Abstract
Two of the most agriculturally important cereal crop plants are wheat (Triticum aestivum) and rice (Oryza sativa). Rice has been shown to produce a number of diterpenoid natural products as phytoalexins and/or allelochemicals – specifically, labdane-related diterpenoids, whose biosynthesis proceeds via formation of an eponymous labdadienyl/copalyl diphosphate (CPP) intermediate (e.g., the ent-CPP of gibberellin phytohormone biosynthesis). Similar to rice, wheat encodes a number of CPP synthases (CPS), and the three CPS characterized to date (TaCPS1,2,&3) all have been suggested to produce ent-CPP. However, several of the downstream diterpene synthases will only react with CPP intermediate of normal or syn, but not ent, stereochemistry, as described in the accompanying report. Investigation of additional CPS did not resolve this issue, as the only other functional synthase (TaCPS4) also produced ent-CPP. Chiral product characterization of all the TaCPS then revealed that TaCPS2 uniquely produces normal, rather than ent-, CPP; thus, providing a suitable substrate source for the downstream diterpene synthases. Notably, TaCPS2 is most homologous to the similarly stereochemically differentiated syn-CPP synthase from rice (OsCPS4), while the non-inducible TaCPS3 and TaCPS4 cluster with the rice OsCPS1 required for gibberellin phytohormone biosynthesis, as well as with a barley (Hordeum vulgare) CPS (HvCPS1) that also is characterized here as similarly producing ent-CPP. These results suggest that diversification of labdane-related diterpenoid metabolism beyond the ancestral gibberellins occurred early in cereal evolution, and included the type of stereochemical variation demonstrated here.
Keywords: Oryza sativa, Triticum aestivum, Hordeum vulgare, copalyl diphosphate synthase
1. Introduction
Wheat and rice provide the bulk of the direct caloric intake for the global human population (Dyson, 1996). Accordingly, their ability to resist diseases is of considerable interest. Notably, a broadly conserved aspect of the plant defense response is the production of phytoalexins, natural products whose biosynthesis is induced by pathogen infection, and which exhibit antibiotic activity against the responsible microbe (VanEtten et al., 1994). Rice has served a model system for the cereal crop plant family, with exhaustive sequence information available (Kikuchi et al., 2003; IRGSP, 2005), which has led to extensive studies of various aspects of its metabolism, including that of phytoalexins. Of particular importance in rice seems to be an array of labdane-related diterpenoids, with over twenty such natural products from rice meeting the definition of phytoalexins, with some also suggested to serve as allelochemicals (Peters, 2006).
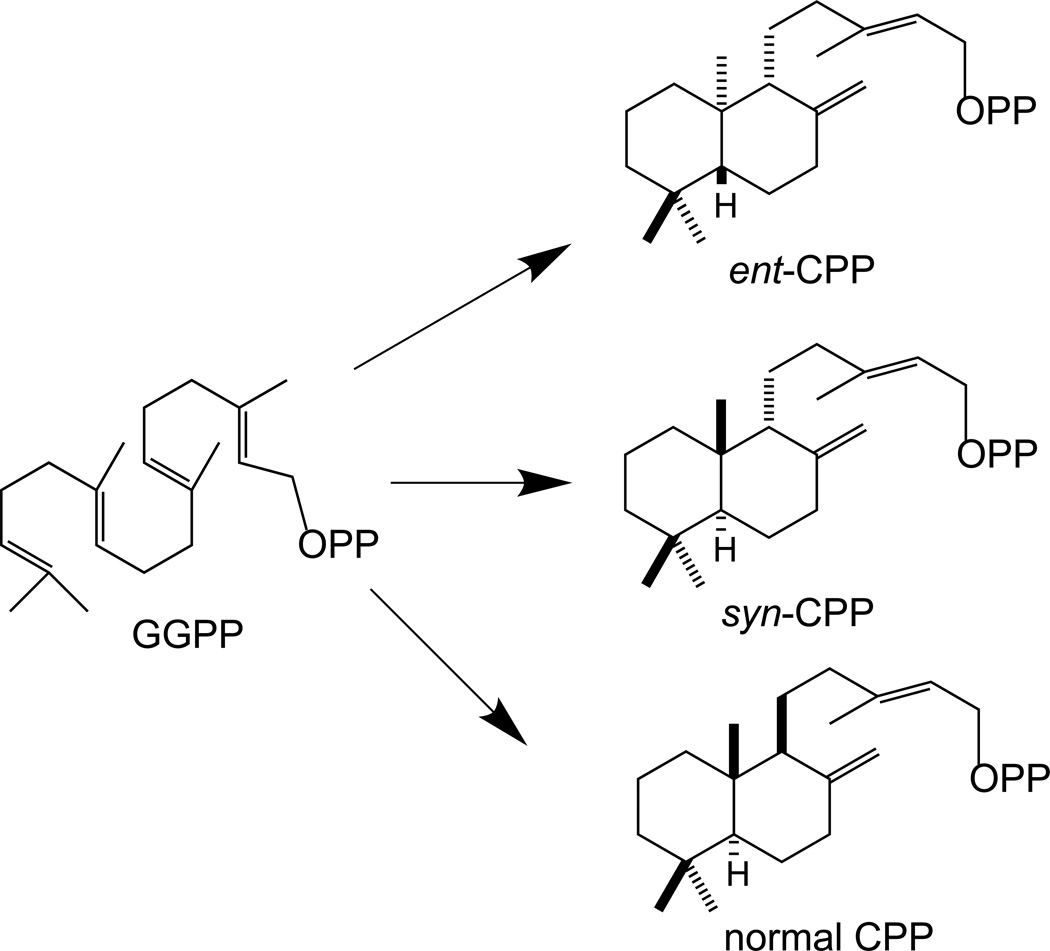
The labdane-related diterpenoids represent a large super-family of natural products, with ~7,000 known, that share a characteristic decalin ring structure in common (Peters, 2010). This core structure is formed by bicyclization of the general diterpenoid precursor (E,E,E)-geranylgeranyl diphosphate (GGPP), in protonation-initiated reactions catalyzed by class II diterpene cyclases (Fig. 1). These generally produce one of the three common stereoisomers of CPP, syn-, ent-, or normal, with the corresponding enzymes then termed CPP synthases (CPSs). This intermediate is then generally further cyclized and/or rearranged to diterpene olefins by CPP specific class I diterpene synthases, which are often termed ent-kaurene synthase-like (KSL) for the presumably ancestral enzyme, at least in plants, where ent-kaurene synthases (KSs) are required for gibberellin phytohormone biosynthesis.
Figure 1.
Cyclization of geranylgeranyl diphosphate (GGPP) to copalyl diphosphate (CPP), which can vary in stereochemistry, with common designations (ent-, syn-, and normal) as shown.
Of particular interest here, the committed step in labdane-related diterpenoid biosynthesis is then catalyzed by CPSs, which appear to be regulated by synergistic inhibitory effects exerted by GGPP and magnesium (Mg2+). Those class II diterpene cyclases involved in gibberellin biosynthesis are so inhibited, whereas those dedicated to secondary/more specialized metabolism are relatively immune to such inhibition (Prisic and Peters, 2007). Notably, this differential susceptibility has been attributed to the exact identity of a particular residue, histidine versus arginine, as found in gibberellin associated CPSs relative to more specialized enzymes (Mann et al., 2010). However, while this single residue regulatory switch was originally hypothesized to play a direct role in catalysis, this has not been born out by the recently determined crystal structure of the gibberellin associated CPS from the dicot Arabidopsis thaliana [i.e., AtCPS (Köksal et al., 2011)], leaving the underlying mechanism opaque.
Consistent with the observed complexity of labdane-related diterpenoid metabolism in rice, its genome contains three functional CPS (OsCPS), all of which have been characterized (Peters, 2006; Toyomasu, 2008). OsCPS1 is required for GA biosynthesis (Sakamoto et al., 2004), produces the relevant ent-CPP, and does not exhibit inducible transcription (Otomo et al., 2004; Prisic et al., 2004). On the other hand, the transcription of OsCPS2 and OsCPS4 (OsCPS3 being a pseudogene) is induced by elicitation with chitin or methyl jasmonate, or by UV-irradiation, with OsCPS2 producing ent-CPP and OsCPS4 producing syn-CPP, and these presumably are involved in more specialized metabolism (Otomo et al., 2004; Prisic et al., 2004; Xu et al., 2004).
Molecular phylogenetic analysis based on characterization of maize (Zea mays) as well as rice CPS (Harris et al., 2005), suggested that CPS gene expansion and functional diversification to more specialized metabolism occurred early in the cereal crop plant family – i.e., the Poaceae (Peters, 2006). Consistent with this hypothesis, gene probing/mapping experiments with a CPS from barley (Hordeum vulgare) indicated that at least barley and wheat also contain an expanded CPS gene family (Spielmeyer et al., 2004). In a preliminary report, it was demonstrated that wheat contains at least three CPS (TaCPS1–3), with TaCPS1 and TaCPS2 exhibiting inducible transcription consistent with a role in more specialized metabolism. All three were suggested to produce ent-CPP, although the possibility that they might produce the enantiomeric normal CPP was acknowledged as well (Toyomasu et al., 2009). As described in the accompanying report on the wheat KSL (TaKSL), some of these downstream enzymes only react with syn- or normal (i.e., not ent-), CPP (Zhou et al., submitted). These results prompted us to look for syn- or normal CPP producing CPS in wheat. Here we report that the only other functional CPS we were able to clone from wheat (TaCPS4), also produced ent- or normal (but not syn-) CPP, as does a previously cloned barley CPS [i.e., HvCPS1 (Spielmeyer et al., 2004)]. Accordingly, wheat does not appear to encode a syn-CPP synthase. We then more specifically analyzed the stereochemistry of the resulting CPP for all the TaCPS, as well as HvCPS1, and found that TaCPS2 uniquely produces normal, rather than ent-, CPP. The implications of these findings for the evolution of diterpenoid metabolism in the cereal crop family, as well as more generally, are discussed.
2. Results
2.1 Identification of copalyl diphosphate synthase genes from wheat and barley
Isolation of HvCPS1 has been previously reported (Spielmeyer et al., 2004), as has that of TaCPS1-3 (Toyomasu et al., 2009). Given the exclusive use of syn- or normal CPP by some of the downstream TaKSL, as reported in the accompanying study (Zhou et al., submitted), along with the previously reported suggestion that TaCPS1-3 all produced ent-CPP (Toyomasu et al., 2009), we sought additional wheat CPS(s). Further cloning efforts yielded two additional CPS gene fragments, albeit these were closely related to TaCPS2 and 3, raising the possibility that they were polyploidy derived homoeologs. Nevertheless, we extended these via RACE to cover sufficient amounts of the corresponding open reading frames for biochemical analysis, and have designated them TaCPS4 and 5 (GenBank accessions AB525387 and AB525388, respectively). These do not appear to be homoeologs of TaCPS2 and 3, as both share less than 90% nucleotide sequence identity with any other TaCPS. Since mRNA levels of TaCPS1 and 2 are increased in response to UV-irradiation (Toyomasu et al., 2009), such elicitation of TaCPS4 and 5 was investigated, but found not to occur (data not shown).
2.2 Functional characterization of TaCPS4-5 and HvCPS1
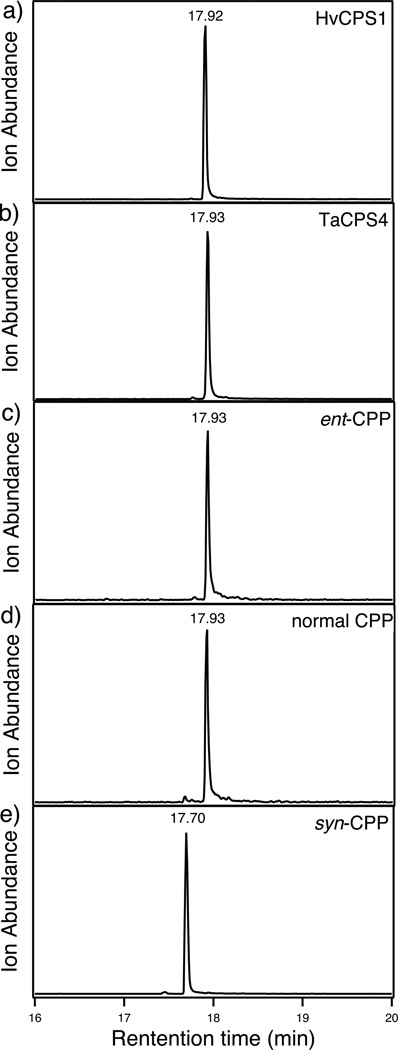
HvCPS1 and TaCPS4 & 5 were functionally characterized via use of a previously developed metabolic engineering system (Cyr et al., 2007) – specifically, by using a vector carrying a GGPP synthase into which a CPS also can be easily incorporated for functional analysis, as previously described (Morrone et al., 2009). Extraction of the resulting recombinant cultures yields copalol released by dephosphorylation of the heterologously produced CPP by endogenous phosphatases. Analysis by gas chromatography with mass spectrometric detection (GC-MS) demonstrated the production of copalol by cultures expressing HvCPS1 and TaCPS4, indicating that these, but not TaCPS5, are functional CPP synthases. Comparison to authentic standards further indicated that HvCPS1 and TaCPS4 produced CPP of ent- or normal stereochemistry (Fig. 2).
Figure 2.
Characterization of TaCPS4 and HvCPS1 activity. GC-MS analysis (275 m/z extracted ion chromatographs) of the dephosphorylated enzymatic products of (a) HvCPS1 and (b) TaCPS4, compared to authentic standards for dephosphorylated (c) ent-, (d) normal, or (e) syn- CPP.
2.3 Stereochemical analysis of TaCPS1-4 and HvCPS product outcome
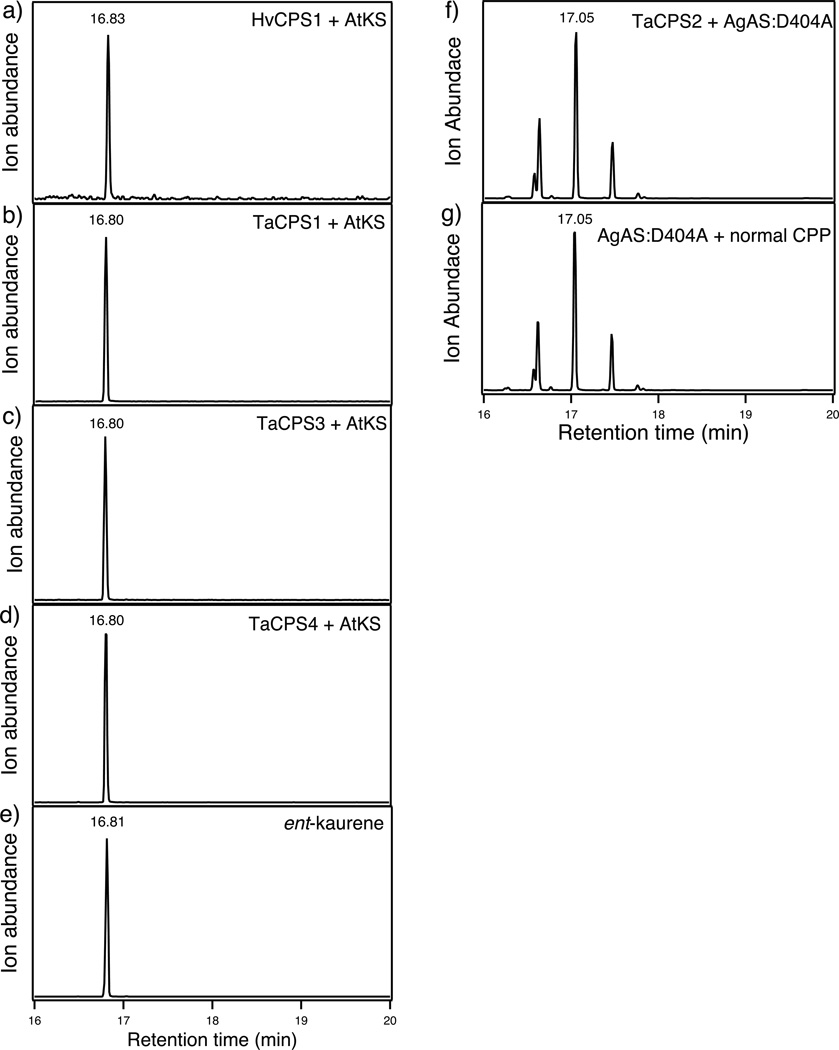
Given the stereospecificity of several TaKSL for syn- or normal, but not ent-, CPP (Zhou et al., submitted), and the notable lack of production of syn-CPP by any of the functional TaCPS, we further investigated the stereochemical outcome mediated by all the TaCPS, as well as HvCPS1. This was accomplished by coupling these to stereospecific KSL, using the metabolic engineering system, much as previously described (Morrone et al., 2009). In particular, each of these CPS were co-expressed with not only a GGPP synthase, but also either the ent-CPP specific kaurene synthase from Arabidopsis thaliana (AtKS), or the normal CPP specific abietadiene synthase from Abies grandis [i.e., the AgAS:D404A mutant that retains only KSL/class I activity (Peters et al., 2001)]. GC-MS analysis of extracts from the resulting recombinant cultures demonstrated that TaCPS1, 3, 4 and HvCPS1 produce ent-CPP, as coexpression of these with AtKS results in production of ent-kaurene (Figure 3a–e), while abietadienes are not observed upon co-expression with AgAS:D404A (data not shown). By contrast, TaCPS2 produces normal CPP, as co-expression of this with AgAS:D404A results in production of abietadienes (Figure 3f&g), while ent-kaurene is not observed upon co-expression with AtKS (data not shown).
Figure 3.
Characterization of product stereochemistry for TaCPS1-4 and HvCPS1. GC-MS analysis (275 m/z extracted ion chromatographs) of the ent-kaurene produced by the coupled action of the ent-CPP specific AtKS with (a) HvCPS1, (b) TaCPS1, (c) TaCPS3, (d) TaCPS4, with comparison to an authentic sample (e), and the production of abietadienes by the normal CPP specific AgAS:D404A coupled with (f) TaCPS2 or fed (g) normal CPP.
2.4 Molecular phylogenetic analysis of cereal CPS
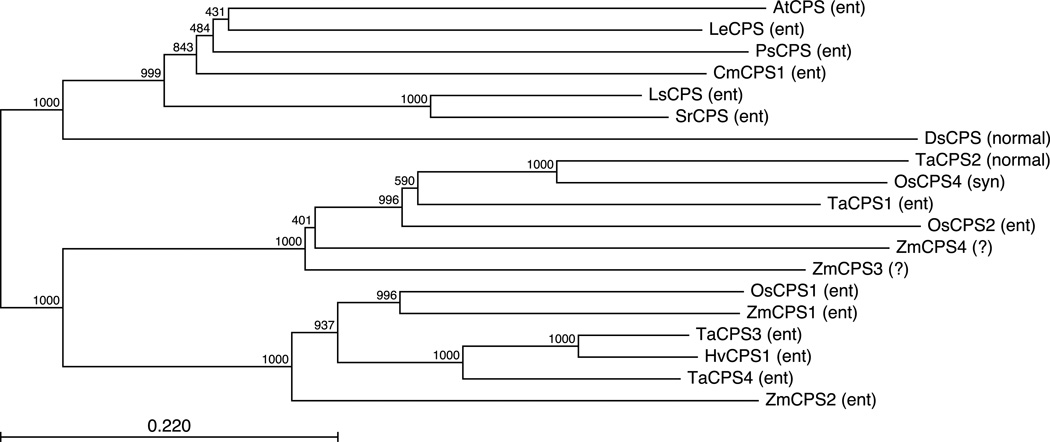
While molecular phylogenetic analysis of TaCPS1-3 was previously described (Toyomasu et al., 2009), we repeated this here to include TaCPS4 and HvCPS1, as well as a wider range of characterized CPSs (Figure 4). In particular, we aligned the full-length amino acid sequences of not only the TaCPS and HvCPS1 characterized here, but also CPSs from rice and the more distantly related cereal Zea mays [i.e., ZmCPS1-4 from the maize genome (Schnable et al., 2009)]. In addition, a number of CPS from dicots also were included to provide a designated outgroup for the resulting phylogenetic tree.
Figure 4.
Phylogenetic analysis of cereal CPS family. (a) Phylogenetic tree constructed from amino acid sequence alignment using the Neighbor Joining method. (b) Amino acid alignment of TaCPS1-4 and HvCPS1, with the catalytic ‘DXDD’ motif underlined and the ‘*’ underneath the regulatory switch position described in the text.
3. Discussion
It has been recently demonstrated that maize produces labdane-related diterpenoids as phytoalexins (Schmelz et al., 2011), much as has been found with rice (Peters, 2006; Toyomasu, 2008). This suggests that such more specialized metabolism may have arisen early in evolution of the Poaceae (i.e., cereal plant family). Consistent with this hypothesis, not only maize and rice, but also barley and wheat, contain expanded CPS and KS(L) gene families encoding the sequentially acting diterpene synthases that are the distinguishing feature of labdane-related diterpenoid biosynthesis. Although no such phytoalexins have yet been identified from wheat, the UV-inducible transcription of TaCPS1 and TaCPS2 suggests that these potentially play a role in phytoalexin biosynthesis. In addition, the demonstration that TaCPS2 produces normal CPP provides direct evidence that wheat can produce labdane-related diterpenoids other than gibberellins. The presence of such biosynthetic capacity is further supported and clarified by the characterization of TaKSLs that similarly do not produce the ent-kaurene intermediate of gibberellin biosynthesis, but alternative tricyclic (pimaradiene) diterpene olefins instead, as described in the accompanying report (Zhou et al., submitted).
Phylogenetic analysis of the characterized CPSs from angiosperms indicates early expansion and functional diversification of those from monocots/cereals (Fig. 4A). These CPSs fall into two distinct clusters, one of which is associated with gibberellin biosynthesis in both maize and rice [i.e., An1/ZmCPS1 and OsCPS1 (Bensen et al., 1995; Sakamoto et al., 2004)]. The grouping of TaCPS3 & 4, as well as HvCPS1, with ZmCPS1 and OsCPS1 suggests that these may similarly play a role in such phytohormone metabolism. However, given the greater evolutionary distance between maize and rice relative to that within the small grain cereal family (i.e., rice, wheat and barley), it is somewhat puzzling that ZmCPS1 and OsCPS1 group separately from these other small grain CPS. This may, in fact, argue against a role for HvCPS1 and TaCPS3 & 4 in gibberellin metabolism. Indeed, while An2/ZmCPS2 falls into the gibberellin associated cluster, it exhibits inducible transcription and presumably functions in maize phytoalexin biosynthesis, while transcription of ZmCPS3 (previously termed Cpsl1), which falls into the other phylogenetic cluster, is not elicited by fungal infection (Harris et al., 2005). This suggests that the use of labdane-realted diterpenoids as phytoalexins may have independently evolved in maize relative to the small grain cereals. In addition, it should be noted that the single residue regulatory switch position in HvCPS1 encodes the arginine associated with CPS devoted to more specialized metabolism rather than the histidine otherwise found in those operating in gibberellin biosynthesis, although both TaCPS3 & 4 do contain a histidine at this position (Fig. 4B). Intriguingly, TaCPS2 contains a glutamine at this position, which presumably is functionally analogous to arginine, conferring resistance to inhibition by the Mg2+ co-factor, as the sterochemical differentiation of its product precludes a role in gibberellin biosynthesis.
The CPSs from the other phylogenetic cluster are presumably involved in more specialized metabolism, based in part on the production of stereochemically differentiated CPP and, at least in the case of the rice and wheat paralogs (i.e., OsCPS2 & 4 and TaCPS1 & 2), UV-inducible transcription (Otomo et al., 2004; Prisic et al., 2004; Toyomasu et al., 2009; Xu et al., 2004). Given the production of normal CPP by TaCPS2 shown here, it is notable that TaCPS2 is most closely related to the similarly stereochemically distinguishable syn-CPP producing OsCPS4. This suggests that such stereochemical differentiation in product outcome might underlie the observation that both wheat and rice contain two CPS associated with more specialized metabolism relative to gibberellin biosynthesis, for which there seems to be additional CPS(s) in each plant species. Moreover, while these pairs of CPS exhibit similarly inducible transcription, one produces ent-CPP (OsCPS2 and TaCPS1) while the other produces a different stereoisomer (OsCPS4 and TaCPS2). Further, the observed phylogenetic groupings suggest that this differentiation of biochemical function (i.e., product stereochemistry) occurred prior to the split between the wheat and rice lineages. Although comparison with the similarly stereochemically differentiated DsCPS, which produces normal CPP (Gao et al., 2009), indicates that such biochemical diversity arose separately in at least the monocots and dicots.
4. Conclusion
The results presented here demonstrate that wheat contains an expanded and functionally diverse family of CPS. While the relevant biological activities of the derived labdane-related diterpenoids remain largely unclear, the retention of an expanded CPS gene family throughout the Poaceae suggests persistent use of these natural products in cereal crop plants; most likely in defense, as found in both rice and maize. More immediately, the biochemical activity exhibited by the CPSs characterized here increases our understanding of the diterpenoid metabolic repertoire of wheat and provides some insight into its evolution. Specifically, all cereal crop plants seem to contain at least two CPSs associated with more specialized metabolism in addition to CPS(s) involved in gibberellin biosynthesis, and our results suggest that such retention of diversity (i.e., at least three CPSs) may be due to stereochemical variation in product outcome as well as association with primary or secondary metabolism. In particular, the two CPSs in rice and wheat that seem to be involved in more specialized metabolism (i.e., as indicated by inducible transcription of the relevant genes) produce distinct isomers of CPP (i.e., OsCPS2 and TaCPS1 produce ent-CPP, while OsCPS4 and TaCPS2 produce syn- and normal CPP, respectively). The conservation exhibited between the biochemically novel OsCPS4 and TaCPS2 suggests that such differentiation arose prior to at least the split between rice and wheat in the small grain cereal lineage. Such stereochemical variation is further reflected in the specificities of the downstream KSL, examples of which from both rice and wheat selectively react with CPP isomers other than the ent-configured form relevant to gibberellin biosynthesis. Thus, our results suggest that expanded roles for labdane-related diterpenoid natural products, beyond formation of the ancestral gibberellin phytohormones, arose early in the Poaceae, including uses for the production of stereochemically distinct isomers of the characteristic CPP intermediate.
5. Experimental
5.1 General
Unless otherwise noted, all chemical reagents were purchased from Fisher Scientific (Loughborough, Leicestershire, UK), and molecular biology reagents from Invitrogen (Carlsbad, CA, USA). All recombinant expression was carried out with the OverExpress C41 strain of E. coli (Lucigen, Middleton, WI, USA). GC-MS analyses were performed as described in the accompanying report (Zhou et al., submitted). Analysis of TaCPS4 and 5 mRNA levels following UV-irradiation also was carried out as described in the accompany report (Zhou et al., submitted), albeit using the primers described in Table 1.
Table 1.
Primers used in RT-PCR analysis of mRNA levels
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TaCPS4 | GGAGGCTGGACGTTGTC | GAATGCAGGATCGTCGTC |
| TaCPS5 | GACCAACGATGCATCTGTC | GGAGGCTTGTCTTTCATGG |
5.2 Cloning
A CPS gene fragment was cloned from T. aestivum cv. Nourin-61-gou via use of previously described degenerate primers based on the ‘SAYDTAW’ and ‘DDTAMA’ amino acid sequence motifs conserved in class II diterpene cyclase (Otomo et al., 2004). This fragment was extended via rapid amplification of cDNA ends (RACE), and designated TaCPS4. Another CPS gene was cloned during isolation of the previously described TaCPS2 [i.e., via end-to-end RT-PCR with putatively gene specific primers (Toyomasu et al., 2009)], and designated here TaCPS5.
Each CPS was truncated to remove the plastid targeting sequence from its N-terminus (HvCPS1 after residue 83 and TaCPS1-5 after residue 61, 59, 89, 40, and 28, respectively) and generate fully active pseudo-mature enzymes. This was accomplished during sub-cloning into the Gateway vector system via PCR amplification and directional topoisomerization insertion into pENTR/SD/D-TOPO, with the ensuing constructs verified by complete gene sequencing. These clones were subsequently transferred via directional recombination into the DEST cassette of a previously described pGG-DEST vector (Morrone et al., 2009).
5.3 Recombinant expression and stereochemical analysis
The activity of TaCPS1-5 and HvCPS1 were investigated via recombinant expression in E. coli. Initial investigations were carried out by co-expression of each CPS with a GGPP synthase. Stereochemistry of the observed CPP products was then determined by co-expression each CPS, with not only a GGPP synthase, but also a class I labdane-related diterpene synthase that only reacts with either ent- or normal CPP (i.e., AtKS or AgAS:D404A, respectively), which was accomplished using previously described pDEST14 based expression vectors (Cyr et al., 2007). The diterpenes produced by the resulting recombinant bacteria were then identified as described in the accompanying report (Zhou et al., submitted). Briefly, by GC-MS analysis of organic extracts from induced cultures and comparison to authentic standards (see Figures 2 and 3).
5.4 Bioinformatic analysis
The alignment presented in Figure 4B was constructed using VectorNTI (software version 11.0). For phylogenetic analysis the CLC Sequence Viewer (Version 6.6.2) software package was used for both generation of an initial alignment, using full-length amino acid sequences, as well as for construction of the tree shown in Figure 4A, which resulted from analysis of this alignment with the nearest neighbor joining method. In addition, this alignment was used as the basis for phylogenetic analysis based on conserved blocks of sequence – i.e., using the G-Block algorithm as implemented at www.phylogeny.fr (Dereeper et al., 2008). However, this led to qualitatively similar results, and only the tree based on the full-length alignment is shown here. The CPS sequences used in this phylogenetic analysis, beyond those from wheat and barley reported here, are those from rice [OsCPS1,2,&4 (Otomo et al., 2004; Prisic et al., 2004; Xu et al., 2004)], maize [ZmCPS1-4 (Bensen et al., 1995; Harris et al., 2005; Schnable et al., 2009)], Arabidopsis [AtCPS (Sun and Kamiya, 1994)], pea [PsCPS (Ait-Ali et al., 1997)], pumpkin [CmCPS1 (Smith et al., 1998)], tomato [LeCPS (Rebers et al., 1999)], Stevia [SrCPS (Richman et al., 1999)], lettuce [LsCPS (Sawada et al., 2008)], and Danshen [DsCPS (Gao et al., 2009)].
Biochemical characterization of copalyl diphosphate synthases from wheat and barley
Stereochemical differentiation provides rationale for expanded gene family
Molecular phylogenetic analysis suggests this arose early in cereal crop evolution
Acknowledgements
We thank Dr. Tsuneo Sasanuma (Yamagata University) for providing the T. aestivum cv. Nourin-61-gou seeds. This study was generously supported in part by grants from the USDANIFA- AFRI (2008-35318-05027) and NIH (GM076324) to R.J.P., and in part by support from the Grant-in-Aid for Scientific Research C (no. 17580093 to T.T.) from the Japanese Society for the Promotion of Science.
Abbreviations
- CPP
copalyl diphosphate
- CPS
CPP synthase
- TaCPS
wheat (Triticum aestivum)CPS
- HvCPS1
barley (Hordeum vulgare) CPS
- OsCPS
rice (Oryza sativa) CPS
- AtCPS
Arabidopsis thaliana CPS
- KS
ent-kaurene synthase
- AtKS
Arabidopsis thaliana KS
- KSL
KS-like
- TaKS(L)
wheat (Triticum aestivum) KSL
- AgAS
grand fir (Abies grandis) abietadiene synthase
- cv
cultivar
- GC-MS
gas chromatography with mass spectrometric detection
- GGPP
((E,E,E)-geranygeranyl diphosphate
- RACE
rapid amplification of cDNA ends
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Ali T, Swain SM, Reid JB, Sun T, Kamiya Y. The LS locus of pea encodes the gibberellin biosynthesis enzyme ent-kaurene synthase A. Plant J. 1997;11:443–454. doi: 10.1046/j.1365-313x.1997.11030443.x. [DOI] [PubMed] [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP. Cloning and characterization of the maize, An1 gene. Plant Cell. 1995;7:75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr A, Wilderman PR, Determan M, Peters RJ. A Modular Approach for Facile Biosynthesis of Labdane-Related Diterpenes. J. Am. Chem. Soc. 2007;129:6684–6685. doi: 10.1021/ja071158n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson T. Population and Food: Global trends and future prospects. 1996. [Google Scholar]
- Gao W, Hillwig ML, Huang L, Cui G, Wang X, Kong J, Yang B, Peters RJ. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org. Lett. 2009;11:5170–5173. doi: 10.1021/ol902051v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, Allard S, Kathiresan A, Ouellet T, Peters RJ. The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol. 2005;59:881–894. doi: 10.1007/s11103-005-1674-8. [DOI] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature. 2005:793–800. doi: 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Satoh K, Nagata T, Kawagashira N, Doi K, Kishimoto N, Yazaki J, Ishikawa M, Yamada H, Ooka H, Hotta I, Kojima K, Namiki T, Ohneda E, Yahagi W, Suzuki K, Li CJ, Ohtsuki K, Shishiki T, Otomo Y, Murakami K, Iida Y, Sugano S, Fujimura T, Suzuki Y, Tsunoda Y, Kurosaki T, Kodama T, Masuda H, Kobayashi M, Xie Q, Lu M, Narikawa R, Sugiyama A, Mizuno K, Yokomizo S, Niikura J, Ikeda R, Ishibiki J, Kawamata M, Yoshimura A, Miura J, Kusumegi T, Oka M, Ryu R, Ueda M, Matsubara K, Kawai J, Carninci P, Adachi J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashidume W, Hayatsu N, Imotani K, Ishii Y, Itoh M, Kagawa I, Kondo S, Konno H, Miyazaki A, Osato N, Ota Y, Saito R, Sasaki D, Sato K, Shibata K, Shinagawa A, Shiraki T, Yoshino M, Hayashizaki Y. Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science. 2003;301:376–379. doi: 10.1126/science.1081288. [DOI] [PubMed] [Google Scholar]
- Köksal M, Hu H, Coates RM, Peters RJ, Christianson DW. Structure of Copalyl Diphosphate Synthase from Arabidopsis thaliana, a Protonation-Dependent Diterpene Cyclase. Nat. Chem. Biol. 2011;7:431–433. doi: 10.1038/nchembio.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann FM, Prisic S, Davenport EK, Determan MK, Coates RM, Peters RJ. A single residue switch for Mg2+-dependent inhibition characterizes plant class II diterpene cyclases from primary and secondary metabolism. J. Biol. Chem. 2010;285:20558–20563. doi: 10.1074/jbc.M110.123307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone D, Chambers J, Lowry L, Kim G, Anterola A, Bender K, Peters RJ. Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum . FEBS Lett. 2009;583:475–480. doi: 10.1016/j.febslet.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Otomo K, Kenmoku H, Oikawa H, Konig WA, Toshima H, Mitsuhashi W, Yamane H, Sassa T, Toyomasu T. Biological functions of ent- and syn-copalyl diphosphate synthases in rice: key enzymes for the branch point of gibberellin and phytoalexin biosynthesis. Plant J. 2004;39:886–893. doi: 10.1111/j.1365-313X.2004.02175.x. [DOI] [PubMed] [Google Scholar]
- Peters RJ. Uncovering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry. 2006;67:2307–2317. doi: 10.1016/j.phytochem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Peters RJ. Two rings in them all: The labdane-related diterpenoids. Nat. Prod. Rep. 2010;27:1521–1530. doi: 10.1039/c0np00019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RJ, Ravn MM, Coates RM, Croteau RB. Bifunctional abietadiene synthase: Free diffusive transfer of the (+)-copalyl diphosphate intermediate between two distinct active sites. J. Am. Chem. Soc. 2001;123:8974–8978. doi: 10.1021/ja010670k. [DOI] [PubMed] [Google Scholar]
- Prisic S, Peters RJ. Synergistic substrate inhibition of ent-copalyl diphosphate synthase: A potential feed-forward inhibition mechanism limiting gibberellin metabolism. Plant Physiol. 2007;144:445–454. doi: 10.1104/pp.106.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisic S, Xu M, Wilderman PR, Peters RJ. Rice contains two disparate ent-copalyl diphosphate synthases with distinct metabolic functions. Plant Physiol. 2004;136:4228–4236. doi: 10.1104/pp.104.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers M, Kaneta T, Kawaide H, Yamaguchi S, Yang YY, Imai R, Sekimoto H, Kamiya Y. Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J. 1999;17:241–250. doi: 10.1046/j.1365-313x.1999.00366.x. [DOI] [PubMed] [Google Scholar]
- Richman AS, Gijzen M, Starratt AN, Yang Z, Brandle JE. Diterpene synthesis in Stevia rebaudiana: recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway. Plant J. 1999;19:411–421. doi: 10.1046/j.1365-313x.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, Miyao A, Hirochika H, Kitano H, Ashikari M, Matsuoka M. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134:1642–1653. doi: 10.1104/pp.103.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Katsumata T, Kitamura J, Kawaide H, Nakajima M, Asami T, Nakaminami K, Kurahashi T, Mitsuhashi W, Inoue Y, Toyomasu T. Germination of photoblastic lettuce seeds is regulated via the control of endogenous physiologically active gibberellin content, rather than of gibberellin responsiveness. J. Exp. Bot. 2008;59:3383–3393. doi: 10.1093/jxb/ern192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PE. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. U.S.A. 2011;108:5455–5460. doi: 10.1073/pnas.1014714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, Minx P, Reily AD, Courtney L, Kruchowski SS, Tomlinson C, Strong C, Delehaunty K, Fronick C, Courtney B, Rock SM, Belter E, Du F, Kim K, Abbott RM, Cotton M, Levy A, Marchetto P, Ochoa K, Jackson SM, Gillam B, Chen W, Yan L, Higginbotham J, Cardenas M, Waligorski J, Applebaum E, Phelps L, Falcone J, Kanchi K, Thane T, Scimone A, Thane N, Henke J, Wang T, Ruppert J, Shah N, Rotter K, Hodges J, Ingenthron E, Cordes M, Kohlberg S, Sgro J, Delgado B, Mead K, Chinwalla A, Leonard S, Crouse K, Collura K, Kudrna D, Currie J, He R, Angelova A, Rajasekar S, Mueller T, Lomeli R, Scara G, Ko A, Delaney K, Wissotski M, Lopez G, Campos D, Braidotti M, Ashley E, Golser W, Kim H, Lee S, Lin J, Dujmic Z, Kim W, Talag J, Zuccolo A, Fan C, Sebastian A, Kramer M, Spiegel L, Nascimento L, Zutavern T, Miller B, Ambroise C, Muller S, Spooner W, Narechania A, Ren L, Wei S, Kumari S, Faga B, Levy MJ, McMahan L, Van Buren P, Vaughn MW, Ying K, Yeh CT, Emrich SJ, Jia Y, Kalyanaraman A, Hsia AP, Barbazuk WB, Baucom RS, Brutnell TP, Carpita NC, Chaparro C, Chia JM, Deragon JM, Estill JC, Fu Y, Jeddeloh JA, Han Y, Lee H, Li P, Lisch DR, Liu S, Liu Z, Nagel DH, McCann MC, SanMiguel P, Myers AM, Nettleton D, Nguyen J, Penning BW, Ponnala L, Schneider KL, Schwartz DC, Sharma A, Soderlund C, Springer NM, Sun Q, Wang H, Waterman M, Westerman R, Wolfgruber TK, Yang L, Yu Y, Zhang L, Zhou S, Zhu Q, Bennetzen JL, Dawe RK, Jiang J, Jiang N, Presting GG, Wessler SR, Aluru S, Martienssen RA, Clifton SW, McCombie WR, Wing RA, Wilson RK. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- Smith MW, Yamaguchi S, Ait-Ali T, Kamiya Y. The first step of gibberellin biosynthesis in pumpkin is catalyzed by at least two copalyl diphosphate synthases encoded by differentially regulated genes. Plant Physiol. 1998;118:1411–1419. doi: 10.1104/pp.118.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmeyer W, Ellis M, Robertson M, Ali S, Lenton JR, Chandler PM. Isolation of gibberellin metabolic pathway genes from barley and comparative mapping in barley, wheat and rice. Theor. Appl. Genet. 2004;109:847–855. doi: 10.1007/s00122-004-1689-6. [DOI] [PubMed] [Google Scholar]
- Sun T-P, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T. Recent Advances Regarding Diterpene Cyclase Genes in Higher Plants and Fungi. Biosci. Biotechnol. Biochem. 2008;72:1168–1175. doi: 10.1271/bbb.80044. [DOI] [PubMed] [Google Scholar]
- Toyomasu T, Kagahara T, Hirose Y, Usui M, Abe S, Okada K, Koga J, Mitsuhashi W, Yamane H. Cloning and characterization of cDNAs encoding ent-copalyl diphosphate synthases in wheat: insight into the evolution of rice phytoalexin biosynthetic genes. Biosci Biotechnol Biochem. 2009;73:772–775. doi: 10.1271/bbb.80781. [DOI] [PubMed] [Google Scholar]
- VanEtten HD, Mansfield JW, Bailey JA, Farmer EE. Two classes of plant antibiotics: phytoalexins versus 'phytoanticipins'. Plant Cell. 1994;6:1191–1192. doi: 10.1105/tpc.6.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Hillwig ML, Prisic S, Coates RM, Peters RJ. Functional identification of rice syn-copalyl diphosphate synthase and its role in initiating biosynthesis of diterpenoid phytoalexin/allelopathic natural products. Plant J. 2004;39:309–318. doi: 10.1111/j.1365-313X.2004.02137.x. [DOI] [PubMed] [Google Scholar]
- Zhou K, Xu M, Tiernan MS, Xie Q, Toyomasu T, Sugawara C, Oku M, Usui M, Mitsuhashi W, Chono M, Chandler PM, Peters RJ. Functional characterization of wheat ent-kaurene(- like) synthases indicates continuing evolution of labdane-related diterpenoid metabolism in the cereals. Phytochemistry. doi: 10.1016/j.phytochem.2012.08.021. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]