Abstract
Understanding substance use disorders (SUDs) and the problems associated with abstinence has grown in recent years. Nonetheless, highly efficacious treatment targeting relapse prevention has remained elusive, and there remains no FDA-approved pharmacotherapy for psychostimulant dependence. Preclinical and clinical investigations assessing the utility of classical antidepressants, which block monoamine reuptake, show mixed and often contradictory results. Mirtazapine (Remeron®) is a unique FDA-approved antidepressant, with negligible affinity for reuptake proteins, indirectly augments monoamine transmission presumably through antagonist activity at multiple receptors including the norepinephrine (NE)α2, and serotonin (5-HT)2A/C receptors. Historically, mirtazapine was also considered to be a 5-HT2C antagonist, but recent evidence indicates that mirtazapine is an inverse agonist at this receptor subtype. Suggesting a promising role for mixed-action serotonergic drugs for addiction pharmacotherapy, mirtazapine attenuates psychostimulant-induced behaviors in several rodent models of substance abuse, and antagonizes methamphetamine-induced biochemical and electrophysiological alterations in rats. Preclinical findings are confirmed through published case studies documenting successful outcomes with mirtazapine therapy across a number of SUDs. To date, a large scale clinical trial assessing the utility of mirtazapine in substance abuse pharmacotherapy has yet to be conducted. However, as reviewed here, accumulating preclinical and clinical evidence argues that mirtazapine, or compounds that emulate aspects of its pharmacological profile, may prove useful in helping treat addictions.
Keywords: methamphetamine, methamphetamine-seeking, cue reactivity, atypical antidepressant, self-administration, serotonin
1. Introduction
Despite the many advances of preclinical neurobiology, substance use disorders (SUDs) remain prevalent with devastating effects on the drug-abusing individual and society. The psychostimulants, cocaine, amphetamine, and methamphetamine are highly addictive with the potential for multiorgan pathogenesis, and the abuse of these drugs continues to be problematic world-wide (Compton and Volkow, 2006b;Compton and Volkow, 2006a). Methamphetamine abuse resulted in a $23.4 billion socioeconomic burden in 2005 (Nicosia et al., 2009). In 2006, cocaine caused over 500,000 emergency department visits, while amphetamine and methamphetamine abuse resulted in over 100,000 visits (SAMHSA, 2008). The trajectory of this problem remains of concern, as can be implied from the use of these drugs by adolescents and young adults. For example, in 2009, 2.4% of 12th grade students tried methamphetamine and 6% tried cocaine (Johnston et al., 2010). Historically, treatment of stimulant abuse and dependence relied almost exclusively on cognitive therapy with advancing efforts incorporating contingency management (Stitzer and Vandrey, 2008). Nonetheless, relapse rates remain high. As there are currently no FDA-approved pharmacotherapeutics for the treatment of stimulant abuse, the identification of such (either as a monotherapy or as adjunct to behavioral/cognitive therapy) remains a critical area of intense research.
Abuse of opioids, including misuse of prescription painkillers and the use of the illicit drug, heroin, also presents a significant burden on the abusing individuals, their families and society. There are several FDA-approved therapies for opioid addiction that have proven efficacy (e.g., methadone, buprenorphine, and naltrexone), but additional regimens may improve the capacity to customize therapeutic approaches to further enhance treatment efficacy. Thus, medication development for opioid addiction continues to be a highly important research endeavor.
SUDs are a chronic disease state, necessitating a well-tolerated, efficacious pharmacotherapy that can be administered for extended durations to provide palliative care and ultimately reverse the persistent aberrant neuroplasticity associated with the disease. To provide a backdrop for medication development, the following sections will first overview neuroplasticity associated with SUDs, with a focus on psyshostimulants. Subsequently, the potential utility of mixed-action serotonergic compounds to ameliorate, or compensate for, these drug-induced states will be discussed. To help illustrate the potential for the serotonergic compounds to provide sustained therapy, effects of both acute and chronic administration will be presented. Finally, preclinical and clinical evidence of the prototype for this review, i.e., mirtazapine, to provide therapeutic benefits in drug-abusing humans, will be highlighted.
2. Monoaminergic Targets for SUD Pharmacotherapy
The dysregulation of dopamine (DA) transmission has been a focus of studies on mechanisms of addiction (Vocci and Ling, 2005). However, capitalizing on this knowledge has not yet produced effective pharmacotherapies. Interest has developed in recent years regarding in the utilization of serotonergic ligands for addiction medication (for reviews see Bubar and Cunningham, 2008;Bubar and Cunningham, 2006), potentially benefiting from the functional interdependence shared between DA and serotonin systems. Accordingly, antidepressants, both tricyclics and selective serotonin reuptake inhibitors (SSRIs), have been investigated in preclinical and clinical settings for SUDs. However, these studies have not been sufficiently compelling so as to yield an FDA-approved treatment (Lima et al., 2003). The “classical” antidepressants bind to serotonergic and adrenergic transporters, which result in increased levels of these transmitters throughout the brain. As dopaminergic transmission is largely unaltered, treatment with the classical antidepressants may further the imbalance that exists among these transmitter systems in the addicted brain during times of protracted withdrawal (Koob and Volkow, 2010). Thus, atypical antidepressants which have little affinity for monoamine transporters may offer improved efficacy and therapeutic benefit to patients with SUDs, and action at receptors that act more to modulate transmission may present more promising therapeutic targets. One candidate with this profile is mirtazapine.
3. Mirtazapine: Pharmacology, Physiology and Behavior
Mirtazapine (Remeron®) is an atypical antidepressant, FDA-approved for the treatment of moderate to severe depression. Several aspects of the pharmacological profile of this compound make it appealing for SUD/addiction therapy. These are overviewed below.
a) Pharmacokinetics
After oral administration of mirtazapine, peak plasma concentrations occur within 0.5–2 hours, and the average elimination half-life in humans is 20–40 hours (Timmer et al., 2000). Mirtazapine is largely metabolized to inactive products by the hepatic cytochrome p450 isoenzymes, CYP2D6, CYP1A2 and CYP3A4 (Spina et al., 2008;Stormer et al., 2000). However, once mirtazapine reaches therapeutic levels, the CYP3A4 isoenzyme is responsible for up to 70% of metabolism and clearance (Stormer et al., 2000). These pharmacokinetic features are particularly relevant when considering the utility of using mirtazapine for long term treatment of SUDs when there is a comorbid condition also managed pharmacologically. For examples, affective disorders, such as depression and/or schizophrenia, are often dually diagnosed with SUDs. Clozapine is an atypical antipsychotic frequently used to treat schizophrenia, and in contrast to mirtazapine, clozapine is primarily metabolized by CYP1A2 (Eiermann et al., 1997;Urichuk et al., 2008). Analogously, fluoxetine, a popular SSRI used to treat depression, is predominantly reliant on CYP2D6 for hepatic metabolism (Mandrioli et al., 2006). The minimally overlapping pharmacokinetic properties of mirtazapine with other clinically used CNS compounds, advance the possible utility of mirtazapine as a SUD pharmacotherapy by minimizing pharmacokinetic drug-drug interactions with medications that may be used to treat comorbid affective disorders, like schizophrenia and depression. HIV/AIDS also frequently co-exists with SUDs. Standard care for AIDS includes use of antiretroviral therapy (HAART), including but not limited to zidovudine, lamivudine, abacavir, and ritonavir. Zidovudine and lumivudine can be largely excreted unchanged in the urine (Blum et al., 1988;Yuen et al., 1995); abacavir is not significantly metabolized by CYP enzymes (Yuen et al., 2008). While CYP3A contributes to ritonavir metabolism, substrates/inhibitors of this enzyme do not completely impede ritonavir metabolism, even at high concentrations (Kumar et al., 1996). Thus, by avoiding the metabolic pathways of these other therapeutics, mirtazapine can be used as an adjunctive treatment without interference with other needed therapeutic regimens.
b) Pharmacodynamics
Unlike classical antidepressants, mirtazapine has negligible affinity for reuptake proteins. It exhibits high affinity antagonism at histamine (H)1, serotonin (5-HT)2A, 5-HT3, and norepinephrine (NE)α2 receptors (Table 1) (de Boer et al., 1988;Hoyer, 1988;Kelder et al., 1997;Kooyman et al., 1994). Historically, also considered to be a 5-HT2C antagonist, recent evidence expands our understanding of the complexity of mirtazapine by the inclusion of inverse agonist properties (i.e., negative intrinsic efficacy) at constitutively active (i.e., agonist-independent activation) 5-HT2C receptors (Labasque et al., 2010;Chanrion et al., 2008). With this unique pharmacological profile, mirtazapine is devoid of many of the complications associated with classical reuptake inhibitors such as unwanted cardiovascular side-effects (Sala et al., 2006;Thanacoody and Thomas, 2005;Stahl et al., 2005). Additionally, mirtazapine has a much more rapid onset of clinical efficacy, with therapeutic benefit being achieved in depressed patients in as few as ten days (Nierenberg, 2001). While mirtazapine seems to be an improvement over classical antidepressants, it does exhibit some side-effects, including sedation, likely resulting from H1 antagonism, (a target not thought to contribute to the therapeutic efficacy), and weight gain, potentially due to 5-HT2C antagonism/inverse agonism. However, with proper dosing, the side-effect profile of mirtazapine is quite safe and well-tolerated across a variety of patient populations.
Table 1.
Receptor affinities for mirtazapine (Ki nM) (de Boeret al., 1988;Hoyer, 1988;Kelder et al., 1997;Kooyman et al., 1994).
| α1 | α2 | 5-HT1A | 5-HT2A | 5-HT2C | 5-HT3 | D1 | D2 | SERT | NET | H1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mirtazapine | 500 | 65 | >1000 | 6 | 12 | 8 | >1000 | >1000 | >1000 | >1000 | 0.5 |
c) Neurochemistry and Physiological Consequences of Mirtazapine Treatment
With such a complex pharmacological profile, mirtazapine has numerous effects throughout the central nervous system. For example, inhibition of 5-HT2A/C receptor activity results in increased excitation of neurons in the dorsal raphe (Haddjeri et al., 1998). Additional influence on the dorsal raphe results from NEα2 antagonism. This disrupts the negative feedback mediated by pre-synaptic NEα2 receptors in the locus coeruleus, and leads to increased adrenergic activation of NEα1 receptors in the dorsal raphe. This ability to stimulate the key serotonergic nucleus in the brain is complemented by antagonism at NEα2 heteroreceptors on serotonergic neurons, also leading to increased activity of the dorsal raphe and increased 5-HT efflux (Kakui et al., 2009;de Boer et al., 1996;de Boer, 1996;Westenberg, 1999;Haddjeri et al., 1998). While mirtazapine has little affinity for 5-HT1A receptors, the result of enhanced 5-HT in the synaptic cleft through the aforementioned mechanisms promotes stimulation of 5-HT1A receptors by endogenous 5-HT. As this mechanism would predict, mirtazapine-dependent activation of the 5-HT1A receptor is verified behaviorally in rodents (Kakui et al., 2009;Berendsen and Broekkamp, 1997;Nakayama et al., 2004).
Mirtazapine also enhances DA and NE signaling, with acute administration increasing DA levels in the prefrontal cortex (Nakayama et al., 2004), and both DA and NE in the frontal-occipital and medial prefrontal cortices (Devoto et al., 2004). These neurochemical effects may be of particular interest given the observed monoamine hypofunction evident during abstinence from psychostimulant abuse, a phenomenon thought to contribute to the negative affect experienced by psychostimulant-withdrawn individuals (Volkow et al., 2003;Volkow et al., 2008;Volkow et al., 2006;Childress et al., 1999;Grant et al., 1996;O'Brien et al., 1998). Moreover, as all psychostimulants excessively enhance DA, 5-HT, and NE transmission (through reuptake inhibition and/or calcium-independent release), the ability of mirtazapine to enhance DA (Nakayama et al., 2004;Devoto et al., 2004), NE (Devoto et al., 2004), and 5-HT (Haddjeri et al., 1998), signaling, but to a lesser extent than do psychostimulants, may provide a means for mirtazapine to “take the edge off” the negative symptoms associated with withdrawal from abused stimulants.
While abstinent stimulant-dependent subjects exhibit cortical and striatal hypo-activity when at rest (see above), these brain regions are hyper-responsive to drug-related stimuli (Volkow et al., 2003;Volkow et al., 2008;Volkow et al., 2006;Childress et al., 1999;Grant et al., 1996;O'Brien et al., 1998). This phenomenon is thought to reflect learned associations between previously neutral cues and the rewarding nature of the abused drug. During drug-taking, these associations are greatly enhanced so that the cues become highly significant to the individual. These drug-associated memories persist long after drug-taking ceases, and re-exposure to cues linked to the memories can trigger craving that may contribute to drug-seeking and relapse. Thus, a primary goal in medication development for SUDs is to provide a therapy that can reduce the salience of the drug memory to deter drug-craving and/or drug-seeking. There is a growing appreciation for the role of 5-HT in drug-related memories (Nic Dhonnchadha and Cunningham, 2008), and the ability of mirtazapine to modify 5-HT signaling in cortical and striatal regions may be useful in down regulating the salience of drug-related cues in the withdrawn addict.
d) How the Serotonergic Pharmacology of Mirtazapine Relates to its Effects on Behavior Induced by Abused Drugs
Of the serotonergic receptors acted upon by mirtazapine, the highest affinity is exhibited for the 5-HT2A/C and 5-HT3 subtypes (Table 1), and several preclinical studies illustrate the importance of these receptor subtypes in regulating monoaminergic signaling. Important considerations involved in helping translate the outcomes of these preclinical studies to the human condition, include the `phases' of the drug addiction profile being evaluated and the animal model employed. Accordingly, the following sections will further explore the functional pharmacology of the 5-HT2A/C and 5-HT3 receptor targets of mirtazapine within the context of the drug-abuse phase, and the behavioral tasks utilized to model particular aspects of the addiction profile.
(i) 5-HT2A/C receptors
Mirtazapine acts on 5-HT2A/C receptors and these receptors are clearly involved in the effects of psychostimulants (Bubar and Cunningham, 2008;Bubar and Cunningham, 2006). Responses of ventral pallidal neurons to iv administered 5-HT2A/C receptor agonist DOI are enhanced in rats chronically treated with methamphetamine (Napier and Istre, 2008). This outcome may reflect a functional upregulation of 5-HT2A/2C receptors, and suggests that these receptors may be dysregulated during psychostimulant abuse. Such observations support the potential of 5-HT2A/C receptors to serve as therapeutic targets for psychostimulant abuse (for reviews see Bubar and Cunningham, 2008;Bubar and Cunningham, 2006). Additional support can be drawn from studies where actions of 5-HT2A receptors are distinguished from those evoked by 5-HT2C.
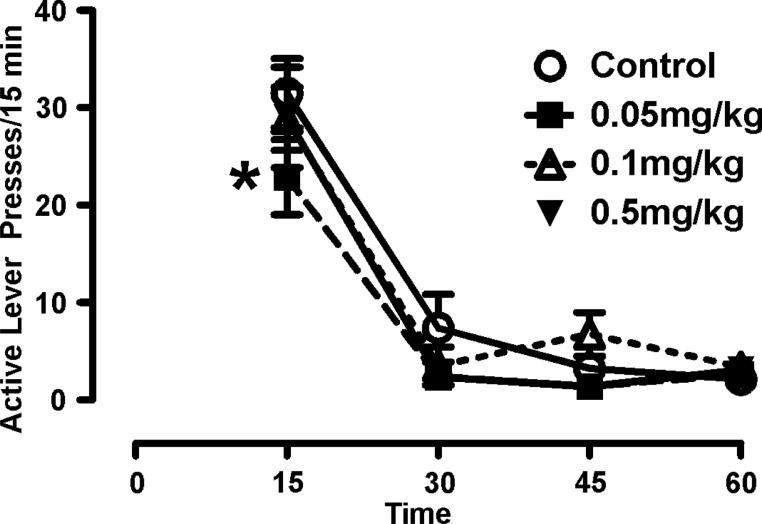
Serotonin2A receptors provide positive regulation of DA in the mesoaccumbens pathway (De Deurwaerdere et al., 2004;De Deurwaerdere and Spampinato, 1999;Lucas and Spampinato, 2000;Alex et al., 2005;Gobert et al., 2000;Di Matteo et al., 1999;Alex et al., 2005). In rodents, 5-HT2A antagonism with M100907 shows promising results in blocking stimulant-induced actions. M100907 attenuates cocaine-evoked motor activity in naïve and sensitized animals (Filip et al., 2004) and attenuates cue-induced reinstatement of cocaine-seeking when administered systemically (Nic Dhonnchadha et al., 2009), as well as when site injected into the prefrontal cortex (Pentkowski et al., 2010). We have observed that M100907 attenuated methamphetamine-seeking behavior in rats (Figure 1). For this assessment, we used a model of drug-seeking wherein rats were trained to self-administer methamphetamine and tested for cue reactivity (without a prior extinction training) (Graves and Napier, 2011;Graves and Napier, 2012). Our findings are consistent with that of Nic Dhonnadchadha et al., 2009 who show suppression of cue-induced reinstatement in rats trained to self-administer cocaine. Taken together, these studies provide compelling preclinical evidence indicating that 5-HT2A receptor antagonism holds promise for addiction medication.
Figure 1. Serotonin2A antagonism attenuates methamphetamine-seeking in a rodent model of substance abuse.
Rats were trained to self-administer methamphetamine and repeatedly tested for “seeking”-like behavior via cue reactivity (CR) assessments using the identical protocol previously described (Graves and Napier, 2011;Graves and Napier, 2012). Prior to CR assessments rats were administered a pretreatment of the 5-HT2A receptor antagonist M100907 (0.05, 0.1, or 1.0mg/kg) or vehicle. Two-way repeated measures ANOVA reveals did not reveal significant Treatment effect (F(3,28)=1.37), a significant Time effect (F(3,84) =71.8), and no Treatment × Time interaction (F(9,84)=1.65); however, a preplanned Newman-Keuls post-hoc assessment indicated that 0.05mg/kg significantly decreased active lever pressing (i.e. index of methamphetamine-seeking) in the first 15 minutes of the CR test (*p<0.05). This effect was lost at higher doses, possibly reflecting a loss of specificity
Serotonin2C receptors contrast the 5-HT2A receptors and have inhibitory influence over DA systems due to extensive (but not exclusive) localization in GABAergic neurons of the mesolimbic and mesocortical circuits (Bubar and Cunningham, 2007;Liu et al., 2007;Serrats et al., 2005;Bubar et al., 2011). As such, 5-HT2C agonists attenuate drug-seeking in rodents (Grottick et al., 2000;Fletcher et al., 2008;Neisewander and Acosta, 2007;Pentkowski et al., 2010) whereas antagonism enhances acute cocaine-evoked motor activity (Fletcher et al., 2002;Fletcher et al., 2006;Filip et al., 2004), the interoceptive cues of cocaine (Filip et al., 2006), and conditioned hyperactivity to cocaine (Liu and Cunningham, 2006). The ability of 5-HT2C antagonism to augment cocaine-mediated behaviors is lost with repeated administration of cocaine (Filip et al., 2004), suggesting 5-HT2C receptors may be dysregulated by repeated psychostimulant administration.
Serotonin2C receptor pharmacology is quite complex, and the tools available to manipulate 5-HT2C receptor function are not limited to simple agonist/antagonist relationships. This metabotropic receptor undergoes mRNA editing which dramatically impacts its function (Niswender et al., 1998;Wang et al., 2000;Werry et al., 2008). The fully unedited 5-HT2C receptor has high levels of constitutive activity (i.e., agonist-independent signaling) and strong coupling efficacy; as editing is increased, constitutive activity is diminished. Various editing patterns of the 5-HT2C receptor are associated with depression (Gurevich et al., 2002), suicide (Gurevich et al., 2002;Niswender et al., 2001;Pandey et al., 2006;Dracheva et al., 2008b;Dracheva et al., 2008a) and schizophrenia (Sodhi et al., 2001) in humans, and these editing patterns are modulated by chronic antidepressant administration in both humans (Gurevich et al., 2002) and rodents (Barbon et al., 2011;Englander et al., 2005). It is not yet known if changes in mRNA editing of the 5-HT2C receptor are involved in SUDs. As DA signaling in the striatum and nucleus accumbens is strongly regulated by constitutively active 5-HT2C receptors (at least in halothane-anesthetized rats) (De Deurwaerdere et al., 2004), if editing does occur with SUDs, this mechanism may contribute to the alterations in DA transmission that are known to occur with these disorders. Furthermore, there is evidence for constitutively active 5-HT2C receptors to regulate accumbal DA release, both at the level of nucleus accumbens (Navailles et al., 2006) and through the medial prefrontal cortex (Leggio et al., 2009), but not via the ventral tegmental area (Navailles et al., 2006). This regional specificity of 5-HT2C constitutive activity is intriguing given that local injection of a 5-HT2C antagonist into the nucleus accumbens attenuates cocaine-mediated behaviors (Filip and Cunningham, 2002); outcomes that contrast those obtained with systemic administration of 5-HT2C antagonists (Fletcher et al., 2002;Fletcher et al., 2006;Filip et al., 2004;Filip et al., 2006;Liu and Cunningham, 2006). Inverse agonists (i.e., ligands with negative intrinsic efficacy) at 5-HT2C receptors may provide more target-directed action and therefore more distinct behavioral effects than antagonists. This prediction is based on the fact that inverse agonists will display negative efficacy in brain regions with constitutively active receptors and competitive antagonism (i.e., orthosteric competition) occurring at receptors that are not constitutively active. This concept is supported by evidence from our laboratory demonstrating that administration of the putative 5-HT2C inverse agonist SB 206553 dose-dependently attenuates methamphetamine-seeking behavior and methamphetamine-evoked rearing in rats trained to self-administer (Graves and Napier, 2012). In contrast, 5-HT2C receptor antagonism does not alter these behaviors (Graves and Napier, 2012), nor cue-induced reinstatement of cocaine-seeking (Neisewander and Acosta, 2007;Filip, 2005). These studies indicate that inverse agonism at 5-HT2C receptors may contribute to the ability of mirtazapine to serve as a pharmacotherapy, and it is of great interest to further explore the role of constitutively active 5-HT2C receptors in SUDs.
(ii) 5-HT3 receptors
Further 5-HT influence by mirtazapine is through antagonism of the 5-HT3 receptor. The 5-HT3 receptor is the only known 5-HT receptor not linked to G-protein signaling. Stimulation of this ion channel results in excitation and depolarization. While the link between 5-HT3 receptors and DA is not completely understood, this receptor exhibits a profile that supports its utility for addiction pharmacotherapy. In rats, methamphetamine-induced sensitization is attenuated after treatment with the DA agonist pergolide plus the 5-HT3 antagonist ondansetron (Davidson et al., 2007). Ondansetron, when administered alone, also ameliorates cocaine-mediated behaviors. In cocaine self-administration paradigms administration of ondansetron 3.5hrs after a self-administration session decreases cocaine self-administration on the subsequent day (Davidson et al., 2002b;Davidson et al., 2002a). When ondansetron is administered for the first five consecutive days of withdrawal from a cocaine sensitization regimen, expression of sensitization is decreased after 7, 10, 14, and 28 days of withdrawal (Davidson et al., 2002b;King et al., 2000). These evidences indicate that 5-HT3 antagonism may be a means to modulate psychostimulant-mediated behaviors.
(iii) Animal models of addiction
Several addiction models are employed to help elucidate the neurobiology of SUDs and to served as screening tools for potential pharmacotherapies (O'Brien and Gardner, 2005). Mirtazapine shows preclinical promise in several models including motor sensitization (McDaid et al., 2007), conditioned place preference (Voigt and Napier, 2011;Herrold et al., 2009;Kang et al., 2008;Voigt et al., 2011;Graves et al., 2012), and models of drug-seeking in rats trained to self-administer (Graves and Napier, 2011). Testing mirtazapine in multiple paradigms strengthens confidence in interpreting outcomes; this serves as the basis of the following discussion.
Motor sensitization
Motor sensitization refers to the phenomenon wherein repeated administration of a substance of abuse, such as psychostimlulants or opioids, leads to an enhancement in motor activity. Motor sensitization provides a useful behavioral manifestation of drug-induced neuronal plasticity. Acute administration of mirtazapine (30min pretreatment) attenuates the expression of morphine-induced motor sensitization (Graves et al., 2012), but has yet to be tested for effects on psychostimulant-induced motor sensitization. However, in a methamphetamine sensitization paradigm, repeated mirtazapine (15 once-daily treatments) administered during a period of withdrawal attenuates the expression of sensitization and reverses associated electrophysiological changes in the ventral pallidum (McDaid et al., 2007). In contrast to the above, when mirtazapine is co-administered with pergolide, a non-selective dopamine agonist, during the last 7 days of a 14 day withdrawal period from methamphetamine, expression of sensitization is not attenuated when tested 14 days after the combination treatment ceased (Bhatia et al., 2011). It is not clear whether differences in these studies are a result of the distinct paradigms, number of mirtazapine administrations during withdrawal, or a potential interference of mirtazapine efficacy at augmenting sensitization by pergolide. Helping shed light on this dilemma are studies with mirtazapine administration in other models of the human addiction process (overviewed below).
Conditioned place preference
Conditioned place preference (CPP) is a behavioral task that indirectly measures drug reward by assessing an animal's preference to spend more time in an environment previously paired with a rewarding substance (Tzschentke, 1998). In brief, CPP paradigms typically will use a multichamber apparatus, and the animal is confined within a chamber with distinct cues (e.g., visual, textile, olfactory cues) immediately following exposure to a rewarding substance such as methamphetamine. Saline is paired with another chamber with unique cues; with repeated pairings, animals learn to associate a particular environment with the subjective aspects of a stimulus. Preference is determined by the amount of time the animals spend in the drug-paired chamber when given access to the entire test apparatus in a drug-free state. When trained with a drug that is a positive reinforcer, such as methamphetamine, subjects will spend more time in the chamber previously associated with methamphetamine (referred to as expression of CPP). CPP as a pharmacotherapy screening tool is best utilized when candidate compounds are tested for their ability to attenuate the expression of CPP, suggesting that the salience of the drug-associated memory has been dampened.
We have tested the efficacy of mirtazapine at attenuating the expression of methamphetamine-induced CPP (Voigt and Napier, 2011;Herrold et al., 2008;Voigt et al., 2011). Interestingly, a 24hr pretreatment of mirtazapine is sufficient to attenuate the expression of methamphetamine-induced place preference using a single-pairing CPP paradigm (Herrold et al., 2008). A single administration of mirtazapine also decreases the magnitude of methamphetamine-induced CPP when mirtazapine is paired with re-exposurew CPP is blocked (Voigt and Napier, 2011) indicating an ability of mirtazapine to disrupt the reconsolidation of salient memories.
These actions of mirtazapine are not limited to psychostimulants, for we (Graves et al., 2012) and others (Kang et al., 2008) also demonstrate mirtazapine efficacy in mitigating morphine-induced place preference. In our hands, using a 10 day paradigm with 4 pairings of morphine, a 30min pretreatment of 5.0mg/kg mirtazapine before the expression test, attenuates place preference (Graves et al., 2012). Similarly, Kang and colleagues reported that 6 pairings of morphine induced place preference is attenuated by larger doses of mirtazapine (10.0 and 30mg/kg) acutely administered before the expression test (Kang et al., 2008). In contrast to findings wherein a 24hr pretreatment of mirtazapine attenuates methamphetamine-induced place preference in a single-pairing CPP paradigm (Herrold et al., 2008), a 24hr pretreatment is not sufficient to attenuate the expression of morphine-induced CPP using a more robust four-pairing CPP paradigm (Graves et al., 2012). At this time it is unclear whether the differences in the ability of a 24hr pretreatment to impact the expression of CPP is a consequence of the CPP protocol used or due to pharmacological differences between methamphetamine and morphine.
Self-administration
Mirtazapine has also been studied in a methamphetamine self-administration paradigm. Self-administration is distinct from CPP in that self-administration is an operant task contingent on the animals executing a particular behavior (e.g., lever pressing, nose poking). Self-administration paradigms also result in much larger total drug exposure over the lifetime of the task than does CPP. Self-administration protocols often employ the use of cues presented during times of drug infusion so that each self-administration session reinforces both contextual and explicit cues which can be used to provoke seeking behavior once the animal is withdrawn from the primary reinforcer.
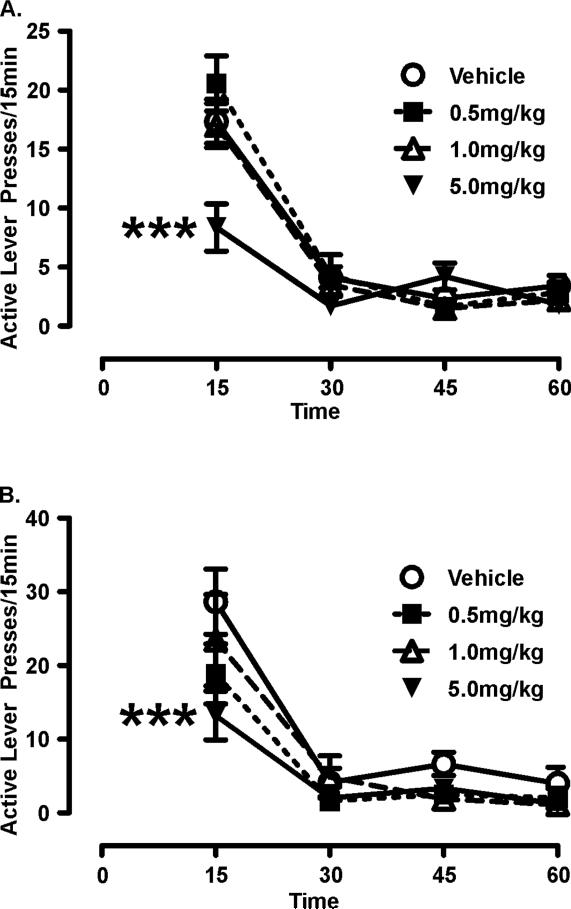
We have employed two self-administration and withdrawal protocols to assess the potential utility of mirtazapine as a substance abuse pharmacotherapy; namely cue reactivity and cue-induced reinstatement (Graves and Napier, 2011). Briefly, both paradigms involve a period of self-administration in which a rat is trained to lever press to receive intravenous infusion of methamphetamine. In cue reactivity testing, rats are subsequently evaluated for lever pressing behavior in the presence of contingently presented cues, in the absence of methamphetamine. In cue-induced reinstatement, lever pressing behavior is first extinguished by repeated testing in the absence of methamphetamine and the explicit cues, and then tested for reinstatement of lever pressing behavior precipitated by exposure to drug-associated (explicit) cues. In both experimental protocols, a 15 minute pretreatment of mirtazapine attenuates lever pressing behavior to approximately 50% of rates obtained following vehicle treatment (Figure 2) (Graves and Napier, 2011). Sedation is a clinically relevant side effect of mirtazapine, and it is important to verify that the dose of mirtazapine used in the cue reactivity/reinstatement protocols was not sufficient to introduce this as a potential confound. Accordingly, we determined that the dose of mirtazapine that reduced drug-seeking had no effect on the rats' ability to execute a rotarod task, nor did it alter motor activity in an open field in rats with a history of methamphetamine self-administration (Graves and Napier, 2011).
Figure 2. Mirtazapine attenuates methamphetamine-seeking in rodent models of substance abuse.
Rats were trained to self-administer methamphetamine and repeatedly tested for “seeking”-like behavior in the presence of cues using two paradigms, a repeated measures cue reactivity (CR) paradigm (A) and a between measures cue-induced reinstatement protocol (B); detailed methods can be found in Graves & Napier 2011. A. During CR testing, cues are contingently presented on a fixed ratio (FR) 1 schedule for 1hr. Two-way rmANOVA reveals significant Treatment effect (F(3,52)=3.80), Time effect (F(3,156)=116.23), and Treatment × Time interaction (F(9,156)=5.09) with 5.0mg/kg mirtazapine attenuating active lever pressing (index of seeking) (***p<0.001 comparing vehicle vs. 5.0 mg/kg mirtazapine pretreatment; Newman-Keuls post-hoc) B. Each reinstatement session consisted of a single, non-contingent presentation of the cue light followed by contingent cue presentations on a fixed ratio FR1 schedule for 1hr. Similar to results seen with CR assessments, twoway rmANOVA reveals a significant Treatment effect (F(3,9)=3.67), Time effect (F(3,124)=45.49), and no Treatment × Time interaction (F(9,124)=1.24) and again, acute pretreatment with 5.0mg/kg mirtazapine decreases active lever pressing in the first 15min (***p<0.001 comparing vehicle vs. 5.0 mg/kg mirtazapine pretreatment; Newman-Keuls post-hoc). Reprinted from Graves & Napier, 2011 with permission from Elsevier.
(iv) Effects of chronic mirtazapine on tasks that model addiction
The above overviewed studies demonstrate the efficacy of acutely administered mirtazapine to attenuate addictive-like behaviors in laboratory animals. However, its utility in the clinical setting will likely require chronic treatments, and it is important to verify that mirtazapine will remain efficacious with such treatments. To provide insights into the effects of chronic mirtazapine, we revealed in a CPP protocol, that ten days of mirtazapine, administered after methamphetamine-conditioning, attenuates the subsequent expression of CPP when the rats are tested in a drug-free state (Voigt et al., 2011). Similarly, rats treated with a motor sensitizing regimen of methamphetamine and subsequently administered mirtazapine for 15 days (M-F for three weeks) fail to express the typical sensitized motor responses to an acute methamphetamine challenge (McDaid et al., 2007). To buttress the behavioral data, we also revealed that repeated administration of mirtazapine reverses methamphetamine-induced neuronal plasticity; i.e., in rats subjected to a motor sensitizing treatment regimen of methamphetamine, the excitatory responses of ventral pallidal typically seen to an intravenous challenge of methamphetamine is absent in rats that also received repeated administration of mirtazapine (McDaid et al., 2007). In summary, behavioral and neuronal alterations established by methamphetamine administration can be abrogated by subsequent treatments with mirtazapine, and in preclinical studies tested thus far, this capacity does not diminish with repeated administration of mirtazapine (Graves and Napier, 2011;Voigt et al., 2011).
4. Clinical Evidence for Mirtazapine Therapy of SUDs
Accumulating clinical evidence supports the preclinical literature urging the consideration of mirtazapine as a substance abuse pharmacotherapy. While a large scale double-blind placebo-controlled study assessing the ability of mirtazapine to provide therapeutic benefit for SUDs has not been reported, there are several ongoing clinical trials that should help fill this knowledge gap (see ClinicalTrials.gov). The following reviews the available clinical literature, as well as our new evidence that indicates the therapeutic potential for mirtazapine to provide relief for patients suffering from SUDs.
One of the many factors thought to contribute to relapse is the inability to properly manage withdrawal symptoms (Brecht et al., 2000). McGregor et al., report in an open label study that mirtazapine can reduce symptoms of methamphetamine withdrawal (McGregor et al., 2008). Mirtazapine decreases the severity of subjective withdrawal symptoms over 10 days of abstinence including reductions in agitation, anxiety, fatigue, irritability, paranoid ideation, anhedonia, vivid dreams, and suicide ideations. Mirtazapine also increases the amount of sleep and results in a trend towards reports of general well-being (McGregor et al., 2008). In a randomized, double-blind, placebo controlled study conducted in men who have sex with men, Colfax and colleagues found that mirtazapine also reduces methamphetamine use in patients receiving counseling (Colfax et al., 2011). Moreover, a meta-analysis conducted by Rose and Grant concluded that mirtazapine has promising potential as a methamphetamine dependence pharmacotherapy (Rose and Grant, 2008)
As can be concluded from the above overview, the current clinical literature regarding mirtazapine and psychostimulants (and opioid) abuse is limited; the clinical use of mirtazapine in alcoholism is better developed and this literature may provide insights into its utility for SUDs in general. Mirtazapine treatment during alcohol detoxification significantly improves scores on the Hamilton Anxiety Rating Scale (HARS), the Hamilton Depression Rating Scale (HDRS) and the Global Assessment Scale (GAS) when compared to patients receiving psychotherapy alone (Liappas et al., 2005). Similar results are found on the HARS and HDRS scores in patients comorbid with alcohol dependence and depression, with additional decreases in alcohol craving as measured by the Obsessive Compulsive Drinking Scale (OCDS) and the Visual Analogue Scale for Craving (VAS) in an open label study (Yoon et al., 2006). Altintoprak et al., also show that mirtazapine treatment decreases HDRS and craving scores in abstinent alcoholics comorbid with depression in an in-patient treatment setting (Altintoprak et al., 2008). Outcomes in alcohol detoxification programs are also improved with mirtazapine administration (Liappas et al., 2004;Liappas et al., 2005) with greater improvement in HDRS, HARS, and GAS scores compared to patients receiving no pharmacotherapy or venlafaxine (Liappas et al., 2004). Taken together, this literature builds a strong case for using mirtazapine to improve outcomes in patients with alcohol dependence, and supports preliminary reports that its use improves withdrawal from psychostimulants as well (McGregor et al., 2008).
In conjunction with Resurrection Behavioral Health (an addiction treatment center in Chicago, IL), a clinical team at the Center for Compulsive Behaviors and Addiction at Rush University has documented the use of mirtazapine for relapse reduction in two case reports of SUDs:
The first patient (36 years of age) had abused opioids for approximately seven years prior to entering treatment and also supplemented with cocaine to counter the fatigue and opioid withdrawal symptoms. The patient entered the inpatient chemical dependency program and relapsed after four months of abstinence. This required a second inpatient treatment including detoxification. Patient history indicated nonspecific depressive symptoms; however it was unclear whether the depression was primary or secondary to the SUD. Eight different treatment programs and several medications including sertraline, fluoxetine, bupropion, thioridazine, naltrexone, and modafinil were used with none of the medications successfully attenuating cravings or maintaining abstinence. After initial evaluation in December 2000 the patient was started on 30mg of mirtazapine and enrolled in a relapse prevention group coupled with individual therapy. Monthly follow-ups were implemented with random urine toxicology screens. The patient was followed until June of 2005 at which time he reported no relapses. Throughout treatment, the mirtazapine dose was titrated up to 90mg at which point the patient experienced amelioration of anxiety symptoms; however, due to increased systolic blood pressure to 160mm/hg, dosage was reduced. The second patient was a 32 year old male referred for continued treatment and monitoring through the state Medical Licensing Board. His abuse began with alcohol and cannabis use in college. Drug use increased and included cocaine and opioids throughout medical school. After treatment based on a 12-step model without opioid replacement therapy, the patient was abstinent for approximately two years after which a relapse required a second inpatient treatment. During and after the second inpatient treatment he was prescribed a number of medications including sertraline, citalopram and lithium with no appreciable reductions in craving. At the time of referral, the patient was taking 100mg nortriptyline at bedtime and 150mg of bupropion twice/day for opioid dependence, cocaine abuse, and generalized anxiety disorder; conditions confirmed by our diagnoses. Under our care, bupropion dosing was decreased to 150mg every morning and nortriptyline increased to 150mg at bedtime. With no appreciable changes in cravings or anxiety symptoms bupropion was discontinued. The patient was started on a relapse prevention program with individual therapy during which the patient exhibited cravings and euphoric recall. Nortriptyline was discontinued and mirtazapine treatment commenced at 30mg at bedtime. Within four weeks the patient reported reduced cravings for his drugs of choice and improved anxiety symptoms with further enhanced benefit at 45mg mirtazapine. Currently the patient continues to be stable on 45mg mirtazapine at bedtime and has adhered to his relapse prevention program.
To further advance this clinical evidence, another team at the Rush University Center for Compulsive Behavior and Addiction, conducted a retrospective study of psychiatric records collected from 2004–2007 at the Ruth M. Rothstein CORE Center. This retrospective analysis focused on the use of mirtazapine in HIV+ patients with SUDs, a particularly vulnerable population. Among 500 unduplicated HIV+ patients, 44 were maintained on mirtazapine for a minimum of 3 months of continuous treatment. Mirtazapine was primarily used to treat anxiety and mood disorders in these 44 patients. Over the course of the 3 years analyzed, only a single patient discontinued use due to daytime sedation. Patients were maintained on doses ranging from 15–60mg/day with no adverse events attributed to drug-drug interactions indicating safe use with antiretroviral therapy such as HAART. While usage of illicit substances was not a primary focus when data was collected, it was validated retrospectively that 27 of the 44 patients had a diagnosis of cocaine, methamphetamine, or polysubstance dependence including at least one stimulant. Of the 27 patients, 16 were on antiretroviral therapy (HAART), and as previously noted (Colfax et al., 2011), mirtazapine was well-tolerated by HIV+ individuals on antiretroviral therapy. Fifteen of the 27 patients were able to maintain their sobriety from cocaine, methamphetamine, or both while being maintained on mirtazapine. Upon discontinuation of care at the CORE Center, documentation of relapse could not be validated. Nonetheless, several patients returned to care and reported relapse on their stimulant of choice when they ceased using or had run out of mirtazapine. Other patients anecdotally acknowledged that while taking mirtazapine, their cravings were substantially reduced.
Such positive outcomes were not reported by Cruickshank et al., who performed a placebo controlled study assessing the use of mirtazapine for the management of acute methamphetamine withdrawal and found no significant differences between mirtazapine and placebo groups in study retention and an increase in anxiety and sleep duration with mirtazapine (Cruickshank et al., 2008). However, this study used a small sample size (13 patients on mirtazapine) with a focus on acute withdrawal states, which are often characterized by anxiety and hypersomnolence. Similarly, a more recent double-blind placebo-controlled study found that mirtazapine is not superior to placebo at decreasing cocaine use in cocaine-dependent subjects comorbid with depression (Afshar et al., 2012); although, similar to the study by Cruickshank et al., the sample size was small with only 11 patients on mirtazapine. Thus, despite two reports where mirtazapine fails to perform as a substance abuse pharmacotherapy, the bulk of the clinical observations suggest that mirtazapine therapy may improve outcomes across a number of SUDs as well as benefit patients with comorbid conditions, including depression, anxiety and/or HIV/AIDS.
Another aspect of mirtazapine treatment that may provide therapeutic benefit for patients with SUDs pertains to cognitive function. There is an extensive literature on neurocognitive dysfunction by those suffering from SUDs (Bowden et al., 2001;Bechara, 2005;Yucel and Lubman, 2007;Nath, 2010). A meta-analysis of studies on the neurocognitive effects of cocaine abuse reported largest effect sizes for measures with attentional and executive components, which included the WAIS-R Arithmetic and Digit Span subtests, the Stroop, the Trail Making Test-B, Symbol Digit Modalities and the Paced Auditory Serial Addition Test (Jovanovski et al., 2005). Cognitive deficits in patients with SUDs are also seen in decision-making processes using delayed discounting paradigms. Delayed discounting paradigms offer the option of choosing an immediate reward or a reward that is delayed and given in the future; future rewards are devalued but are robustly affected by drugs of abuse which promote the devaluation of future rewards (Setlow et al., 2009;Bickel et al., 2007;Bickel and Marsch, 2001). In opioid-dependent humans, a future monetary reward is much more rapidly devalued compared to non-dependent controls (Madden et al., 1997). Additionally, opioid-dependent patients rapidly devalue heroin indicating the importance of the immediacy of the reward (Madden et al., 1997), i.e. no amount of heroin in the future is worth a smaller amount now. Similarly, monetary rewards are more rapidly devalued in current cigarette smokers compared to both smokers who have successfully quit and in controls who did not have a history of smoking (Bickel et al., 1999). The decision of an immediate, smaller reward versus a larger reward in the future is regulated by competing neuronal systems, namely the nucleus accumbens and cortical regions such as the prefrontal cortex (McClure et al., 2004;Bickel et al., 2009) and these reports are complemented by imaging studies revealing altered function in both cortical and accumbal regions at various stages of addiction (Kalivas and Volkow, 2005;Koob and Volkow, 2010;Goldstein and Volkow, 2011). Collectively, the available data suggest a dominance of the accumbal pathway guiding immediate reward choices over cortical function. This leads to impaired decision making and increased devaluation of future rewards. Potential effects of mirtazapine have not been tested in delayed discounting paradigms nor have there been reports assessing potential cognitive enhancing effects of mirtazapine in patients with SUDs. However, a number of clinical studies find significant improvements in cognitive performance in other patient populations.
In patients with major depression, mirtazapine improves measures of executive function after 3 and 6 months of treatment (Borkowska et al., 2007). Cognitive performance is also improved in patients with schizophrenia; in an 8-week, open-label trial in schizophrenic patients mirtazapine improved immediate and delayed verbal memory (Delle et al., 2007). In contrast, a 6-week, randomized, double-blind, placebo-controlled trial of adjunctive mirtazapine in patients with schizophrenia being treated with clozapine finds no effect on digit span, word learning, trail making or verbal fluency (Berk et al., 2009). However, improvements are seen in memory and vocabulary with an 8-week, randomized, double-blind, placebo-controlled trial of mirtazapine in patients with schizophrenia who were treated with risperidone (Cho et al., 2011). Overall, these studies provide evidence of improved cognitive function with mirtazapine in patients with psychiatric illnesses. These studies also indicate the importance of treatment duration for cognitive enhancements. Further efforts are needed to determine if the above findings extend to patients with SUDs as well as in patients with comorbidities, a common occurrence with SUDs.
5. Future Directions
Mirtazapine has a complex pharmacological profile, and many of the direct and indirect targets impacted by mirtazapine may contribute to decreasing opioid– and psychostimulant-mediated behaviors. However, we contend that inverse agonism at the 5-HT2C receptor may provide an efficacious target with should not exhibit some of the unwanted side effects that result from the other receptors engaged by mirtazapine. In brief, inverse agonism may be a particularly important and intriguing component that needs to be more fully explored.
Spampinato et al., (De Deurwaerdere et al., 2004) show that regulation of DA efflux in the nucleus accumbens and striatum is heavily influenced by constitutively active 5-HT2C receptors. We recently assessed the ability of a 5-HT2C inverse agonist (SB 206553) to augment methamphetamine-seeking behavior in rats and found a robust, dose-dependent inhibition of seeking behavior (Graves and Napier, 2012). In contrast to the above, administration of two chemically distinct 5-HT2C antagonists (SDZ Ser 082 and SB 242084) had no effect on methamphetamine-seeking (Graves and Napier, 2012). These findings are supported by cocaine studies wherein SDZ Ser 082 and SB 2420 84 also have no effect on cue-induced reinstatement of cocaine-seeking (Filip, 2005;Neisewander and Acosta, 2007).
To better understand the mechanism(s) resulting in SB 20653 effects on psychostimulant-mediated behaviors we recently explored the effects of SB 206553 on neuronal function in the nucleus accumbens shell. Whole-cell current clamp recordings were conducted in brain slices from rats trained to self-administer methamphetamine (after 1–4 days withdrawal from operant procedures) and compared to saline-yoked rats. Similar to the results found with cocaine sensitization (Kourrich and Thomas, 2009;Zhang et al., 1998) and self-administration (Mu et al., 2010;Schramm-Sapyta et al., 2006), excitability of neurons in the accumbens shell is decreased (i.e. fewer action potentials generated in response to depolarizing current injection) after 1–4 days withdrawal from methamphetamine self-administration (unpublished data). In contrast, bath application of the putative inverse agonist SB 206553 increased neuronal excitability (unpublished data) with no evidence of functional dysregulation of 5-HT2C receptors by methamphetamine self-administration. Additional studies are needed to determine potential psychostimulant-induced plasticity in other regions such as the medial prefrontal cortex.
The aforementioned studies were the first to assess the effect of the putative 5-HT2C receptor inverse agonist, SB 206553, on methamphetamine-induced behaviors and find a dramatic decrease in seeking (Graves and Napier, 2012). Moreover, in the accumbens shell, neuronal excitability was decreased by methamphetamine self-administration, an effect that was abrogated by acute bath application of SB 206553. Further study is needed to determine signaling mechanisms, potential plasticity, and ability to maintain efficacy with chronic therapy. Nonetheless, inverse agonism at 5-HT2C receptors has provided impressive initial results and is an exciting avenue for continued medications development.
6. Conclusions
In summary, mirtazapine has multiple targets likely contributing either additively or synergistically to its ability to serve as pharmacotherapy to SUDs including 5-HT3 and 5HT2A antagonism, indirect 5-HT1A agonism, and 5-HT2C inverse agonism. While mirtazapine appears to be very promising both preclinically and clinically, large double-blind placebo-controlled trials are needed to validate the summarized data. Finally, additional investigations that utilize the knowledge gained from mirtazapine and engage in de novo synthesis of novel compounds may aid in the discovery of medications with enhanced efficacy and improved side-effect profiles.
Acknowledgements
Work reported in this review was supported by USPHSGs R01 DA015760, F31 DA024923, and the Daniel F. and Ada L. Rice Foundation. The authors thank the Program Officer of our R01, Dr. Nathanial Appel for his insightful discussions on this topic throughout the life of the project. The authors also wish to thank Dr. Kathryn Cunningham for her valuable discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: TCN and SMG have nothing to disclose. RR is on the speakers' bureau and advisory boards for Eli Lilly, Bristol Meyers Squibb, Otsuka, Astra Zeneca, Janssen, Forest, Sonovian, Pfizer, Wyeth and receives research support from Serpacor and Janssen. JW is on the BMS Virology Speakers' Bureau.
Reference List
- 1.Afshar M, Knapp CM, Sarid-Segal O, Devine E, Colaneri LS, Tozier L, Waters ME, Putnam MA, Ciraulo DA. The efficacy of mirtazapine in the treatment of cocaine dependence with comorbid depression. Am J Drug Alcohol Abuse. 2012;38:181–186. doi: 10.3109/00952990.2011.644002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA. Modulation of dopamine release by striatal 5-HT2C receptors. Synapse. 2005;55:242–251. doi: 10.1002/syn.20109. [DOI] [PubMed] [Google Scholar]
- 3.Altintoprak AE, Zorlu N, Coskunol H, Akdeniz F, Kitapcioglu G. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: a randomized, double-blind study. Hum Psychopharmacol. 2008;23:313–319. doi: 10.1002/hup.935. [DOI] [PubMed] [Google Scholar]
- 4.Barbon A, Orlandi C, La VL, Caracciolo L, Tardito D, Musazzi L, Mallei A, Gennarelli M, Racagni G, Popoli M, Barlati S. Antidepressant treatments change 5-HT2C receptor mRNA expression in rat prefrontal/frontal cortex and hippocampus. Neuropsychobiology. 2011;63:160–168. doi: 10.1159/000321593. [DOI] [PubMed] [Google Scholar]
- 5.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 6.Berendsen HH, Broekkamp CL. Indirect in vivo 5-HT1A-agonistic effects of the new antidepressant mirtazapine. Psychopharmacology (Berl) 1997;133:275–282. doi: 10.1007/s002130050402. [DOI] [PubMed] [Google Scholar]
- 7.Berk M, Gama CS, Sundram S, Hustig H, Koopowitz L, D'Souza R, Malloy H, Rowland C, Monkhouse A, Monkhouse A, Bole F, Sathiyamoorthy S, Piskulic D, Dodd S. Mirtazapine add-on therapy in the treatment of schizophrenia with atypical antipsychotics: a double-blind, randomised, placebo-controlled clinical trial. Hum Psychopharmacol. 2009;24:233–238. doi: 10.1002/hup.1017. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia KS, Szabo ST, Fowler JC, Wetsel WC, Lee TH. Reversal of long-term methamphetamine sensitization by combination of pergolide with ondansetron or ketanserin, but not mirtazapine. Behav Brain Res. 2011;223:227–232. doi: 10.1016/j.bbr.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 10.Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 12.Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum MR, Liao SH, Good SS, de MP. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988;85:189–194. [PubMed] [Google Scholar]
- 14.Borkowska A, Drozdz W, Ziolkowska-Kochan M, Rybakowski J. Enhancing effect of mirtazapine on cognitive functions associated with prefrontal cortex in patients with recurrent depression. Neuropsychopharmacol Hung. 2007;9:131–136. [PubMed] [Google Scholar]
- 15.Bowden SC, Crews FT, Bates ME, Fals-Stewart W, Ambrose ML. Neurotoxicity and neurocognitive impairments with alcohol and drug-use disorders: potential roles in addiction and recovery. Alcohol Clin Exp Res. 2001;25:317–321. [PubMed] [Google Scholar]
- 16.Brecht ML, von MC, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32:211–220. doi: 10.1080/02791072.2000.10400231. [DOI] [PubMed] [Google Scholar]
- 17.Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- 18.Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–297. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- 20.Bubar MJ, Stutz SJ, Cunningham KA. 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS ONE. 2011;6:e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanrion B, Mannoury la CC, Gavarini S, Seimandi M, Vincent L, Pujol JF, Bockaert J, Marin P, Millan MJ. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol Pharmacol. 2008;73:748–757. doi: 10.1124/mol.107.041574. [DOI] [PubMed] [Google Scholar]
- 22.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho SJ, Yook K, Kim B, Choi TK, Lee KS, Kim YW, Lee JE, Suh S, Yook KH, Lee SH. Mirtazapine augmentation enhances cognitive and reduces negative symptoms in schizophrenia patients treated with risperidone: a randomized controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:208–211. doi: 10.1016/j.pnpbp.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Colfax GN, Santos GM, Das M, Santos DM, Matheson T, Gasper J, Shoptaw S, Vittinghoff E. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68:1168–1175. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, Shand D. A placebo-controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug Alcohol Rev. 2008;27:326–333. doi: 10.1080/09595230801935672. [DOI] [PubMed] [Google Scholar]
- 26.Davidson C, Gopalan R, Ahn C, Chen Q, Mannelli P, Patkar AA, Weese GD, Lee TH, Ellinwood EH. Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Eur J Pharmacol. 2007;565:113–118. doi: 10.1016/j.ejphar.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 27.Davidson C, Lazarus C, Xiong XY, Lee TH, Ellinwood EH. 5-HT2 receptor antagonists given in the acute withdrawal from daily cocaine injections can reverse established sensitization. Eur J Pharmacol. 2002a;453:255–263. doi: 10.1016/s0014-2999(02)02390-7. [DOI] [PubMed] [Google Scholar]
- 28.Davidson C, Lee TH, Xiong Z, Ellinwood EH. Ondansetron given in the acute withdrawal from a repeated cocaine sensitization dosing regimen reverses the expression of sensitization and inhibits self-administration. Neuropsychopharmacology. 2002b;27:542–553. doi: 10.1016/S0893-133X(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 29.de Boer TH. The pharmacologic profile of mirtazapine. J Clin Psychiatry. 1996;57(Suppl 4):19–25. [PubMed] [Google Scholar]
- 30.de Boer TH, Maura G, Raiteri M, de Vos CJ, Wieringa J, Pinder RM. Neurochemical and autonomic pharmacological profiles of the 6-aza- analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology. 1988;27:399–408. doi: 10.1016/0028-3908(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 31.de Boer TH, Nefkens F, van Helvoirt A, Van Delft AM. Differences in modulation of noradrenergic and serotonergic transmission by the alpha-2 adrenoceptor antagonists, mirtazapine, mianserin and idazoxan. J Pharmacol Exp Ther. 1996;277:852–860. [PubMed] [Google Scholar]
- 32.De Deurwaerdere P, Spampinato U. Role of serotonin(2A) and serotonin(2B/2C) receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- 33.De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delle CR, Salviati M, Fiorentini S, Biondi M. Add-on mirtazapine enhances effects on cognition in schizophrenic patients under stabilized treatment with clozapine. Exp Clin Psychopharmacol. 2007;15:563–568. doi: 10.1037/1064-1297.15.6.563. [DOI] [PubMed] [Google Scholar]
- 35.Devoto P, Flore G, Pira L, Longu G, Gessa GL. Mirtazapine-induced corelease of dopamine and noradrenaline from noradrenergic neurons in the medial prefrontal and occipital cortex. Eur J Pharmacol. 2004;487:105–111. doi: 10.1016/j.ejphar.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 37.Dracheva S, Chin B, Haroutunian V. Altered serotonin 2C receptor RNA splicing in suicide: association with editing. Neuroreport. 2008a;19:379–382. doi: 10.1097/WNR.0b013e3282f556d2. [DOI] [PubMed] [Google Scholar]
- 38.Dracheva S, Patel N, Woo DA, Marcus SM, Siever LJ, Haroutunian V. Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol Psychiatry. 2008b;13:1001–1010. doi: 10.1038/sj.mp.4002081. [DOI] [PubMed] [Google Scholar]
- 39.Eiermann B, Engel G, Johansson I, Zanger UM, Bertilsson L. The involvement of CYP1A2 and CYP3A4 in the metabolism of clozapine. Br J Clin Pharmacol. 1997;44:439–446. doi: 10.1046/j.1365-2125.1997.t01-1-00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filip M. Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep. 2005;57:35–46. [PubMed] [Google Scholar]
- 42.Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol Exp Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- 43.Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology (Berl) 2006;183:482–489. doi: 10.1007/s00213-005-0197-y. [DOI] [PubMed] [Google Scholar]
- 44.Filip M, Cunningham KA. Serotonin 5-HT(2C) receptors in nucleus accumbens regulate expression of the hyperlocomotive and discriminative stimulus effects of cocaine. Pharmacol Biochem Behav. 2002;71:745–756. doi: 10.1016/s0091-3057(01)00741-9. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonost M100,907 and the 5-HT(2C) receptor antagonist SB242,084 on cocaine-iduced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropschopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 46.Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher PJ, Sinyard J, Higgins GA. The effects of the 5-HT(2C) receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology (Berl) 2006;187:515–525. doi: 10.1007/s00213-006-0453-9. [DOI] [PubMed] [Google Scholar]
- 48.Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, dhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graves SM, Napier TC. Mirtazapine Alters Cue-Associated Methamphetamine Seeking in Rats. Biol Psychiatry. 2011;69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graves SM, Napier TC. SB 206553, a putative 5-HT2C inverse agonist, attenuates methamphetamine-seeking in rats. BMC Neurosci. 2012;13:65. doi: 10.1186/1471-2202-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graves SM, Persons AL, Riddle JL, Celeste NT. The atypical antidepressant mirtazapine attenuates expression of morphine-induced place preference and motor sensitization. Brain Res. 2012;1472:45–53. doi: 10.1016/j.brainres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- 55.Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- 56.Haddjeri N, Blier P, De Montigny C. Acute and long-term actions of the antidepressant drug mirtazapine on central 5-HT neurotransmission. J Affect Disord. 1998;51:255–266. doi: 10.1016/s0165-0327(98)00223-7. [DOI] [PubMed] [Google Scholar]
- 57.Herrold AA, Shen F, Graham MP, Harper LK, Specio SE, Tedford CE, Napier TC. Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2009;99:231–239. doi: 10.1016/j.drugalcdep.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Herrold AA, Shen F, Grahma MP, Harper LK, Specio SE, Tedford CE, Napier TC. Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2008 doi: 10.1016/j.drugalcdep.2008.08.005. in press. [DOI] [PubMed] [Google Scholar]
- 59.Hoyer D. Functional correlates of serotonin 5-HT1 recognition sites. 1988:59–71. doi: 10.3109/10799898809048978. [DOI] [PubMed] [Google Scholar]
- 60.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2009. NIH Publication No 10-7584 Volume I: Secondary school students. 2010 [Google Scholar]
- 61.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 62.Kakui N, Yokoyama F, Yamauchi M, Kitamura K, Imanishi T, Inoue T, Koyama T. Anxiolytic-like profile of mirtazapine in rat conditioned fear stress model: Functional significance of 5-hydroxytryptamine 1A receptor and alpha1-adrenergic receptor. Pharmacol Biochem Behav. 2009;92:393–398. doi: 10.1016/j.pbb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 64.Kang L, Wang D, Li B, Hu M, Zhang P, Li J. Mirtazapine, a noradrenergic and specific serotonergic antidepressant, attenuates morphine dependence and withdrawal in Sprague-Dawley rats. Am J Drug Alcohol Abuse. 2008;34:541–552. doi: 10.1080/00952990802183921. [DOI] [PubMed] [Google Scholar]
- 65.Kelder J, Funke C, De Boer T, Delbressine L, Leysen D, Nickolson V. A comparison of the physicochemical and biological properties of mirtazapine and mianserin. J Pharm Pharmacol. 1997;49:403–411. doi: 10.1111/j.2042-7158.1997.tb06814.x. [DOI] [PubMed] [Google Scholar]
- 66.King GR, Xiong ZP, Douglass S, Ellinwood EH., Jr. Long-term blockade of the expression of cocaine sensitization by ondansetron, a 5-HT3 receptor antagonist. Eur J Pharmacol. 2000;394:97–101. doi: 10.1016/s0014-2999(99)00926-7. [DOI] [PubMed] [Google Scholar]
- 67.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kooyman AR, Zwart R, Vanderheijden PM, Van Hooft JA, Vijverberg HP. Interaction between enantiomers of mianserin and ORG3770 at 5-HT3 receptors in cultured mouse neuroblastoma cells. Neuropharmacology. 1994;33:501–507. doi: 10.1016/0028-3908(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 69.Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 71.Labasque M, Meffre J, Carrat G, Becamel C, Bockaert J, Marin P. Constitutive Activity of Serotonin2C Receptors at G Protein-Independent Signaling: Modulation by RNA Editing and Antidepressants. Mol Pharmacol. 2010 doi: 10.1124/mol.110.066035. [DOI] [PubMed] [Google Scholar]
- 72.Leggio GM, Cathala A, Neny M, Rouge-Pont F, Drago F, Piazza PV, Spampinato U. In vivo evidence that constitutive activity of serotonin(2C) receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06356.x. [DOI] [PubMed] [Google Scholar]
- 73.Liappas J, Paparrigopoulos T, Malitas P, Tzavellas E, Christodoulou G. Mirtazapine improves alcohol detoxification. J Psychopharmacol. 2004;18:88–93. doi: 10.1177/0269881104040241. [DOI] [PubMed] [Google Scholar]
- 74.Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:55–60. doi: 10.1016/j.pnpbp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Lima MS, Reisser AA, Soares BG, Farrell M. Antidepressants for cocaine dependence. Cochrane Database Syst RevCD002950. 2003 doi: 10.1002/14651858.CD002950. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Cunningham KA. Serotonin2C receptors (5-HT2C R) control expression of cocaine-induced conditioned hyperactivity. Drug Alcohol Depend. 2006;81:275–282. doi: 10.1016/j.drugalcdep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Lucas G, Spampinato U. Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. Journal of Neurochemistry. 2000;74:693–701. doi: 10.1046/j.1471-4159.2000.740693.x. [DOI] [PubMed] [Google Scholar]
- 79.Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- 80.Mandrioli R, Forti GC, Raggi MA. Fluoxetine metabolism and pharmacological interactions: the role of cytochrome p450. Curr Drug Metab. 2006;7:127–133. doi: 10.2174/138920006775541561. [DOI] [PubMed] [Google Scholar]
- 81.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 82.McDaid J, Tedford CE, Mackie AR, Dallimore JE, Mickiewicz AL, Shen F, Angle JM, Napier TC. Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 2007;86:55–66. doi: 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 83.McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat. 2008;35:334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schluter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakayama K, Sakurai T, Katsu H. Mirtazapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Brain Res Bull. 2004;63:237–241. doi: 10.1016/j.brainresbull.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 86.Napier TC, Istre ED. Methamphetamine-induced sensitization includes a functional upregulation of ventral pallidal 5-HT2A/2C receptors. Synapse. 2008;62:14–21. doi: 10.1002/syn.20460. [DOI] [PubMed] [Google Scholar]
- 87.Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- 88.Navailles S, Moison D, Ryczko D, Spampinato U. Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J Neurochem. 2006;99:1311–1319. doi: 10.1111/j.1471-4159.2006.04188.x. [DOI] [PubMed] [Google Scholar]
- 89.Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- 90.Nic Dhonnchadha BA, Cunningham KA. Serotonergic mechanisms in addiction-related memories. Behav Brain Res. 2008;195:39–53. doi: 10.1016/j.bbr.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-ht2a receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic cost of Methamphetamine Use in the United States, 2005. Rand Corporation; Santa Monica, CA: 2009. [Google Scholar]
- 93.Nierenberg AA. Do some antidepressants work faster than others? J Clin Psychiatry. 2001;62(Suppl 15):22–25. [PubMed] [Google Scholar]
- 94.Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–491. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- 95.Niswender CM, Sanders-Bush E, Emeson RB. Identification and characterization of RNA editing events within the 5-HT2C receptor. Ann N Y Acad Sci. 1998;861:38–48. doi: 10.1111/j.1749-6632.1998.tb10171.x. [DOI] [PubMed] [Google Scholar]
- 96.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 97.O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 98.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M. Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res. 2006;31:167–176. doi: 10.1007/s11064-005-9006-6. [DOI] [PubMed] [Google Scholar]
- 99.Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of Medial Prefrontal Cortex Serotonin 2C (5-HT(2C)) Receptors Attenuates Cocaine-Seeking Behavior. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- 101.Sala M, Coppa F, Cappucciati C, Brambilla P, d'Allio G, Caverzasi E, Barale F, De Ferrari GM. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs. 2006;7:256–263. [PubMed] [Google Scholar]
- 102.SAMHSA . Drug Abuse Warning Network, 2006: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: 2008. (DAWN DAWN Series D-30). DHHS Publication No. (SMA) 08-4339. [Google Scholar]
- 103.Schramm-Sapyta NL, Olsen CM, Winder DG. Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmacology. 2006;31:1444–1451. doi: 10.1038/sj.npp.1300918. [DOI] [PubMed] [Google Scholar]
- 104.Serrats J, Mengod G, Cortes R. Expression of serotonin 5-HT2C receptors in GABAergic cells of the anterior raphe nuclei. J Chem Neuroanat. 2005;29:83–91. doi: 10.1016/j.jchemneu.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Setlow B, Mendez IA, Mitchell MR, Simon NW. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav Pharmacol. 2009;20:380–389. doi: 10.1097/FBP.0b013e3283305eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 2001;6:373–379. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- 107.Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206–1227. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]
- 108.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 109.Stitzer ML, Vandrey R. Contingency management: utility in the treatment of drug abuse disorders. Clin Pharmacol Ther. 2008;83:644–647. doi: 10.1038/sj.clpt.6100508. [DOI] [PubMed] [Google Scholar]
- 110.Stormer E, von Moltke LL, Shader RI, Greenblatt DJ. Metabolism of the antidepressant mirtazapine in vitro: contribution of cytochromes P-450 1A2, 2D6, and 3 A4. Drug Metab Dispos. 2000;28:1168–1175. [PubMed] [Google Scholar]
- 111.Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning : cardiovascular toxicity. Toxicol Rev. 2005;24:205–214. doi: 10.2165/00139709-200524030-00013. [DOI] [PubMed] [Google Scholar]
- 112.Timmer CJ, Sitsen JM, Delbressine LP. Clinical pharmacokinetics of mirtazapine. Clin Pharmacokinet. 2000;38:461–474. doi: 10.2165/00003088-200038060-00001. [DOI] [PubMed] [Google Scholar]
- 113.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 114.Urichuk L, Prior TI, Dursun S, Baker G. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008;9:410–418. doi: 10.2174/138920008784746373. [DOI] [PubMed] [Google Scholar]
- 115.Vocci F, Ling W. Medications development: Successes and challenges. Pharmacol Ther. 2005 doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 116.Voigt RM, Mickiewicz AL, Napier TC. Repeated mirtazapine nullifies the maintenance of previously established methamphetamine-induced conditioned place preference in rats. Behav Brain Res. 2011;225:91–96. doi: 10.1016/j.bbr.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voigt RM, Napier TC. Context-dependent effects of a single administration of mirtazapine on the expression of methamphetamine-induced conditioned place preference. Front Behav Neurosci. 2011;5:92. doi: 10.3389/fnbeh.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Q, O'Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- 122.Werry TD, Loiacono R, Sexton PM, Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 123.Westenberg HG. Pharmacology of antidepressants: selectivity or multiplicity? J Clin Psychiatry. 1999;60(Suppl 17):4–8. [PubMed] [Google Scholar]
- 124.Yoon SJ, Pae CU, Kim DJ, Namkoong K, Lee E, Oh DY, Lee YS, Shin DH, Jeong YC, Kim JH, Choi SB, Hwang IB, Shin YC, Cho SN, Lee HK, Lee CT. Mirtazapine for patients with alcohol dependence and comorbid depressive disorders: a multicentre, open label study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1196–1201. doi: 10.1016/j.pnpbp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: implications for diagnosis, treatment and prevention. Drug Alcohol Rev. 2007;26:33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- 126.Yuen GJ, Morris DM, Mydlow PK, Haidar S, Hall ST, Hussey EK. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35:1174–1180. doi: 10.1002/j.1552-4604.1995.tb04043.x. [DOI] [PubMed] [Google Scholar]
- 127.Yuen GJ, Weller S, Pakes GE. A review of the pharmacokinetics of abacavir. Clin Pharmacokinet. 2008;47:351–371. doi: 10.2165/00003088-200847060-00001. [DOI] [PubMed] [Google Scholar]
- 128.Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: Reduced sodium currents in nucleus accumbens neurons. Journal of Neuroscience. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]