Abstract
Background
The nature of the relationship between major depression (MD) and phenotypes related to smoking behavior, including nicotine dependence (ND), is complicated. We present results from analyses comparing models wherein MD and ND are influenced by a shared latent factor to one in which causal pathways between phenotypes are examined.
Method
Data were collected for 2906 adult male twins from a population-based sample. Structural equation modeling was used to derive path estimates for shared liability and causal models. MD was assessed according to DSM-III-R diagnostic criteria; ND was assessed using the Fagerstrom Test for Nicotine Dependence (FTND).
Results
The best fitting shared liability model included genetic, but not environmental, influences shared between MD and FTND; a small proportion of these shared influences were also common to smoking initiation. The best fitting causal model included a unidirectional causal path from FTND to MD, with no direct genetic correlation between MD and smoking initiation. Model fit statistics indicated that these models provided nearly identical fits to the data, with the causal model providing a slightly superior AIC value.
Conclusions
The phenotypic association between MD and FTND is likely due to both a causal relationship, wherein increasing levels of nicotine dependence increase one’s risk for depression, and to a shared genetic liability between the two.
Limitations
This sample consists of Caucasian males born in Virginia, and findings might not be generalizable to others. Statistical power was less than ideal.
Keywords: nicotine dependence, major depression, shared liability, causal relationship
Introduction
Major depression and tobacco use both represent substantial health burdens worldwide. The Centers for Disease Control and Prevention reported that between 2000 and 2004, smoking accounted for over 400,000 annual deaths in the United States alone, and cost nearly $100 billion in productivity losses annually during that period (CDC, 2008). Depression, too, exacts a steep economic cost: the economic burden of depression was estimated at $83 billion in the U.S. in 2000 (Greenberg et al., 2003); over 7% of this estimate is attributed to suicide-related costs. The lifetime prevalence of major depression in the U.S., as assessed between 2001 and 2002, was 16.2% (Kessler et al., 2003); approximately 20% of U.S. adults currently smoke (CDC, 2011).
Phenotypic Associations
Multiple researchers have reported an association between tobacco use and depression. For example, Breslau et al. (1991) found that the lifetime prevalence of major depression (MD) increased across groups with increasing levels of nicotine dependence (ND). A study of a large, population-based sample found that individuals with depression were more likely to be current or past smokers than those without a history of depression (Breslau et al., 2004). These findings were supported and complemented by a more recent report (Swendsen et al., 2010) that used the National Comorbidity Survey Replication sample, in which non-nicotine dependent individuals with a lifetime history of major depression at time 1 were at an increased risk (OR=1.4) of nicotine dependence at time 2 (the reciprocal relationship was not reported). John et al. (2004) reported that current daily smokers were more likely to have an affective disorder diagnosis than individuals who had never been regular smokers (OR=1.8); former daily smokers’ risk, though elevated, was not significant. Studies also support a positive association between smoking and suicide ideation, attempts, and completion (Breslau et al., 2005; Döme et al., 2010; Döme et al., 2011). This relationship is independent of a history of MD, with both MD and daily smoking significantly associated with suicidality (Breslau et al., 2005). Thus, the potential consequences of smoking on public health outcomes are potentially dire.
Causal relationships
Investigators have explored the possibilities that MD leads to smoking, typically through a self-medication mechanism; that smoking leads to MD, potentially through neurobiological or other physiological effects of nicotine and smoke exposure; or that both directions of effect are relevant. In the context of the self-medication hypothesis, nicotine could be used to treat depressive symptoms or negative affect, with continued use leading to nicotine dependence (Fergusson et al., 2003). There is some subjective evidence for such a relationship: Lerman and colleagues (1996) found that people who were depressed were more likely to report that they smoked for stimulation and negative affect regulation. Results from a longitudinal study of young adults suggest that a reciprocal causal relationship could also contribute to the observed association (Breslau et al., 1998): the authors suggested that individuals might progress to daily smoking in an effort to medicate negative affect, while nicotine acts on dopaminergic neurons in the nucleus accumbens that have also been linked to depressed mood.
Other studies have also explored the physiological effects of nicotine exposure. Balfour and Ridley (2000) reported that adrenocortical and serotonergic functioning are compromised with nicotine exposure, which could have subsequent effects on depression. In addition to the potential for influences on neural pathways implicated in both nicotine administration and depression, many constituents of the smoke produced by cigarettes and cigars – including heavy metals and ammonium products – have toxic effects on the brain (Swan and Lessov-Schlaggar, 2007), including oxidative stress. Increased oxidative stress, as assessed across a variety of measures, has been associated with depression (Hovatta et al., 2010). Thus, even apart from the effects of nicotine itself, the neurotoxicity of tobacco smoke might present its own risk.
Evidence of a shared genetic or environmental liability
Many studies that are designed to test for causal pathways between MD and ND are insufficient for ruling out the possibility of a shared liability between phenotypes in the form of genetic or environmental influences common to both. These different etiological relationships are not mutually exclusive. Twin studies on depression and smoking-related phenotypes, including ND, largely support the existence of a shared liability. Kendler and colleagues (1993) reported a genetic correlation between phenotypes in a population-based female sample (see below). Similarly, a genetic correlation has been reported between MD and ND in a population-based sample of men (Lyons et al., 2008), and between MD and regular smoking in a sample including both sexes (Edwards et al., 2010). Korhonen and colleagues (2007) reported evidence of a causal relationship between smoking persistence – a slightly different nicotine-related phenotype – and depression in a sample of Finnish male twins; a modest genetic correlation between traits (rG=0.25) was also reported. Unfortunately, the nature of the data precluded testing the hypothesis that earlier depression caused later smoking.
Previously, a cotwin control analysis was conducted using the female portion of our population-based twin sample, and results indicated that shared genetic liability accounted for the phenotypic association between major depression and regular smoking (Kendler et al. 1993). The current report seeks to follow-up on those findings in male twins. Using this genetically informative sample, we aim to compare two models of the relationship between MD and nicotine dependence (rather than regular smoking, which was used in the prior study), while modeling the contingency of nicotine dependence on initiation of tobacco use. The first is a shared liability model, in which the phenotypic association between MD and nicotine dependence is a function of common genetic and/or environmental influences. The second models reciprocal causation between depression and nicotine dependence. By comparing the fit of the models, we can examine whether the association between MD and ND is better explained by genetic and/or environmental influences shared across phenotypes, by causal relationships between them, or whether both provide plausible explanations of the data.
Methods
Data collection and sample
Participants were part of the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD), which has been previously described (Kendler and Prescott, 2006). The current sample consisted of male-male pairs who participated in Wave 2. Data were available for 2906 individuals, including 697 complete monozygotic (MZ) twin pairs and 487 complete dizygotic (DZ) twin pairs. The remaining 538 individuals were members of twin pairs wherein data were not available for the co-twin. The mean age for these participants was 36.5 (SD=9.21). Zygosity was determined using a combination of self-report measures, photographs, and genotyping (Kendler and Prescott, 2006). The project received human subjects approval from Virginia Commonwealth University and participants provided informed consent prior to all interviews. Diagnostic interviews were based on the Structured Clinical Interview for DSM-III-R (Spitzer et al., 1987), and were conducted by trained interviewers who held a master’s degree in a mental health-related field, or who held a bachelor’s degree in such a field and had two years of clinical experience.
Measures
A lifetime diagnosis of major depression was determined using criteria from the Diagnostic and Statistical Manual of Mental Disorders, edition III-R. Because of the time-intensive nature of the interview process, only individuals who had experienced feelings of sadness/depression and/or a loss of interest for at least 7 days in a row were administered the entire depression section of the interview. For the purposes of the current analyses, those who did not meet either of those criteria were coded as not having had a depressive episode. The depression phenotype used in these analyses is binary, where a score of 0 indicated that the participant had no history of a depressive episode, and a score of 1 indicated that the participant met DSM-III-R diagnostic criteria for a major depressive episode.
Participants were asked whether they had ever used tobacco products. Those who endorsed tobacco use initiation (INIT) were further asked whether they had ever used tobacco regularly for at least a month. Participants who endorsed regular smoking (current or past) were administered items from the Fagerstrom Test for Nicotine Dependence that they rated for the period of their heaviest lifetime tobacco use (FTND, Heatherton et al., 1991). Participants could receive a raw FTND score from 0–10. For twin modeling, these scores were converted into an ordinal variable with five levels (scored 0–4).
Statistical analyses
Descriptive statistics were obtained using JMP 8.0 and SAS 9.1. Preliminary analyses were conducted in SAS. Where appropriate, PROC GENMOD was used to correct for twinship.
Twin modeling was conducted in Mx (Neale et al., 2006) using the raw ordinal data option. The use of ordinal data assumes that the categories are representative of an underlying normal distribution of liability, with thresholds in liability discriminating between categories. All models included age as a covariate. In twin modeling, liability to phenotypes such as MD can be attributed to several latent sources of variance: additive genetic factors (A), shared environment (C), and unique environment (E). The C variance component represents environmental exposures and experiences that are shared by both members of a twin pair and contribute to twins’ increased similarity in a given phenotype, irrespective of zygosity. Environmental factors that are unique to one twin are accounted for by the E component; these factors reduce twin similarity for a given phenotype. The E component also includes measurement error. Estimates of each of these variance components are calculated by comparing the phenotypic correlation between monozygotic twins, who share all their genes, to dizygotic twins, who share half of their genes on average identical by descent. In the current sample, phenotypic correlations were higher for MZ twins than for DZ twins, suggesting that genetic factors contribute to manifestation of these phenotypes.
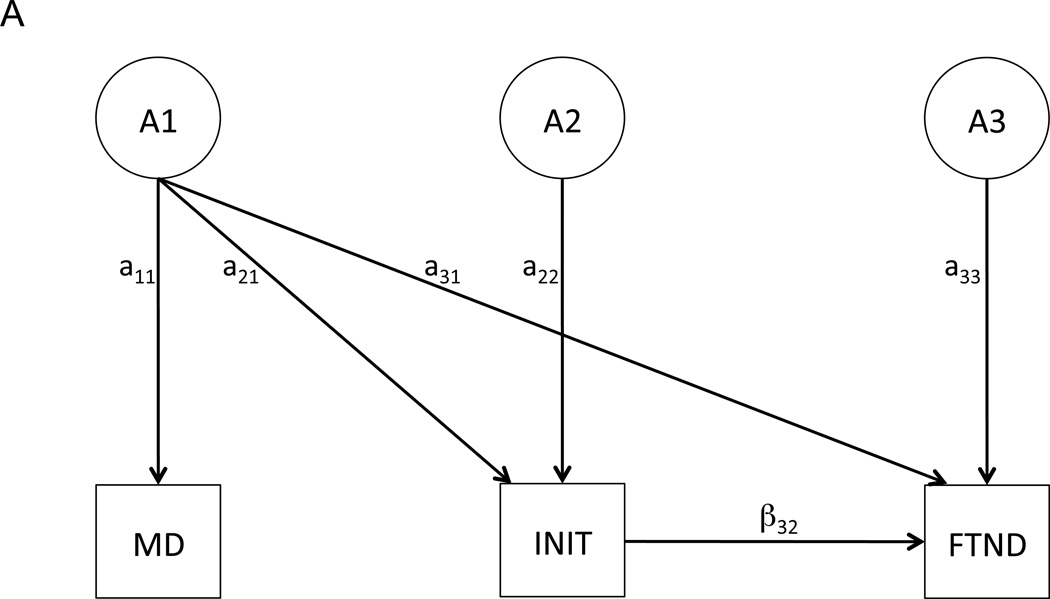
We began with two different (and non-nested) full models: a shared liability model (Figure 1A) and a reciprocal causation model (Figure 1B). Both models included age at interview as a covariate. Because shared liability and causal paths between the same variables cannot be modeled simultaneously, model fitting proceeded separately for each, and model fit statistics for the best-fitting shared liability and causal models were compared. As depicted in Figure 1A, the shared liability model allows for a single latent genetic/environmental factor (e.g., A1) to influence each phenotype (MD, INIT, and FTND). However, because FTND is contingent upon initiation of tobacco use, a contingency pathway is included in the model from INIT to FTND (see Kendler et al., 1999 for further information on this model structure). The shared liability model is used to test the hypothesis that MD and FTND are influenced in part by a common subset of genetic and/or environmental factors, leading to their phenotypic association.
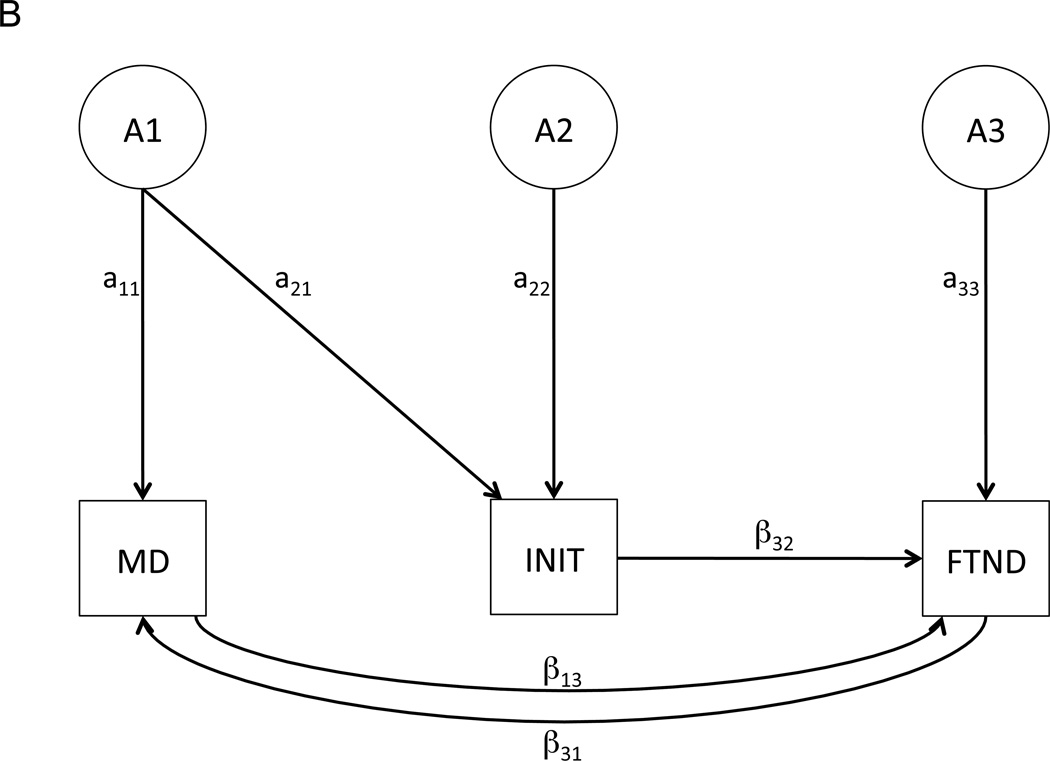
Figure 1.
Shared liability (A) and reciprocal causation (B) models depicting potential relationships among MD, INIT, and FTND. For the sake of simplicity, only the causal paths (labeled with “β”) and latent genetic paths (labeled with “a”) are depicted; however, the genetic paths are accompanied by shared and unique environmental paths (described as “C” and “E”, respectively, in the Methods).
Like the shared liability model, the reciprocal causation model allows for shared genetic/environmental influences between MD and INIT. The contingency pathway between INIT and FTND is also retained in this model. However, the only direct relationships modeled between MD and FTND are in the form of causal pathways from MD to FTND and vice versa. This model enables us to test the hypothesis that MD, once manifest, directly influences FTND; we can also test the reverse, that FTND directly influences MD.
Model fitting proceeded by first testing the significance of sources of variance (A and C: E cannot be removed from the model since it encompasses measurement error). Next, individual paths were tested. Model fit was assessed using the change in −2 times the log likelihood of the model, which is distributed as a χ2 statistic with degrees of freedom (df) equal to the change in the number of paths estimated between the full and nested models. A significant Δχ2 resulted in rejection of the submodel. We also used Akaike’s Information Criterion (AIC, Akaike, 1987) in model selection: a lower AIC value indicates a better balance between the explanatory value of the model and parsimony.
Results
Descriptive Statistics and Preliminary Analyses
Of the 2906 individuals in the sample, 84.2% (N=2446) had ever used a tobacco product. Of those who had used a tobacco product, 72.7% (N=1777) were current or past regular users. These individuals were administered the FTND. The mean FTND score was 4.66, and a median of 5. The lifetime prevalence of a major depressive episode was 28.8% (N=834).
The mean age of onset for regular tobacco use was 17.0; the mean age of onset for a depressive episode was 23.5 years. The phenotypic association between INIT and MD was positive and significant (OR=1.38 [CI=1.09, 1.75], p<0.01), as was the association between regular use and MD (OR=1.52 [CI=1.23, 1.87], p<0.01). Similarly, FTND scores and MD were positively and significantly associated (b=0.14 [SE=0.02], Z=6.85, p<0.01). The mean FTND score among MD+ individuals was 5.33 (SE=0.11), while among MD− individuals the mean was 4.34 (SE=0.08). The age of onset for regular tobacco use did not differ across those who met MD criteria versus those who did not (p=0.43); likewise, the age of first depressive episode did not differ across those who had used tobacco regularly versus those who had not (p=0.54).
Twin modeling
Shared liability model
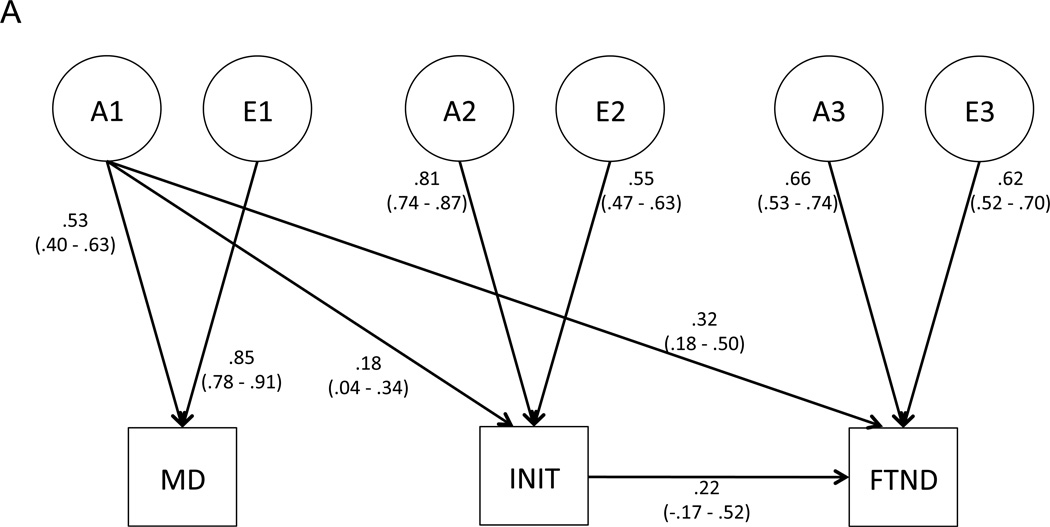
We first tested whether shared environmental influences could be removed from the model (Table 1, top panel, Model 2), and found that this did not reduce model fit significantly. Next, we tested whether setting the genetic or environmental covariance between MD and INIT to 0 reduced fit (paths a21 and e21, respectively); the genetic path could not be removed (top panel, Model 3) but the environmental path could (top panel, Model 4). Accordingly, the environmental path was dropped from subsequent models. Subsequent tests revealed that the direct genetic correlation between MD and FTND (a31) was significant (top panel, Model 5); however, the corresponding environmental correlation (e31) was not (top panel, Model 6). Thus, the best-fitting shared liability model includes a genetic correlation between both MD and INIT (path a21), as well as a genetic relationship between MD and FTND that results from two sources: the genetic correlation between MD and INIT (path a21) that flows through the contingency pathway (path β32) from INIT to FTND, and the direct genetic relationship between MD and FTND (path a31) (Figure 2A).
Table 1.
Model Fitting Procedure.
| Shared Liability Model | ||||||
| # | Model Description | Comparison | Δdf | Δχ2 | p-value | ΔAIC |
| 1 | Full model | n/a | (6109) | (−2LL=9012.77) | n/a | (−3205.23) |
| 2 | Set shared environmental influences to 0 | 2 vs. 1 | 5 | 0.12 | 1.00 | −9.88 |
| 3 | Drop genetic correlation between MD and INIT (a21) | 3 vs. 2 | 1 | 5.00 | 0.03 | 3.00 |
| 4 | Drop environmental correlation between MD and INIT (e21) | 4 vs. 2 | 1 | 0.17 | 0.68 | −1.83 |
| 5 | Drop direct genetic correlation between MD and FTND (a31) | 5 vs. 4 | 1 | 8.29 | <.01 | 6.29 |
| 61 | Drop direct environmental correlation between MD and FTND (e31) | 6 vs. 4 | 1 | 1.48 | 0.23 | −0.53 |
| Reciprocal Causation Model | ||||||
| # | Model Description | Comparison | Δdf | Δχ2 | p-value | ΔAIC |
| 1 | Full model | n/a | (6110) | (−2LL=9010.91) | n/a | (−3209.09) |
| 2 | Set shared environmental influences to 0 | 2 vs. 1 | 4 | 2.08 | 0.72 | −5.92 |
| 3 | Drop genetic correlation between MD and INIT (a21) | 3 vs. 2 | 1 | 0.48 | 0.49 | −1.52 |
| 4 | Drop environmental correlation between MD and INIT (e21) | 4 vs. 3 | 1 | 0.60 | 0.44 | −1.40 |
| 52 | Test causal path from MD to FTND (b31) | 5 vs. 4 | 1 | 1.10 | 0.29 | −0.90 |
| 6 | Test causal path from FTND to MD (b13) | 6 vs. 5 | 1 | 34.92 | <0.01 | 32.92 |
| 7 | Test causal path from FTND to MD (b13) with b31 intact | 7 vs. 4 | 1 | 10.80 | <0.01 | 8.80 |
Final models are in italicized text.
Fit statistics for final shared liability model: −2LL=9014.54; degrees of freedom=6116; AIC=−3217.46.
Fit statistics for final causal model: −2LL=9015.17; degrees of freedom=6117; AIC=−3218.83.
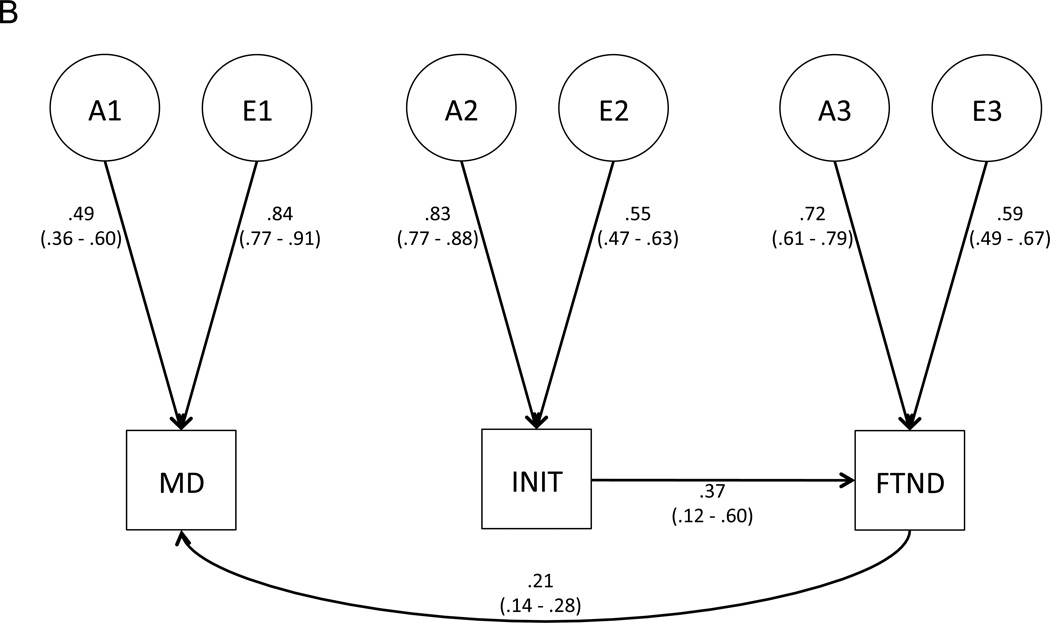
Figure 2.
Best-fitting, final models for (A) the shared liability model, and (B) the causal model. Path estimates (95% confidence intervals) are provided.
Causal model
As with the shared liability model, we first tested whether shared environmental influences could be dropped from the model, and found that they could (Table 1, bottom panel, Model 2). We next tested the direct genetic and environmental correlations between MD and INIT (a21 and e21, respectively) and found that both could be set to 0 without a significant deterioration in model fit (bottom panel, Models 3 and 4). The causal path from MD to FTND (b31) could be set to 0 (bottom panel, Model 5); however, the reciprocal causal path (b13) could not, regardless of whether b31 was left intact (bottom panel, Models 6 and 7). Thus, the final (best-fitting) causal model indicated that no direct genetic or environmental relationship exists between MD and INIT, and that there is a unidirectional causal relationship from FTND to MD (Figure 2B).
Comparison of shared liability and causal models
The final models have very similar fit statistics. The AIC value for the causal model is slightly superior to that of the shared liability model (AIC=−3218.83 [df=6117] vs. AIC=−3217.46 [df=6116]). Thus, relative to the explanation embodied by the simpler shared liability model, we can be only slightly more confident that nicotine dependence and depression are phenotypically associated due to a causal relationship rather than to a shared liability. In the context of this causal model, once nicotine dependence has been established through tobacco use initiation, this dependence actually causally influences depression, a relationship that becomes more pronounced at higher levels of dependence.
Genetic and environmental influences on MD, INIT, and FTND
The shared liability and causal models produced quite similar estimates of the total genetic and environmental variances of MD, INIT, and FTND. Table 2 provides estimates of the proportions of total phenotypic variance attributable to each latent genetic and environmental factor for both models. As expected, the models partition the sources of genetic and environmental variance somewhat differently. For example, in the shared liability model, 22.1% of the total genetic variance of FTND is attributable to latent factor A1, while that factor does not account for any of the total genetic variance of FTND under the causal model.
Table 2.
Sources of genetic and environmental variance for each phenotype.
| Shared Liability Model | |||
| Genetic Influences | MD | INIT | FTND |
| Total genetic variance* | 0.28 | 0.70 | 0.60 |
| % from A1 | 100 | 4.8 | 22.1 |
| % from A2 | n/a | 95.2 | 5.3 |
| % from A3 | n/a | n/a | 72.6 |
| Environmental Influences | |||
| Total environmental variance | 0.72 | 0.30 | 0.40 |
| % from E1 | 100 | n/a | n/a |
| % from E2 | n/a | 100 | 3.6 |
| % from E3 | n/a | n/a | 96.4 |
| Causal Model | |||
| Genetic Influences | MD | INIT | FTND |
| Total genetic variance | 0.27 | 0.69 | 0.61 |
| % from A1 | 90.1 | n/a | n/a |
| % from A2 | 1.5 | 100 | 15.5 |
| % from A3 | 8.4 | n/a | 84.5 |
| Environmental Influences | |||
| Total environmental variance | 0.73 | 0.31 | 0.39 |
| % from E1 | 97.6 | n/a | n/a |
| % from E2 | <1 | 100 | 10.5 |
| % from E3 | 2.2 | n/a | 89.5 |
Note that, because twin modeling was conducted using ordinalized variables, the total phenotypic variance for each trait is constrained to 1; thus, these values are actually proportions of variance rather than true variance. However, totals might not sum to 1 due to rounding.
Discussion
We sought to use a genetically informative, population-based sample to investigate the phenotypic association between major depression and nicotine dependence. Specifically, we asked whether a shared liability model or a causal model provided a better fit to the data. Our results indicate that these models are nearly equally supported by the data, although the fit of the causal model is slightly – but not substantially – better. A reasonable interpretation of these results is that both mechanisms (a causal relationship and a shared liability) contribute to the phenotypic association between major depression and nicotine dependence, perhaps in different individuals or within the same individual at different times in their lives.
Previous studies support the existence of a genetic correlation between MD and nicotine dependence. In a large Swedish twin population, a modest genetic correlation specific to these phenotypes existed beyond the genetic relationship between depression and regular cigarette use (Edwards et al., 2010). Other twin studies have also found evidence of low to modest genetic and/or environmental correlations between MD or depressive symptoms and nicotine dependence (Fu et al., 2002; Korhonen et al., 2007; Lyons et al., 2008; McCaffery et al., 2008). Previously, shared genetic influences were implicated in an analysis of the relationship between MD and regular smoking in female twins from Virginia (Kendler et al., 1993).
The implication of such studies is that a subset of the genetic (and in some cases, though not in the current report, environmental) influences underlying liability to depression also underlies the liability to nicotine dependence. Since we identified a genetic correlation between depression and both tobacco use initiation and nicotine dependence beyond that which is conveyed through initiation, we conclude that the nature of these shared genetic influences varies. Those that are primarily relevant to depression and initiation of tobacco use might be generally related to internalizing and externalizing, which previous studies have been found to be modestly genetically related (Kendler et al., 2003, Kendler et al., 2011). Previous work in a female sample from this population has demonstrated that some psychological or personality constructs (e.g., self-esteem and locus of control) are associated with nicotine dependence but not with initiation, while others (e.g., neuroticism and extraversion) are related to both (Kendler et al., 1999). Perhaps the genetic variation underlying such constructs contributes to the different sources of genetic covariation between MD and FTND shown here. Another possibility is that the direct genetic covariation between depression and nicotine dependence represents genetic variation affecting neural systems that are common to both depression and the establishment or maintenance of nicotine dependence (e.g., dopaminergic or glutamatergic neurotransmission, Lajtha and Sershen, 2010).
It is impossible to determine exactly which variants underlie the genetic correlation between MD and FTND – or, for that matter, between MD and INIT. Indeed, the identification of such variants is the goal of ongoing molecular genetic research. Even without this information, we can conclude that individuals who carry a genetic liability to MD also harbor a genetic liability to FTND. Still, a substantial proportion of the genetic liability to depression is not shared with either tobacco use phenotype, so it is possible for someone carrying a high genetic load for depression to carry few (or none) of the risk variants that predispose to both phenotypes. The absence of significant environmental correlation indicates that environmental variance influencing MD is largely irrelevant to FTND.
The implications of the model wherein nicotine dependence causes depression are quite distinct from those of the shared liability model. This model excludes direct genetic and environmental correlations between MD and FTND: the only correlations between these phenotypes are transmitted through the causal path between FTND and MD. The causal path between FTND and MD indicates that, as FTND increases, the risk of MD increases. This is true irrespective of the sources of variance influencing FTND, as the risk pathway is a function of the manifested FTND phenotype itself. Under this model, if smoking (and, by extension, nicotine dependence) is reduced in a population, rates of MD should subsequently decline so long as the causal process is reversible. These consequences differ from those expected from the relationship between MD and FTND described by the shared liability model. In that model, environmental influences on MD would not affect FTND: in the causal model, both genetic and environmental influences on FTND can increase risk of MD. In addition, a decrease in smoking across the population would not impact rates of MD.
Given the absence of a significant causal pathway between MD and FTND, the “self-medication” hypothesis is not clearly supported by these data. There are other reports in the literature of a lack of evidence of self-medication: Johnson and Breslau (2006) found that, among a cohort of older smokers – those who began smoking prior to the public health campaign to reduce smoking – there was no evidence for the self-medication hypothesis, inasmuch as smoking persistence was unrelated to history of depression. Kang and Lee (2010) examined MD and smoking amount across two years and did not find a significant association between year one depression and year two smoking. However, other longitudinal studies (Breslau et al., 1998; Chaiton et al., 2009) support the existence of a bidirectional causal relationship.
Furthermore, other studies support the existence of a causal relationship between FTND, or in some cases regular smoking, and MD. Using a longitudinally assessed sample, and controlling for unobserved genetic and environmental influences, Boden and colleagues (2010) found evidence of a causal relationship from nicotine dependence to depression, but no evidence of the opposite causal path. Likewise, Kang and Lee (2010), also using a large longitudinally evaluated sample, reported a significant association between the smoking amount in year one and depression in year two. A critical implication of the causal relationship detected here is that a reduction in FTND would have a positive impact on depression, so long as the risk conferred by increasing FTND is reversible. Some studies have reported an improvement in depression after smoking cessation (Berlin et al., 2010; Shahab and West, 2009), but others have not (Glassman et al., 2001; Glassman et al., 1990). More research is needed to clarify the relationship among nicotine dependence, history of depression, probability of success at smoking cessation, nicotine withdrawal, and post-withdrawal depression.
As detailed previously, one potential contributor to the increase in risk of depression due to nicotine dependence is that many constituents of the smoke produced by cigarettes and cigars – including heavy metals and ammonium products – have toxic effects on the brain (Swan and Lessov-Schlaggar, 2007). If tobacco smoke were responsible for the increased depression risk, we would expect that the causal pathway from FTND to MD would be non-significant in men who only used smokeless tobacco and were therefore not exposed to the harmful effects of their own cigarette/cigar/pipe smoke. Unfortunately, only 29 pairs of twins were concordant in their use of only smokeless tobacco products; were a larger sample available, we could meaningfully compare the shared liability and causal models between a group that used smoking tobacco versus one that used only smokeless tobacco.
Another limitation is that many of the regular tobacco users in this sample used a variety of tobacco products (e.g., cigarettes, cigars, smokeless tobacco). Because the wording of most tobacco use items referred to products that are both smokeless and smoked, it was not possible to determine whether the relationship between nicotine dependence and depression differs across these two types of tobacco use. Given the potential physiological effects of smoke on the brain, this is a pertinent and interesting question, but one that cannot be answered with the current data.
The findings reported here are not entirely consistent with an earlier report on the relationship between regular smoking and depression among women in the same sample (Kendler et al., 1993). Although both studies support the existence of a shared genetic liability between phenotypes, the previous study did not support a causal relationship between phenotypes in either direction. The methods employed differed across studies: a co-twin control analysis was initially used to explore the relationship between phenotypes in women, while the current report relied on structural equation modeling. In addition, we modeled INIT in the context of a pathway to FTND, while the previous study examined INIT (called “ever smoked” in that study) on its own. Another difference between the studies is the measure of smoking: we used FTND score whereas the previous study used average daily cigarette consumption. However, since cigarette consumption is an important component of FTND score (accounting for up to 3 of the 10 possible points), the measures are certainly correlated. It is worth noting the sex differences in prevalence for the phenotypes of interest: depression is more prevalent in women, and regular smoking is more common among men. Future studies should model these phenotypes in both sexes simultaneously so that sex differences can be tested directly.
In summary, these results imply that the phenotypic relationship between depression and nicotine dependence in men is complex. It is likely attributable to multiple mechanisms – a causal relationship and a shared genetic liability – that could differ across individuals in a population, and/or could manifest within the same individual concurrently or across different periods in time. The causal model indicates that, as one’s nicotine dependence increases, so does their risk of depression; whether this is due to physiological (e.g., neurobiological) effects of nicotine or some other factor remains unclear. The shared liability model indicates that a modest genetic correlation (0.32) exists between depression and nicotine dependence. The majority of this correlation is direct, while a small proportion is also correlated with smoking initiation. Molecular genetic studies are needed to elucidate the nature of these genetic influences; for example, some might be related to neural pathways or neurotransmission, while others influence internalizing.
Limitations
The findings presented herein should be considered in the context of several limitations. The primary limitation is the lack of statistical power to determine which model (causal or shared liability) provides a better fit to the data. As determined by Heath and colleagues (1993), power to make this distinction is limited in situations where the underlying sources of variance do not differ across phenotypes. Had we detected significant shared environmental effects on one of the phenotypes, the difference in fit between causal and shared liability models might have been more pronounced. Furthermore, there are limitations to this approach to model fit comparisons when attempting to discriminate between causal and shared liability models (Rhee et al., 2004; Rhee et al., 2005).
One potentially complicating factor is the relationship between bipolar disorder and smoking. Previous studies (Zimmermann et al., 2009; Jabben et al., 2011; Hu et al., 2012) have demonstrated that a substantial proportion of individuals diagnosed with MD also have unrecognized subthreshold, or in some cases clinical, symptoms of mania. Furthermore, nicotine dependence is higher among individuals with MD and subthreshold bipolar disorder than among those with pure MD (Zimmermann et al., 2009). It is possible that the relationship between depression and smoking differs among individuals with pure depression versus those who also experience symptoms of mania. Unfortunately, we were unable to explore these relationships here because manic symptoms were not assessed in the current sample, though bipolar disorder has been examined in the female portion of the sample (Karkowski and Kendler, 1997), and its prevalence was consistent with prior reports (e.g., Kessler et al., 1994). The complex relationships among smoking and symptoms of depression and mania should be explored further where data is available.
Another potential limitation is the age range of the sample. Smoking prevalence has changed rather dramatically in the U.S. over the lifetime of some of the participants, and we considered the possibility that the relationship between MD and FTND might differ across different cohorts. To test this hypothesis, we conducted a series of analyses wherein the sample was divided into 2–3 cohorts. Under both conditions (2 or 3 cohorts), we were able to constrain the parameter estimates to be equal across the cohorts, suggesting that, despite differences in smoking behavior over time, the underlying sources of variance did not differ significantly. In the analyses presented here, age is included as a covariate in an effort to account for its potential impact on the relationship between MD and FTND. However, these models should be replicated in either larger samples that would be more statistically powerful in cohort tests, or in a population that has not experienced pronounced changes in smoking behavior over time. Finally, since the sample is limited to Caucasian males, these results might not be generalizable to other ethnicities.
Acknowledgements
The authors would like to thank Charles O. Gardner, Ph.D., and Hermine H. Maes, Ph.D., for their technical input.
Funding Sources
This research was supported by funding from the National Institute of Alcohol Abuse and Alcoholism (Grants F32AA19849 [to Alexis C. Edwards], R37AA011408, and P20AA017828 [to Kenneth S. Kendler]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
A.C.E. and K.S.K. designed the study. A.C.E. conducted the statistical analyses and wrote the initial draft of the manuscript. A.C.E. and K.S.K. finalized the manuscript. Both authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol. Biochem. Behav. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Berlin I, Chen H, Covey LS. Depressive mood, suicide ideation and anxiety in smokers who do and smokers who do not manage to stop smoking after a target quit day. Addiction. 2010;105:2209–2216. doi: 10.1111/j.1360-0443.2010.03109.x. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br. J. Psychiatry. 2010;196:440–446. doi: 10.1192/bjp.bp.109.065912. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch. Gen. Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychol. Med. 2004;34:323–333. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking. A longitudinal investigation. Arch. Gen. Psychiatry. 1998;55:161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz LR, Johnson EO, Peterson EL, Davis GC. Smoking and the risk of suicidal behavior: a prospective study of a community sample. Archives of General Psychiatry. 2005;62:328–334. doi: 10.1001/archpsyc.62.3.328. [DOI] [PubMed] [Google Scholar]
- CDC. Morbidity and Mortality Weekly Report. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses --- United States, 2000--2004. 2008:1226–1228. [PubMed]
- CDC. Morbidity and Mortality Weekly Report. Current Cigarette Smoking Prevalence Among Working Adults --- United States, 2004--2010. 2011:1305–1309. [PubMed]
- Chaiton MO, Cohen JE, O'Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health. 2009;9:356. doi: 10.1186/1471-2458-9-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dome P, Kapitany B, Ignits G, Porkolab L, Rihmer Z. Tobacco consumption and antidepressant use are associated with the rate of completed suicide in Hungary: an ecological study. Journal of Psychiatric Research. 2011;45:488–494. doi: 10.1016/j.jpsychires.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Dome P, Lazary J, Kalapos MP, Rihmer Z. Smoking, nicotine and neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews. 2010;34:295–342. doi: 10.1016/j.neubiorev.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Maes HH, Pedersen NL, Kendler KS. A population-based twin study of the genetic and environmental relationship of major depression, regular tobacco use and nicotine dependence. Psychol. Med. 2010:1–11. doi: 10.1017/S0033291710000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol. Med. 2003;33:1357–1367. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch. Gen. Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional family data. Behav. Genet. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010;68:261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Hu C, Xiang YT, Ungvari GS, Dickerson FB, Kilbourne AM, Si TM, Fang YR, Lu Z, Yang HC, Chiu HF, Lai KY, Hu J, Chen ZY, Huang Y, Sun J, Wang XP, Li HC, Zhang JB, Wang G. Undiagnosed bipolar disorder in patients treated for major depression in China. Journal of Affective Disorders. 2012;140:181–186. doi: 10.1016/j.jad.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Jabben N, Penninx BW, Beekman AT, Smit JH, Nolen WA. Co-occurring manic symptomatology as a dimension which may help explaining heterogeneity of depression. Journal of Affective Disorders. 2011;131:224–232. doi: 10.1016/j.jad.2010.12.012. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity--a population-based study including smoking cessation after three years. Drug Alcohol Depend. 2004;76:287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Breslau N. Is the association of smoking and depression a recent phenomenon? Nicotine Tob. Res. 2006;8:257–262. doi: 10.1080/14622200600576644. [DOI] [PubMed] [Google Scholar]
- Kang E, Lee J. A longitudinal study on the causal association between smoking and depression. J Prev Med Public Health. 2010;43:193–204. doi: 10.3961/jpmph.2010.43.3.193. [DOI] [PubMed] [Google Scholar]
- Karkowski LM, Kendler KS. An examination of the genetic relationship between bipolar and unipolar illness in an epidemiological sample. Psychiatric Genetics. 1997;7:159–163. [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Roysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am. J. Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch. Gen. Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol. Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology. The Guilford Press; 2006. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch. Gen. Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Broms U, Varjonen J, Romanov K, Koskenvuo M, Kinnunen T, Kaprio J. Smoking behaviour as a predictor of depression among Finnish men and women: a prospective cohort study of adult twins. Psychol. Med. 2007;37:705–715. doi: 10.1017/S0033291706009639. [DOI] [PubMed] [Google Scholar]
- Lajtha A, Sershen H. Nicotine: alcohol reward interactions. Neurochem. Res. 2010;35:1248–1258. doi: 10.1007/s11064-010-0181-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict. Behav. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, Grant MD, Rende R, Eisen S, Eaves L, Tsuang MT. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine Tob. Res. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Stanton C, Lloyd-Richardson EE, Niaura R. Depressive symptoms and cigarette smoking in twins from the National Longitudinal Study of Adolescent Health. Health Psychol. 2008;27:S207–S215. doi: 10.1037/0278-6133.27.3(suppl.).s207. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Richmond, VA: Dept. of Psychiatry, Virginia Commonwealth University; 2006. [Google Scholar]
- Rhee SH, Hewitt JK, Corley RP, Willcutt EG, Pennington BF. Testing hypotheses regarding the causes of comorbidity: examining the underlying deficits of comorbid disorders. J. Abnorm. Psychol. 2005;114:346–362. doi: 10.1037/0021-843X.114.3.346. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Lessem JM, Stallings MC, Corley RP, Neale MC. The validity of the Neale and Kendler model-fitting approach in examining the etiology of comorbidity. Behav. Genet. 2004;34:251–265. doi: 10.1023/B:BEGE.0000017871.87431.2a. [DOI] [PubMed] [Google Scholar]
- Shahab L, West R. Do ex-smokers report feeling happier following cessation? Evidence from a cross-sectional survey. Nicotine Tob. Res. 2009;11:553–557. doi: 10.1093/ntr/ntp031. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M. Biometrics Research Department. New York: New York State Psychiatric Institute; 1987. Structured Clinical Interview for DSM-III-R (SCID) [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, Sampson N, Kessler RC. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–1128. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]