should be considered as a proof of concept awaiting larger studies. The single-center study, the small sample size and the lack of a control arm are obvious limitations. We did not assess dyssynchrony by other modalities, since all patients had normal LV function and a narrow QRS. The direction of change in dyssynchrony variables is more important than absolute changes in dyssynchrony variables. Nevertheless, this is the first study which showed improvement in LV dyssynchrony in patients with normal LV systolic function after a short term treatment with ranolazine. The ease of phase analysis and its non-user dependence, coupled with simultaneous ischemia/scar assessment suggests that this could be an attractive methodology for exploring the mechanisms by which ranolazine produces clinical benefits, especially since it has now assumed a first line status in the management of symptomatic patients with CAD.
Acknowledgement
We acknowledge the help of Misty Pruitt, RN in data collection.
Funding Sources:
The study was funded by a grant from Gilead Sciences Inc, Foster City, CA.
The study design, data collection, interpretation and reporting were the responsibility of the investigators at UAB.
The phase analyses of the data were done by the investigators at Emory University, who were supported in part by an NIH grant (1R01HL094438, PI: Ji Chen, PhD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: AEI is a consultant to Gilead Sciences Inc, Foster City, CA and LB is an employee of Gilead Sciences Inc, Foster City, CA. JC and EVG receive royalties from the sale of the Emory Cardiac Toolbox. All other authors have no conflicts of interest to disclose.
References
- 1.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol. 2008;44:954–967. doi: 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. ERICA investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–575. doi: 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Allely MC, Alps BJ. Prevention of myocardial enzyme release by ranolazine in a primate model of ischaemia with reperfusion. Br J Pharmacol. 1990;99:5–6. doi: 10.1111/j.1476-5381.1990.tb14641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Hale SL, Leeka JA, Kloner RA. Improved left ventricular function and reduced necrosis after myocardial ischemia/reperfusion in rabbits treated with ranolazine, an inhibitor of the late sodium channel. J Pharmacol Exp Ther. 2006;318:418–423. doi: 10.1124/jpet.106.103242. [DOI] [PubMed] [Google Scholar]
- 7.Venkataraman R, Belardinelli L, Blackburn B, Heo J, Iskandrian AE. A study of the effects of ranolazine using automated quantitative analysis of serial myocardial perfusion images. JACC Cardiovasc Imaging. 2009;2:1301–1309. doi: 10.1016/j.jcmg.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Venkataraman R, Aljaroudi W, Belardinelli L, Heo J, Iskandrian AE. The effect of ranolazine on the vasodilator-induced myocardial perfusion abnormality. J Nucl Cardiol. 2011;18:456–462. doi: 10.1007/s12350-011-9364-1. [DOI] [PubMed] [Google Scholar]
- 9.Iskandrian AE, Garcia EV. Nuclear Cardiac Imaging: Principles and Applications. 4 ed. Oxford: Oxford University Press; 2008. [Google Scholar]
- 10.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, Iskandrian AE. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Kalogeropoulos A, Verdes L, Butler J, Garcia EV. Left-ventricular systolic and diastolic dyssynchrony as assessed by multi-harmonic phase analysis of gated SPECT myocardial perfusion imaging in patients with end-stage renal disease and normal LVEF. J Nucl Cardiol. 2011;18:299–308. doi: 10.1007/s12350-010-9331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura H, Hara A, Hashizume H, Maruyama K, Abiko Y. Protective effects of ranolazine, a novel anti-ischemic drug, on the hydrogen peroxide-induced derangements in isolated, perfused rat heart:comparison with dichloroacetate. Jpn J Pharmacol. 1998;77:31–39. doi: 10.1254/jjp.77.31. [DOI] [PubMed] [Google Scholar]
- 14.Sossalla S, Wagner S, Rasenack ECL, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts-role of late sodium current and intracellular ion accumalation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8:741–747. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- 16.Morrow DA, Scirica BM, Sabatine MS, de Lemos JA, Murphy SA, Jarolim P, Theroux P, Bode C, Braunwald E. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2010;55:1189–1196. doi: 10.1016/j.jacc.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 17.Figueredo VM, Pressman GS, Romero-Corral A, Murdock E, Holderbach P, Morris DL. Improvement in left ventricular systolic and diastolic performance during ranolazine treatment in patients with stable angina. J Cardiovasc Pharmacol Ther. 2011;16:168–172. doi: 10.1177/1074248410382105. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed on October 23, 2011]; http://clinicaltrials.gov/ct2/show/NCT00574756.
- 19.Jacobshagen C, Belardinelli L, Hasenfuss G, Maier LS. Ranolazine for the treatment of heart failure with preserved ejection fraction: background, aims, and design of the RALI-DHF study. Clin Cardiol. 2011;34:426–432. doi: 10.1002/clc.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, Stokkel M, van der Wall EE, Garcia EV, Bax JJ. Phase analysis of gated myocardial perfusion SPECT compared to tissue doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–1714. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 21.Boogers MJ, Chen J, Veltman CE, van Bommel RJ, Mooyaart EA, Al Younis I, van der Hiel B, Dibbets P, van der Wall EE, Schalij MJ, Garcia EV, Bax JJ, Delgado V. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: a comparison with tissue Doppler imaging. Eur J Nucl Med Mol Imaging. 2011;38:2031–2039. doi: 10.1007/s00259-011-1870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


