Abstract
New therapeutic approaches that eliminate or reduce the occurrence of intimal hyperplasia following balloon angioplasty could improve the efficacy of vascular interventions and improve the quality of life of patients suffering from vascular diseases. Here, we report that treatment of arteries using catheter balloons coated with thin polyelectrolyte-based films (‘polyelectrolyte multilayers’, PEMs) can substantially reduce intimal hyperplasia in an in vivo rat model of vascular injury. We used a layer-by-layer (LbL) process to coat the surfaces of inflatable catheter balloons with PEMs composed of nanolayers of a cationic poly(β-amino ester) (polymer 1) and plasmid DNA (pPKCδ) encoding the δ isoform of protein kinase C (PKCδ), a regulator of apoptosis and other cell processes that has been demonstrated to reduce intimal hyperplasia in injured arterial tissue when administered via perfusion using viral vectors. Insertion of balloons coated with polymer 1/pPKCδ multilayers into injured arteries for 20 min resulted in local transfer of DNA and elevated levels of PKCδ expression in the media of treated tissue 3 days after delivery. IFC and IHC analysis revealed these levels of expression to promote downstream cellular processes associated with up-regulation of apoptosis. Analysis of arterial tissue 14 days after treatment revealed polymer 1/pPKCδ-coated balloons to reduce the occurrence of intimal hyperplasia by ~60% compared to balloons coated with films containing empty plasmid vectors. Our results demonstrate the potential therapeutic value of this nanotechnology-based approach to local gene delivery in the clinically important context of balloon-mediated vascular interventions. These PEM-based methods could also prove useful for other in vivo applications that require short-term, surface-mediated transfer of plasmid DNA.
Keywords: Layer-by-Layer, Polyelectrolyte Multilayers, Thin Films, Gene Delivery, Intimal Hyperplasia
Introduction
Atherosclerosis is a common and often life-threatening vascular condition. Several different interventional approaches, including balloon angioplasty and the implantation of intravascular stents, are currently used clinically to remove atherosclerotic plaques and/or address complicating factors associated with other vascular diseases and conditions [1]. These interventional procedures are considerably less invasive than some other treatment options (e.g., bypass grafting, etc.) and have resulted in significant improvements in the quality of life of patients suffering from vascular diseases [2]. Unfortunately, however, these procedures are not perfect – for example, approximately 40% of all patients that undergo balloon angioplasty procedures experience post-operative restenosis, a re-narrowing of a treated artery due to vascular collapse, thrombosis, or intimal hyperplasia that can lead to reduced blood flow to critical organs [3–6]. Intimal hyperplasia, in particular, is a major cause of restenosis and is estimated to occur following 30–50% of all angioplasty procedures [7].
The clinical use of intravascular stents that locally deliver small-molecule antiproliferative drugs to arterial tissue has been demonstrated to reduce the occurrence of intimal hyperplasia to ~10% in coronary arteries [8–10]. Unfortunately, however, these drug-eluting stents are ineffective in preventing intimal hyperplasia in peripheral arteries. In addition, conventional drug-eluting stents can also prevent healthy re-endothelialization of the arterial wall and subsequently cause life-threatening thrombosis [11–13]. Several studies have demonstrated that coated catheter balloons can also be used to transfer antiproliferative drugs to the vascular wall, and that this approach can address other concerns and potential limitations associated with the implantation of drug-eluting stents [14–16]. The continued development of new approaches that eliminate or reduce the occurrence of intimal hyperplasia would represent a significant step toward improving the overall efficacy of vascular interventions and, as a result, substantially improve the well being of patients receiving these treatments [17].
Intimal hyperplasia is a complex pathological process trigged by injuries associated with vascular interventions. The neointimal lesions that result are highly cellular and composed of cells that resemble vascular smooth muscles cells (VSMCs), cells that normally reside in the medial layer of the vessel wall. It is believed that the release of signaling molecules (e.g., growth factors, cytokines, chemokines, etc.) at sites of injury causes VSMCs to switch from a quiescent phenotype to a proliferative phenotype and migrate from the media to the sub-intimal space [18]. Recent experimental evidence has also demonstrated that the recruitment of progenitor cells (and the subsequent differentiation of these cells to VSMC-like cells) can contribute significantly to intimal hyperplasia [19]. Many years of investigations into the molecular basis of intimal hyperplasia have identified key cellular and molecular events that could potentially be targeted using protein- or gene-based agents (e.g., using antibodies, DNA, siRNA, etc.) to provide control over SMC and progenitor cell proliferation, migration, and differentiation [17, 20–22]. The work reported here takes a step toward a minimally-invasive, gene-based approach to preventing intimal hyperplasia by using polymer-coated catheter balloons to transfer a potential therapeutic DNA construct to injured vascular tissue [23]. Our approach is based on materials-based methods developed for the stepwise, ‘layer-by-layer’ assembly of nanostructured, polyelectrolyte-based thin films and coatings [24, 25].
Methods for the alternating, ‘layer-by-layer’ deposition of positively and negatively charged polymers (polyelectrolytes) on surfaces have been used to fabricate thin films (called ‘polyelectrolyte multilayers’, or PEMs) of interest in a broad range of biomedical contexts, including the design of functional biointerfaces and the development of new platforms for drug delivery [26–29]. In the context of drug delivery, this approach is attractive because it can be used to control film thickness and, thus, drug loading levels, precisely (e.g., at the nanometer scale, by control over the number of polymer layers deposited during assembly). This approach is also well suited for the fabrication of thin and conformal films on the surfaces of objects with complex shapes (including those of common interventional devices, such as stents) [30–34]. Of particular importance to the work reported here, the negatively charged nature of DNA (an anionic polyelectrolyte) permits incorporation of large DNA constructs directly into the structures of these multilayered assemblies (e.g., as ‘layers’). Finally, this ‘multilayered’ approach to fabrication also leads to thin films that contain intimately mixed nano-layers of DNA and cationic polymers – the latter of which is a class of materials used broadly for the non-viral delivery of DNA to cells [35–37]. The ability to incorporate and subsequently control the release of DNA from surfaces coated with DNA-containing multilayers has opened the door to new approaches to the localized and surface-mediated delivery of DNA, both in vitro and in vivo. A variety of different layer-by-layer approaches have been developed for this purpose; these methods and other biomedical applications of PEMs have recently been reviewed comprehensively [26–29, 38, 39]. Other studies of particular relevance to this current investigation are described briefly below.
We have reported in several past studies on the design and characterization of PEMs using plasmid DNA and polymer 1 [23, 34, 40–45], a model hydrolytically degradable poly(β-amino ester) originally developed for the polyplex-mediated delivery of DNA [46, 47]. That past work demonstrated (i) that these materials (referred to hereafter as ‘polymer 1/DNA films’) erode and release DNA gradually in physiologically relevant media (e.g., over several days) [34, 40, 43, 45], (ii) that surfaces coated with films ~100 nm thick can be used to promote the localized and surface-mediated delivery of DNA to cells in vitro [34, 40, 41, 45], and (iii) that, in addition to promoting the release of DNA, polymer 1 can also play a role in promoting the internalization and trafficking of DNA by cells [45]. As a first step toward evaluating the potential of these materials to deliver DNA and promote cell transfection in vivo, we recently reported that inflatable catheter balloons coated with polymer 1/DNA films can promote the transfection of arterial tissue in a rat model of balloon-mediated arterial injury [23]. That work used catheter balloons coated with films fabricated using reporter plasmids encoding β-galactosidase (β-gal) or enhanced green fluorescent protein (EGFP) to investigate the initial feasibility of that approach; expression of β-galactosidase and EGFP was observed in VSMCs in the medial layers of arteries 72 hours after treatment with film-coated balloons.
While the results of that prior study provide important proof of concept with respect to potential therapeutic applications of this PEM-based approach to balloon-mediated delivery, it remains unclear whether these methods could be used to promote levels of transgene expression that would be either (i) elevated enough to achieve potential therapeutic effects (e.g., using a potential therapeutic gene), or (ii) sustained enough to inhibit longer-term physiological processes associated with intimal hyperplasia (e.g., over periods of several weeks). This current study sought to investigate the potential of this ‘multilayered’ approach to prevent or reduce the occurrence of intimal hyperplasia in balloon-injured arteries using films fabricated using plasmid DNA encoding the δ isoform of protein kinase C (PKCδ), a regulator of apoptosis and other cell processes [48] that has been demonstrated by our group to reduce the extent of intimal hyperplasia in balloon injured arterial tissue when administered via perfusion using viral methods [22].
Materials and Methods
Materials and General Considerations
Linear poly(ethyleneimine) (LPEI, MW = 25,000) was purchased from Polysciences, Inc. (Warrington, PA). Sodium poly(4-styrenesulfonate) (SPS, MW = 70,000) was purchased from Acros Organic (Geel, Belgium). Plasmid DNA encoding luciferase (pCMV-Luc; 6201 b.p.) was purchased from Elim Biopharm (San Francisco, CA). pcDNA3 (5446 b.p.) and pcDNA3-PKCδ (7471 b.p.; referred to hereafter as pPKCδ) plasmids were amplified using E. coli DH5α and extracted using a Bio-Rad Maxi Prep kit. Purified DNA was greater than 80% supercoiled. The size and functionality of each plasmid DNA construct was verified by restriction enzyme digest and western blot analysis, respectively. Polymer 1 and a fluorescent analog of polymer 1 end-labeled with Oregon Green 488 (polymer 1OG) were synthesized as previously described (Mn ~15,000) [44]. Fogarty arterial embolectomy catheters (2-French diameter) were purchased from Edwards Lifesciences, LLC (Irvine, CA). Rabbit polyclonal anti-PKCδ IgG and goat polyclonal anti-MCP-1 IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-Ki67 was purchased from AbCam (Cambridge, MA). DAPI, Oregon Green 488 cadaverine, Alexa Fluor 488 donkey anti-goat IgG, Alexa Fluor 488 donkey anti-mouse, Alexa Fluor 546 donkey anti-rabbit IgG, and Alexa Fluor 488 donkey anti-rabbit IgG antibodies were purchased from Invitrogen (Carlsbad, CA). Rabbit cleaved caspase-3 monoclonal antibody was purchased from Cell Signaling Technology (Danvers, MA). Goat anti-rabbit IgG (H+L)-HRP conjugate was purchased from Bio-Rad (Hercules, CA). Hematoxylin, eosin, and Pierce Metal Enhanced DAB Substrate Kits were both purchased from Thermo Scientific (Rockford, IL). In situ cell death detection kits were purchased from Roche Applied Science (Indianapolis, IN). For experiments requiring fluorescently labeled DNA, a tetramethylrhodamine (TMR) Label-IT nucleic acid kit was purchased from Mirus Bio Corporation (Madison, WI) and used according to the manufacturer’s instructions. Deionized water (18 MΩ) was used to prepare all buffer and polymer solutions. All materials were used as received unless otherwise noted. Solutions of LPEI and SPS used to fabricate polymer multilayers were filtered through a 0.2 µm nylon membrane syringe prior to use. Fluorescence microscopy and phase contrast microscopy images were acquired using either an Olympus IX70 fluorescence microscope, Nikon Eclipse E600, or a Ti-U Eclipse fluorescence microscope using Metavue 7.1.2.0, cellSens, or Nikon Elements software packages, respectively. Laser scanning confocal microscopy (LSCM) images were collected using a Nikon A1R high speed confocal microscope using appropriate excitation lasers and emission filters. LSCM images were processed using the NIS-Elements C imaging software. Fluorescence, LSCM, and optical microscopy images were analyzed using ImageJ Software (NIH).
Preparation of Polyelectrolyte Solutions
Solutions of LPEI and SPS used for fabrication of LPEI/SPS precursor layers (20 mM with respect to the repeat unit molecular weight of the polymer) were prepared using a 13 mM NaCl solution, as previously described [23, 40, 41]. LPEI solutions contained 5 mM HCl to aid polymer solubility. Solutions of polymer 1 and polymer 1OG (5 mM with respect to the repeat unit molecular weight of the polymer) were prepared using 100 mM sodium acetate buffer (pH 4.9). Solutions of plasmid DNA were prepared in 100 mM acetate buffer (pH 4.9) but were not filtered prior to use. For experiments requiring fluorescently labeled DNA, labeled DNA was added to a solution of unlabeled DNA to give a 50% or 20% (w/w) labeled/unlabeled plasmid solution (see text for details).
Fabrication of Multilayered Films on the Surfaces of Inflatable Catheter Balloons
Prior to the fabrication of DNA-containing films, embolectomy catheter balloons were pre-coated with 10 bilayers of a multilayered polyelectrolyte film composed of LPEI and SPS as described previously [23]. Polymer 1/DNA layers were then deposited on top of these precursor layers according to the following protocol: 1) catheter balloons were completely immersed in a solution of polymer 1 for 5 minutes, 2) substrates were removed and immersed in two wash baths of 100 mM acetate buffer for one minute each, 3) substrates were then immersed in a solution of DNA for 5 min, and 4) substrates were rinsed again as described in step 2. This sequence was repeated until the desired number of polymer 1/DNA layer pairs or “bilayers” was deposited on the surface of the catheter balloon. Following the final rinse step, balloons were rinsed with 18 MΩ deionized water and allowed to air dry. Embolectomy catheters were then stored in their original packaging in a dry, dark location prior to use. All films were fabricated and stored at ambient room temperature.
General Surgical Procedures
After induction of anesthesia with 2.5% isoflurane, arterial injury was induced in male Sprague-Dawley rats (2–3 months old, ~350–400 g) by means of carotid balloon angioplasty as described previously [22, 23]. In brief, a longitudinal incision was made in the neck of the rat in order to isolate the left external, internal, and common carotid arteries. Three passages of an uncoated angioplasty balloon inflated to a pressure of 2 atm were then used to denude the common carotid artery of the endothelial layer. Next, a balloon coated with a polymer 1/DNA film (either 16 or 32 bilayers thick, see text) was inserted and inflated until it was observed to expand against the arterial wall (~2 atm). After a 20-minute incubation period, the balloon was deflated and removed from the artery. The external carotid artery was then ligated and blood flow was restored to the common and internal carotid arteries and the surgical wound was closed layer-to-layer. The animals were sacrificed post-operatively at a predetermined time depending on the timescale of the experiment (see text for details). All experimental protocols were approved by the Institute Animal Care and Use Committee at University of Wisconsin-Madison (#M002285) and conformed to the Guide for the Care and Use of Laboratory Animals published by the NIH Publication No. 85-23, 1996 revision.
Characterization of in vivo Delivery and Expression of DNA in Rat Carotid Arteries
For experiments designed to characterize the extent of delivery of DNA to rat arterial tissue immediately following balloon-mediated delivery, the common carotid artery was treated with an angioplasty balloon coated with a (polymer 1/pCMV-LucTMR)16 film (for this experiment, films were fabricated using solutions of DNA containing 20% (w/w) TMR-labeled DNA). To evaluate the delivery of both polymer 1 and DNA to rat arterial tissue at 10 hours and 23 hours following surgery, the common carotid artery was treated with an angioplasty balloon coated with a (polymer 1OG/pCMV-LucTMR)16 film (for this experiment, films were fabricated using solutions of DNA containing 50% (w/w) TMR-labeled DNA). Following sacrifice, the common carotid arteries were embedded and frozen in OCT compound and cut into 5 µm sections for analysis by fluorescence microscopy and/or LSCM. For experiments designed to characterize PKCδ expression and other cellular markers associated with PKCδ expression and apoptosis, the common carotid artery was treated with an angioplasty balloon coated with a (polymer 1/pPKCδ)32 film. Rats were sacrificed at 3 days and arteries were immediately embedded and frozen in OCT compound and cut into 5 µm sections for analysis by fluorescence microscopy and/or LSCM. For experiments designed to investigate PKCδ–mediated inhibition of intimal hyperplasia, the common carotid artery was treated with an angioplasty balloon coated with a (polymer 1/pPKCδ)32 film or a balloon coated with a (polymer 1/pcDNA3)32 film as a negative control. Rats were sacrificed 14 days post-operation and harvested common carotid arteries were immediately fixed and embedded in paraffin. To investigate the amount of DNA incorporated into films during fabrication, a balloon coated with a (polymer 1/pPKCδ)32 film was incubated in 1 mL of PBS buffer in a plastic UV/Vis cuvette, as previously described [23]. The DNA solution absorbance was measured until no additional change in DNA solution absorbance was observed.
Morphometric Analysis and Immunofluorescence Staining
Morphometric analysis was performed using hematoxylin and eosin (H&E)-stained arteries or elastin-stained arteries. Briefly, the areas encompassed by the (1) lumen surface (luminal area), (2) internal elastic lamina minus lumen surface (intimal area), and (3) EEL minus internal elastic lamina (medial area), and (4) the EEL length were measured using ImageJ software as previously described [49, 50]. The intima:media ratio (I/M ratio) was calculated as the intimal area divided by the medial area. To determine the I/M ratio for a specific area of a treated artery, three arbitrarily chosen sets of tissue (containing three consecutive tissue sections each) were selected from three regions of the carotid artery (each region represented approximately ~1.5 mm of the harvested artery): one region (II) in the area of the artery treated most directly by the film-coated angioplasty balloon and two regions (I and III) proximal to this region (see Figure 5A for a schematic illustration and additional explanation of the differences in these regions used for analysis). The I/M ratio, lumenal area, and EEL length for each region (i.e., regions I, II, and III) are reported as the average (with standard deviation) of all tissue sections measured for the region. For statistical analyses of these values using ANOVA Single Factor analysis of variance, the average of the three consecutive tissue sections was used to determine the I/M ratio, lumenal area, and EEL for each tissue set. The average values for the three tissue sets of each region were then used to determine the statistical significance of measured values for the same regions of rats treated with either pPKCδ or pcDNA3 [e.g. for 3 pPKCδ rats: 3 sections/region X 3 rats/region = n = 9 measurements/region and for 2 pcDNA3 rats (3 sections/region X 2 rats/region = n = 6 measurements/region)]. TUNEL staining was performed using a Roche in situ Cell Death Detection Kit according to the manufacturer’s protocol. Immunofluorescence staining was performed using rabbit anti-PKCδ, rabbit anti-Ki67, and goat anti-MCP-1. All primary antibodies were diluted (v/v) 1:100 in 0.5% bovine serum albumin/0.3% normal donkey serum in phosphate-buffered saline. Rabbit primary antibodies were detected using either Alexa Fluor 488 donkey anti-rabbit IgG or Alexa Fluor 546 donkey anti-rabbit IgG antibodies. Goat primary antibodies were detected using Alexa Fluor 546 donkey anti-goat antibodies. Immunohistochemistry (IHC) staining was performed using rabbit anti-cleaved caspase-3 (1:500) and goat anti-rabbit HRP conjugate IgG(H+L) (1:300), followed by the counterstaining of nuclei with hematoxylin.
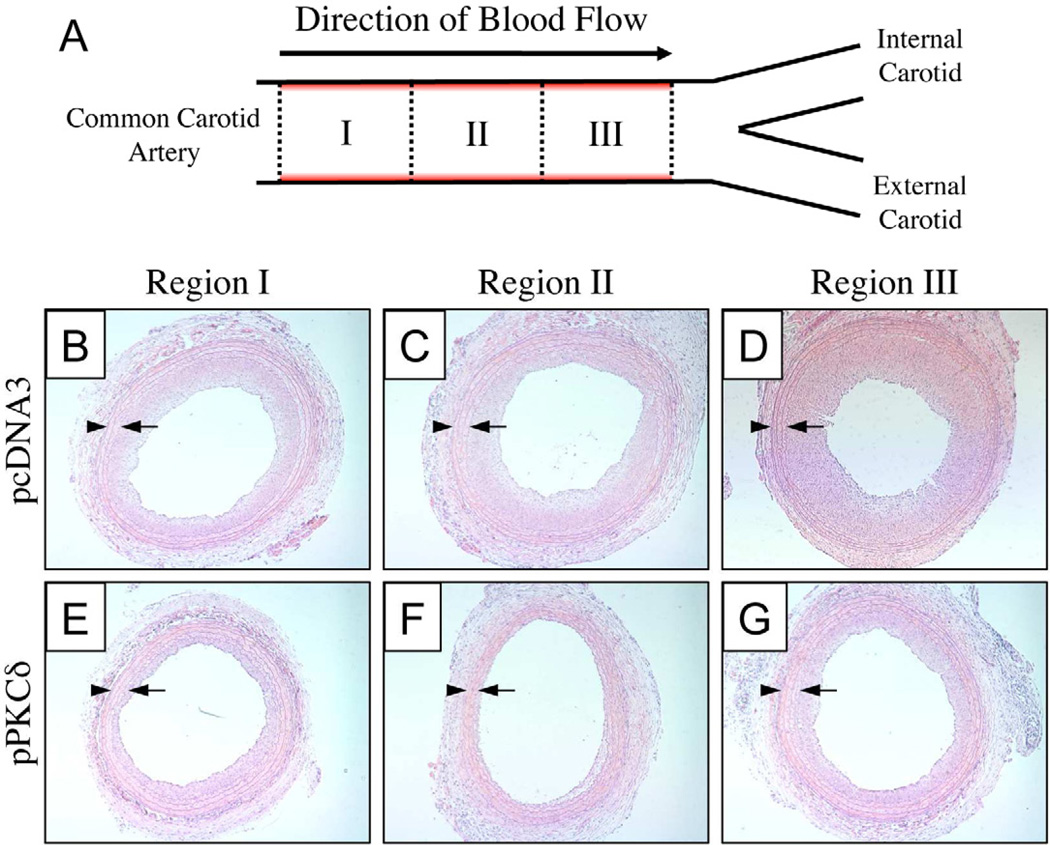
Figure 5.
(A) Schematic showing the area of the common carotid artery that was injured and/or balloon-treated. Red shading indicates areas of the artery where initial injury was induced by three passages of an inflated angioplasty balloon (see text for details). Regions I, II, and III denote three different regions of arterial tissue used to characterize intimal hyperplasia 14 days after treatment with balloons coated with polymer 1/DNA films 32-bilayers thick. Panels B–D show representative images of H&E-stained tissue sections from Regions I-III for a rat treated with a balloon coated with a film fabricated using pcDNA3 (as a control). Panels E–G show representative images of tissue sections from Regions I-III for a rat treated with a balloon coated with a film fabricated using pPKCδ. The locations of the internal and external elastic lamina are indicated by arrows and arrowheads, respectively.
Results and Discussion
Our recent study demonstrated that catheter balloons coated with polymer 1/DNA films can be used to deliver and promote the expression of plasmid DNA in balloon-injured rat arteries [23]. The results of that study also demonstrated (i) that these thin films are robust enough to withstand challenges associated with the insertion of the balloons into arteries during surgery, (ii) that DNA can be released rapidly after balloon inflation (e.g., over the 20 minute duration of the procedure used in that study), and (iii) that DNA can be released in amounts sufficient to promote the expression of two common reporter plasmid constructs (encoding β-gal or EGFP) in VSMCs residing in the media of injured arteries [23]. As noted in the Introduction above, PEM-based release of DNA can, in general, be accomplished using a range of other polymers and film architectures [28, 38, 39]. However, we selected this polymer 1-based system as a materials platform for use in this current study on the basis of those past in vivo results and because this model film system has been particularly well studied both in vitro and from a physicochemical standpoint [23, 34, 40–45, 51–53].
Transfer and Dissemination of DNA Following Balloon-Mediated Delivery
This investigation sought to determine whether polymer 1/DNA multilayers could be used to promote levels of protein expression in arterial tissue sufficient to modulate cellular pathways that are important in the context of reducing or preventing post-operative intimal hyperplasia and restenosis. To provide a basis for these studies, we first conducted a series of experiments to characterize in greater detail the initial transfer and subsequent dissemination of DNA and polymer 1 in arterial tissue following balloon-mediated delivery. These initial studies were performed using catheter balloons coated with films fabricated using a fluorescently labeled plasmid DNA construct encoding luciferase (DNATMR) and a recently reported fluorescently end-labeled derivative of polymer 1 (polymer 1OG) [44]. These experiments were performed using a rat arterial injury model similar to that used in our past studies (see Materials and Methods for additional details) and catheter balloons coated with polymer 1/pPKCδ films 16 bilayers thick (the term ‘bilayer’, as used here, refers to one polymer 1/DNA layer pair deposited during layer-by-layer fabrication) [23]. For these and all other experiments described below, film-coated balloons were inflated and left in contact with injured arterial tissue for 20 minutes prior to deflation and removal.
Figures 1A–B show the result of an initial experiment using a balloon coated with a film fabricated using DNATMR and (unlabeled) polymer 1. Figure 1A shows a high magnification LSCM image of a cross-section of the left common carotid artery of a rat harvested immediately after treatment with the film-coated balloon (i.e., prior to the restoration of blood flow); the image in Figure 1B shows the right common carotid (uninjured, untreated) artery harvested from the same rat. Inspection of Figure 1A reveals the presence of red, DNA-associated fluorescence that was transferred to the luminal side of the arterial wall during treatment with the film-coated balloon. Further inspection reveals (i) that red fluorescence is not uniformly distributed and (ii) that it is limited largely to the inner elastic lamina forming the boundary between the lumen and the media (that is, no DNA is observed to have penetrated into the first medial layer of the artery during the initial balloon-treatment procedure). This initial result is generally consistent with those of our past study [23].
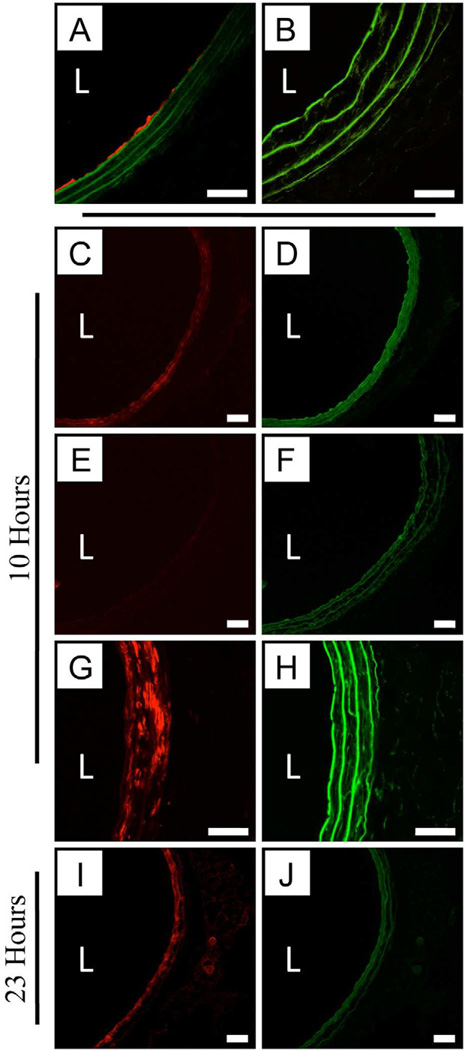
Figure 1.
Representative low magnification fluorescence microscopy images (C–F, I–J) and high magnification LSCM images (A–B,G–H) of rat carotid arteries treated with balloons coated with either (A) a polymer 1/DNATMR film 16 bilayers thick, or (C–D, G–J) a polymer 1OG/DNATMR film 16 bilayers thick. TMR fluorescence is false-colored red and Oregon Green (OG) fluorescence is false-colored green; additional green fluorescence arises from natural tissue autofluorescence (e.g., as shown in panel B). Tissue sections shown in A–B were harvested for analysis immediately following treatment with film-coated balloons (i.e., prior to restoration of blood flow; see text). Tissue sections shown in C–H and I–J were harvested 10 hours and 23 hours, respectively, following treatment with film coated balloons. Panels B and E–F show sections of uninjured and untreated artery sections used as negative controls. For all images, the designation ‘L’ indicates the location of the artery lumen. Scale bars for LSCM images (A–B, G–H) are 40 µm. Scale bars for fluorescence microscopy images (C–F, I–J) are 200 µm.
Figures 1C–J show the results of additional experiments using balloons coated with films fabricated using both DNATMR and polymer 1OG (i.e., DNATMR/polymer 1OG films), and provide additional insight into the locations and distributions of both of these film components at later time points following the initial balloon treatment. Figures 1C–D show two fluorescence microscopy images of a single tissue sample showing the locations of DNATMR (red) and polymer 1OG (green) respectively, 10 hours after initial treatment. These images reveal both DNA and polymer 1OG to be present throughout the media and around the circumference of the artery (a section of untreated contra-lateral artery, imaged through red and green fluorescence channels, is included in Figures 1E–F for comparison; the bright green fluorescence in 1F is the result of normal arterial autofluorescence).
Figures 1G and 1H show higher magnification LSCM images of a section of tissue immediately adjacent to the section shown in panels 1C–D. Inspection of Figure 1G reveals DNA to be distributed throughout the media (albeit non-uniformly), and that it does not appear to be present in the adventitia (the region of tissue outside the external elastic lamina forming the outer boundary of the media) after 10 hours. Fluorescence associated with polymer 1OG was not as intense in these LSCM images (Figure 1H) as it was in the lower magnification fluorescence images (e.g., see Figure 1D), but it was higher than levels of green fluorescence in LSCM images of untreated negative control arteries (data not shown). Figures 1I–J show images of tissue harvested 23 hours after treatment using a balloon coated with an otherwise identical DNATMR/polymer 1OG film. The image in Figure 1I suggests that DNA is distributed more uniformly around the circumference of the artery than it was after 10 hours; this image also reveals low levels of DNA-associated fluorescence in adventitial tissue. We did not observe significant levels of polymer 1OG-associated fluorescence in the media in these 23-hour samples (e.g., see Figure 1J; levels of red and green fluorescence in negative arterial controls at 23 hours were similar to those shown in Figures 1E–F for tissues harvested at 10 hours; data not shown).
When combined, the data in Figure 1 are consistent with a physical picture that involves the initial transfer of film from the surface of a balloon to the luminal side of the arterial wall, followed by the subsequent time-dependent transport of both polymer and DNA deeper into the tissue (that is, initial treatment with film-coated balloons does not itself appear to result in the immediate transfer or delivery of significant amounts of DNA into the media). Local variations in the intensity of DNA-associated fluorescence on the luminal surface (1A) and in the media of the artery in images 1C, 1G, and 1I are consistent with variations in local levels of transgene expression observed in our past short-term studies on expression of β-gal and EGFP [23]. Although the reasons for these variations are not completely understood, they could arise from initial non-uniform transfer of film from the surfaces of the coated balloons or from local variations in the extents of the initial arterial injury (or, likely, a combination of these two influences).
In general, polymer 1OG appeared to be more evenly distributed around the circumference of the artery (as compared to DNATMR) after 10 hours (Figure 1C–D). This observation is consistent with the substantially smaller size of this polymer (as compared to plasmid DNA), which could, in general, allow it to diffuse or be transported through tissue more readily than DNA. The apparent absence of significant amounts of polymer 1OG in tissue after 23 hours (Figure 1J) is also consistent with this proposition, although we note that the hydrolytically degradable nature of polymer 1 [46] could also play a role in promoting the more rapid clearance of this material. In general, we conclude from these studies that balloon-mediated delivery of polymer 1/DNA films results in the simultaneous transfer and time-dependent transport of both polymer 1 and DNA into the media of injured arteries over a period of approximately one day following delivery. The co-localization of both of these film components in medial tissue is potentially significant in the context of promoting subsequent cell transfection (discussed below). The results of a recent in vitro study demonstrate that the presence of polymer 1 in these materials can help promote more effective internalization of DNA by cells following surface-mediated delivery [45].
Characterization of the Delivery and Expression of pPKCδ in Balloon-Treated Arteries
To evaluate this balloon-mediated approach further and establish proof of concept in a potential therapeutic context, we performed a second series of experiments using balloons coated with films fabricated using a plasmid DNA construct (pPKCδ) encoding the protein PKCδ. PKCδ plays an active role in mediating cellular apoptosis in a wide range of cell types [48], and, in addition to its role in regulating apoptosis, can up-regulate the production of proteins such as monocyte chemoattractant protein-1 (MCP-1) [54]. Si et al. reported recently that adenovirus-mediated expression of PKCδ in rat VSMCs following balloon angioplasty mediated the recruitment of adventitial cells to the neointima through the upregulation of the MCP-1/CCR2 axis [55]. Other research also indicates that MCP-1 potentially plays a role in the recruitment of progenitor cells to injured vessel walls [54, 56, 57]. As such, cell apoptosis, proliferation, and MCP-1 accumulation are used to measure downstream effects of PKCδ expression in the discussion below.
Experiments to characterize the delivery and relative levels of local expression of PKCδ in VSMCs in the medial layers of balloon-injured rat arterial tissue were performed using catheter balloons coated with polymer 1/pPKCδ films 32 bilayers thick (as opposed to films 16 bilayers thick used in the experiments described above). Our past study demonstrated that balloons coated with polymer 1/DNA films 16 bilayers thick contain ~21 µg of DNA and are sufficient to promote reporter gene expression in injured arterial tissue [23]. We measured balloons coated with films 32 bilayers thick to release ~26 µg of DNA, as determined by exhaustive erosion of film-coated balloons in PBS buffer, as previously described [23]. Because the minimal or optimal levels of pPKCδ expression needed to induce robust downstream responses were not known at the outset of these studies, we used 32-bilayer films here and in all other studies described below in an effort to increase the amount of DNA delivered during the initial balloon treatment procedure. For these experiments, we also used balloons coated with otherwise identical polymer 1/DNA films fabricated using the pcDNA3 construct (the plasmid vector used to express PKCδ) as an empty vector control. Rats were sacrificed three days after balloon treatment to allow sufficient time for transgene expression and the onset of potential downstream physiological and/or cellular processes.
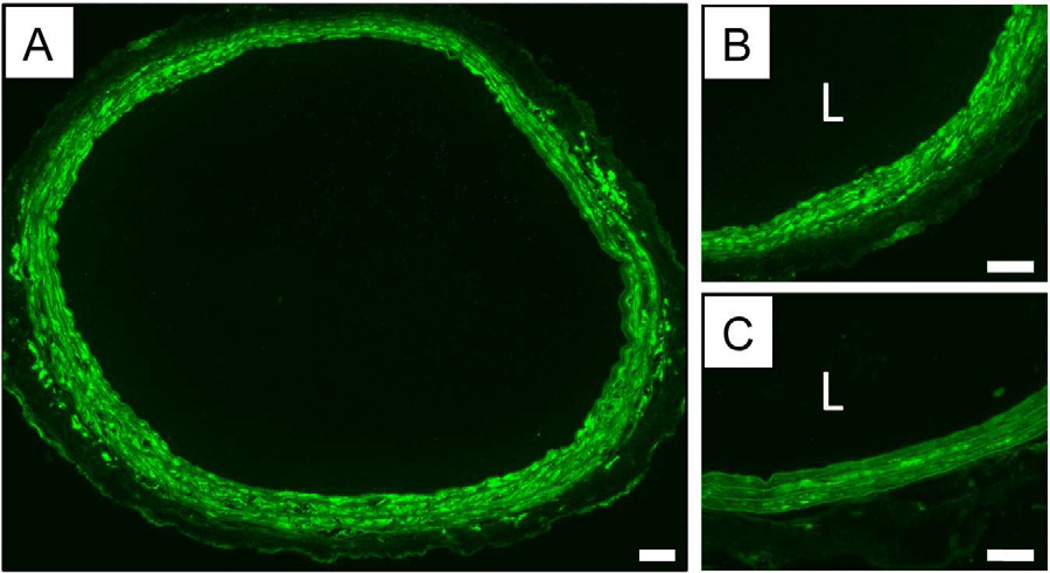
Figures 2A–B show low and high magnification fluorescence microscopy images of PKCδ expression (as indicated by IFC staining, shown as green) in a cross-section of rat arterial tissue harvested three days after treatment with a balloon coated with a polymer 1/pPKCδ film. Figure 2C shows a high magnification image of the cross-section of an artery of a different rat treated with a control balloon coated with a polymer 1/pcDNA3 film. Inspection of these images reveals significantly higher levels of PKCδ expression in arterial tissue that was treated with the polymer 1/pPKCδ film. Further inspection of Figures 2A–B reveals high levels of PKCδ expression throughout the media (i.e., the area between the internal and external elastic laminae). These results are generally consistent with those of our past studies on balloon-mediated expression of reporter genes [23] and the results of our initial fluorescence-based transfer and dissemination studies described above (in Figure 1).
Figure 2.
Representative fluorescence microscopy images of rat carotid arteries stained for PKCδ using immunofluorescence staining. The image in (A) shows a montage of low magnification images showing the locations and relative levels of PKCδ expression in a cross-section of an injured artery treated with a balloon coated with a polymer 1/PKCδ film 32 bilayers thick (see text). (B) A higher magnification image of a section of a PKCδ-treated artery. (C) A high magnification image of a control section of tissue from an injured artery that was treated with a polymer 1/pcDNA3 film 32 bilayers thick. This image shows native levels of PKCδ expression in vascular tissue induced by the original balloon injury and subsequent treatment with a film-coated balloon containing a control plasmid. In panels B and C, the designation ‘L’ indicates the location of the artery lumen. Scale bars = 100 µm.
Characterization of the Effects of pPKCδ Delivery on Cellular Processes
We performed additional analyses of tissue samples arising from the experiments above to determine if levels of PKCδ expression promoted by these materials (e.g., as shown in Figure 2) were sufficient to promote the up-regulation of specific proteins and apoptotic processes generally associated with increased PKCδ expression. One indicator that is present and can be readily detected during apoptosis in cells is the fragmentation of nuclear DNA. IFC staining and in situ modification and labeling of fragmented DNA (TUNEL staining) were used to identify the levels of these various proteins and DNA fragmentation in arterial tissue three days after treatment with balloons coated with either polymer 1/pPKCδ or polymer 1/pcDNA3 (control) films.
Figure 3A shows a representative high magnification LSCM image showing PKCδ expression (green) and TUNEL staining used to identify fragmented chromosomal DNA (red) for injured tissue treated with a polymer 1/pPKCδ film-coated balloon. To aid in the analysis of these images, cell nuclei were stained using DAPI (shown as blue in these images) and the boundaries of the inner elastic lamina and outer elastic lamina are shown as white dashed lines to indicate the location of the medial tissue. Initial inspection of Figure 3A shows that nearly all cells in the field of view (as indicated by the presence of blue nuclei) exhibited higher levels of PKCδ expression than injured tissue treated with a polymer 1/pcDNA3 film-coated balloon (e.g., shown in Figure 3D).
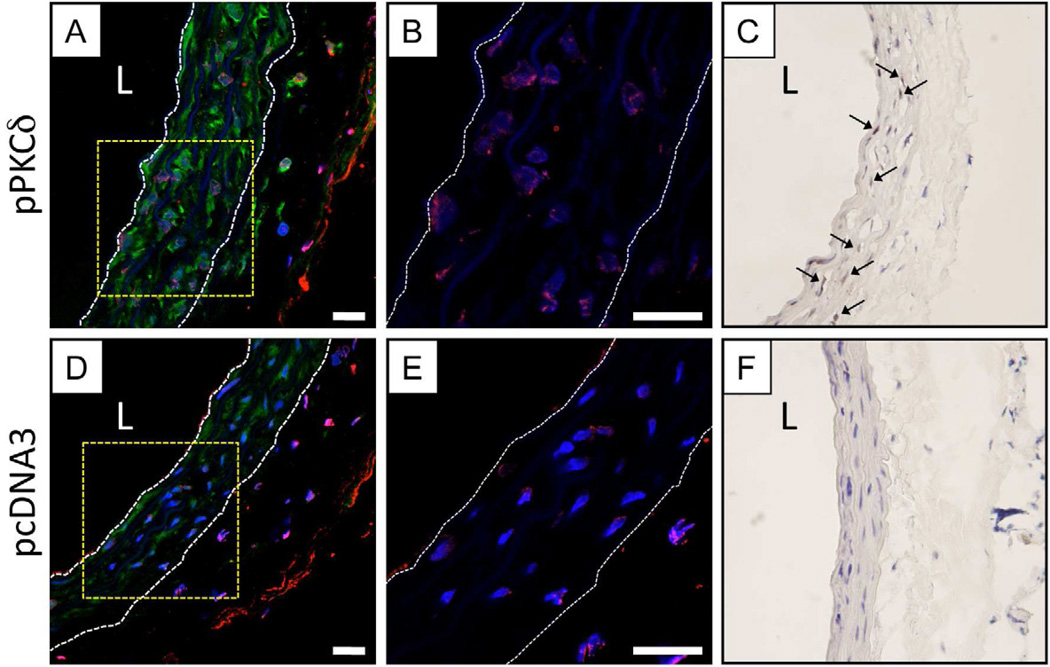
Figure 3.
Representative high magnification LSCM images showing TUNEL staining (false-colored red, A-B, D-E) or IHC staining for activated (cleaved) caspase-3 (stained brown, C, F) for sections of injured arterial tissue treated with a balloon coated with either a polymer 1/PKCδ film (A–C) or a polymer 1/pcDNA3 film (D–F) 32 bilayers thick. Images in panels A and D show IFC staining of PKCδ expression (false-colored green). In images A, B, D, and E, cell nuclei are stained and false-colored blue, and the locations of the internal and external elastic lamina defining the boundaries of the media are indicated by white dashed lines. In images C and F, cell nuclei are stained blue with hematoxylin; the locations of representative cells positive for cleaved caspase-3 (brown) are indicated by black arrows. Panels B and E show enlarged images of the regions enclosed by the yellow dashed-line boxes shown in A and C (without the green channel showing PKCδ expression, for clarity). In panels A, C, E, and F, the designation ‘L’ indicates the location of the artery lumen. Scale bars = 20 µm.
Figure 3B shows an enlarged image of the area indicated by the yellow box shown in panel A and reveals punctate red TUNEL staining, characteristic of DNA fragmentation associated with apoptosis, in the nuclei of cells in tissue treated with the polymer 1/pPKCδ film-coated balloons (green PKCδ-associated fluorescence is removed in this enlarged image for clarity). An equivalent enlarged image of the injured artery shown in panel D treated with a polymer 1/pcDNA3 (control) film-coated balloon is also included in panel E. Inspection of the image in panel E also reveals the presence of nuclei positive for TUNEL staining in this section of injured tissue. This staining was not generally as intense (or present in as many nuclei) as that observed in panel B, and the TUNEL-stained nuclei in these samples were not as extensively co-localized with expression of PKCδ. We note that both (i) the initial injury of tissue and (ii) the subsequent 20-minute treatment of the artery with inflated balloons are themselves likely sufficient to induce some variable level of cellular apoptosis in both of these samples. Analysis of 12 to 15 different images of three different sections taken from different regions of film-treated arteries revealed that ~30% (±12%) of the nuclei were positive for TUNEL staining in the polymer 1/pPKCδ-treated tissue, as compared to ~13% (±5%) for tissue treated with polymer 1/pcDNA3 balloons (additional representative images can be found in Figure S1 of the Supporting Information). These results (p < 0.02) are consistent with the induction of higher levels of apoptosis in tissue treated with pPKCδ [22, 55, 58]. In general, cell nuclei in pPKCδ-treated tissue (shown in panels A and B of Figure 3 and in Figure 4 below) were also smaller and more rounded than the nuclei observed for control pcDNA3-treated tissue.
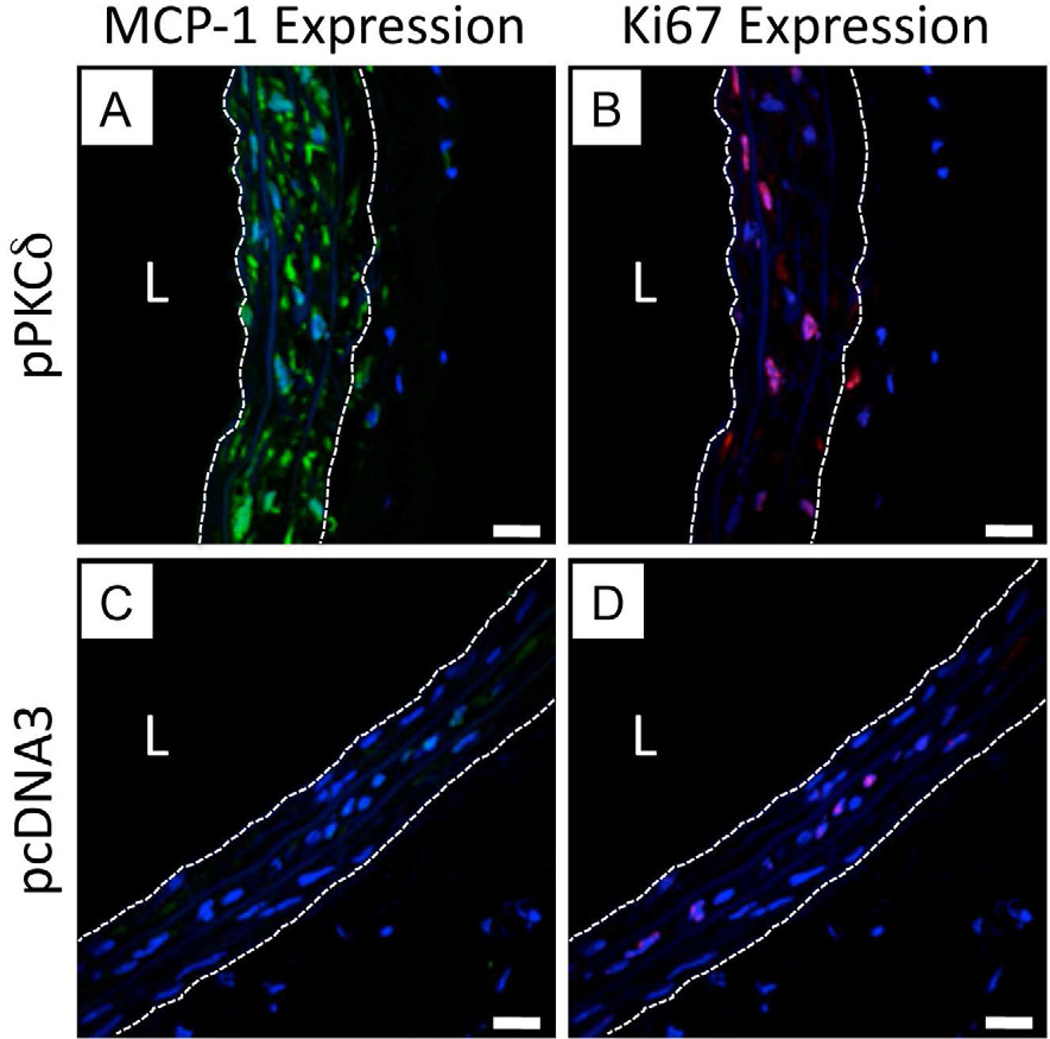
Figure 4.
Representative high magnification LSCM images showing IFC staining for MCP-1 expression (A and C, false-colored green) and Ki67 expression (B and D, false-colored red) for sections of injured arterial tissue treated with a balloon coated with either a polymer 1/PKCδ film (A, B) or a polymer 1/pcDNA3 film (C, D) 32 bilayers thick. In all images, cell nuclei are stained and false-colored blue, and the locations of the internal and external elastic lamina defining the boundaries of the media are indicated by white dashed lines. Additional IFC staining of tissue sections immediately adjacent to those shown in images A and B showed high levels of PKCδ expression; tissue sections immediately adjacent to those shown in images C and D showed relatively low levels of PKCδ expression (data not shown). In each image, the designation ‘L’ indicates the location of the artery lumen. Scale bars = 20 µm.
We also characterized cell apoptosis by performing IHC using antibodies against activated (cleaved) caspase-3, a protein fragment associated with the activation of cellular pathways involved in apoptosis [59]. Representative micrographs of polymer 1/pPKCδ-treated tissue and polymer 1/pcDNA3-treated tissue are shown in panels C and F of Figure 3 (nuclei are stained blue and cleaved caspase-3 is stained brown). Analysis of images arising from these experiments revealed ~23% of cells in pPKCδ-treated tissue to be positive for cleaved caspase-3; the percentage of cleaved caspase-3-positive cells in pcDNA3-treated tissue was generally negligible. These IHC results demonstrate clearly that the transfer and expression of pPKCδ increases levels of apoptosis in VSMCs significantly, and they provide additional confirmation of the TUNEL staining results described above.
While PKCδ is an active promoter of apoptotic pathways that could be used to limit VSMC accumulation, it also is involved in pathways that up-regulate the production of monocyte chemoattractant protein-1 (MCP-1), which is classically associated with recruitment of monocytes and, as established more recently, progenitor cells [19, 56, 57, 60–62]. Although the precise range of functions of MCP-1 in injured arteries remains to be elucidated, levels of MCP-1 are used here as a measure of PKCδ-mediated downstream events. In addition to MCP-1 expression, we characterized levels of Ki67, a marker of cellular proliferation that was previously found to be unaffected by adenovirus-mediated PKCδ expression [22].
Figure 4 shows representative high magnification LSCM images showing MCP-1 expression (4A and 4C, green fluorescence) and Ki67 expression (4B and 4D, red fluorescence) in arteries treated with polymer 1/DNA film-coated balloons incorporating either pPKCδ (4A and 4B) or pcDNA3 (4C and 4D). To aid in the analysis of these images, cell nuclei were stained using DAPI and the boundaries of the inner elastic lamina and outer elastic lamina are shown as white dashed lines to indicate the locations of the medial tissue. Inspection of these images reveals nearly all cells in pPKCδ-treated tissue to have higher levels of MCP-1 as compared to the pcDNA3-treated control, consistent with the increased levels of expression of PKCδ in these tissues, as discussed above. Unexpectedly, however, we also observed levels of Ki67 expression in the nuclei of pPKCδ-treated tissue to be higher (~44 ±7%) than that of the control tissue treated with pcDNA3 (~9 ±2%) (p < 0.002; from inspection of 12 to 15 different images from sections from three different regions of the treated tissue. Additional representative images can be found in Figure S2 of the Supporting Information). Although the proliferation rate in pcDNA-treated arteries is comparable to that observed in past literature reports of balloon-treated arteries, the level of proliferation in PKCδ-treated arteries observed here is significantly higher than levels observed in arteries treated with adenoviral vectors promoting expression of PKCδ used in our past study [22, 56]. The reason for this apparent discrepancy is not clear. Taken together, however, the results shown in Figures 3 and 4 reveal that the balloon-mediated delivery of pPKCδ to injured arterial tissue results in higher levels of PKCδ expression in all layers of the media, and suggest that the levels of expression observed are sufficient to influence downstream cellular processes associated with the up-regulation of apoptosis and MCP-1 expression.
Reduction of Intimal Hyperplasia in Arteries Treated with Polymer 1/pPKCδ Films
We performed a final series of experiments to evaluate the ability of our film-coated balloons to inhibit intimal hyperplasia in balloon-injured arterial tissue. These studies were also performed using catheter balloons coated with either polymer 1/pPKCδ films or polymer 1/pcDNA3 (control) films 32 bilayers thick. For these experiments, rats were sacrificed 14 days after balloon treatment to allow sufficient time for neointimal growth to occur.
Figure 5A shows a schematic illustrating the area of the carotid arteries treated in these experiments. The area of the common carotid artery shown in red corresponds to the region of tissue injured during initial balloon-mediated denuding of the endothelial layer (see Materials and Methods for additional details of surgical procedures). For the purposes of harvesting and characterizing sections of tissue after balloon treatment, we divided this injured section of artery into three regions approximately 1.5 mm in length: Region I (the region proximal to the aorta and upstream of the balloon relative to the direction of blood flow), Region II (an intermediate area of tissue), and Region III (the region proximal to the bifurcation of the common carotid artery and downstream of the balloon relative to blood flow). During surgery, film-coated balloons were inflated approximately in the middle of the region of injured tissue, corresponding to Region II. We note, however, that the nature of the surgical procedure used in these experiments makes it difficult to determine with absolutely certainty which portion of injured tissue was in direct contact with the balloon during treatment. We return to a consideration of this point again in the discussion below.
Panels B–D of Figure 5 show representative sections of tissue (H&E-stained to aid in identification of the adventitia, media, and intima) for regions I, II, and III (5B, 5C, and 5D, respectively) for a rat treated with a balloon coated with a polymer 1/pcDNA3 (control) film. These images show a gradual increase in the extent of intimal hyperplasia progressing from Region I toward the arterial bifurcation (Region III), and are, in general, similar to the extent of intimal hyperplasia observed at 14 days following injury in the absence of any therapeutic intervention (as reported in past studies) [22]. Panels E–G of Figure 5 show images of tissue from a rat that was treated with a balloon coated with a polymer 1/pPKCδ film. Inspection of these images reveals levels of intimal hyperplasia that are, overall, qualitatively lower than those shown in panels B-D. The extents of intimal hyperplasia here are again observed to be higher in Region III (the region of tissue closest to the arterial bifurcation); the lowest levels of intimal hyperplasia are observed in Regions I and II. These results suggest that balloons coated with polymer 1/pPKCδ films can promote levels of PKCδ expression that are sufficient to inhibit processes that lead to intimal hyperplasia.
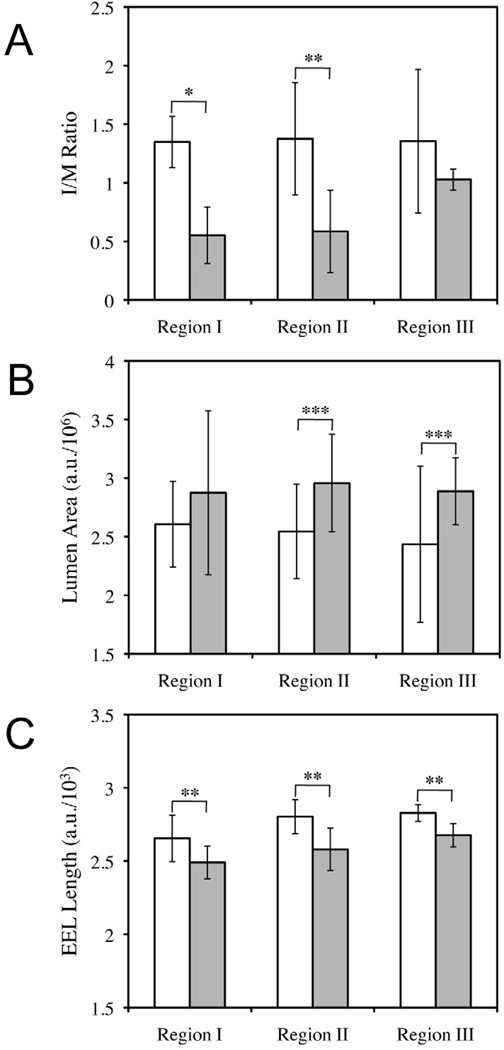
To facilitate quantitative interpretations of these results, we measured the intima/media ratio (I/M ratio), lumen area, and external elastic lamina (EEL) length of multiple different samples arising from these experiments (see Materials and Methods for details). The results of these analyses are summarized in Figure 6 and are reported as the average (with standard deviation) for each region in all rats. ANOVA Single Factor analysis of variance was used to determine the statistical significance of differences in I/M ratio, lumen area, and EEL length for each region. As shown in Figure 6A, rats treated with polymer 1/pPKCδ films had lower I/M ratios in all regions of the artery, however, this difference was only determined to be statistically significant in Regions I and II (p < 0.001 and p < 0.01, respectively). These results suggest that increased expression of PKCδ following polymer 1/DNA film treatment can reduce the occurrence of restenosis following initial balloon injury by ~60%.
Figure 6.
Plots showing average I/M ratio (A), lumen area (B), and external elastic lamina (EEL) length (C) measured for Regions I, II, and III (as defined above in Figure 5) of injured arteries treated with balloons coated with polymer 1/DNA films (32 bilayers thick) fabricated using either pcDNA3 (control; white bars) or pPKCδ (gray bars). Average values and error bars (shown as standard deviations) were calculated using measurements for three arbitrarily chosen sections from each Region (I, II, and III) depicted in Figure 5A. Two rats treated with polymer 1/pcDNA3 films (n = 6 for statistical analysis for each region) and three rats treated with polymer 1/PKCδ films (n = 9 for statistical analysis for each region) were used to calculate averages, standard deviations, and statistical significance of the data. Statistical analysis was conducted using ANOVA Single Factor analysis of statistical variance with the following reported p values: * p < 0.001; ** p < 0.01; and *** p < 0.10. See Materials and Methods for additional details on calculation of values and analysis of statistical variance.
The reduction of intimal hyperplasia observed in Regions I and II observed in this study is higher than that reported in a recent study investigating the potential therapeutic effects of adenovirus-induced expression of PKCδ in injured rat arterial tissue [22]. That study demonstrated ~40% reductions in intimal hyperplasia 14 days after the perfusion of balloon-injured arteries using solutions containing an adenoviral vector encoding PKCδ (a substantially different mode of delivery as compared to the film/balloon-based approach investigated here). As described above, Region II is the section of arterial tissue that we estimate was most directly in contact with film-coated balloons during treatment. Significant reductions in intimal hyperplasia in Region I could, however, have resulted from direct contact of this region of tissue with the balloon during treatment, or, potentially, from other proximity-related influences [e.g., longer-range transport of DNA, PKCδ, or other biomolecular signals that arise from the initial treatment of adjacent tissue, etc.]. Additional studies will be required to investigate these possible affects further.
We also measured the average lumen size for rats in each treatment group as shown in Figure 6B. In general, rats treated with balloons coated with polymer 1/pPKCδ films had larger lumenal areas than those treated with balloons coated with polymer 1/pcDNA3 films (observed to be statistically relevant for Regions II and III; p < 0.10). To more fully understand the mechanism responsible for this lumenal gain, we examined the lengths of the external elastic laminae, an indicator of vascular remodeling [63]. As shown in Figure 6C, the lengths of external elastic laminae in rats treated with polymer 1/pPKCδ films were significantly longer than those of rats treated with the control films (p < 0.01). This result suggests that arteries treated with polymer 1/pPKCδ films underwent an outward or adaptive remodeling. We note that in a past study we observed little influence of adenovirus-induced expression of PKCδ on vascular remodeling [55]. Although the reasons for this discrepancy are not clear, the beneficial effect of polymer 1/pPKCδ films on vascular remodeling, along with its inhibition of intimal hyperplasia, demonstrates the therapeutic potential of this approach.
Conclusions
We have demonstrated that treatment of injured arterial tissue with catheter balloons coated with DNA-containing PEMs can reduce intimal hyperplasia in a rat model of vascular injury. Insertion of balloons coated with polymer 1/pPKCδ multilayers into injured arteries resulted in local transfer of DNA and elevated levels of PKCδ expression that were sufficient to promote downstream cellular processes associated with up-regulation of apoptosis and the production of MCP-1 three days after treatment. Characterization of balloon-treated tissue 14 days after initial treatment also revealed polymer 1/pPKCδ-coated balloons to reduce intimal hyperplasia by ~60% compared to balloons coated with control films containing empty plasmid vectors. The results of this study advance proof-of-concept in vitro and in vivo results reported in past studies and demonstrate the potential therapeutic value of this ‘polyelectrolyte multilayer’ approach to the local transfer of DNA in the clinically important context of balloon-mediated vascular intervention. In addition to potential therapeutic benefits, the materials and approaches reported here could lead to new gene-based methods for the investigation of fundamental aspects of intimal hyperplasia. Our results suggest that this PEM-based approach could also prove useful in other in vivo applications that require short-term, surface-mediated transfer of DNA or other therapeutic agents.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (R01 EB006820 to D.M.L. and R01 HL081424 to B.L.). The authors are grateful to Lance Rodenkirch and Michael Hendrickson at the W. M. Keck Center for Biological Imaging for technical support of instrumentation and numerous helpful discussions associated with LSCM experiments. We are also grateful to Selin Aytar for assistance with collecting confocal microscopy images, Daniel Agnew for assistance with producing the pcDNA3 and pPKCδ plasmids, Amy Suwanabol for assistance with animal surgeries and many helpful discussions, and to Drew Roennenburg for processing of arteries used to analyze levels of intimal hyperplasia and the presence of activated caspase-3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information. Supporting information including additional representative microscopy images used to quantify TUNEL staining and Ki67 expression can be found in online version at doi:
References
- 1.Mueller RL, Sanborn TA. The history of interventional cardiology - cardiac-catheterization, angioplasty, and related interventions. Am Heart J. 1995;129:146–172. doi: 10.1016/0002-8703(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (tasc ii) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Liu MW, Roubin GS, King SB. Restenosis after coronary angioplasty - potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 4.Elezi S, Kastrati A, Hadamitzky M, Dirschinger J, Neumann FJ, Schomig A. Clinical and angiographic follow-up after balloon angioplasty with provisional stenting for coronary in-stent restenosis. Catheter Cardiovasc Interv. 1999;48:151–156. doi: 10.1002/(sici)1522-726x(199910)48:2<151::aid-ccd6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Sharif F, Daly K, Crowley J, O'Brien T. Current status of catheter- and stent-based gene therapy. Cardiovasc Res. 2004;64:208–216. doi: 10.1016/j.cardiores.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 9.Degertekin M, Regar E, Tanabe K, Smits PC, van der Giessen WJ, Carlier SG, et al. Sirolimus-eluting stent for treatment of complex in-stent restenosis - the first clinical experience. J Am Coll Cardiol. 2003;41:184–189. doi: 10.1016/s0735-1097(02)02704-3. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 11.McFadden EP, Stabile E, Regar E, Cheneau E, Ong ATL, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 12.Virmani R, Farb A, Guagliumi G, Kolodgie FD. Drug-eluting stents: Caution and concerns for long-term outcome. Coron Artery Dis. 2004;15:313–318. doi: 10.1097/00019501-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Tung R, Kaul S, Diamond GA, Shah PK. Narrative review: Drug-eluting stents for the management of restenosis: A critical appraisal of the evidence. Ann Intern Med. 2006;144:913–919. doi: 10.7326/0003-4819-144-12-200606200-00009. [DOI] [PubMed] [Google Scholar]
- 14.De Labriolle A, Pakala R, Bonello L, Lemesle G, Scheinowitz M, Waksman R. Paclitaxel-eluting balloon: From bench to bed. Catheter Cardiovasc Interv. 2009;73:643–652. doi: 10.1002/ccd.21895. [DOI] [PubMed] [Google Scholar]
- 15.Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation. 2010;121:2672–2680. doi: 10.1161/CIRCULATIONAHA.110.936922. [DOI] [PubMed] [Google Scholar]
- 16.Granada JF, Milewski K, Zhao H, Stankus JJ, Tellez A, Aboodi MS, et al. Vascular response to zotarolimus-coated balloons in injured superficial femoral arteries of the familial hypercholesterolemic swine. Circ Cardiovasc Interv. 2011;4:447–455. doi: 10.1161/CIRCINTERVENTIONS.110.960260. [DOI] [PubMed] [Google Scholar]
- 17.Kent KC, Liu B. Intimal hyperplasia - still here after all these years! Ann Vasc Surg. 2004;18:135–137. doi: 10.1007/s10016-004-0019-4. [DOI] [PubMed] [Google Scholar]
- 18.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 19.Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009;49:502–510. doi: 10.1016/j.jvs.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, et al. Local gene transfer of phvegf- plasmid by gene-eluting stents - an alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 21.Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, et al. Arterial gene transfer of the tgf-beta signalling protein smad3 induces adaptive remodelling following angioplasty: A role for ctgf. Cardiovasc Res. 2009;84:326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanouchi D, Kato K, Ryer EJ, Zhang F, Liu B. Protein kinase c delta mediates arterial injury responses through regulation of vascular smooth muscle cell apoptosis. Cardiovasc Res. 2010;85:434–443. doi: 10.1093/cvr/cvp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saurer EM, Yamanouchi D, Liu B, Lynn DM. Delivery of plasmid DNA to vascular tissue in vivo using catheter balloons coated with polyelectrolyte multilayers. Biomaterials. 2011;32:610–618. doi: 10.1016/j.biomaterials.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 25.Bertrand P, Jonas A, Laschewsky A, Legras R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol Rapid Commun. 2000;21:319–348. [Google Scholar]
- 26.Ai H, Jones SA, Lvov YM. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem Biophys. 2003;39:23–43. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 27.Tang ZY, Wang Y, Podsiadlo P, Kotov NA. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv Mater. 2006;18:3203–3224. [Google Scholar]
- 28.Jewell CM, Lynn DM. Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Adv Drug Deliv Rev. 2008;60:979–999. doi: 10.1016/j.addr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudou T, Crouzier T, Ren KF, Blin G, Picart C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv Mater. 2010;22:441–467. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- 30.Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules. 2003;4:1564–1571. doi: 10.1021/bm0341834. [DOI] [PubMed] [Google Scholar]
- 31.Etienne O, Gasnier C, Taddei C, Voegel JC, Aunis D, Schaaf P, et al. Antifungal coating by biofunctionalized polyelectrolyte multilayered films. Biomaterials. 2005;26:6704–6712. doi: 10.1016/j.biomaterials.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 32.Etienne O, Schneider A, Taddei C, Richert L, Schaaf P, Voegel JC, et al. Degradability of polysaccharides multilayer films in the oral environment: An in vitro and in vivo study. Biomacromolecules. 2005;6:726–733. doi: 10.1021/bm049425u. [DOI] [PubMed] [Google Scholar]
- 33.Schultz P, Vautier D, Richert L, Jessel N, Haikel Y, Schaaf P, et al. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-msh, for coating a tracheal prosthesis. Biomaterials. 2005;26:2621–2630. doi: 10.1016/j.biomaterials.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 34.Jewell CM, Zhang JT, Fredin NJ, Wolff MR, Hacker TA, Lynn DM. Release of plasmid DNA from intravascular stents coated with ultrathin multilayered polyelectrolyte films. Biomacromolecules. 2006;7:2483–2491. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 36.Amiji M. Polymeric gene delivery: Principles and applications. New York, New York: CRC Press; 2004. [Google Scholar]
- 37.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 38.Jewell CM, Lynn DM. Surface-mediated delivery of DNA: Cationic polymers take charge. Curr Opin Colloid In. 2008;13:395–402. doi: 10.1016/j.cocis.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelikin AN. Drug releasing polymer thin films: New era of surface-mediated drug delivery. ACS Nano. 2010;4:2494–2509. doi: 10.1021/nn100634r. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JT, Chua LS, Lynn DM. Multilayered thin films that sustain the release of functional DNA under physiological conditions. Langmuir. 2004;20:8015–8021. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 41.Jewell CM, Zhang JT, Fredin NJ, Lynn DM. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Control Release. 2005;106:214–223. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Saurer EM, Jewell CM, Kuchenreuther JM, Lynn DM. Assembly of erodible, DNA-containing thin films on the surfaces of polymer microparticles: Toward a layer-by-layer approach to the delivery of DNA to antigen-presenting cells. Acta Biomater. 2009;5:913–924. doi: 10.1016/j.actbio.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saurer EM, Flessner RM, Sullivan SP, Prausnitz MR, Lynn DM. Layer-by-layer assembly of DNA- and protein-containing films on microneedles for drug delivery to the skin. Biomacromolecules. 2010;11:3136–3143. doi: 10.1021/bm1009443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechler SL, Lynn DM. Design and synthesis of a fluorescently end-labeled poly(beta-amino ester): Application to the characterization of degradable polyelectrolyte multilayers. J Polym Sci Part A: Polym Chem. 2011;49:1572–1581. doi: 10.1002/pola.24578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bechler SL, Lynn DM. Characterization of degradable polyelectrolyte multilayers fabricated using DNA and a fluorescently-labeled poly(beta-amino ester): Shedding light on the role of the cationic polymer in promoting surface-mediated gene delivery. Biomacromolecules. 2012;13:542–552. doi: 10.1021/bm2016338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynn DM, Langer R. Degradable poly(beta-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768. [Google Scholar]
- 47.Lynn DM, Anderson DG, Akinc A, Langer R. Degradable poly(beta-amino ester)s for gene delivery. In: Amiji M, editor. Polymeric gene delivery: Principles and applications. New York: CRC Press; 2004. pp. 227–241. [Google Scholar]
- 48.Yoshida K. Pkc delta signaling: Mechanisms of DNA damage response and apoptosis. Cell Signal. 2007;19:892–901. doi: 10.1016/j.cellsig.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 49.Prus K, Edstrom A, Wallin M. Actin-induced stimulation of microtubule-associated atpase activity. FEBS Lett. 1981;125:49–52. doi: 10.1016/0014-5793(81)80993-3. [DOI] [PubMed] [Google Scholar]
- 50.Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, et al. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic rho-kinase inhibition. J Vasc Surg. 2005;42:757–764. doi: 10.1016/j.jvs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 51.Fredin NJ, Zhang JT, Lynn DM. Surface analysis of erodible multilayered polyelectrolyte films: Nanometer-scale structure and erosion profiles. Langmuir. 2005;21:5803–5811. doi: 10.1021/la050596+. [DOI] [PubMed] [Google Scholar]
- 52.Fredin NJ, Zhang JT, Lynn DM. Nanometer-scale decomposition of ultrathin multilayered polyelectrolyte films. Langmuir. 2007;23:2273–2276. doi: 10.1021/la0624182. [DOI] [PubMed] [Google Scholar]
- 53.Fredin NJ, Flessner RM, Jewell CM, Bechler SL, Buck ME, Lynn DM. Characterization of nanoscale transformations in polyelectrolyte multilayers fabricated from plasmid DNA using laser scanning confocal microscopy in combination with atomic force microscopy. Microsc Res Techniq. 2010;73:834–844. doi: 10.1002/jemt.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubl S, Tsai S, Ryer EJ, Wang CJ, Hu J, Kent KC, et al. Upregulation of protein kinase c delta in vascular smooth muscle cells promotes inflammation in abdominal aortic aneurysm. J Surg Res. 2009;153:181–187. doi: 10.1016/j.jss.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Si Y, Ren J, Wang P, Rateri DL, Daugherty A, Shi XD, et al. Protein kinase c-delta mediates adventitial cell migration through regulation of monocyte chemoattractant protein-1 expression in a rat angioplasty model. Arterioscler Thromb Vasc Biol. 2012;32:943–54. doi: 10.1161/ATVBAHA.111.244921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, et al. Anti-monocyte chemoattractant protein- monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 57.Itoh H, Yamamura S, Ware JA, Zhuang SB, Mii S, Liu B, et al. Differential effects of protein kinase c on human vascular smooth muscle cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2001;281:H359–H370. doi: 10.1152/ajpheart.2001.281.1.H359. [DOI] [PubMed] [Google Scholar]
- 58.Bai X, Margariti A, Hu Y, Sato Y, Zeng L, Ivetic A, et al. Protein kinase c{delta} deficiency accelerates neointimal lesions of mouse injured artery involving delayed reendothelialization and vasohibin- accumulation. Arterioscler Thromb Vasc Biol. 2010;30:2467–2474. doi: 10.1161/ATVBAHA.110.215723. [DOI] [PubMed] [Google Scholar]
- 59.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Leonard EJ, Skeel A, Yoshimura T. Biological aspects of monocyte chemoattractant protein- (mcp-) Adv Exp Med Biol. 1991;305:57–64. doi: 10.1007/978-1-4684-6009-4_7. [DOI] [PubMed] [Google Scholar]
- 61.Yoshimura T, Leonard E. Human monocyte chemoattractant protein-: Structure and function. Cytokines. 1992;4:131–152. [PubMed] [Google Scholar]
- 62.Zhang F, Tsai S, Kato K, Yamanouchi D, Wang CJ, Rafii S, et al. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of mcp- production in vascular smooth muscle cells. J Biol Chem. 2009;284:17564–17574. doi: 10.1074/jbc.M109.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward MR, Pasterkamp G, AC Y, CB Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–1191. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.