Abstract
Objective
Bone turnover markers (BTMs) predict fracture in older women, whereas data on younger women are lacking. To test the hypothesis that BTMs measured before and after menopause predict fracture risk, we performed a cohort study of 2,305 women.
Methods
Women attended up to nine clinic visits for an average of 7.6 ± 1.6 years; all were aged 42 to 52 years and were premenopausal or early perimenopausal at baseline. Incident fractures were self-reported. Serum osteocalcin and urinary cross-linked N-telopeptide of type I collagen (NTX) were measured at baseline. NTX was measured at each annual follow-up. Interval-censored survival models or generalized estimating equations were used to test whether baseline BTMs and changes in NTX, respectively, were associated with fracture risk. Hazard ratios (HRs) or odds ratios were calculated with 95% CIs.
Results
Women who fractured (n = 184) had about a 10% higher baseline median NTX (34.4 vs 31.5 nanomoles of bone collagen equivalents per liter per nanomole of creatinine per liter; P = 0.001), but there was no difference in osteocalcin. A 1-SD decrease in lumbar spine bone mineral density (BMD) measured premenopausally was associated with a higher fracture risk during menopause (HR, 1.55; 95% CI, 1.32–1.73). Women with a baseline NTX greater than the median had a 45% higher risk of fracture, multivariable-adjusted (HR, 1.45; 95% CI, 1.04–2.23). The HR of fracture among women with both the lowest spine BMD (quartile 1) and the highest NTX (quartile 4) at baseline was 2.87 (95% CI, 1.61–6.01), compared with women with lower NTX and higher BMD. Women whose NTX increased more than the median had a higher risk of fracture (odds ratio, 1.51; 95% CI, 1.08–2.10). Women who had baseline NTX greater than the median experienced greater loss of spine and hip BMD.
Conclusions
A higher urinary NTX excretion measured before menopause and across menopause is associated with a higher risk of fracture. Our results are consistent with the pathophysiology of transmenopausal changes in bone strength.
Keywords: Bone resorption, Fracture, Menopause, Osteoporosis, Bone mineral density
Osteoporotic fractures are a major public health problem with important morbidity and mortality consequences. At the age of 50 years, the lifetime risk of a woman experiencing a fracture due to osteoporosis is estimated to be at least 40%. Osteoporotic fractures, including hip,1 wrist,2 and vertebral fractures,3 have important consequences, including increased risks of disability, hip and vertebral fractures, and mortality.4,5 Fractures are more common than the combined numbers of myocardial infarction, stroke, and breast cancer in women of all ethnicities.6
Menopause represents a vulnerable time in a woman’s life for a number of reasons but particularly for her skeletal health. Estrogen deficiency associated with menopause increases bone remodeling, leading to an imbalance between bone formation and bone resorption.7,8 This increase in bone remodeling persists for several years and becomes associated with an increased rate of bone loss.9,10 In the Study of Women’s Health Across the Nation (SWAN), women who transitioned through menopause experienced a significantly higher rate of bone loss than women who remained premenopausal, independent of age.11 This accelerated bone loss could place women at a higher risk for fractures.
Markers of bone turnover have been shown to predict the risk of fractures in many, but not all, prospective studies.12–15 Associations are generally stronger for bone resorption markers than for bone formation markers16; in some studies, these associations are independent of bone mineral density (BMD).15 However, all of these studies have been carried out in postmenopausal women, particularly in women aged 65 years or older,15,17 or in older men.18 To our knowledge, no study has examined the ability of bone turnover markers (BTMs) measured premenopausally or during the menopausal transition to predict the subsequent risk of fracture. The US Preventive Services Task Force most recently noted that no study has addressed screening for high risk in the premenopausal or early perimenopausal period.19 Clearly, we need more knowledge on the factors that might identify women who are at high risk for fracture as they enter menopause.
The objectives of the current analyses are to test the following hypotheses: (1) BTMs measured before the final menstrual period will predict fractures and changes in BMD during the menopausal transition; (2) changes in bone resorption across the menopausal transition are associated with the risk of subsequent fracture; and (3) these associations are independent of baseline BMD, estradiol (E2), and follicle-stimulating hormone (FSH). These analyses were carried out in SWAN, a longitudinal cohort study of the menopausal transition in a community-based sample of women from multiple ethnic groups. SWAN is the first large-scale, multiethnic, longitudinal cohort study to assess BMD, bone resorption, and fractures across the menopausal transition.
METHODS
Study sample
SWAN is a multisite, longitudinal, cohort study of midlife that is being conducted in a community-based sample of 3,302 women at seven clinical sites.20 Enrollees aged 42 to 52 years were still menstruating 3 months before screening and were not using oral contraceptives or postmenopausal hormone therapy. The SWAN bone substudy included women from five of the seven SWAN clinical sites located in Boston, the Detroit area, Los Angeles, Pittsburgh, and Oakland. At each site, half of the women were white. The other half were African American (at the Boston, Detroit, and Pittsburgh field sites) and Japanese and Chinese (at the Los Angeles and Oakland sites, respectively). BMD was measured annually in 2,413 women. Of these, 2,305 (96%) women had at least one measure of cross-linked N-telopeptide of type I collagen (NTX) and fracture data. Women were censored at the first report of hormone therapy or at the time of hysterectomy or bilateral oophorectomy. Individual annual data were excluded because they indicated the use of medications known to affect bone or calcium metabolism. Written informed consent form was obtained from all participants, and each site’s protocol was conducted with approval from an institutional review board.
Bone turnover measurements
Women were asked to collect fasting blood and urine samples before 10:00 AM on days 2 to 5 of the menstrual cycle if menstrual periods were still occurring.21 If a woman could not provide blood and urine samples before 10:00 AM, we recorded the time that the specimens were collected. Because BTMs show diurnal variation,22,23 we adjusted for the time of collection in our analyses. Samples were stored at −80° until the assay. Funding was initially acquired for baseline osteocalcin; subsequent funding was used for annual NTX measures. We had no funding for longitudinal measures of osteocalcin. The annual NTX values were all batched and sent to the laboratory at one time. In the current study, NTX was measured in 15,942 urine samples from 2,305 women (an average of seven annual samples per woman from a possible nine annual visits). Specimen retrieval and assay were conducted without knowledge of menopausal transition stage, race, BMD, fracture status, or other potentially important covariates.
Osteocalcin was measured in duplicate using an immunoradiometric assay (ELSA-OSTEO; CisBio International, Bagnols/Ceze, France) that measures both the 1- to 49-amino-acid intact human osteocalcin molecule and the 25- to 37-amino-acid fragment. The lower limit of detection of the assay is 0.4 ng/mL, and the intra-assay and interassay coefficients of variation are both less than 6%. Reactivity with uncarboxylated and/or partially carboxylated osteocalcin was not defined in this assay.
NTX was measured using an automated immunoassay (Vitros ECi; Ortho Clinical, Rochester, NY) and expressed as nanomoles of bone collagen equivalents per liter per nanomole of creatinine per liter (nM BCE/nM Cr). The lower limit of detection was 10 nM BCE/nM Cr, and the intra-assay and interassay coefficients of variation for NTX/Cr were 2.8% and 4.8%, respectively, across the assay range. Samples (>3,000 nM BCE/nM Cr) were diluted 1:20 before measurement. NTX values greater than 3 SDs from the overall mean (>94.7 nM BCE/nM Cr) were considered outliers and excluded. Creatinine was measured with the Cobas Mira autoanalyzer (Horiba ABX, Montpellier, France) based on the Jaffé reaction. The lower limit of detection was 0.014 mM. The intra-assay and interassay coefficients of variation were 0.6% and 4.1%, respectively, across the assay range.
Hormone measures
E2 concentrations were assayed with an ACS-180 automated analyzer (Bayer Diagnostics, Norwood, MA) at the SWAN Core Laboratory, University of Michigan. Serum E2 concentrations were measured in duplicate with a modified offline ACS-180 (E2-6) immunoassay. The interassay and intra-assay coefficients of variation averaged 10.6% and 6.4%, respectively, across the assay range.24 Serum FSH concentrations were measured with a two-site chemiluminometric immunoassay. The interassay and intra-assay coefficients of variation were 12.0% and 6.0%, respectively.25
Assessment of BMD
BMDs of the posterior-anterior lumbar spine and femoral neck were measured by dual-energy x-ray absorptiometry (DXA) with a Hologic QDR 2000 densitometer (Hologic, Inc., Waltham, MA) in Pittsburgh and Oakland, or with a Hologic QDR 4500A densitometer in Boston, the Detroit area, and Los Angeles. Osteodyne positioners (Osteodyne, Research Triangle, NC) were used to position the participants for all measurements of the proximal femur.26
All five centers used a standard quality control program that involved the following: training, certification, and recertification of DXA operators by Synarc, Inc. (Waltham, MA); daily measurement of a Hologic anthropomorphic spine phantom at each site; cross-site calibration with a single anthropomorphic spine phantom; and random review of 5% of scans plus all problem scans by Synarc. Measurements of the local spine phantoms and the circulating spine phantom were analyzed by Synarc and used to adjust DXA measurements for minor temporal or geographic variations.
Determination of menopause stage
At each annual visit, menopause stage was determined based on reports of the frequency and regularity of menstrual bleeding, as previously described.11 Women were classified as premenopausal if they had experienced at least one menstrual period in the last 3 months with no change in the regularity of their menstrual bleeding during the last year. Women were classified as early perimenopausal if they had experienced at least one menstrual period in the last 3 months with some change in the regularity of their menstrual bleeding during the last year. Women were classified as late perimenopausal if they had experienced no menstrual bleeding in the last 3 months but some menstrual bleeding during the last 11 months. Women were classified as postmenopausal once they had experienced at least 12 consecutive months of amenorrhea. The postmenopausal stage was defined as beginning at the time of the woman’s final menstrual period. Once a woman had transitioned to a more advanced menopause stage, she was not reclassified into an earlier stage.
Fractures
Incident fractures that occurred after baseline were ascertained at each annual visit by self-report. The accuracy of the self-report of fractures was verified during visits 7 to 10 by review of radiology reports. The false-positive rate was 0.9%. Twelve percent of fractures could not be confirmed because we were unable to obtain the radiographic reports. We included both traumatic and nontraumatic fractures because both have been linked to a lower BMD.27 The average follow-up for this analysis was 7.6 ± 1.6 years (range, 0.6–9.4 y). All self-reported fractures are included in the primary analysis. Because digit and facial fractures are not typically related to low BMD, we performed a sensitivity analysis after excluding these fractures. We calculated fracture rates per 1,000 person years for each ethnic group, both with and without digit and facial fractures.
Statistical analyses
The characteristics of women who experienced an incident fracture during the follow-up period were compared with those of women who did not fracture using t test and χ2 test. Because the distributions of BTMs were skewed, we compared the median osteocalcin and NTX in women who experienced a fracture with those in women who did not fracture. To determine whether BTMs measured at baseline during the premenopausal or early perimenopausal period predicted incident fractures during the transition, we used interval-censored survival models. These models are similar to Cox proportional hazards models but are approximate when the exact date of the event is not known (only that the event occurred between certain timepoints). We calculated the hazard ratios (HRs) and 95% CIs for fracture per 1-SD decrease in BMD, and for NTX and osteocalcin greater than the median.
We developed a series of models adding in important covariates. We initially adjusted only for time of collection then age, and then for age, race, and clinic site. To determine whether the associations were independent of lumbar spine BMD, we adjusted for spine BMD. The full multivariate model included time of collection, age, race, clinic site, spine BMD, fracture history, height, weight, menopause status at baseline, education, diabetes, baseline smoking, and alcohol use. Subsequent models added these baseline hormones to the full multivariable models to test whether the associations were mediated by E2 or FSH.
To test for an interaction between baseline NTX and baseline spine BMD, we formed four groups: “high” NTX (quartile 4; >41.5 nM BCE/nM Cr) and “low” spine BMD (quartile 1; <0.98 g/cm2); “high” NTX (quartile 4) and “normal” BMD (quartiles 2, 3, and 4); “normal” NTX (quartiles 1, 2, and 3) and “low” BMD (quartile 1); and “normal” NTX (quartiles 1, 2, and 3) and “normal” BMD (quartiles 2, 3, and 4). We calculated the incidence of fracture across the four groups. We estimated the HR (95% CI) of fracture in each group in comparison with the referent group, “normal” NTX, and “normal” BMD.
The changes in NTX from baseline to each follow-up visit were calculated. The median change in NTX across all years combined was calculated. If any value was greater than the median, the participant was considered to have experienced an increase in NTX greater than the median. To determine whether changes in NTX during the menopausal transition are associated with fracture, we used generalized estimating equations with time-varying covariates to calculate the odds ratio and 95% CI.
To determine whether changes in NTX during the menopausal transition predict fracture, we used a similar modeling strategy in which we additionally adjusted for baseline NTX. In addition, posterior-anterior spine BMD, weight, menopause status, smoking, alcohol, diabetes, E2, and FSH were added as time-varying covariates.
To test whether baseline osteocalcin or NTX was associated with rates of BMD change, we used repeated measures of generalized estimating equations. Osteocalcin and NTX were entered into the models as values less than the median. We also calculated the mean change in BMD among those less than, equal to, or greater than the median.
All data analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). For longitudinal analyses, data were censored at the time that a woman began hormone therapy or antiresorptive therapy or reported hysterectomy and bilateral oophorectomy. In sensitivity analyses, we also excluded women who reported a fracture before the baseline visit.
RESULTS
Over an average follow-up of 7.6 years, 184 (8.7%) women experienced at least one incident fracture. The most common fracture sites were the ankle (n = 33), arm (n = 14), foot (n = 26), wrist (n = 20), and toe (n = 38). Women who reported a fracture were more likely to be white and more likely to report a history of fracture before baseline (Table 1).
TABLE 1.
Characteristics of women who experienced an incident fracture and women who did not fracture
| No fracture during follow-up (n = 1,921) | Any fracture during follow-up (n = 184) | Pa | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | ||
| Baseline characteristics | |||||||
| Age, y | 45.7 | 2.7 | 46.0 | 45.7 | 2.52 | 46.00 | 0.743 |
| Weight, kg | 73.4 | 21.2 | 68.1 | 78.0 | 20.22 | 76.10 | 0.005 |
| Height, cm | 162.3 | 6.9 | 162.3 | 163.3 | 6.55 | 163.40 | 0.057 |
| Estradiol, pg/mLa | 75.4 | 81.1 | 55.6 | 77.7 | 93.73 | 54.33 | 0.900 |
| FSH, mIU/mLa | 23.5 | 25.0 | 15.4 | 22.8 | 21.85 | 15.15 | 0.518 |
| Posterior-anterior lumbar spine BMD, g/cm2 | 1.08 | 0.14 | 1.08 | 1.05 | 0.14 | 1.04 | 0.005 |
| Hip BMD, g/cm2 | 0.96 | 0.15 | 0.95 | 0.95 | 0.14 | 0.94 | 0.19 |
| Osteocalcin, ng/mL | 16.0 | 6.1 | 15.1 | 16.6 | 5.53 | 15.7 | 0.06 |
| NTX, nM BCE/nM Cr | 33.6 | 14.1 | 31.5 | 37.1 | 14.53 | 34.4 | 0.001 |
| Change in spine BMD/y, mg/cm2 per year | −0.05 | 0.07 | −0.05 | −0.05 | 0.07 | −0.04 | 0.920 |
| Change in hip BMD/y, mg/cm2 per year | −0.02 | 0.05 | −0.01 | −0.02 | 0.06 | −0.02 | 0.280 |
| No fracture during follow-up, n (%) | Incident fracture during followup, n (%) | P | |||||
| Ethnicity | |||||||
| Black | 529 (27.5) | 57 (30.1) | 0.002 | ||||
| Chinese | 221 (11.5) | 12 (6.5) | |||||
| Japanese | 251 (13.1) | 10 (5.4) | |||||
| White | 920 (47.9) | 105 (57.1) | |||||
| Education | |||||||
| College or more | 854 (44.81) | 75 (40.8) | 0.29 | ||||
| Positive fracture history | 316 (16.5) | 69 (37.9) | <0.0001 | ||||
| Current smoker | 292 (15.3) | 33 (17.9) | 0.35 | ||||
| Alcohol use at baseline | 925 (48.4) | 90 (48.9) | 0.89 | ||||
| Menopause status at baseline | |||||||
| Early perimenopausal | 828 (43.7) | 82 (45.8) | 0.58 | ||||
| Premenopausal | 1,069 (56.4) | 97 (54.2) | |||||
| Menopause status on the last follow-up visit | |||||||
| Postmenopausal | 952 (59.7) | 95 (60.1) | 0.90 | ||||
| Late perimenopausal | 166 (10.4) | 19 (12.0) | |||||
| Early perimenopausal | 428 (26.9) | 40 (25.3) | |||||
| Premenopausal | 48 (3.0) | 4 (2.5) | |||||
| Diabetes | 85 (4.5) | 18 (9.8) | 0.001 | ||||
FSH, follicle-stimulating hormone; BMD, bone mineral density; NTX, cross-linked N-telopeptide of type I collagen; nM BCE/nM Cr, nanomoles of bone collagen equivalents per liter per nanomole of creatinine per liter.
P values for estradiol, FSH, osteocalcin, and NTX were based on log-transformed values.
The rates of fracture per 1,000 person years were as follows: 16.9 (95% CI, 13.1–21.9) for black women, 8.2 (95% CI, 4.7–14.5) for Chinese women, 6.2 (95% CI, 3.3–11.4) for Japanese women, and 19.0 (95% CI, 15.7–23.0) for white women. Patterns of fracture rates by ethnicity were similar when we excluded digit and facial fractures.
Women who reported an incident fracture were heavier and had lower lumbar spine BMD. There was no significant difference in age, baseline E2, FSH, hip BMD, smoking, or alcohol use between women who did and did not report an incident fracture (Table 1). Women who reported a fracture were slightly taller at baseline than women who did not report a fracture (P = 0.06). There was no difference in the change in spine or hip BMD by fracture status.
The average menopause status at the last SWAN follow-up did not differ by fracture status (Table 1). By the ninth annual SWAN visit, most women (60%) had transitioned through menopause, and only about 3% remained premenopausal. The median NTX on the last follow-up visit was greatest in women who were postmenopausal (45.4 nM BCE/nM Cr) compared with women who were late perimenopausal (37.9 nM BCE/nM Cr), early perimenopausal (31.2 nM BCE/nM Cr), or premenopausal (35.2 nM BCE/nM Cr; P ≤ 0.0001).
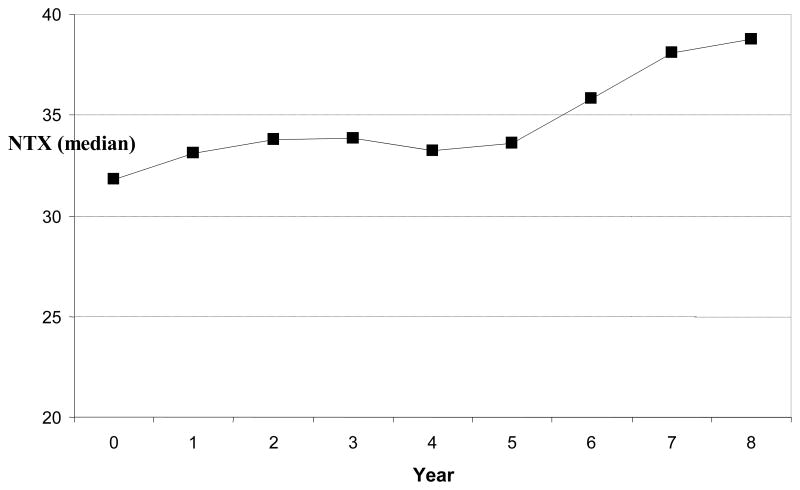
The median NTX value was fairly consistent across the first 4 years of the follow-up period and then began to increase (Fig. 1).
FIG. 1.
Median cross-linked N-telopeptide of type I collagen (NTX; nanomoles of bone collagen equivalents per liter per nanomole of creatinine per liter) by visit year.
Baseline BTMs, fracture, and bone loss
The median baseline osteocalcin was slightly higher in women who experienced an incident fracture than in those who did not (15.7 vs 15.1 ng/mL, respectively; P = 0.06). Women whose baseline osteocalcin was greater than the median (>15.1 ng/mL) were more likely to experience a fracture (HR, 1.33; 95% CI, 0.99–1.97; Table 2). However, the association diminished after adjustment for age, race, and other covariates.
TABLE 2.
HR and 95% CI of fracture by baseline osteocalcin: HR per value greater than the median (15.1 ng/mL)
| HR | 95% CI | P | |
|---|---|---|---|
| Unadjusted | 1.33 | 0.99–1.97 | 0.060 |
| Adjustments | |||
| Model 1: age | 1.49 | 0.99–2.53 | 0.057 |
| Model 2: +race and site | 1.42 | 0.94–2.45 | 0.102 |
| Model 3: +fracture history | 1.11 | 0.84–1.60 | 0.52 |
| Model 4: +lumbar spine BMD | 0.98 | 0.75–1.41 | 0.92 |
| Model 5: +height and weight | 1.04 | 0.78–1.52 | 0.81 |
| Model 6: +menopause status at baseline | 1.06 | 0.79–1.56 | 0.72 |
| Full multivariate modela | 1.11 | 0.85–1.67 | 0.53 |
Each consecutive model includes variables in the previous model. Time of collection was adjusted for in each model.
HR, hazard ratio; BMD, bone mineral density.
Full multivariate model covariates included time of collection, baseline age, race, site, fracture history, lumbar spine BMD, height, weight, menopause status, education, alcohol use, smoking, and diabetes.
The median baseline NTX was 9.9% higher in women who subsequently reported fractures than in those who did not fracture (34.4 vs 31.5 nM BCE/nM Cr; P = 0.001). Urinary NTX measured before the final menstrual period was associated with the risk of subsequent fracture. Women whose baseline NTX was above the median (>31.9 nM BCE/nM Cr) had a 59% greater risk of fracture than women whose baseline NTX was below the median (HR, 1.59; 95% CI, 1.15–2.43; Table 3). This association was independent of age, race, site, and prior fracture. Lumbar spine BMD, but not hip BMD, was related to fracture risk: a 1-SD decrease in lumbar spine BMD was associated with an HR of 1.50 and a 95% CI of 1.28 to 1.68.
TABLE 3.
HR and 95% CI of fracture by baseline NTX: HR per value greater than the median (31.9 nM BCE/nM Cr)
| HR | 95% CI | P | |
|---|---|---|---|
| Unadjusted | 1.59 | 1.15–2.43 | 0.003 |
| Adjustments | |||
| Model 1: age | 1.44 | 1.05–2.17 | 0.003 |
| Model 2: +race and site | 1.43 | 1.04–2.16 | 0.024 |
| Model 3: +fracture history | 1.36 | 1.00–2.06 | 0.054 |
| Model 4: +lumbar spine BMD | 1.42 | 1.03–2.16 | 0.030 |
| Model 5: +height and weight | 1.45 | 1.05–2.24 | 0.023 |
| Full multivariate modela | 1.46 | 1.05–2.26 | 0.021 |
| Full multivariate model + FSH | 1.49 | 1.06–2.31 | 0.017 |
| Full multivariate model + E2 | 1.46 | 1.05–2.26 | 0.022 |
Each consecutive model includes variables in the previous models. Time of collection was adjusted for in each model.
HR, hazard ratio; NTX, cross-linked N-telopeptide of type I collagen; nM BCE/nM Cr, nanomoles of bone collagen equivalents per liter per nanomole of creatinine per liter; BMD, bone mineral density; FSH, follicle-stimulating hormone; E2, estradiol.
Full multivariate model covariates included time of collection, baseline age, race, site, fracture, history, lumbar spine BMD, height, weight, menopause status, education, alcohol use, smoking, and diabetes.
Addition of lumbar spine BMD to the model slightly attenuated the association between baseline NTX and subsequent fractures (P = 0.064), but this association remained significant in the full multivariate model (HR, 1.45; 95% CI, 1.04–2.23). Further adjustment for baseline FSH or E2 had no effect on these results. Similar results were observed when we adjusted for hip BMD, instead of lumbar spine BMD (data not shown).
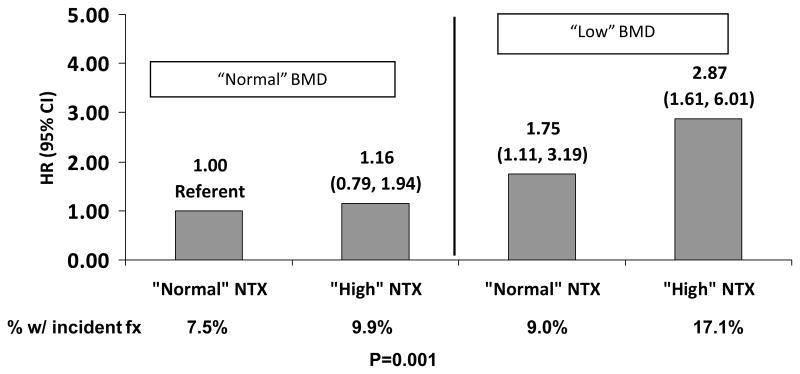
A greater proportion of women with both the lowest lumbar spine BMD (quartile 1) and the greatest NTX (quartile 4) at baseline had a fracture during follow-up (17.1%) compared with women with “low” BMD (quartile 1) but “normal” NTX (quartiles 1, 2, and 3; 9.9%); women with “normal” BMD (quartiles 2, 3, and 4) but “high” NTX (quartile 4; 9.04%); and women with both BMD and NTX in the “normal” range (7.5%; P = 0.001). A “low” baseline lumbar spine BMD, irrespective of NTX value, was associated with a higher risk of fracture (Fig. 2). The combination of both “low” spine BMD and “high” NTX was associated with a 2.87-fold (95% CI, 1.61–6.01) increased risk of fracture compared with women with normal levels of both NTX and lumbar spine BMD, but the interaction was not significant (P interaction = 0.32; Fig. 2).
FIG. 2.
Multivariable-adjusted hazard ratio (HR) and 95% CI of fracture by baseline cross-linked N-telopeptide of type I collagen (NTX) and lumbar spine bone mineral density (BMD; P interaction = 0.32). fx, fracture.
Normal BMD = Quartile 2, 3 or 4 (> 0.98 g/cm2); Low BMD = Quartile 1 (≤0.98g/cm2); Normal NTX = Quartile 1, 2 or 3 (≤ 41.5 nM BCE/nMCr); High NTX = Quartile 4(> 41.5nM BCE/nMCr). Models adjusted for baseline age, race, site, fracture history, education, smoking, diabetes, menopausal status, height and weight.
Loss in total hip and lumbar spine BMD was greater for women with baseline NTX greater than the median. The mean (SD) change in total hip BMD from baseline to visit 8 was −0.014 (0.058) and −0.024 (0.058) in women with baseline NTX less than or equal to the median NTX versus women with baseline NTX greater than the median NTX, respectively (P = 0.002). The mean (SD) change in spine BMD from baseline to visit 8 was −0.047 (0.067) and −0.059 (0.066) in women with baseline NTX less than or equal to the median NTX versus women with baseline NTX greater than the median NTX, respectively (P = 0.002). These associations were significant in the multivariate repeated-measures models that account for the annual BMD measures (β ≈ −0.005, P < 0.01).
Longitudinal fracture results
Women whose urinary NTX excretion increase was above the median had a 29% higher risk of fracture compared with women whose urinary NTX excretion increase was below the median (odds ratio, 1.29; 95% CI, 0.95–1.78; P = 0.103; Table 4). In models adjusting for baseline NTX, both the baseline NTX and the changes in NTX were significant independent predictors of incident fracture. Women who experienced increases in their NTX greater than the median during follow-up had a 54% increased risk of fracture. In the full multivariable model, changes in NTX greater than the median were associated with about a 50% increased risk of fracture independent of baseline NTX. Further addition of FSH or E2 as time-varying covariates had no important effect on the results (data not shown).
TABLE 4.
Longitudinal association between changes in NTX and incident fractures during followup: OR and 95% CI for change in NTX greater than the median and for baseline NTX greater than the mediana
| OR | 95% CI | P | |
|---|---|---|---|
| Model 1: adjusted for urine collection time | |||
| Change in NTX > median | 1.29 | 0.95–1.75 | 0.103 |
| Model 2: model 1 + baseline NTX | |||
| Change in NTX > median | 1.54 | 1.13–2.11 | 0.007 |
| Model 3: model 2 + age | |||
| Change in NTX > median | 1.51 | 1.10–2.07 | 0.012 |
| Model 4: model 3 + race and site | |||
| Change in NTX > median | 1.56 | 1.13–2.14 | 0.006 |
| Model 5: model 4 + fracture history | |||
| Change in NTX > median | 1.50 | 1.10–2.05 | 0.011 |
| Model 6: model 5 + lumbar spine BMD | |||
| Change in NTX > median | 1.49 | 1.09–2.04 | 0.013 |
| Model 7: model 6 + height and weight | |||
| Change in NTX > median | 1.50 | 1.08–2.08 | 0.015 |
| Model 8: model 7 + menopause status | |||
| Change in NTX > median | 1.46 | 1.06–2.03 | 0.022 |
| Model 9: model 8 + education, alcohol, smoking, and diabetes | |||
| Change in NTX > median | 1.51 | 1.08–2.10 | 0.015 |
The median baseline NTX is 31.9 nM BCE/nM Cr.
NTX, cross-linked N-telopeptide of type I collagen; OR, odds ratio; BMD, bone mineral density; nM BCE/nM Cr, nanomoles of bone collagen equivalents per liter per nanomole of creatinine per liter.
Median change in NTX greater than 3.1 nM BCE/nM Cr.
Sensitivity analyses
Sensitivity analyses that excluded toe, finger, and facial fractures (n = 34) had little effect on either baseline or longitudinal results (data not shown). We also excluded women who had a previous fracture, and the results were similar: women with baseline NTX greater than the median had a 60% increased risk of fracture (HR, 1.60; 95% CI, 1.04–2.91).
DISCUSSION
Evidence from prospective studies of older postmenopausal women13–17,28 and older men18 has shown that BTMs are associated with fracture risk and may be useful for fracture prediction. To our knowledge, no previous study has assessed whether BTMs measured in premenopause and early perimenopause predict fractures during the menopausal transition. Our results demonstrate that higher levels of the bone resorption marker NTX and greater increases in NTX during the menopausal transition are independently associated with an increase in fracture risk. These associations were independent of BMD, serum E2, FSH, and other potential covariates. The magnitude of the association was similar to that of the association between a 1-SD decrease in spine BMD.29 Serum osteocalcin level, a marker of osteoblast activity, was also associated with fracture risk in unadjusted analyses, but not in the full multivariate models. The stronger association observed with NTX (a bone resorption marker) than with osteocalcin (an osteoblast marker) is consistent with previous reports.16 The observation that the association of NTX with fracture risk is independent of BMD is also consistent with prior studies.15,30,31 Our results extend these earlier findings on older women to younger premenopausal women.
The underlying mechanisms whereby higher levels of NTX predict a higher risk of fractures could reflect faster rates of bone loss. Consistent with this observation, we showed that women with higher NTX experienced a significantly greater loss of both spine and hip BMD. We have previously shown that bone loss accelerates substantially in late perimenopause and continues at a similar pace in the initial postmenopausal years.11 However, our fracture models adjusted for baseline BMD and changes in BMD, suggesting that there must be other mechanisms that underlie our findings. We have recently shown that composite strength indices that integrate body size and bone size with bone density to capture the major contribution to bone strength relative to loads applied to bone during falls have the potential to explain racial/ethnic differences in fracture risk.32 In addition, deterioration of bone architecture may contribute independently to skeletal fragility, above and beyond the decrease in BMD. A greater proportion of the fracture efficacy of antiresorptive drugs is explained by reductions in bone turnover rather than by improvement in BMD.33–35 Resorption cavities surrounding individual trabeculae lead to stress concentrators, which in turn lead to a local weakening of the trabeculae that is disproportionate to the small amount of bone lost.36 Finally, a high rate of bone turnover increases the proportion of newly synthesized bone, which is less mineralized.37 All of these factors could underlie the association between higher bone resorption and fracture.
Fracture rates were higher in white women and lower in Asian women, consistent with previous studies.6 Fracture rates were comparable in African-American women and white women, a finding inconsistent with studies of older women. The reason for this is unclear, but it could reflect an earlier age at menopause observed in African-American women in some studies.38 In SWAN, we found no difference in age at menopause for African-American women, but a much greater proportion of African-American women had surgical amenorrhea and were therefore censored in our analyses.39 In addition, these results are based on a relatively small number of African-American women who fractured.
Our results were also independent of baseline serum FSH and E2 and changes in these reproductive hormones. This finding was somewhat surprising because we have previously shown that higher levels of FSH, but not other reproductive hormones, are positively associated with bone turnover before the final menstrual period.24 We hypothesized that addition of these hormones to our models would attenuate the association between NTX and fracture. However, there was little, if any, attenuation, suggesting that these reproductive hormones may not be correlated with fracture across the menopause.
Lumbar spine BMD was associated with an increased risk of fracture across menopause, and the magnitude of the association was similar to what has been reported for older women and men.40,41 We found no association with total hip BMD. This may reflect the higher proportion of the more metabolically active trabecular bone in the spine versus the hip. The new paradigm has suggested that trabecular bone loss begins well before menopause and thus may reflect the strength of bone upon entry into menopause.
Women with both “low” BMD and “high” NTX at study entry had almost a threefold greater risk of fracture during the transition than women with “normal” levels of BMD and NTX. We defined these categories by quartiles; they are not clinical cutoffs. Indeed, the cutoff for lumbar spine BMD as “low” corresponds to a t score of about −0.6. Nevertheless, our results suggest that women who enter menopause with the lowest spine BMD and the highest NTX have a higher risk of fracture and could be targeted for preventive efforts.
Recent national guidelines have commented on the utility of BTMs in predicting fracture risk. Both the 2008 US National Osteoporotic Foundation guidelines and the 2008 UK guidelines note that BTMs can be measured to predict fracture risk and have potential for risk assessment, but that more research is needed.
Serum BTMs are primarily used in clinical practice for women undergoing postmenopausal osteoporosis therapy. In bisphosphonate-treated women, BTMs are useful in providing early indications of treatment efficacy, are predictors of BMD response and fracture risk reduction, and may be useful for monitoring compliance.42 However, a recent systematic review noted that pretreatment values are not helpful in selecting women for treatment.43 Although we noted that women with higher NTX values (>median) were at a higher risk for fracture and experience faster rates of bone loss, the positive predictive power was low, and it is improbable that they will be useful in individual women. Nevertheless, our findings are consistent with the pathophysiology of menopausal changes in the bone and show for the first time that BTMs measured premenopausally predicts fractures. Women who experienced greater increases in NTX have a higher risk of fracture and experience faster rates of bone loss.
Some limitations of our study deserve mention. We studied a large population of multiethnic women. Previous SWAN analyses suggested that the rate of bone loss across menopause was somewhat greater in Japanese women.21 We adjusted for race/ethnicity but had no power to examine whether NTX predicts fractures differently across race/ethnicity. NTX levels are sensitive to the time of collection. We attempted to collect urine samples before 10:00 AM on days 2 to 5 of the menstrual cycle. However, we collected and stored specimens even if they were collected outside this window. Over time in SWAN, menstrual cycles became increasingly variable, and standardizing the day of collection became more difficult. Therefore, we adjusted for the time of collection in our analysis, and it had no effect on our results. We relied on self-reported fracture, but a later validity study suggested the confirmation of about 88% of all fractures. In addition, we found the expected relationship between age, race, BMD, and fracture history. Previous analyses of selfreports of fracture have also shown a greater than 88% confirmation.44,45 We measured NTX in urine. Urinary measures of NTX are more variable than serum measures of NTX because they must be corrected for creatinine level. However, this variability would bias our findings toward null. On the other hand, women may prefer urine samples to blood draws, generating greater clinical acceptance. Finally, we measured osteocalcin as a marker of bone formation. Newer, more sensitive markers of bone formation (eg, bone-specific alkaline phosphatase) may have been more strongly associated with fracture.
CONCLUSIONS
Baseline measurements of NTX and lumbar spine BMD during premenopause and early perimenopause and changes in NTX across menopause are associated with subsequent fracture risk. The combination of both low spine BMD and high NTX is associated with almost a threefold increased risk of fracture.
Acknowledgments
University of Michigan, Ann Arbor, MI: MaryFran Sowers, principal investigator (PI); Massachusetts General Hospital, Boston, MA: Joel Finkelstein, PI, 1999-present; Robert Neer, PI, 1994–1999; Rush University, Rush University Medical Center, Chicago, IL: Howard Kravitz, PI, 2009-present; Lynda Powell, PI, 1994–2009; University of California, Davis/Kaiser: Ellen Gold, PI; University of California, Los Angeles: Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY: Rachel Wildman, PI, 2010-present; Nanette Santoro, PI, 2004–2010; University of Medicine and Dentistry-New Jersey Medical School, Newark, NJ: Gerson Weiss, PI, 1994–2004; and the University of Pittsburgh, Pittsburgh, PA: Karen Matthews, PI.
National Institutes of Health Program Office: National Institute on Aging, Bethesda, MD: Sherry Sherman, PI, 1994-present; Marcia Ory, PI, 1994–2001; National Institute of Nursing Research, Bethesda, MD: program officers.
Central Laboratory: University of Michigan: Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh: Kim Sutton-Tyrrell, PI, 2001-present; New England Research Institutes, Watertown, MA: Sonja McKinlay, PI, 1995–2001.
Steering Committee: Susan Johnson, current chair; Chris Gallagher, former chair.
We thank the study staff at each site and all the women who participated in SWAN.
Funding/support: The Study of Women’s Health Across the Nation received grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health (grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495). Measures of cross-linked N-telopeptide of type I collagen were funded by the Swiss Precision Diagnostics Development Company Limited Priory (Business Park Bedford, UK) under the direction of Dr. Sarah Johnson. This work was also supported by Department of Defense grant DAMD17-96-6118; NIH grant K24-DK02759 (to J.S.F.); the Iris Cantor-University of California, Los Angeles Women’s Health Center; University of California, Los Angeles Center of Excellence in Women’s Health grant RFP 282-97-0025 (to G.A.G.); and NIH grant RR-1066 (to Massachusetts General Hospital, Boston, MA).
Footnotes
Financial disclosure/conflicts of interest: Dr. Cauley has received research support and consulting fees from Novartis Pharmaceuticals and Merck. Drs. Chang, Crandall, Danielson, Lo, Finkelstein, Greendale, Neer, Prairie, and Ruppert, as well as Ms. Meyn, have no conflicts of interest to report. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Nursing Research, the Office of Research on Women’s Health, or the NIH.
References
- 1.Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157:1023–1031. doi: 10.1093/aje/kwg081. [DOI] [PubMed] [Google Scholar]
- 2.Edwards B, Song J, Dunlop P, Fink H, Cauley JA. Functional decline after incident wrist fractures—Study of Osteoporotic Fractures: prospective cohort study. BMJ. 2010;341:c3324. doi: 10.1136/bmj.c3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nevitt MC, Ross PD, Palermo L, et al. Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. The Fracture Intervention Trial Research Group. Bone. 1999;25:613–619. doi: 10.1016/s8756-3282(99)00202-1. [DOI] [PubMed] [Google Scholar]
- 4.Magaziner J, Lydick E, Hawkes W, et al. Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health. 1997;87:1630–1636. doi: 10.2105/ajph.87.10.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Danielson M, Boudreau R, et al. Serum 25 hydroxyvitamin (OH)D and fracture risk in multi-ethnic women: the Women’s Health Initiative (WHI) J Bone Miner Res. 2009;24(suppl 1):S451. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby AJ. Bone formation and resorption in postmenopausal osteoporosis. Lancet. 1981;2:536. doi: 10.1016/s0140-6736(81)90931-4. [DOI] [PubMed] [Google Scholar]
- 8.Jilka RL. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med Pediatr Oncol. 2003;41:182–185. doi: 10.1002/mpo.10334. [DOI] [PubMed] [Google Scholar]
- 9.Reeve J, Pearson J, Mitchell A, et al. Evolution of spinal bone loss and biochemical markers of bone remodeling after menopause in normal women. Calcif Tissue Int. 1995;57:105–110. doi: 10.1007/BF00298429. [DOI] [PubMed] [Google Scholar]
- 10.Cosman F, Nieves J, Wilkinson C, et al. Bone density change and biochemical indices of skeletal turnover. Calcif Tissue Int. 1996;58:236–243. doi: 10.1007/BF02508642. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 13.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 14.Tromp AM, Ooms ME, Popp-Snijders C, Roos JC, Lips P. Predictors of fractures in elderly women. Osteoporos Int. 2000;11:134–140. doi: 10.1007/PL00004174. [DOI] [PubMed] [Google Scholar]
- 15.Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386–393. doi: 10.1359/JBMR.0301244. [DOI] [PubMed] [Google Scholar]
- 16.Looker AC, Bauer DC, Chesnut CH, III, et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int. 2000;11:467–480. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 17.Ross PD, Kress BC, Parson RE, et al. Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int. 2000;11:76–82. doi: 10.1007/s001980050009. [DOI] [PubMed] [Google Scholar]
- 18.Bauer DC, Garnero P, Harrison SL, et al. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res. 2009;24:2032–2038. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:99–111. doi: 10.7326/0003-4819-153-2-201007200-00262. [DOI] [PubMed] [Google Scholar]
- 20.Sowers MF, Crawford S, Sternfeld B. Design, survey sampling and recruitment methods of SWAN: a multicenter, multi-ethnic, community-based cohort study of women at the menopausal transition. In: Wren JLR, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. New York, NY: Academic Press; 2000. [Google Scholar]
- 21.Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 22.Seibel MJ. Biochemical markers of bone turnover, 1: biochemistry and variability. Clin Biochem Rev. 2005;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- 23.Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int. 2000;11(suppl 6):S30–S44. doi: 10.1007/s001980070004. [DOI] [PubMed] [Google Scholar]
- 24.Sowers MR, Greendale GA, Bondarenko I, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14:191–197. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 25.Sowers MR, Randolph JF, Zheng H, et al. Genetic polymorphisms and obesity influence estradiol decline during the menopause. Clin Endocrinol (Oxf) 2011;74:618–623. doi: 10.1111/j.1365-2265.2010.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hans D, Duboeuf F, Schott AM, et al. Effects of a new positioner on the precision of hip bone mineral density measurements. J Bone Miner Res. 1997;12:1289–1294. doi: 10.1359/jbmr.1997.12.8.1289. [DOI] [PubMed] [Google Scholar]
- 27.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 28.Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporos Int. 2000;11(suppl 6):S55–S65. doi: 10.1007/s001980070006. [DOI] [PubMed] [Google Scholar]
- 29.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 30.Johnell O, Oden A, De Laet C, et al. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002;13:523–526. doi: 10.1007/s001980200068. [DOI] [PubMed] [Google Scholar]
- 31.Meier C, Liu PY, Handelsman DJ, Seibel MJ. Endocrine regulation of bone turnover in men. Clin Endocrinol (Oxf) 2005;63:603–616. doi: 10.1111/j.1365-2265.2005.02333.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishii S, Cauley JA, Greendale GA, et al. Ethnic differences in composite indices of femoral neck strength. Osteoporos Int. 2012;23:1381–1390. doi: 10.1007/s00198-011-1723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer DC, Garnero P, Hochberg MC, et al. Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res. 2006;21:292–299. doi: 10.1359/JBMR.051018. [DOI] [PubMed] [Google Scholar]
- 34.Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 35.Chapurlat RD, Bauer DC, Cummings SR. Association between endogenous hormones and sex hormone-binding globulin and bone turnover in older women: Study of Osteoporotic Fractures. Bone. 2001;29:381–387. doi: 10.1016/s8756-3282(01)00584-1. [DOI] [PubMed] [Google Scholar]
- 36.Dempster DW. The contribution of trabecular architecture to cancellous bone quality. J Bone Miner Res. 2000;15:20–23. doi: 10.1359/jbmr.2000.15.1.20. [DOI] [PubMed] [Google Scholar]
- 37.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34:783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Bromberger JT, Matthews KA, Kuller LH, et al. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 39.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 40.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 41.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22:211–219. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 42.Baim S, Wilson CR, Lewiecki EM, et al. Precision assessment and radiation safety for dual-energy x-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom. 2005;8:371–378. doi: 10.1385/jcd:8:4:371. [DOI] [PubMed] [Google Scholar]
- 43.Funck-Brentano T, Biver E, Chopin F, et al. Clinical utility of serum bone turnover markers in postmenopausal osteoporosis therapy monitoring: a systematic review. Semin Arthritis Rheum. 2011;41:157–169. doi: 10.1016/j.semarthrit.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Nevitt MC, Cummings SR, Stone KL, et al. Risk factors for a first-incident radiographic vertebral fracture in women > or = 65 years of age: the Study of Osteoporotic Fractures. J Bone Miner Res. 2005;20:131–140. doi: 10.1359/JBMR.041003. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11:264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]