Abstract
Paraoxonase 2 deficiency (PON2-def) alters mitochondrial function and exacerbates the development of atherosclerosis in mice. PON2 overexpression protects against ER stress in cell culture. In this paper, we examined the role of PON2 in the unexplored link between ER stress and mitochondrial dysfunction and tested whether restoration of PON2 in macrophages is sufficient to reduce aggravated atherosclerosis in PON2-def/apoE−/− mice on a Western diet. ER stress response genes, intracellular calcium levels, and apoptotic nuclei were significantly elevated in PON2-def/apoE−/− macrophages compared to apoE−/− macrophages in response to ER stressors, but not at the basal level. In contrast, PON2-def/apoE−/− macrophages exhibited greater mitochondrial stress at the basal level, which was further worsened in response to ER stressors. There was no difference in ER stress response genes and apoptotic nuclei between apoE−/− and PON2-def/apoE−/− macrophages when pretreated with xestospongin (which blocks the release of calcium from ER) suggesting that PON2 modulates cell survival and ER stress by maintaining calcium homeostasis. Treatment with a mitochondrial calcium uptake inhibitor, RU360, attenuated ER stressor mediated mitochondrial dysfunction in PON2-def/apoE−/− macrophages. CHOP expression (ER stress marker) and apoptotic nuclei were significantly higher in aortic lesions of PON2-def/apoE−/− mice compared to apoE−/− mice fed a Western diet. Restoration of PON2 in macrophage reduced ER stress, mitochondrial dysfunction and apoptosis in response to ER stressors. Furthermore, restoration of PON2 in macrophages reduced lesional apoptosis and atherosclerosis in PON2-def/apoE−/− mice on a Western diet. Our data suggest that macrophage PON2 modulates mechanisms that link ER stress, mitochondrial dysfunction and the development of atherosclerosis.
Keywords: Paraoxonase 2, Mitochondria, Endoplasmic reticulum, Macrophages, Oxidative stress
1. Introduction
Atherosclerosis is a chronic inflammatory condition in which the migration of circulating monocytes into the vessel wall is an important step. According to the lipid oxidation hypothesis of atherosclerosis, low-density lipoprotein (LDL) is a major target of oxidation and minimally oxidized lipids in LDL recruit monocytes into the sub endothelial space where they differentiate into macrophages. Subsequent uptake of oxidized lipids by macrophages causes the formation of foam cells and atherosclerosis[1, 2].
Mitochondrial respiratory chain dysfunction resulting in increased production of superoxides causes the oxidation of low-density lipoprotein (LDL) [3], vascular cell apoptosis, and increased atherosclerotic lesion formation[4]. Unfolded protein response (UPR) leading to endoplasmic reticulum (ER) stress induces oxidative stress, vascular cell apoptosis, and the development of atherosclerosis[5, 6]. Emerging evidence suggests that there is an extensive crosstalk between ER and mitochondria under both physiological and pathophysiological conditions[7]. Understanding the key players in the crosstalk between the two organelles is an important area of investigation.
PON2 is a membrane associated protein ubiquitously expressed including macrophages within many tissues[8, 9]. At the subcellular level, PON2 is localized to both the ER[10] and mitochondria[11], and protects against oxidative stress[12]. We have shown that in PON2-def mice, enhanced mitochondrial oxidative stress was accompanied by reduced electron transport chain (ETC) complex I + III activities, oxygen consumption, adenosine triphosphate (ATP) levels and further exacerbates the development of atherosclerosis[11]. Using cell culture models, we and Altenhofer et al. have shown that overexpression of PON2 prevents the generation of ubisemiquinone-induced mitochondrial superoxide formation[11, 13]. In this report, we show that macrophages from PON2-def/apoE−/− mice show altered calcium handling in response to ER stressors, enhanced UPR, and a worsened mitochondrial oxidative stress in a calcium dependent manner. We further demonstrate that restoration of PON2 in macrophages reduces mitochondrial dysfunction, ER stress, ER stress-mediated mitochondrial dysfunction and diet-induced atherosclerosis in PON2-def/apoE−/− mice.
2. Materials and methods
2.1. Mice and diet
Female PON2-def mice were crossed with apoE−/− mice on a C57BL/6J background to generate PON2-def/apoE−/− and PON2+/+/apoE−/− littermate controls. Mice were started on a Western diet containing 0.15% cholesterol providing 42% calories as fat (TD 88137; Harlan Teklad, Madison, WI) at 6 weeks of age, and maintained on this diet for an additional 16 weeks. In separate studies, LysMCre mice were crossed with PON2-def/apoE−/− to obtain PON2-def/LysMCre/apoE−/− mice[14] and were placed on a Western diet for 16 weeks. At the end of the study, the mice were fasted overnight and organs were collected. The Animal Research Committee at UCLA approved all animal protocols.
2.2. Atherosclerosis studies
Aortic root lesion analysis, CD68 quantification and En face lesion analysis were carried out in a blinded fashion by light microscopy as described previously[15, 16] and lipid peroxide content in the lipoproteins was analyzed as described previously[15].
2.3. Macrophage harvest and treatments
Peritoneal macrophages were harvested from mice three days after the animals received an i.p injection of thioglycolate, and the cells were plated in 10% FBS in DMEM media as previously described[15]. Adherent macrophages were treated with ER stressor namely, tunicamycin or thapsigargin or Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (Ox-PAPC) as described in the figure legends. ER stressor concentrations and treatment times were chosen based on previous reports [17–20]. Higher concentrations (1mM tunicamycin; 25 μM thapsigargin) were chosen for the short incubation times. The outcomes measured for these experiments were intracellular calcium flux (30 seconds to 4 mintutes; Figs 1B [upper panel], 2A, 3A and 6B), mitochondrial calcium (1hr; Figs 3C and 4A) and UPR gene (1hr; Fig 3B). Lower concentrations of ER stressors (0.35 μM tunicamycin; 0.25 μM thapsigargin; and 50 μg/ml Ox-PAPC) were chosen for the longer incubation times (5–24hrs). The outcomes measured for these experiments were expression of UPR genes (5 hrs; Figs 1A, 1E [left panel], 2B and 6A), mitochondrial superoxide (5 hrs; Figs 3G, 4B and 6C), ROS (5 hrs; Figs 1C, 1E (middle panel) and 2C), apoptosis (24 hrs; Figs 1D, 1E (right panel), 2D, 4C and 6D), calcium levels at specified time point (Figs 1B (lower panel) and 3D) and mitochondrial funcion (5 hrs; Figs 3E–F).
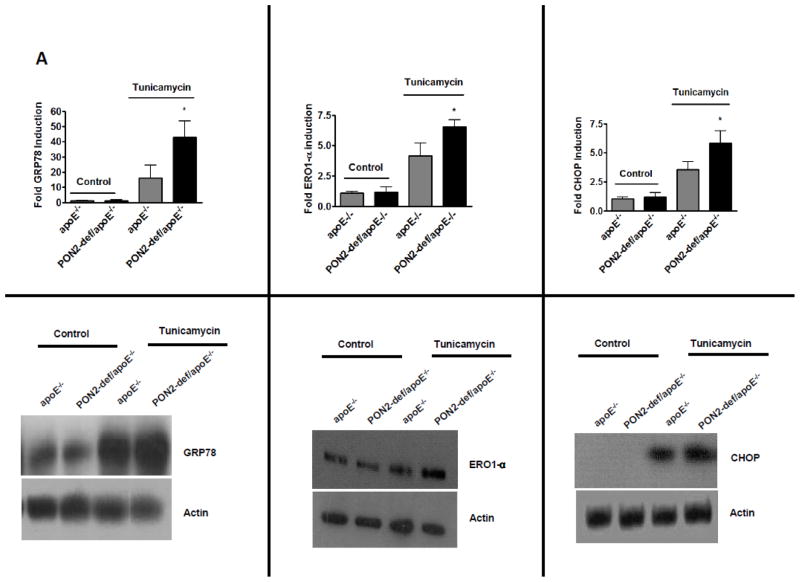
Fig. 1. PON2 deficiency alters the intracellular calcium handling and enhances the unfolded protein response in macrophages in response to ER stress.
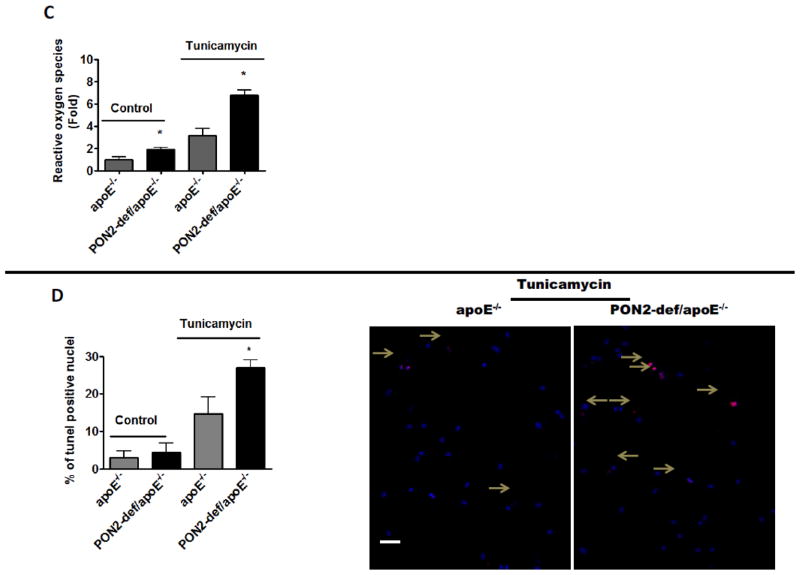
Peritoneal macrophages were isolated from the indicated experimental groups and treated with tunicamycin (0.35 M) for 5 hours. (A) ER stress genes GRP78, ERO1-α and CHOP mRNA were quantified by real-time PCR and normalized to cyclophilin (upper panel). Fold changes in gene expression relative to apoE−/− (untreated) cells are reported as the mean ± SD (n=3). Immunoblots showing protein (lower panel) levels (in 50 μg of total lysates) of the corresponding genes from the same experiment. (B) Intracellular calcium was quantified in macrophages as described in the methods. For measuring intracellular calcium macrophages were first pretreated with indo-1 (2μM) for 20 mins and calcium was quantified at basal level for 30 seconds. Tunicamycin (1mM) was added to the cells and calcium was quantified after 4 minutes and 30 seconds using flow cytometer. Indo-1 violet/blue ratio was analysed using flowJo software. Raw data was presented as a graph (upper right panel). Values were expressed as fold change over tunicamycin treated apoE−/− macrophages (upper left panel). Macrophages were treated with 0.35 μM tunicamycin for 1 hour. At the end of each incubation, 10 μM Indo 1, an intracellular-specific fluorescent calcium dye, was added to the media, and the cells were incubated on ice for 15 mins at 4°C. The cells were then washed and incubated for an additional 4 hours with tunicamycin, such that total incubation time for tunicamycin was 5 hours and intracellular calcium was measured (lower left panel). (C) Macrophages were treated with tunicamycin (0.35μM) for 5 hrs and reactive oxygen species were quantified using DCF assay as described under materials and methods. The Relative fluorescence unit (RFU) is proportional to ROS levels and values were expressed as fold changes in the ROS level over the control apoE−/− macrophages (n=3). (D) Macrophages were treated with tunicamycin (0.6μM) for 24hrs and then apoptotic cells were quantified (left panel, n=3 independent experiments). Representative micrographs of TUNEL-positive signal (red) in nuclei of peritoneal macrophages treated with tunicamycin are shown in the right panel. Bar 10μm. (E) Macrophages were treated with ox-PAPC at final concentration of 50μg/mL for 4hr, CHOP was quantified by real-time PCR and normalized to cyclophilin (left panel) and ROS were quantified (middle panel) using DCF assay as described under materials and methods and presented as described above (1C). Macrophages were treated with ox-PAPC at final concentration of 50 μg/ml for 24hrs and then TUNEL - positive nuclei were quantified (right panel, n=3 independent experiments). *p<0.05 relative to control.
Fig. 3. ER stress influences mitochondrial function in PON2-def/apoE−/− macrophages.
(A). Macrophages were isolated from the indicated experimental groups and intracellular and mitochondrial calcium levels were quantified. For intracellular calcium, macrophages were treated with indo-1(2μM) and calcium was quantified using a flow cytometer at basal level for 30 seconds and then followed by thapsigargin treatment (25μM) for 4 minutes and 30 seconds. Indo violet (calcium bound dye) was quantified as mean fluorescence intensity and the results were presented as fold change over the thapsigargin treated control apoE−/− macrophages. (B) Macrophages were treated with thapsigargin (25μM) for 1 hr and ERO1-α mRNA was quantified by real-time PCR and normalized to cyclophilin. (C) For measuring mitochondrial calcium levels, macrophages were treated with thapsigargin (25μM) for 1 hr and calcium was measured in the macrophages using Rhodo-2 as described in material and methods and results were presented as fold change in the mitochondrial calcium level over the control apoE−/− macrophages (left panel) and representative histograms of flow cytometry experiments are shown (right panel). (D) Macrophages were treated with medium alone as control or with 0.25 μM thapsigargin for 1 hour. At the end of each incubation, 10 μM Rhod-2, a mitochondrial-specific fluorescent calcium dye, was added to the media, and the cells were incubated on ice for 15 mints at 4°C. The cells were then washed and incubated for an additional 4 hours with thapsigargin, such that total incubation time for thapsigargin was 5 hours and mitochondrial calcium was measured as described in the methods section. Macrophages were isolated from apoE−/− and PON2-def/apoE−/− mice (n =6 per group). For each experiment macrophages from each group (n=2) were pooled (n=2 per group) and treated with thapsigargin at a final concentration 0.25μM for 6hrs. Mitochondrial and cytosolic fractions were isolated and assayed for cytochrome c by western blotting (E), Complex IV activity (F), and mitochondrial superoxide levels presented as fold change over thapsigarigin treated control apoE−/− macrophages (G), as described under materials and methods section. *p<0.05 relative to control.
Fig. 4. ER stress influences mitochondrial function in PON2-def/apoE−/− macrophages and induces apoptosis in a calcium dependent manner.
(A) Macrophages were isolated from experimental groups and pretreated with RU360 (10 μM) for 1 hr followed by thapsigargin 25μM for an additional hour, and mitochondrial calcium was quantified as described in material and methods. Values was expressed as fold change in the mitochondrial calcium level over the untreated control apo E−/−. (B) Macrophages were isolated from experimental groups and pretreated with RU360 at the concentration of 10 μM for 1 hr followed by thapsigargin (0.25 μM) treatment for 6 hrs and mitochondrial superoxide was quantified as described. The RFU is proportional to mitochondrial superoxide level and value was expressed as fold change in the mitochondrial superoxide level over the untreated control apoE−/− macrophages. (C) Macrophages were pretreated with RU360 followed by thapsigargin (2μM) for 24 hrs and then apoptotic nuclei were quantified. n= 3 independent experiments. *p<0.05 relative to control.
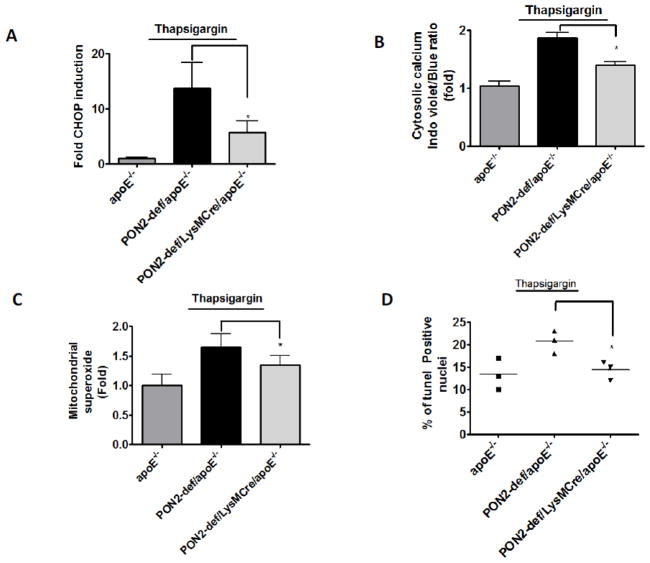
Fig. 6. Restoring macrophage PON2 expression in PON2-def/apoE−/− mice reduces mitochondrial superoxide levels and maintains cell survival under ER stress conditions.
Peritoneal macrophages were obtained from apoE−/−, PON2-def/apoE−/−, and PON2-def/LysMCre/apoE−/− mice on a standard chow diet. (A) Macrophages were treated with thapsigargin at final concentration of 0.25 μM for 6 hrs, CHOP was quantified by real-time PCR. (B) For intracellular calcium, macrophages were pretreated with indo-1(2μM) and intracellular calcium was quantified using a flow cytometer at basal level for 30 seconds followed by thapsigargin treatment (25μM) for 4 minutes and 30 seconds. Indo-1 violet/blue ratio was analysed using flowJo software. Values were expressed as fold change over the control apoE−/− macrophages. (C) Mitochondrial superoxide was quantified in thapsigargin treated cells using fluorescence microplate reader as described. The result were presented as fold change over the control apoE−/− macrophages. (D) TUNEL-positive nuclei were quantified after treatment with thapsigargin (0.25 μM for 24 hrs).) Horizontal lines represent mean values. Each symbol represents results obtained from individual mice. n= 3 independent experiments. *p<0.05 relative to PON2-def/apoE−/− mice.
2.4. Quantification of mitochondrial superoxide
Mitochondrial enriched fraction was isolated from peritoneal macrophages as described by Graham[21]. Mitochondrial superoxide assay was carried out by incubating 4 μg of mitochondrial protein with 5 μM dihydroethidium in a 96-well plate format at 37°C for 60 mins. The reaction was stopped by the addition of 0.6% Triton X-100 and 10 μg of sonicated salmon sperm DNA and the ethidium bromide/DNA fluorescence was measured by excitation at 544 nm and emission at 612 nm using a BMG Labtech fluorescence microplate reader[22].
2.5. Mitochondrial Cytochrome Oxidase (complex IV) activity Assay
Mitochondrial cytochrome oxidase activity assay was carried out as described by the manufacturer’s protocol (Sigma Aldrich, St. Louis, USA). Briefly, 0.22mM of cytochrome c was prepared in water and was reduced (ferrocytochrome c) by adding dithiothreitol (DTT) to the final concentration of 0.5mM. Mitochondrial suspension (100 μg protein) was added into a cuvette containing 0.95 ml assay buffer (10mM Tris-HCl, pH7.0, containing 120 mM KCl) and brought the reaction volume to 1.05ml with dilution buffer (10mM Tris-HCl, pH 7.0, containing 250mM sucrose). Reaction was started by adding 50 μl of 0.22mM ferrocytochrome c and measured at 550 nm at room temperature using kinetic program (10 second interval; 6 reading) with a spectrophotometer. Enzyme activity was expressed as nmol cytochrome c oxidized/mg protein.
2.6. Measurement of calcium level
Intracellular calcium was measured in peritoneal macrophages before and after treatment with tunicamycin or thapsigargin using Indo-1 AM as described[23]. Changes in the intracellular calcium concentration were quantified by a shift in the indo-1 emission peak from 485 nm (indo-blue) for unbound dye to 405 nm (indo-violet) when the indo-1 molecule was bound to calcium. Mitochondrial calcium was measured with Rhod-2/AM. Briefly, peritoneal macrophages in phenol red-free medium were incubated with 1 μM Rhod-2/AM at 37° C for 20 mins followed by thapsigargin (25μM) treatment for 1 hr, then mean fluorescence intensity was analyzed by flow cytometry at 549 nm (excitation) and 581 nm (emission)[24].
2.7. Intracellular Reactive oxygen species (ROS) measurement
A 2′,7′-dichlorofluorescein (DCF) assay was used for the quantification of intracellular ROS as previously described[25]. Briefly, cell viability was assessed with trypan blue on a hemacytometer. Viable cells were plated onto 96-well plates (7×104 cells/well) and loaded with 100 μM DCF (Invitrogen) for 1 hour at 37°C. Cells were subsequently washed using Krebs-Ringer buffer and treated with ER stressor as described in the figure legends. Fluorescence was measured at the indicated time using a fluorescence microplate reader (Spectra Max Gemini XS, Molecular Devices, Sunnyvale, CA) with an excitation filter of 485 nm and an emission filter at 530 nm.
2.8. Quantitative RT-PCR analysis
Total RNA was isolated from peritoneal macrophages of experimental groups by RNeasy mini kit (Qiagen, Valencia, CA, USA), and 0.1 μg RNA was used for reverse transcription with a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA). Two microliter of the cDNA was used for PCR reaction with gene specific primers and Q SYBR Green Supermix (BIO-RAD, Hercules, CA, USA) in a MyiQ Single-Color Real-Time PCR Detection System (BIO-RAD, Hercules, CA, USA). Quantification of mRNA normalization to mouse cyclophilin levels. The primers pairs were as follows (1) C/EBP homologous protein (CHOP) (Forward) 5′-TATCTCATCCCCAGGAAACG-3′ and (Reverse) 5′-CTGCTCCTTCTCCTTCATGC-3′; (2) ER oxidase 1 alpha (ERO1-α) (Forward) 5′-AGCAAACAGCACCAAAGAA-3′ and (Reverse) 5′-TGGTCCTGCGAATCATCATA-3′, (3) GRP78 (Forward) 5′-ACCTGGGTGGGGAAGACTTT -3′ and (Reverse) 5′-TCTTCAAATTTGGCCCGAGT--3′.
2.9. Immunoblotting
Mitochondria-enriched and cytosol fractions were isolated from macrophages, and 50 μg of cytosolic or mitochondrial protein or total protein from macrophages treated with tunicamycin or thapsigargin were loaded on 4%–20% gradient SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked for 1 hour at room temperature in Tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% (w/v) nonfat milk. The following primary antibodies were used at the indicated dilution: Cytochrome c antibodies (Cell Signaling Technology, Danvers, MA, USA) at 1:500 dilution, prohibitin antibodies (Abcam, Cambridge, MA, USA) at 1: 500 dilution, GRP78 antibodies (Santa Cruz Biotechnology Inc, Delaware, Santa Cruz, USA) at 1: 2000, CHOP antibodies (Santa Cruz Biotechnology Inc, Delaware, Santa Cruz, USA) at 1:250 dilution, ERO1-α antibodies (Novus Biologicals, Littleton, CO, USA) at 1: 500 dilution, and β-actin antibodies (Sigma Aldrich, St. Louis, MO, USA) at 1:5000 dilution. Primary antibodies were diluted in TBS containing 3% milk protein at 4°C overnight followed by incubation with appropriate secondary antibody coupled to horseradish peroxidase. The proteins were detected using an ECL or ECL Plus (GE Healthcare, UK).
2.10. Apoptosis assay
The presence of apoptotic cells was assessed by a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay of frozen tissue sections and peritoneal macrophages treated with tunicamycin or thapsigargin or OxPAPC as described under figure legends. For macrophage apoptosis, atleast three separate fields from triplicate wells for each treatment condition were randomly selected and counted for tunel positive nuclei with Nikon E-600 microscope (Nikon, Melville, NY) equipped with a cooled, charge coupled device (CCD) camera (CoolSNAP HQ Monochrome, Roper Scientific, Tucson, AZ). For frozen sections, five random fields from atleast 3 sections of the aortic root from each of the apoE−/− or from PON2-def/apoE−/− were analyzed for apoptotic TUNEL-positive and total (DAPI-stained) cells (6 mice per genotype) or apoE−/−, PON2-def/apoE−/− and PON2-def/LysMCre/apoE−/− mice were analyzed for apoptotic TUNEL-positive and total (DAPI-stained) cells (6 mice per genotype). Percentage of apoptotic cells were calculated using the formula “(Number of apoptotic cells/Total number)×100”
2.11. Analysis of ER stress marker CHOP in atherosclerotic lesions
CHOP expression was analyzed using the immunofluorescence method. The sections were blocked with 5 % sheep serum and were incubated with primary antibodies against CHOP (rabbit antibody, 1:250 in 5% sheep serum) for 3 hrs at 37 °C. After three washes with PBS, the sections were incubated with texas red (Sheep anti-rabbit IgG; Jackson ImmunoResearch, West Grove, PA) in 1:250 dilution in 5 % sheep serum, for 1hr at room temperature. Replacements of the primary antibody with IgG isotypes were performed as negative controls. Sections were then washed and mounted using mounting medium (Vector Laboratories, INC, Burlingame, CA) and examined with a Nikon E-600 microscope (Nikon, Melville, NY) equipped with a cooled, charge coupled device (CCD) camera (CoolSNAP HQ Monochrome, Roper Scientific, Tucson, AZ). Images were obtained and quantified using the Metamorph Image analysis software program.
2.12. Protein estimation
Protein was measured by Bradford method (Sigma Aldrich, St. Louis, USA) as described in commercial kit.
2.13. Statistical analysis
All values are expressed as mean ± standard deviation (SD). Difference between the two groups were analyzed by using a student t test. Differences among the groups were analyzed using One-way ANOVA followed by a Bonferroni adjustment for selected pair of columns and Newman-keu’s for multiple comparisons by Graphpad prisms 5. Images were quantified using image J software. The data shown are mean and Standard deviation (SD). Differences were considered statistically significant at p<0.05.
3. Results
3.1. PON2 deficiency alters the intracellular calcium handling and enhances the unfolded protein response in macrophages in response to ER stress
PON2 is expressed in the ER[10], which is the site of quality control for membrane and secretory proteins[6]. Accumulation of unfolded proteins in the ER causes oxidative stress via the UPR and induces atherogenesis[5]. We first examined, whether PON2 deficiency affects ER stress in the macrophages. Fig. 1A shows a significant increase in tunicamycin-induced gene expression of GRP78, CHOP and ERO1-α (P<0.05) in macrophages isolated from PON2-def/apoE−/− mice compared to control apoE−/− macrophages. The protein levels of GRP78, CHOP and ERO1-α (Fig. 1A) were increased in PON2-def/apoE−/− macrophages when compared to apoE−/− macrophages after treatment with tunicamycin. We did not see any change in the cyclophilin level before or after tunicamycin treatment between the groups (data not shown).
We next examined calcium handling in PON2-def apoE−/− macrophages in response to ER stressor. Tunicamycin-induced calcium flux in short time period (30 seconds to 4 mintutes) was significantly increased in PON2-def/apoE−/− macrophages compared to apoE−/− (Fig. 1B, upper left panel). Tunicamycin-induced intracellular calcium levels at 6hrs point were significantly increased in PON2-def/apoE−/− macrophages compared to apoE−/− macrophages (Fig. 1B, lower panel). However, under untreated conditions, there was no difference in either ER stress response gene expression or intracellular calcium levels between apoE−/− and PON2-def/apoE−/− macrophages (Fig. 1A–B). Moreover, tunicamycin caused a substantial increase in total ROS (Fig. 1C) and apoptosis (Fig. 1D) in PON2-def/apoE−/− macrophages. Fig. 1D right panel shows representative images of apoptotic nuclei from apoE−/− and PON2-def/apoE−/− macrophages upon treatment with tunicamycin. To study the biological significance of ER stress in PON2-def mice in the context of atherosclerosis, macrophages were treated with Ox-PAPC, which is an active component of oxidized LDL and a known inducer of ER stress. As seen in Fig. 1E, Ox-PAPC-induced expression of CHOP, ROS, apoptosis, and caspase 3/7 activity (data not shown) were significantly increased in PON2-def/apoE−/− macrophages. Similar results were observed for ER stress gene expression and ROS production in PON2-def/apoE−/ macrophages were treated with thapsigargin another ER stressor (data not shown).
3.2. PON2 modulates cell survival by regulating intracellular calcium in response to ER stress
To determine whether calcium plays a role in PON2-dependent cell survival following ER stress, macrophages from apoE−/− and PON2-def/apoE−/− mice were pretreated with the inositol 1,4,5 triphosphate receptor blocker, namely xestospongin C. As expected, intracellular calcium levels were reduced when pretreated with xestospongin C; however, there was no difference in tunicamycin-induced intracellular calcium levels between PON2-def/apoE−/− and apoE−/− macrophages when pretreated with xestospongin C (Fig. 2A). Pretreatment with xestospongin C dramatically reduced tunicamycin-induced CHOP expression, ROS, and apoptosis (Fig. 2B–D) in both apoE−/− and PON2-def/apoE−/− macrophages suggesting that intracellular calcium levels are important mediators of PON2-dependent cell survival and UPR under ER stress.
Fig. 2. PON2 modulates cell survival by regulating the intracellular calcium in response to ER stress.
(A) Macrophages were isolated from the indicated experimental groups and pretreated with 0.5 μM xestospongin for 1hr followed by tunicamycin (1mM) and calcium levels was quantified after 4 minutes and 30 seconds as described in fig 1B. (B) Macrophages were pretreated with xestospongin followed by tunicamycin (0.35μM) for 5 hrs then CHOP expression was quantified using qPCR and (C) ROS was quantified using DCF as described in the method section. The results were expressed as fold change in ROS levels over the untreated control (apoE−/−). (D) Macrophages were pretreated with 0.5μM xestospongin for 1 hr followed by tunicamycin (0.6μM) for 24hrs then apoptotic nuclei were quantified. n=3 independent experiments. *p<0.05 relative to control.
3.3. ER stress influences mitochondrial function in PON2-def/apoE−/− macrophages
Mitochondria and the ER maintain a local Ca2+ communication pathway that allows highly effective Ca2+ transfer between the two organelles[7]. Since PON2 deficiency resulted in an elevation of intracellular calcium, we examined whether PON2 deficiency also altered mitochondrial calcium levels and function following ER stress.
PON2-def/apoE−/− macrophages and apoE−/− macrophages were treated with thapsigargin, an ER stress inducer. Similar to our observation with the ER stress inducer tunicamycin in Figure 1B, thapsigargin treatment resulted in elevated intracellular calcium levels in PON2-def/apoE−/− macrophages when compared to apoE−/− macrophages (Fig. 3A). Thapsigargin treatment (25 M, 1hr) increased ERO1-α (ER stress marker) gene expression (Fig. 3B). Mitochondrial calcium levels were significantly increased in PON2-def/apoE−/− macrophages when compared to apoE−/− macrophages at both 1hr (Fig. 3C) and 6hr (Fig. 3D) following thapsigargin treatment. However in untreated cells, there was no difference in mitochondrial calcium between the two groups (data not shown). It has been shown that accumulation of Ca2+ in the mitochondria is known to dissociate cytochrome c from cardiolipin[26]. As seen in Fig. 3E, thapsigargin treatment caused an increase in cytosolic cytochrome c and a corresponding decrease in mitochondrial cytochrome c in PON2-def/apoE−/− macrophages. To further determine whether increased accumulation of mitochondrial calcium alters mitochondrial function, macrophages from both groups were treated with thapsigargin and mitochondrial functions were analyzed. Relative to apoE−/−, after 6 hrs of treatment with thapsigargin mitochondrial complex IV activity was significantly decreased (apoE−/− 140±26, PON2-def/apoE−/− 91±7, p<0.05) in PON2-def/apoE−/− macrophages (Fig. 3F). In untreated cells, complex IV activity and cytochrome c level was not altered between macrophages from apoE−/− and PON2-def/apoE−/− mice (data not shown). Fig. 3G shows that mitochondrial superoxide levels were significantly increased in PON2-def/apoE−/− macrophages following treatment with thapsigargin. Similarly, tunicamycin and Ox-PAPC treatment caused significantly higher mitochondrial calcium accumulation and increased mitochondrial superoxide levels in PON2-def/apoE−/− macrophages (data not shown).
3.4. ER stress influences the mitochondrial function in PON2-def/apoE−/− macrophages and induces apoptosis in a calcium dependent manner
To determine whether calcium mobilization modulates mitochondrial function upon ER stress, macrophages from both groups of mice were pretreated with a mitochondrial calcium uptake blocker namely RU360. RU360 pretreatment caused a pronounced reduction in thapsigargin-induced mitochondrial calcium levels (Fig. 4A) and resulted in complete loss of the PON2-def-dependent increase in thapsigargin-induced mitochondrial superoxide levels and apoptosis in both PON2-def/apoE−/− and apoE−/− macrophages (Fig. 4B–C).
3.5. Intimal cell apoptosis and ER stress are enhanced in the lesions of PON2-def/apoE−/− mice
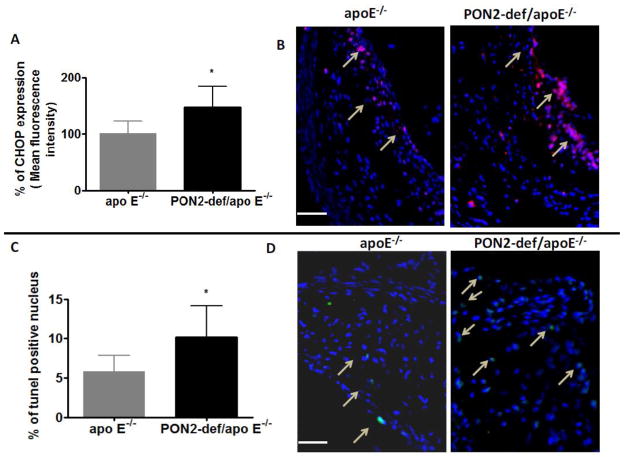
We next examined the effect of PON2 deficiency on ER stress and apoptosis in vivo. PON2-def/apoE−/− and apoE−/− mice were fed a Western diet for 12 weeks and ER stress marker CHOP expression was analyzed in the aortic sections. CHOP was increased in the aorta of PON2-def/apoE−/− mice relative to apoE−/− (Fig. 5A–B). In order to further assess apoptosis in situ, aortic lesions from the two groups of mice were subjected to tunel staining. The number of apoptotic nuclei was significantly higher in PON2-def/apoE−/− mice compared to apoE−/− mice (apoE−/−5.8±2.07, PON2-def/apoE−/−10.5±3.5) (Fig. 5C). Fig. 5D shows the representative images of tunel positive cells in the lesion area.
Fig. 5. Intimal cell ER stress and apoptosis is increased in lesions of PON2-def/apoE−/− mice.
(A) CHOP expression was quantified in the atherosclerotic lesion area of apoE−/− and PON2-def/apoE−/− mice on a Western diet (n=6 per group), as described under material and methods. (B) Representative photographs of CHOP expression in the lesion areas are shown. (C) TUNEL-positive nuclei were quantified in atherosclerotic lesions from PON2-def/apoE−/− mice and apoE−/− on a Western diet (n=6 per group). (D) Representative images show TUNEL-positive signal (indicated by arrows) in nuclei of aortic root lesions. Bar 50μm. *p < 0.05 relative to control.
3.6. Restoring PON2 expression in PON2-def/apoE−/− macrophages reduces the UPR, mitochondrial superoxide levels, and maintains cell survival in response to ER stress
To determine whether macrophage PON2 directly modulates the superoxide levels or maintains cell survival under ER stress conditions, we generated PON2def/LysMCre/apoE−/− mice in which macrophage PON2 expression is partially restored as described previously[14]. Restoring PON2 in macrophages reduces the CHOP gene expression (Fig. 6A) compared to macrophages from PON2-def/apoE−/− mice on treatment with thapsigargin. We did not see any change in the cyclophilin level before or after thapsigargin treatment among the groups (data not shown). In addition, intracellular calcium (Fig. 6B) was restored in PON2def/LysMCre/apoE−/− macrophages compared to PON2-def/apoE−/− macrophages upon treatment with thapsigargin. Mitochondrial superoxide levels were restored in PON2def/LysMCre/apoE−/− macrophages in thapsigargin treated (Fig. 6C) cells. Furthermore, the number of apoptotic nuclei was also reduced in PON2def/LysMCre/apoE−/− macrophages compared to PON2-def/apoE−/− (Fig. 6D).
3.7. Restoration of PON2 in macrophages reduces LDL oxidation, lesion apoptosis, and atherosclerosis
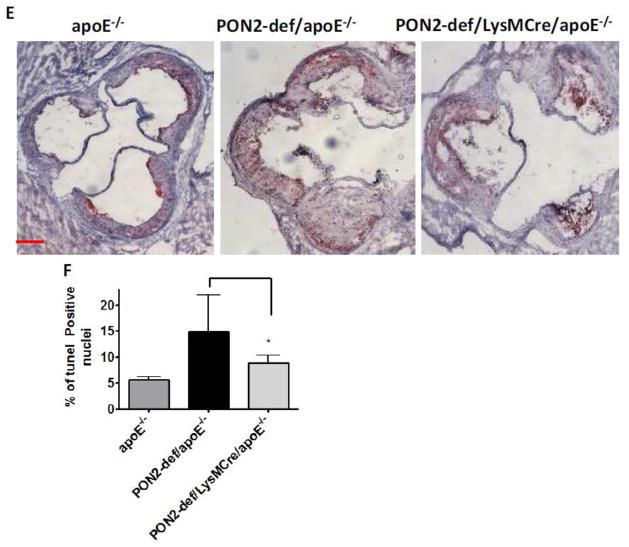
In an ex vivo model, we have previously demonstrated that control LDL incubated with peritoneal macrophages isolated from PON2-def/apoE−/− mice accumulated a significantly increased amount of lipid oxidation, relative to macrophages isolated from apoE−/− [11]. In addition, LDL isolated from PON2-def/apoE−/− mice was associated with increased lipid oxidation[11]. In order to determine whether restoration of PON2 in the macrophages reduces the lipoprotein oxidation in vivo, LDL was isolated from experimental groups and lipid peroxide (LOOH) was measured. Fig. 7A shows that LDL oxidation is significantly lower in PON2-def/LysMCre/apoE−/− compared to PON2-def/apoE−/− mice. It has been shown that oxidized LDL recruits monocytes into the sub-endothelial space where they differentiate into macrophages, which in turn leads to the formation of foam cells and atherosclerosis. Since LDL oxidation was lower in the serum of PON2-def/LysMCre/apoE−/− mice, additional aortic sections from experimental groups were stained for macrophages using CD68 as the marker. Indeed, there were lower levels of macrophage immunoreactivity in the aortic sections of PON2-def/LysMCre/apoE−/− as compared to PON2-def/apoE−/− mice (Fig. 7B). Moreover, on a Western diet PON2-def/LysMCre/apoE−/− mice developed significantly lowered en face lesions along the whole aorta compared to PON2-def/apoE−/− (Fig. 7C). In the current study, since ER stress was reduced in the macrophages of PON2-def/LysMCre/apoE−/− mice in ex vivo model, we examined whether the lesion area and lesional apoptosis were also reduced in PON2-def/LysMCre/apoE−/− mice. As seen in Fig. 7D, atherosclerotic lesions were significantly decreased in PON2-def/LysMCre/apoE−/− mice compared to PON2-def/apoE−/− mice. Fig. 7E shows the representative images of aortic lesions from experimental groups. Apoptotic nuclei were significantly decreased in PON2-def/LysMCre/apoE−/− compared to PON2-def/apoE−/− mice (Fig. 7F).
Fig. 7. Restoring macrophage PON2 expression in PON2-def/apoE−/− mice reduces LDL oxidation, apoptosis and atherosclerosis.
(A) LDL fractions were isolated from experimental groups, pooled, and lipid extracted and LOOH content quantified by Auerbach method. B) Serial aortic root sections (10μm) from apoE−/−, PON2-def/apoE−/− and PON2def/LysMCre/apoE−/− mice administered a high-fat Western diet were immunostained for macrophage-specific marker CD68. Data was presented as morphometric analysis of stained area of lesions from each group. (n=8 per group) Horizontal lines represent mean values. Each symbol represents results obtained from individual mice. (C) En face analysis of atherosclerotic lesions of female apoE−/−(n=9), PON2-def/apoE−/−(n=5), and PON2def/LysMCre/apoE−/− mice (n=7), placed on a high-fat Western diet. Horizontal lines represent mean values. Circles represent results obtained from individual mice. (D) Analysis of aortic root lesions in female apoE−/− (n=8), PON2-def/apoE−/− (n=8), and PON2def/LysMCre/apoE−/− (n=8), mice placed on a high-fat Western diet for 16 weeks. Horizontal lines represent mean values. Each symbol represents results obtained from individual mice. (E) Representative root lesion from the experimental groups. Bar 0.5mm. (F) TUNEL-positive nuclei were quantified in lesions from the experimental groups placed on a Western diet. (n=6 per group). p<0.05 relative to PON2-def/apoE−/− mice.
4. Discussion
Atherosclerosis is the primary mechanism underlying the development of coronary heart disease[2]. Cell culture studies and mouse models of atherosclerosis have revealed a critical role for UPR in lesional macrophage apoptosis and atherosclerotic lesion formation[27]. Perturbations of the ER leads to an evolutionarily conserved cell stress response, the UPR, which is aimed initially at compensating for damage. It can however, eventually trigger cell death if ER dysfunction is severe or prolonged. These perturbations include increased protein synthesis, disturbance of ER calcium homeostasis, disturbances in the redox environment of the ER, alteration in the physical property of the ER membrane bilayer and misfolded proteins[28]. Studies from genetically modified mice or cells demonstrated a role for CHOP, Activating transcription factor 4 (ATF4), inositol 1,4,5-triphosphate receptor (IPT3), ERO1-α, and Bcl-2, in UPR in response to ER stress and atherosclerosis[27–33].
PON2 is a member of PON gene family which consists of PON1, PON2, and PON3. In humans, these three genes are 65% similar at the level of amino acids and 70% similar at the level of nucleotides[8]. In contrast to PON1 and PON3, PON2 is localized in the arterial wall, including macrophages[9]. We have shown that stably transfected cells overexpressing PON2 exhibit significantly lower levels of intracellular oxidative stress when exposed to oxidized phospholipids[12]. PON2 deficiency in mice increases susceptibility to atherosclerosis and this has primarily been associated with increased liver and macrophage oxidative stress which leads to LDL oxidation[15]. Further, LDL from PON2-def mice induced significantly more monocyte chemotaxis than LDL from their control littermates and it was associated with higher levels of macrophage immunoreactivity in the aortic sections of PON2-def mice compared to their wild type controls[15]. Recently, we have shown that PON2 deficiency results in enhanced oxidative stress accompanied by increased mitochondrial oxidative stress with reduced ETC complex I + III activities, oxygen consumption, and ATP levels and exacerbated atherosclerosis[11]. In addition to the mitochondria, PON2 is also found in the ER. PON2 overexpression reduces ER stressor mediated UPR[10]. It is known that exogenous antioxidants reduce UPR upon ER stress[34]. However, it is not known whether the protective effect of PON2 on the ER is due to a direct effect of PON2 on the ER or as a result of the generalized antioxidant properties of PON2. Our current study reveals that PON2 can affect calcium levels and thereby reduces the UPR and improves cell survival under ER stress conditions. Calcium in the ER lumen acts as a cofactor in the regulation of membrane and secretory protein synthesis, protein folding including post-translational modifications, sterol synthesis, and lipid synthesis[5, 28, 35]. Aberrant Ca2+ regulation in the ER causes the protein unfolding leading to UPR. The initial intent of the UPR is to adapt to the changing environment and reestablish normal ER function. Prolonged perturbation of ER by deregulation of calcium level leads to oxidative stress and apoptosis[6, 27, 28, 35]. Thus, our results implicate that there is a novel PON2-dependent calcium mediated cell death pathway in macrophages.
In the current study, we observed increased mitochondrial superoxide formation following ER stress in PON2-def/apoE−/− macrophages (Fig. 3E). This could be due to increased cytosolic calcium that enters into mitochondria and opens the permeability transition pore to release cytochrome c from the inner mitochondrial membrane[36] into the cytosol, where it is no longer able to transfer electrons from complex III (cytochrome bc1 complex) to complex IV (cytochrome oxidase) within the ETC[37]. Indeed, our results show increased cytochrome c in the cytosol, together with decreased complex IV activity, in macrophages stimulated with ER stressor. With decreased complex IV activity, complex III is unable to transfer its electrons to complex IV as effectively. As a result, more ubisemiquinone radical is released from complex III, which is capable of giving its unpaired electron to molecular O2 to form superoxide[38]. PON2 deficiency can further enhance the production of superoxide, as we have previously reported it is the role of PON2 to sequester ubisemiquinone and thereby protect the mitochondria from oxidative damage[11]. Current studies explain the previously unexplored mechanism of mitochondrial superoxide generation under ER stress condition. Within this model, this crosstalk will trigger the production and accumulation of superoxide, which oxidizes cardiolipin and leads to the dissociation of cytochrome c. Thus, ROS generation can further induce the generation of ROS via a mechanism proposed as “ROS induced ROS generation”. Oxidative stress resulting from such feed-forward loops could be devastating by causing cellular damage and apoptosis. Although emerging evidence suggests that there exists an ER-mitochondrial cross-talk in cells under stress[7] the mechanism(s) remain less clear. In the present study, inducers of ER stress including tunicamycin, thapsigargin and ox-PAPC induced UPR (Figs 1–2 and 6), cell death (Figs. 1–2 and 6), and mitochondrial dysfunction (Figs. 3–4 and 6). However, both ER stress, mitochondrial dysfunction, and ER stress induced mitochondrial dysfunction were clearly modulated by PON2, suggesting that PON2 is a key player in the cross talk between ER and mitochondria. Further research will determine the molecular mechanisms by which PON2 modulates calcium handling under ER stress conditions. Nevertheless, the present functional data demonstrates that PON2 maintains the calcium homeostasis and protects the ER and mitochondrial function under ER stress conditions atleast in the macrophages.
Guns et al reported that PON1 gene transfer (adenoviral) regulates calcium homeostasis in smooth muscle cells, and improves vasomotor function in apo E−/− deficient mice with pre-existing atherosclerosis [39]. Using cell culture models and PON3 knockout mouse model Schweikert et al reported that PON3 modulates the mitochondrial oxidative stress during adverse condition and further PON3 deficiency impaired the cell survival upon ER stress[40]. Studies from various laboratories suggest that all three PONs protects against oxidative stress and may function similarly[8, 41]. The question arises as to why there are three PONs? Sequence analysis of PON genes suggest that the PON family evolved by gene duplication with PON2 being the first and PON1 the most recent member. PON1 is produced in the liver and found exclusively associated with high density lipoprotein (HDL) particles in the circulation, and, in part, confers the anti-oxidant and anti-inflammatory properties associated with HDL [9]. PON2 and PON3, expressed in some common as well as distinct cell types, are predominantly localized to intracellular compartments (ER and mitochondria) and modulate cellular oxidative stress generated both by intracellular mechanisms and in response to extracellular stimuli. Thus, there is a clear spatial difference between the three PONs [9, 42]. Additionally, a recent study suggests that PON1 mRNA expression in mouse liver was very low in the fetus, but rapidly increased at birth, reaching adult levels at 15 days of age [43]. In contrast, both PON2 and PON3 expression levels were high in the livers of mouse fetuses while they were decreased after birth (3). Previous reports suggest that oxidative stress induces PON2 and PON3 expression whereas it reduces PON1 gene expression [9]. Thus, there also appears to be a temporal regulation of the PON gene family. Understanding the spatiotemporal molecular targets of PON proteins will unravel novel markers and therapeutic targets for the treatment of inflammatory diseases associated with ER and mitochondrial dysfunction mediated oxidative stress including atherosclerosis.
Macrophages are hallmark of inflammation and plays an important role in the progression of atherosclerosis[44]. Our finding suggest that restoring PON2 in macrophages is sufficient to inhibit aggravated atherosclerosis in PON2-def/apoE−/− mice. It has been documented that expression of an individual gene in a given cell type has resulted in unexpected observations. For example, Abca1 gene has been well studied for its role in cholesterol efflux in macrophages; however, hepatic Abca1 but not the macrophage Abca1 is atheroprotective in mice[45]. Cyclo oxygenase-2 (COX-2) inhibitor usage resulted in cardiovascular side effects prompting extensive research into the contribution to atherogenesis of COX-2 in different cell types. Although COX-2 and its metabolites/products are implicated in every aspect of acute and chronic inflammation including resolution phase, deletion of myeloid COX-2 does not influence the development of atherosclerosis in mice[46]. In this regard, our finding suggest that the protective mechanism of action of PON2 is likely dependent on its function in the myeloid cell type involved in the artery wall milieu.
Conclusion
In conclusion, we have demonstrated that macrophage PON2 is sufficient to reduce the lesion apoptosis and the development of atherosclerosis in PON2-def/apoE−/− mice on a Western diet. We further postulate that ER stress, and ER stress mediated mitochondrial dysfunction is regulated, in part, by PON2.
Highlights.
Background: Paraoxonase 2 (PON2) deficiency causes impaired macrophage function and exacerbates the development of atherosclerosis.
Results: In this report, we show, for the first time, that PON2 modulates calcium homeostasis and the cross talk between ER and mitochondria in macrophages. We further demonstrate that restoration of macrophage PON2 in PON2-def/apoE−/− mice is sufficient to reduce atherosclerosis.
Conclusion: Our results suggest that i) PON2 in macrophages plays an important role in attenuating the development of atherosclerosis in mice and ii) is a key player in the crosstalk between ER and mitochondria during oxidative stress.
Acknowledgments
We thank Dr. Gao Feng for valuable discussions. We also thank Ani Shabazian, and Yuen Lin Lee for their expert technical assistance. This work was supported by the National Heart, Lung and Blood Institute grant 1RO1HL71776 (S.T.R).
Abbreviations
- ATF4
Activating transcription factor 4
- CHOP
C/EBP homologous protein
- DCF
Dichlorofluorescein
- ER
Endoplasmic Reticulum
- ERO1-α
ER oxidase 1 alpha
- ETC
Electron Transport Chain
- IPT3
Inositol 1,4,5-triphosphate receptor
- LOOH
Lipid peroxide
- LDL
Low Density Lipoprotein
- IMM
Inner Mitochondrial Membrane
- UPR
Unfolded Protein Response
- Ox-PAPC
Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine
- PON2-def
Paraoxonase 2-deficiency
Footnotes
Conflicts of interest
There are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mabile L, Meilhac O, Escargueil-Blanc I, Troly M, Pieraggi MT, Salvayre R, Negre-Salvayre A. Mitochondrial function is involved in LDL oxidation mediated by human cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1575–1582. doi: 10.1161/01.atv.17.8.1575. [DOI] [PubMed] [Google Scholar]
- 4.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margittai E, Sitia R. Oxidative protein folding in the secretory pathway and redox signaling across compartments and cells. Traffic. 2011;12:1–8. doi: 10.1111/j.1600-0854.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 7.Walter L, Hajnoczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- 8.Reddy ST, Devarajan A, Bourquard N, Shih D, Fogelman AM. Is it just paraoxonase 1 or are other members of the paraoxonase gene family implicated in atherosclerosis? Curr Opin Lipidol. 2008;19:405–408. doi: 10.1097/MOL.0b013e328304b64e. [DOI] [PubMed] [Google Scholar]
- 9.Ng CJ, Shih DM, Hama SY, Villa N, Navab M, Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med. 2005;38:153–163. doi: 10.1016/j.freeradbiomed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 10.Horke S, Witte I, Wilgenbus P, Kruger M, Strand D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 11.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke CF, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid Redox Signal. 2011;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 13.Altenhofer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Forstermann U, Horke S. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourquard N, Ng CJ, Reddy ST. Impaired hepatic insulin signalling in PON2-deficient mice: a novel role for the PON2/apoE axis on the macrophage inflammatory response. Biochem J. 2011;436:91–100. doi: 10.1042/BJ20101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 16.Tangirala RK, Rubin EM, Palinski W. Quantitation of Atherosclerosis in Murine Models - Correlation between Lesions in the Aortic Origin and in the Entire Aorta, and Differences in the Extent of Lesions between Sexes in Ldl Receptor-Deficient and Apolipoprotein E-Deficient Mice. Journal of Lipid Research. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 17.Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley BJ, Whorton AR. Tunicamycin increases intracellular calcium levels in bovine aortic endothelial cells. Am J Physiol. 1997;273:C1298–1305. doi: 10.1152/ajpcell.1997.273.4.C1298. [DOI] [PubMed] [Google Scholar]
- 20.Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–17630. [PubMed] [Google Scholar]
- 21.Graham JM. Isolation of mitochondria from tissues and cells by differential centrifugation Current protocols in cell biology/editorial board. In: Bonifacino Juan S, et al., editors. Unit 3. Chapter 3. 2001. p. 3. [DOI] [PubMed] [Google Scholar]
- 22.Morten KJ, Ackrell BA, Melov S. Mitochondrial reactive oxygen species in mice lacking superoxide dismutase 2: attenuation via antioxidant treatment. J Biol Chem. 2006;281:3354–3359. doi: 10.1074/jbc.M509261200. [DOI] [PubMed] [Google Scholar]
- 23.MacFarlane AWt, Oesterling JF, Campbell KS. Measuring intracellular calcium signaling in murine NK cells by flow cytometry Methods. Mol Biol. 2010;612:149–157. doi: 10.1007/978-1-60761-362-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y, Tombran-Tink J. Mitochondrial Decay and Impairment of Antioxidant Defenses in Aging RPE Cells. Adv Exp Med Biol. 2010;664:165–183. doi: 10.1007/978-1-4419-1399-9_20. [DOI] [PubMed] [Google Scholar]
- 25.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. Journal of Biological Chemistry. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 26.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 29.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein. Bim Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto M, Nagata E. Type 1 inositol 1,4,5-trisphosphate receptor knock-out mice: their phenotypes and their meaning in neuroscience and clinical practice. J Mol Med (Berl) 1999;77:406–411. doi: 10.1007/s001090050370. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 37.Duan Y, Gross RA, Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol. 2007;585:741–758. doi: 10.1113/jphysiol.2007.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 39.Guns PJ, Van Assche T, Verreth W, Fransen P, Mackness B, Mackness M, Holvoet P, Bult H. Paraoxonase 1 gene transfer lowers vascular oxidative stress and improves vasomotor function in apolipoprotein E-deficient mice with pre-existing atherosclerosis. British journal of pharmacology. 2008;153:508–516. doi: 10.1038/sj.bjp.0707585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweikert EM, Devarajan A, Witte I, Wilgenbus P, Amort J, Forstermann U, Shabazian A, Grijalva V, Shih DM, Farias-Eisner R, Teiber JF, Reddy ST, Horke S. PON3 is upregulated in cancer tissues and protects against mitochondrial superoxide-mediated cell death Cell death and differentiation. 2012 doi: 10.1038/cdd.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witte I, Foerstermann U, Devarajan A, Reddy ST, Horke S. Protectors or Traitors: The Roles of PON2 and PON3 in Atherosclerosis and Cancer. Journal of lipids. 2012;2012:342806. doi: 10.1155/2012/342806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicology and applied pharmacology. 2011;256:369–378. doi: 10.1016/j.taap.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng X, Klaassen CD. Hormonal and Chemical Regulation of Paraoxonases in Mice The Journal of pharmacology and experimental therapeutics. 2012 May 31; doi: 10.1124/jpet.112.194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabas I. Macrophage Apoptosis in Atherosclerosis: Consequences on Plaque Progression and the Role of Endoplasmic Reticulum Stress. Antioxid Redox Sign. 2009;11:2333–2339. doi: 10.1089/ars.2009.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 46.Narasimha AJ, Watanabe J, Ishikawa TO, Priceman SJ, Wu L, Herschman HR, Reddy ST. Absence of myeloid COX-2 attenuates acute inflammation but does not influence development of atherosclerosis in apolipoprotein E null mice. Arterioscler Thromb Vasc Biol. 2010;30:260–268. doi: 10.1161/ATVBAHA.109.198762. [DOI] [PMC free article] [PubMed] [Google Scholar]