Abstract
Objective
To compare the effects of combinatorial therapy with low-dose arginine and a nitrogen scavenging agent (sodium phenylbutyrate) vs. monotherapy with high-dose arginine on liver function tests in patients with argininosuccinic aciduria (ASA).
Study design
Twelve patients with ASA were enrolled in a double-blind, placebo-controlled, cross-over study design. Subjects were randomized to receive either a low-dose of arginine therapy (100 mg•kg−1•d−1) combined with sodium phenylbutyrate (500 mg•kg−1•d−1) (LDA arm) or a high-dose of arginine alone (500 mg•kg−1•d−1) (HDA arm) for one week. At the end of one week of therapy, liver function tests were assessed and metabolite fluxes were measured using a multi-tracer stable isotope protocol.
Results
Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), and measures of synthetic functions of the liver were the primary outcomes. Subjects had significantly increased levels of argininosuccinate (P <0.03) and AST levels (P<0.01) after treatment with high-dose arginine. In the subset of subjects with elevated AST or ALT, treatment with high-dose of arginine was associated with further increases in plasma levels of both aminotransferases. Whereas subjects had increased arginine and citrulline flux with high-dose arginine therapy, the glutamine flux was not different between the two treatment arms. The synthetic liver functions as assessed by prothrombin time, INR, and coagulation factor levels were not different between the HDA and LDA arms.
Conclusions
Administering higher doses of arginine in subjects with ASA results in increases in AST and ALT levels, especially in the subset of patients with elevated baseline aminotransferases. Hence, low-dose arginine sufficient to normalize arginine levels in serum combined with nitrogen scavenging therapy should be considered as a therapeutic option for treatment of ASA in patients with elevations of hepatic aminotransferases.
Keywords: Rare disease clinical research, argininosuccinate lyase, argininosuccinic aciduria, arginine therapy, hepatic disease
1. Introduction
The urea cycle is the principal mechanism for disposal of waste-nitrogen in mammals. Primary and secondary defects of urea cycle enzymes or transporters result in urea cycle disorders (UCDs), a group of inborn errors of hepatic metabolism that often result in life-threatening hyperammonemia [1]. Argininosuccinic aciduria (ASA), caused by deficiency of the enzyme argininosuccinate lyase (ASL), is the second most common UCD [1, 2]. In addition to hyperammonemia, the classic feature observed in all UCDs, subjects with ASA can develop chronic complications that include hepatic disease, neurocognitive deficiencies, and hypertension [2–6]. Hepatic involvement ranging from asymptomatic hepatomegaly and elevation of aspartate and alanine aminotransferases to liver fibrosis can occur even in subjects without significant hyperammonemic episodes [2, 3, 7]. The pathogenesis of liver involvement in ASA is presently unknown. However, the accumulation of argininosuccinate upstream of the metabolic block, the deficiency of arginine and its metabolites downstream of the block, or nitric oxide deficiency may have a role in causation of hepatic complications.
Traditionally, subjects with ASA have been supplemented with high doses of arginine (400–700 mg•kg−1•d−1) to replenish the arginine pool and to facilitate nitrogen excretion via conversion to argininosuccinate [1]. While arginine supplementation prevents metabolic decompensations, its effects on the chronic complications including hepatic disease is unknown [8]. We hypothesized that if argininosuccinate is hepatotoxic, administering higher doses of arginine for purposes of nitrogen excretion would lead to increased production of argininosuccinate, thus resulting in increased hepatic injury. If this were to be true, diverting nitrogen flux away from the urea cycle by the use of a nitrogen scavenging agent in combination with a low-dose of arginine would lead to decreased generation of argininosuccinate and reduced hepatic injury.
Here, we report a randomized, double-blind, placebo-controlled, cross-over trial evaluating the effect of two treatment modalities i.e., a low-dose of arginine combined with sodium phenylbutyrate (low-dose arginine or LDA arm) vs. a high-dose of arginine alone (high-dose arginine, HDA arm) on liver function tests, nitrogen excretion and urea flux in subjects with ASA.
2. Methods
This was a single center study conducted at Texas Children’s Hospital and Baylor College of Medicine (BCM), Houston, TX, USA. The protocol was approved by the Institutional Review Boards of BCM and the Urea Cycle Disorders Consortium. An independent data monitoring and safety committee managed by the NIH Rare Disease Clinical Research Network oversaw the conduct of the trial. Subjects with a confirmed diagnosis of ASA, weight > 10 kg, and serum creatinine less than 1.5 mg/dL were included in the study.
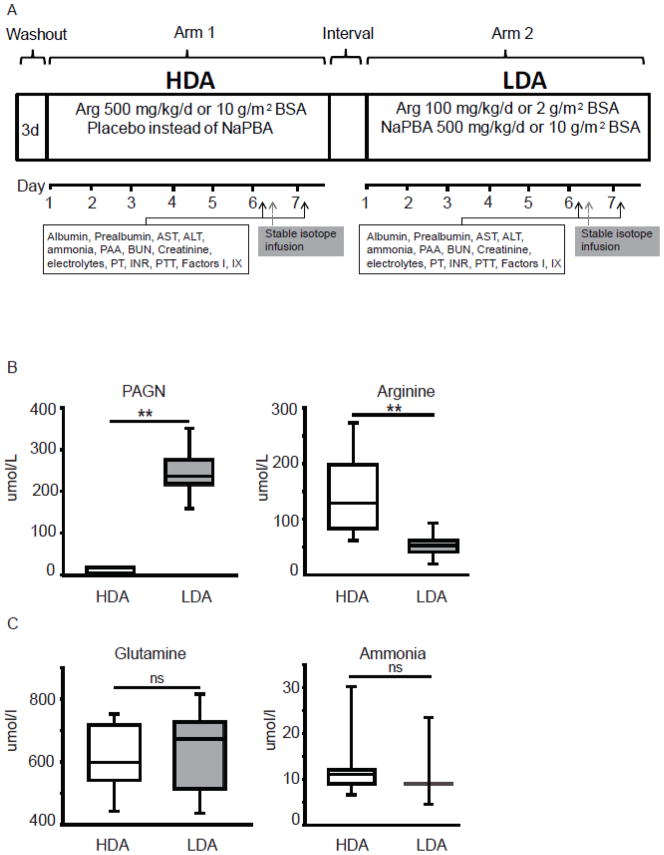
Participants were studied as inpatients where they were randomized to either the LDA or HDA arms. In the LDA arm, subjects received a low-dose of arginine (100 mg•kg−1•d−1 if weight < 20 kg, or 2 g/m2 of body surface area (BSA)/day if weight > 20 kg) combined with sodium phenylbutyrate (500 mg•kg−1•d−1 if weight < 20 kg, or 10 g/m2 of BSA/day if weight > 20 kg). In the HDA arm, they were administered a high-dose of arginine (500 mg•kg−1•d−1 if weight < 20 kg or 10 g/m2 of BSA/day if weight > 20 kg) with placebo instead of sodium phenylbutyrate. The duration of each arm was seven days. On days six and seven, study procedures were performed and subjects were discharged home. After a median period of 54 (range 4–77) days, subjects were re-admitted and crossed over to the alternative arm of the study (Figure 1A).
Figure 1. Study design.
A. Illustrative example of the depicting the study design and study procedures. In this depiction, the subject was randomized to high-dose arginine as the first treatment arm. (PAA – plasma amino acids, BUN – blood urea nitrogen, PT-prothrombin time, PTT- partial thromboplastin time, INR-International normalized ratio) B. Plasma PAGN levels are elevated in patients while on the LDA arm due to conjugation of phenylbutyrate with glutamine to form PAGN. Treatment with high-dose of arginine leads to increase in plasma arginine levels (** P<0.001). C. Short-term nitrogen balance was comparable in both treatment arms as evidenced by normal plasma ammonia and glutamine levels (ns - not significant). The box plots depict the 25th and 75th centile along with the median whereas the error bars depict the 5th and 95th centile.
2.1 Study procedures
Subjects were maintained on a protein-restricted diet (0.6 g•kg−1•d−1) or their currently recommended metabolic diet (Table 1) and were randomized to either arm of the study according to standard procedures. On days six and seven, liver function tests, plasma amino acids and serum chemistries were assayed using standard techniques in the CLIA certified laboratory at TCH (Figure 1A). On day six, the subjects consumed two-thirds of their total daily intake of protein as meals fed every two hours at 0, 2, 4, 6, and 8 hours. The remaining one-third of the day’s protein intake was provided during dinner. At time 0, a baseline blood sample was collected which was followed by primed-constant infusions of [5-15N] glutamine (2 mg•kg−1;2 mg•kg−1•hr−1), [18O][13C]urea (1 mg•kg−1; 0.1 mg•kg−1•hr−1), [1,2-13C2]arginine (0.69 mg•kg−1; 0.69 mg•kg−1 hr−1), and [5-13 C,4,4,5,5-D4]citrulline (0.18 mg•kg−1; 0.18 mg•kg−1•hr−1). The priming dose was administered in 10 minutes whereas the constant infusion continued for 8 hours. Blood samples for isotopic enrichment were drawn at 0, 6, 7 and 7.5 hours of infusion. Primed-constant infusion of a metabolite tracer (i.e. stable isotopic form of a metabolite) in the above-mentioned doses allows for successful detection of isotopic enrichment and calculation of the flux of the respective metabolite. These stable isotopes were chosen to measure the flux of arginine, citrulline, glutamine, and urea. The isotope infusion protocol has been previously validated for sensitivity in six control and three ASA subjects [9]. The entry rate of urea, arginine, citrulline and glutamine were calculated from the isotopic dilution of the infused tracer at plateau enrichment. The plasma phenylacetyl-glutamine (PAGN) was measured by high-performance liquid chromatography at the diagnostic laboratories of Vanderbilt Medical Center, Nashville, TN, USA.
Table 1.
Subject characteristics prior to the enrollment and during each arm of the study.
| Prior to enrollment | HDA arm | LDA arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Arginine dose (mg/kg/day) | Phenylbutyrate dose (mg/kg/d) | Age (yr) | Ht (cm) | Wt (kg) | BMI | Average protein intake # | Age (yr) | Ht (cm) | Wt (kg) | BMI | Average protein intake # |
| 100006 | 150 | None | 13.76 | 169.3 | 63 | 21.98 | 0.67 | 13.73 | 169.3 | 63 | 21.98 | 0.70 |
| 100551 | 180 | 400 | 4.53 | 98 | 18.1 | 18.85 | 0.79 | 4.36 | 98 | 17.4 | 18.12 | 0.79 |
| 101037 | 400 | None | 22.33 | 164.7 | 61.7 | 22.75 | 0.73 | 22.50 | 165 | 60 | 22.04 | 0.69 |
| 101880 | 450 | None | 10.12 | 130 | 28.8 | 17.04 | 0.83 | 10.00 | 130 | 28 | 16.57 | 0.88 |
| 102107 | 125 | None | 20.29 | 182 | 70 | 21.13 | 0.63 | 20.26 | 183 | 71 | 21.20 | 0.65 |
| 102108 | 150 | None | 23.33 | 158 | 61 | 24.44 | 0.60 | 23.29 | 157 | 60 | 24.34 | 0.70 |
| 102635 | 350 | None | 9.06 | 131 | 26.9 | 15.68 | 0.92 | 9.10 | 131 | 26.9 | 15.68 | 0.91 |
| 102747 | 200 | None | 11.15 | 127 | 23.3 | 14.45 | 0.83 | 11.34 | 127 | 23.5 | 14.57 | 0.89 |
| 104591 | 50 | None | 13.44 | 155 | 38.1 | 15.86 | 0.87 | 13.63 | 156 | 41.7 | 17.14 | 0.75 |
| 105458 | 60 | None | 28.61 | 173 | 89 | 29.74 | 0.59 | 28.72 | 173 | 93 | 31.07 | 0.65 |
| 106088 | 135 | None | 13.83 | 160 | 51 | 19.92 | 0.78 | 13.93 | 160 | 51 | 19.92 | 0.83 |
average intake during the 7 days inpatient stay.
2.2 Outcomes
The primary outcome measures were plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), and synthetic function of the liver as assessed by prothrombin time (PT), international normalized ratio (INR), partial thromboplastin time (PTT), and plasma levels of coagulation factors I and IX. The exploratory endpoints were the plasma levels of argininosuccinate, arginine and citrulline as well as the fluxes of arginine, citrulline and urea.
2.3 Statistical Analysis
For calculation of sample size, we used the means and standard deviations (SD) for PT, PTT, the primary outcome measures as follows, mean (SD): 15.7 (0.62) for PT and 32.8 (3.02) for PTT. Assuming r=0.5 between two measurements from the same subject, 12 subjects would provide a power of 0.8 to detect a 10% reduction in primary endpoints at an alpha value of 0.05. The interval period between the two admissions did not allow for any carry-over effect between the two treatment arms. However, we performed two-way repeated measures ANOVA to test for 1) the effect of order of treatment, 2) the treatment, and 3) the interaction between the two factors. The analysis did not show any significant effect of order of treatment on the primary endpoints. When the data were normally distributed, a paired t-test was used for comparison while Wilcoxon Signed Rank Test was used to compare non-normative data between the two arms. Regression analysis was performed using generalized estimating equations (GEE) in order to account for repeated measures on subjects. Partial η2 (analogous to r2) is reported as the proportion of variation accounted for by the independent variable after accounting for subject. A Spearman rank correlation was performed for calculation the correlation co-efficient.
3. Results
Twelve subjects (five males, seven females) were enrolled in the trial. Six subjects were randomized to the LDA arm and six to the HDA arm as the initial treatment arm. One subject was excluded from the analysis as he developed mild hyperammonemia on both arms of the trial and could not complete the study procedures. The median age of the subjects was 13.7 years (range 4.3–23.25). Only one subject had neonatal-onset disease. The subject characteristics are summarized in Table 1.
The plasma PAGN levels were elevated in subjects on the LDA arm, whereas the plasma arginine was significantly higher on the HDA arm. This is consistent with the physiological effects of the treatments in the respective arms (Figure 1B). The plasma glutamine and ammonia levels were normal and comparable in both arms implying that neither intervention adversely affected the short-term nitrogen balance in steady-state and stable subjects (Figure 1C). The plasma levels of albumin, and prealbumin were normal and comparable in both arms of the study (data not shown), suggesting that neither intervention adversely affected nutritional status in the short-term.
3.1 Effects on metabolite flux and concentration
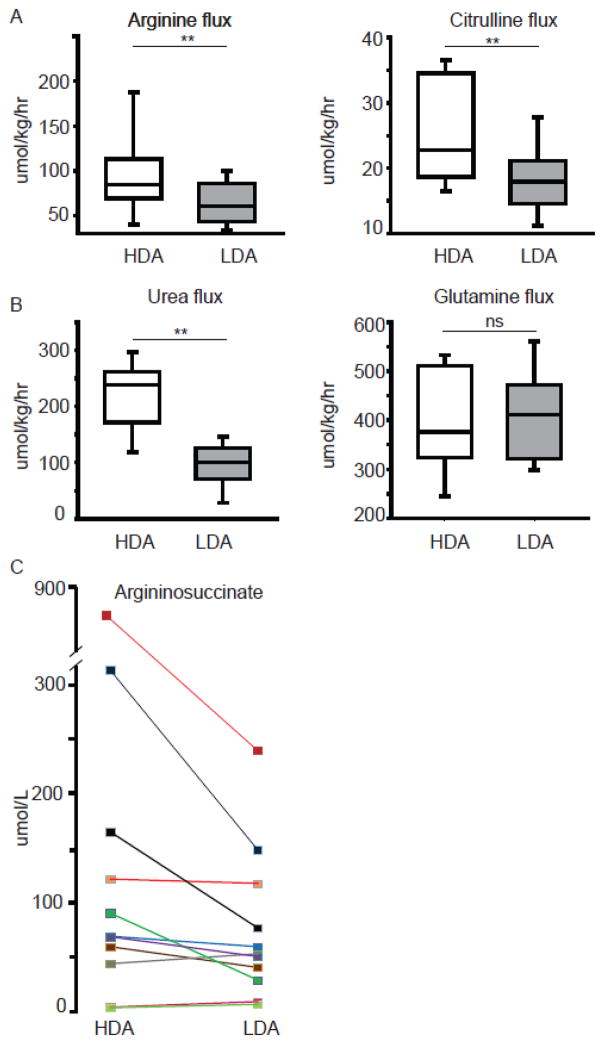
To understand the effects of the two treatments on the dynamic fluxes of metabolites, we used primed-constant infusions of multiple stable isotope tracers. As expected, treatment with the high-dose of arginine was associated with significantly increased fluxes of arginine and citrulline (Figure 2A). The high-dose of arginine increased the urea flux, but the glutamine flux, was similar in both arms, supporting that neither intervention adversely affected nitrogen balance (Figure 2B). Treatment with the high-dose of arginine resulted in higher plasma argininosuccinate levels (P=0.024; Figure 2C).
Figure 2. Effect of the two treatment arms on metabolite fluxes and argininosuccinate levels.
The arginine, citrulline (A) and the urea (B, left panel) fluxes are increased with the high-dose arginine treatment while glutamine flux (B, right panel) is comparable in both arms (**P<0.001, ns - not significant). C. Treatment with the high-dose of arginine results in increased plasma argininosuccinate (P<0.05). Each data-point on depicts the mean of two values measured on days 6 and 7.
3.2 Effects on aspartate and alanine aminotransferases and hepatic synthetic function
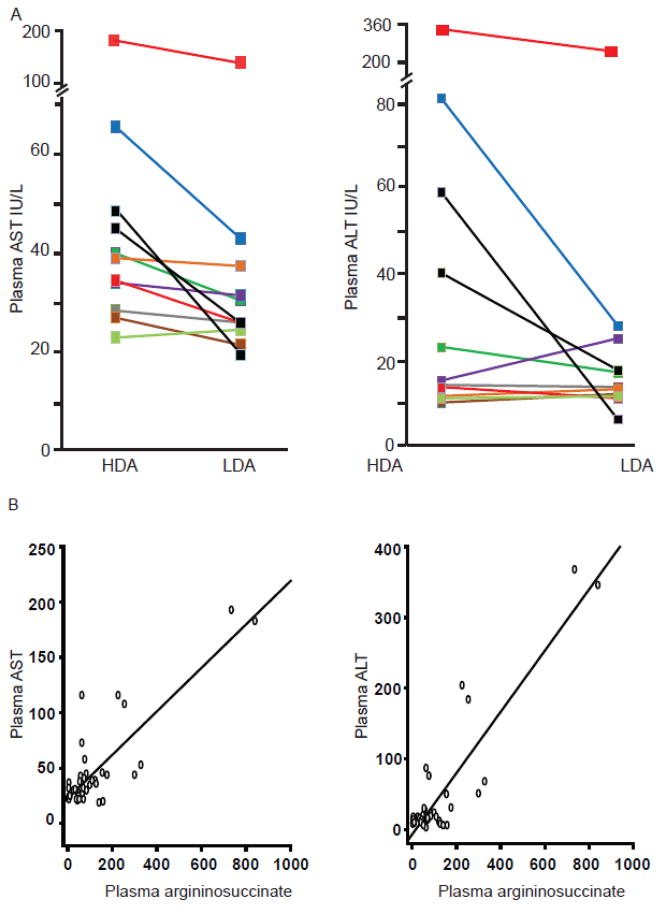
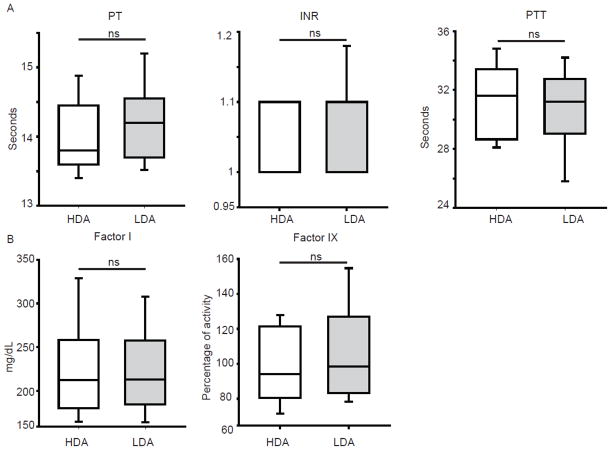
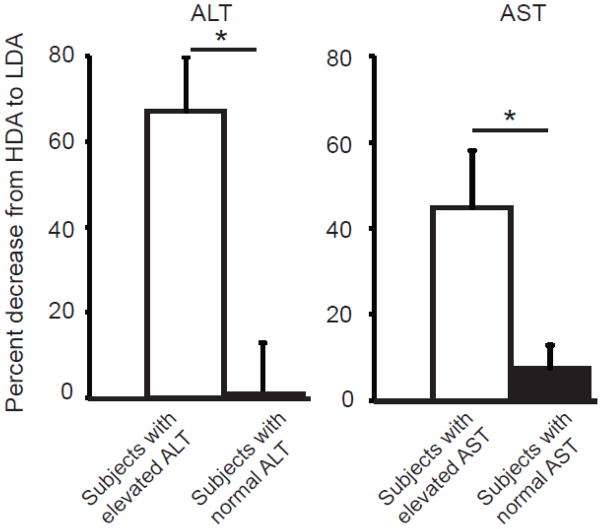
The plasma AST levels were significantly higher (P=0.002) when subjects were treated with the high-dose of arginine (Figure 3A, Table 2). A similar trend was observed in the plasma ALT levels but this did not reach statistical significance (Figure 3A, Table 2). To assess the correlation between the plasma levels of aminotransferases and argininosuccinate, we conducted linear regression analysis. The analysis showed significant correlation (P<0.001) between plasma argininosuccinate and aminotransferase levels (Figure 3B); however, this result must be viewed with caution, as a single subject (100551) had a large influence. The hepatic synthetic functions as assessed by PT, INR, PTT, factor I, and factor IX levels were normal and similar in both treatment arms (Figure 4). In an effort to address a clinically relevant question regarding the effects of high-dose arginine therapy in subjects with pre-existing hepatic disease, we performed a stratified analysis of subjects with elevations of alanine and aspartate aminotransferase levels. In the subset of patients with abnormal aminotransferases on at least one arm of the study, treatment with combinatorial therapy with low-dose of arginine and sodium phenylbutyrate resulted in statistically significant decreases in both AST and ALT as compared to high-dose arginine (Figure 5)
Figure 3. Effect of the two treatment arms on aspartate and alanine aminotransferases.
A. Treatment with the high-dose of arginine leads to increase in plasma AST and ALT. The increased AST levels on HDA arm were statistically significant (P=0.002). Each data-point on the graph depicts the mean of two values measured on days 6 and 7. B. Linear regression using GEE and Spearman rank correlation found a significant relationship between plasma argininosuccinate and AST (partial η2 = 0.635, P<0.001; r2=0.568) and ALT levels (partial η2 = 0.794, P<0.001; r2=0.474).
Table 2.
Mean AST and ALT levels on days 6 and 7 along in individual subjects along with age and sex appropriate normal values.
| Subject | Sex | Mean ALT on HDA arm | Mean ALT LDA arm | Normal range | Mean AST on HDA arm | Mean AST on LDA arm | Normal range |
|---|---|---|---|---|---|---|---|
| 100006 | M | 81.5 | 28 | 10–55 | 65.5 | 43 | 15–40 |
| 100551 | M | 357 | 194 | 10–25 | 188 | 112 | 15–50 |
| 101037 | F | 14 | 13.5 | 9–52 | 28.5 | 26 | 14–36 |
| 101880 | F | 23 | 17 | 10–30 | 40 | 30.5 | 10–40 |
| 102107 | M | 15 | 25 | 21–72 | 34 | 31.5 | 17–59 |
| 102108 | F | 10 | 12 | 9–52 | 27 | 21.5 | 14–36 |
| 102635 | F | 11.5 | 13 | 10–35 | 39 | 37.5 | 15–40 |
| 102747 | F | 13.5 | 11 | 10–30 | 34.5 | 26 | 10–40 |
| 104591 | F | 11 | 11.5 | 10–30 | 23 | 24.5 | 10–30 |
| 105458 | M | 40.5 | 17.5 | 21–72 | 45 | 26 | 17–59 |
| 106088 | F | 59.5 | 6 | 10–30 | 48.5 | 19.5 | 10–30 |
Figure 4. Effect of two treatment arms on hepatic synthetic functions.
A. Coagulation parameters evaluating the extrinsic and the intrinsic pathway show no differences between the two arms. B. Plasma levels of coagulation factors are similar with both treatments.
Figure 5. Stratified analysis of aminotransferase levels in subjects with elevations of AST and ALT.
The subset of patients with elevation of aminotransferases on at least one arm of the study had significant reduction of ALT (*P<0.05) and AST (*P<0.05) with low-dose of arginine as compared to those who had normal aminotransferase levels on both arms of the study.
4. Discussion
Many subjects with ASA receive high doses of arginine supplementation (400–700 mg/kg/day) as this therapy had been considered safe and efficacious [10]. However, recent observations have shown that chronic complications such as hepatic dysfunction, cognitive impairment, and hypertension can occur in spite of arginine therapy and the absence of hyperammonemia [3, 4, 6, 11]. Long-term studies in ASA subjects identified through newborn screening programs have not detected an improved outcome in subjects supplemented with arginine as compared to those who were not [4, 11]. Recent evidence shows that arginine supplementation in ASA does not correct the deficiency of one of its downstream metabolites, nitric oxide (NO) [9]. It is presently unclear whether arginine supplementation restores the other downstream metabolites of arginine. In addition, higher doses of arginine would lead to increased generation of ASA which has been suggested to be hepatotoxic. This raises an important question as to whether high-dose arginine therapy should be used as the sole treatment for nitrogen excretion when there is the theoretical risk of increased generation of argininosuccinate.
In this trial, we show that subjects with ASA had higher AST and ALT levels when treated with a high-dose of arginine. In particular, subjects with elevations of AST and ALT had significant increase in aminotransferases when treated with high-dose of arginine. While serum aminotransferase levels are a sensitive indicator of hepatic cell injury, their elevations can be found in conditions not involving the liver such as disorders of skeletal muscle [12–14]. Our data cannot conclusively prove that the liver was the source for elevated levels of elevated ALT and AST. However, none of the subjects had any clinical evidence suggestive of skeletal muscle disease and hence it is not unreasonable to ascribe liver as the source of the aminotransferases. The increase in the plasma aminotransferases correlated with increases in argininosuccinate levels suggesting that argininosuccinate may have a role in hepatic injury. Whereas the correlation data are interesting, they do not imply causality. Establishing causality of argininosuccinate in pathogenesis of hepatic disease in ASA would need detailed studies involving animal models. Hypomorphic models of ASL and argininosuccinate synthase 1 (ASS1) deficiencies have been developed with the former having significant hepatic dysfunction and the latter with no reported hepatic involvement [9, 15]. Hence, a double knockout model with loss of both ASL and ASS1 may help dissect the role of argininosuccinate in hepatic disease.
Our study did not show any discernible differences in the hepatic synthetic function between the two treatment arms. We used coagulation factor levels and coagulation parameters as measures of hepatic synthetic function since the treatment duration was short. While coagulation parameters are sensitive indicators of decreased hepatic function, they are only altered in the presence of significant hepatic injury. The lack of a sensitive marker for assessment of hepatic synthetic reserve in milder forms of hepatocellular injury limits our ability to understand whether the increase in serum aminotransferases translates into decreased hepatic reserve. We have recently shown that ASL is required for NO production at the level of individual tissues as well as the whole organism [9, 16]. Because the NO deficiency in microvasculature may contribute to liver fibrosis, long-term hepatic injury may be further modified by NO status.
Our study also showed that in the short-term, both treatment modalities were efficacious in maintaining the nitrogen balance. The increase in urea flux in the HDA arm was largely due to efficient conversion of arginine to urea. However, it is possible that the diversion of nitrogen away from the urea cycle in the LDA arm by sodium phenylbutyrate may have also contributed to the difference in the urea flux between the two treatment modalities.
The duration of our study was for one week and the median plasma arginine level of the subjects was in the normal ranges even with the dose of arginine being 100 mg•kg−1•d−1. However, the dose of arginine required for maintaining the plasma arginine levels in the normal ranges with chronic therapy is likely to differ in individual patients. The primary goal of arginine therapy in ASA subjects is to prevent hyperammonemia and the minimal dose needed to accomplish this while maintaining normal plasma levels of arginine should be used. However, if a high-dose of arginine is being used for the sole purpose of nitrogen excretion, especially in subjects with hepatic disease, lowering the dose of arginine and addition of a nitrogen scavenger should be considered.
5. Conclusion
In summary, the pathogenesis of hepatic disease in ASA is likely multi-factorial, with elevations of argininosuccinate being one important factor. Our results suggest that administering high-dose of arginine in ASA subjects can result in abnormalities in the liver function tests. Hence in subjects with preexisting hepatic disease, low-dose arginine sufficient to normalize arginine levels in serum, combined with nitrogen scavenging therapy should be considered as an alternative therapeutic option for chronic treatment. However, in the context of acute hyperammonemia, intravenous infusion of high-dose arginine should still be used because of its efficacy in clearing excess nitrogen in the form of ASA.
Highlights.
We describe the first investigator initiated randomized double-blind study in argininosuccinic aciduria.
The effects of combinatorial therapy with a low-dose of arginine and sodium phenylbutyrate vs. a high-dose of arginine alone on hepatic function tests in argininosuccinic aciduria were compared.
We show that treatment with high-dose of arginine leads to increase in plasma argininosuccinate and hepatic aminotransferases.
A lower dose of arginine that is sufficient to normalize plasma arginine levels, combined with nitrogen scavenging therapy should be considered as an alternative therapeutic option for chronic treatment, especially in patients with hepatic disease.
Acknowledgments
We thank the subject families and the referring physicians for their kind participation. We are grateful A. Tran, J. Stuff, and the nursing staff of the General Clinical Research Center at Texas Children’s Hospital. Ucyclyd Pharma provided sodium phenylbutyrate for the study. This work was made possible by the Urea Cycle Disorders Consortium (UCDC). This consortium is a part of the NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by U54HD061221 from the National Institute of Child Health and Human Development (NICHD) and the NIH Office of Rare Diseases Research (ORDR). The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
This work was supported by the NIH (DK54450, RR19453, RR00188, GM90310, to B. Lee, GM07526, DK081735 to A. Erez, RR024173 to J. Marini), Baylor College of Medicine General Clinical Research Center (RR00188), Intellectual and Developmental Disabilities Research Center (HD024064), The Texas Medical Center Digestive Disease Center, and the Urea Cycle Disorders Rare Disease Clinical Research Network, NIH (HD061221 to BL). SCS Nagamani, OA Shchelochkov and A Erez are awardees of the National Urea Cycle Disorders Foundation Research Fellowship. OA Shchelochkov is an awardee of the O’Malley Fellowship of the UCDC RDCRN.
We thank the Members of the UCDC (Mark L. Batshaw, Mendel Tuchman, Marshall L. Summar, Matthias R. Baumgartner, Susan A. Berry, Stephen Cederbaum, George A. Diaz, Renata C. Gallagher, Cary O. Harding, George Hoffmann, Douglas S. Kerr, Uta Lichter-Konecki, Shawn E. McCandless, J. Lawrence Merritt, Andreas Schulze, Margretta R. Seashore, Tamar Stricker, Susan Waisbren, Derek Wong, and Mark Yudkoff) for their valuable assistance.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brusilow HASW. Online Metabolic and Molecular Basis of Inherited Disease. 2009 [Google Scholar]
- 2.Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori T, Nagai K, Mori M, Nagao M, Imamura M, et al. Progressive liver fibrosis in late-onset argininosuccinate lyase deficiency. Pediatr Dev Pathol. 2002;5:597–601. doi: 10.1007/s10024-002-0109-7. [DOI] [PubMed] [Google Scholar]
- 4.Ficicioglu C, Mandell R, Shih VE. Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening. Mol Genet Metab. 2009;98:273–7. doi: 10.1016/j.ymgme.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunetti-Pierri N, Erez A, Shchelochkov O, Craigen W, Lee B. Systemic hypertension in two patients with ASL deficiency: a result of nitric oxide deficiency? Mol Genet Metab. 2009;98:195–7. doi: 10.1016/j.ymgme.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagamani SC, Erez A, Lee B. Argininosuccinate lyase deficiency. Genet Med. 2012 doi: 10.1038/gim.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann A, Bachmann C, Baumgartner R. Severe liver fibrosis in argininosuccinic aciduria. Arch Pathol Lab Med. 1986;110:136–40. [PubMed] [Google Scholar]
- 8.Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr. 2001;138:S46–54. doi: 10.1067/mpd.2001.111836. discussion S54–5. [DOI] [PubMed] [Google Scholar]
- 9.Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 2011;17:1619–26. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widhalm K, Koch S, Scheibenreiter S, Knoll E, Colombo JP, et al. Long-term follow-up of 12 patients with the late-onset variant of argininosuccinic acid lyase deficiency: no impairment of intellectual and psychomotor development during therapy. Pediatrics. 1992;89:1182–4. [PubMed] [Google Scholar]
- 11.Mercimek-Mahmutoglu S, Moeslinger D, Haberle J, Engel K, Herle M, et al. Long-term outcome of patients with argininosuccinate lyase deficiency diagnosed by newborn screening in Austria. Mol Genet Metab. 100:24–8. doi: 10.1016/j.ymgme.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Ellis G, Goldberg DM, Spooner RJ, Ward AM. Serum enzyme tests in diseases of the liver and biliary tree. Am J Clin Pathol. 1978;70:248–58. doi: 10.1093/ajcp/70.2.248. [DOI] [PubMed] [Google Scholar]
- 13.Nathwani RA, Pais S, Reynolds TB, Kaplowitz N. Serum alanine aminotransferase in skeletal muscle diseases. Hepatology. 2005;41:380–2. doi: 10.1002/hep.20548. [DOI] [PubMed] [Google Scholar]
- 14.Wroblewski F. The clinical significance of transaminase activities of serum. Am J Med. 1959;27:911–23. doi: 10.1016/0002-9343(59)90175-5. [DOI] [PubMed] [Google Scholar]
- 15.Perez CJ, Jaubert J, Guenet JL, Barnhart KF, Ross-Inta CM, et al. Two hypomorphic alleles of mouse Ass1 as a new animal model of citrullinemia type I and other hyperammonemic syndromes. Am J Pathol. 2011;177:1958–68. doi: 10.2353/ajpath.2010.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagamani SC, Campeau PM, Shchelochkov OA, Premkumar MH, Guse K, et al. Nitric-oxide supplementation for treatment of long-term complications in argininosuccinic aciduria. Am J Hum Genet. 2012;90:836–46. doi: 10.1016/j.ajhg.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]