Abstract
Longitudinal studies of the association of estimated glomerular filtration rate (eGFR) and albuminuria with coronary artery calcium, a measure of cardiovascular disease (CVD) burden, are few and contradictory. In this study, 421 community-dwelling men and women (mean age 67 years) without known heart disease had eGFR estimated by the Modification of Diet in Renal Disease equation and albuminuria assessed by urine albumin/creatinine ratio (ACR) between 1997–1999. Mean eGFR was 78 mL/min/1.73m2, median ACR was 10 mg/g. Coronary artery calcium (CAC) was measured by electron beam computed tomography between 2000–2001 when median total Agatston CAC score was 77; 4.5 years later 338 participants still without heart disease had a repeat scan (median CAC score 112); 46% of participants showed CAC progression, defined as an increase ≥2.5 mm3 in square-root transformed CAC volume score. Cross-sectional and longitudinal logistic regression analyses showed no separate or joint association between eGFR or ACR with CAC severity or progression. In conclusion, this study does not support the use of eGFR or ACR to identify asymptomatic older adults who should be screened for subclinical CVD with initial or sequential scanning for CAC. In the elderly, kidney function and CAC may not progress together.
Keywords: albuminuria, chronic kidney disease, coronary artery calcium, coronary heart disease, elderly, glomerular filtration rate
Coronary artery calcium (CAC) has been shown to predict coronary heart disease death, non-fatal myocardial infarction, and coronary revascularization in the general population.1 CAC progression predicts cardiovascular disease (CVD) events2 and may be a better predictor of all-cause mortality than a single measure of CAC.3 Evidence is conflicting for an association between estimated glomerular filtration rate (eGFR),4, 5, 6 albuminuria,7, 8 or both,9, 10, 11, 12 with CAC in cohorts of community-dwelling participants; only three prior studies have included CAC progression.6, 8, 12 The present study was designed to evaluate the association of eGFR and urine albumin/creatinine ratio (ACR), separately and combined, with CAC severity and CAC progression over a 4.5-year period in community-dwelling older adults with no history of heart disease.
METHODS
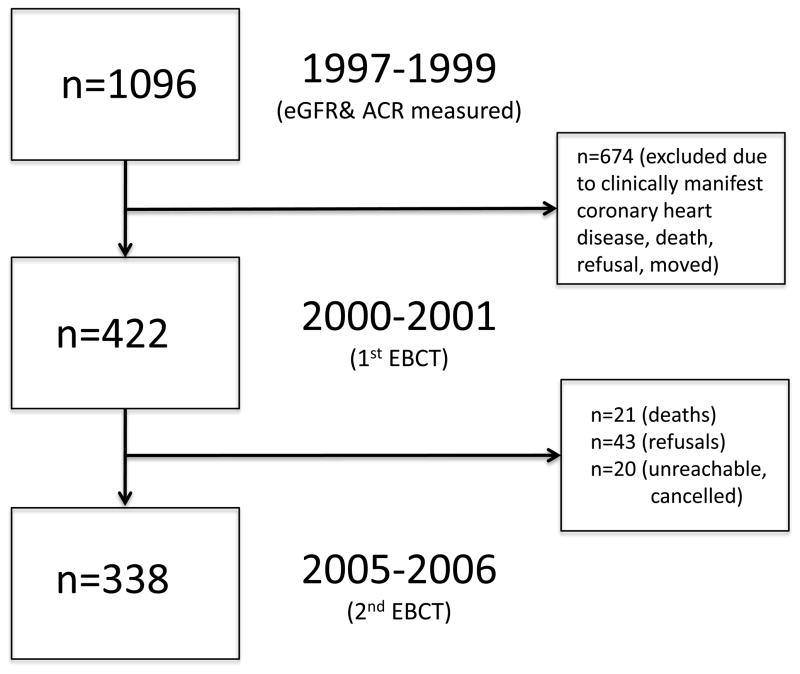
The Rancho Bernardo Study, a cohort of Caucasian, middle class, community-dwelling adults living in southern California, was established in 1972. Between 1997 and 1999, 77% (n=1096) of all surviving community-dwelling participants from the Rancho Bernardo Study attended a research clinic visit where blood and urine were obtained for measurement of CVD risk factors and kidney function. A mean (SD) of 2.3 (0.9) years later, between 2000 and 2001, 422 men and women who were free of clinically manifest coronary heart disease (no physician-diagnosed angina, myocardial infarction, or coronary artery revascularization) had electron beam computed tomography for CAC. 421 of these participants (199 men and 222 women) had blood for assessment of serum creatinine and calculation of eGFR and 362 (172 men and 190 women) had urine for ACR from the 1997–1999 research clinic visit. Between 2005 and 2006, 338 participants still without known heart disease (156 men and 182 women) returned 4.5 (0.5) years later for a repeat CAC scan (Figure 1). Those who did not return for follow-up (n=84) included refusals (n=43), deaths (n=21), and participants who were unreachable or who had cancelled their appointment for unknown reasons (n=20). The study protocol was approved by the Human Research Protection Program at the University of California, San Diego; all participants gave written informed consent.
FIGURE 1.
Study flow diagram
At the 1997–1999 visit, participants completed standardized questionnaires about their medical history, current medications, and dietary supplement use; current medications and prescriptions were brought to the clinic for validation. Cigarette smoking and physical exercise were assessed using standard questionnaires. Height and weight were measured using a calibrated stadiometer and balance-beam scale with participants wearing light clothing and no shoes. Body mass index was calculated as weight (kg)/(height [m])2. Systolic and diastolic blood pressures were measured twice in seated resting subjects using the Hypertension Detection and Follow-up Program protocol.13 Hypertension was defined by patient report of anti-hypertensive medication and/or measured systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
A blood sample was obtained by venipuncture after a requested overnight fast (12–16 hours). Serum was separated and stored at −70° C within 30 minutes of collection. A single, clean-catch, untimed morning urine sample (usually second void) was obtained. Glucose was measured by the glucose oxidase method. Diabetes was defined by patient report of physician diagnosis and/or fasting plasma glucose >126 mg/dL.14 Plasma cholesterol, triglycerides, and high density lipoprotein cholesterol were measured in a Center for Disease Control Certified Lipid Research Clinic laboratory according to the standardized procedures of the Lipid Research Clinics manual15 and low density lipoprotein cholesterol was estimated using the Friedewald equation.16 Serum 25(OH)D (25[OH]D2+25[OH]D3) and 1,25(OH)2D were measured using vitamin D competitive binding protein recognition and chemiluminescence detection. The intra- and inter-assay coefficients of variation for the 25(OH)D assay were 8% and 10%, respectively; the limit of detection was 5 ng/mL. The intra- and inter-assay coefficients of variation for 1,25(OH)2D were 5% to 10% and 10% to 15%, respectively; the limit of detection was 5 pg/mL. The intact parathyroid hormone values were determined in the same laboratory using a chemiluminescence assay kit (Nichols Institute Diagnostics, San Juan Capistrano, California). The intra- and inter-assay coefficients of variation were 6%. Serum creatinine was measured using a variation of the Jaffe enzymatic method on a Hitachi 911 analyzer (Roche Diagnostics Corp., Indianapolis, Indiana), with inter- and intra-assay coefficients of variation of 4%. Urine albumin was measured using the Behring Nephelometer BNA (Dade Behring GmbH, Marburg, Germany) in the National Institutes of Health laboratory (Phoenix, Arizona). The interassay coefficient of variation was 5%. The lower limit of detection of the assay was 6.8 mg/dL; values <6.8 mg/dL were assigned a value of 6.7 mg/dL. Urine creatinine was measured by the kinetic alkaline picrate method using the Ciba-Corning Express (Corning, Medfield, Massachusetts).
CAC was determined in the Lifescore Laboratory of Michael Wright using an Imatron C-150 ultrafast computed tomography scanner (GE Imatron, San Francisco, California), a stationary source detector combination, and a rotating electron beam that produces serial contiguous thin section (100ms) scans. Scans were obtained at end-diastole, usually during a single breath hold. The Agatston method17 was used to quantify the total CAC score, defined as the product of the area of calcium per coronary tomographic segment and a factor rated 1 through 4 depending on the maximal calcium x-ray density in that segment.
eGFR was calculated by the Modification of Diet in Renal Disease equation for Caucasians 18: eGFR (mL/min/1.73m2) = 186 × (serum creatinine [mg/dL])−1.154 × (Age[years])−0.203 × (0.742 if female). ACR was calculated as: ACR (mg/g) = urine albumin (mg/dL)/urine creatinine (g/dL).
CAC scores from the 2000–2001 scan were categorized according to the Rumberger criteria,19 which defines a calcium score of ≤10 as absent/minimal, 11–100 as mild, 101–399 as moderate, and ≥400 as severe. CAC progression was analyzed as a categorical outcome (CAC progression, yes/no) and was defined as a difference ≥2.5 mm3 between the follow-up and the baseline square-root transformed CAC volume score.20 Because interscan variability and error depend on absolute CAC value (CAC variability and score error are larger in patients with higher baseline CAC scores than in those with lower scores), this definition provides an estimate of change that is unbiased with respect to baseline CAC.20
Baseline characteristics at the 1997–1999 clinic visit were compared by CAC category at the 2000–2001 visit, and by CAC progression status at the 2005–2006 visit, using Kruskal-Wallis test for continuous variables and Pearson chi-square test for categorical variables. The association for 1-SD decrease in eGFR or 1-SD increase ACR with baseline CAC category was examined by ordinal logistic regression analysis with lesser severity as the reference. In ordinal logistic regression, the outcome variable is interpreted as a set of cumulative logits based on an increasingly severe CAC score, i.e., the odds of greater severity compared to lesser severity. First, the independent and joint crude associations for eGFR and ACR with CAC category were determined. Next, traditional CVD risk factors (age, sex, body mass index, systolic blood pressure, fasting glucose, high density lipoprotein cholesterol, and triglycerides)21 were entered in blocks, and finally additional CVD and kidney covariates biologically implicated in vascular calcium (smoking, low density lipoprotein cholesterol, 25(OH)D, 1,25(OH)2D, intact parathyroid hormone, lipid-lowering medications, anti-hypertensive medications, calcium and vitamin D supplements)21 were added to a fully adjusted, multivariable model. Secondary analyses included interactions between eGFR and ACR with sex and age to test for effect modification. Analyses were repeated using logistic regression with the categorical outcomes CAC <100 or CAC≥101, and linear regression using continuous CAC as the outcome.
For longitudinal analyses, the independent and joint associations for 1-SD decrease in baseline eGFR or 1-SD increase in ACR with categorical CAC progression were examined in logistic regression models before and after adjustment for the same confounders as above. An additional time interval variable was added to the progression models to account for differences in the time between the baseline and follow-up scans. Analyses were repeated using linear regression with absolute and relative change in CAC volume scores as the outcome, and using logistic regression dividing CAC progressors into initiators (those without CAC at baseline) and progressors (those with CAC at baseline).
The proportional odds assumption was tested using the methods proposed by Brant.22 Model diagnostics failed to indicate violations of the model assumptions. P-values <0.05 were considered statistically significant for all analyses including interaction terms. Statistical analyses were performed with the Statistical Package for the Social Sciences (Base 15.0 for Windows; SPSS Inc, Chicago, Illinois) and STATA 11.1 for Windows (Stata Corp, College Station, Texas).
RESULTS
Baseline characteristics from 1997–1999 for all participants who participated in the 2000–2001 CAC visit are summarized in Table 1. 43 (12%) participants had eGFR<60 mL/min/1.73m2; 31 (9%) had ACR≥30 mg/g and 181 (50%) had ACR≥10 mg/g. 292 (81%) had both eGFR≥60 mL/min/1.73m2 and ACR<30 mg/g, 27 (8%) had both eGFR≥60 mL/min/1.73m2 and ACR≥30 mg/g, 39 (11%) had both eGFR<60 mL/min/1.73m2 and ACR<30 mg/g, and 4 (1%) had both eGFR<60 mL/min/1.73m2 and ACR≥30 mg/g.
Table 1.
Baseline Characteristics in 1997–1999 of Participants of the Rancho Bernardo Study
|
|
||||||
|---|---|---|---|---|---|---|
| Coronary artery calcium score (from 2000–2001) | ||||||
|
| ||||||
| Characteristic | All (n=421) | ≤ 10 n=141(33%) | 11–100 n=89 (21%) | 101–399 n=92 (22%) | ≥ 400 n=99 (24%) | P value for trend |
| Coronary Artery Calcium Scorea | 77 (1.0–357) | 0 (0–1.2) | 43 (22–73) | 185 (141–254) | 815 (523–1389) | n/a |
|
| ||||||
| Age (years) | 67 (7) | 63 (6) | 68 (7) | 69 (7) | 72 (6) | <0.001 |
| Systolic Blood Pressure (mmHg) | 130 (19) | 125 (17) | 130 (18) | 132 (17) | 137 (24) | <0.001 |
| Body Mass Index (kg/m2) | 26 (4) | 26 (4) | 26 (4) | 27 (4) | 27 (4) | 0.13 |
| Fasting Plasma Glucose (mg/dL) | 104 (22) | 98 (10) | 103 (15) | 108 (25) | 109 (33) | <0.001 |
| Low Density Lipoprotein (mg/dL) | 123 (30) | 121 (29) | 123 (29) | 118 (30) | 129 (32) | 0.13 |
| High Density Lipoprotein (mg/dL) | 59 (17) | 64 (17) | 60 (18) | 54 (14) | 55 (15) | <0.001 |
| Triglycerides (mg/dL) | 129 (68) | 128 (70) | 124 (64) | 140 (76) | 126 (62) | 0.26 |
| Serum Calcium (mg/dL) | 9.0 (0.4) | 9.0 (0.3) | 9.1 (0.4) | 9.1 (0.3) | 9.1 (0.4) | 0.02 |
| 25(OH)D (ng/mL) | 44 (15) | 43 (13) | 44 (12) | 44 (18) | 45 (14) | 0.37 |
| 1,25(OH)2D (pg/mL) | 34 (19) | 34 (14) | 36 (20) | 36 (24) | 33 (18) | 0.68 |
| Intact Parathyroid Hormone (pg/mL) | 49 (23) | 48 (19) | 55 (36) | 49 (20) | 47 (18) | 0.73 |
| Serum Creatinine (mg/dL) | 0.9 (0.4) | 0.9 (0.2) | 1.0 (0.8) | 0.9 (0.2) | 1.0 (0.2) | <0.001 |
| Glomerular Filtration Rate (mL/min/1.73m2) | 78 (17) | 78 (17) | 79 (18) | 78 (16) | 77 (17) | 0.70 |
| Urine Albumin/Creatinine (mg/g)a | 10 (6–17) | 11 (6–18) | 10 (6–17) | 9 (6–14) | 10 (6–17) | 0.70 |
|
| ||||||
| Female | 222 (53%) | 108 (77%) | 51 (57%) | 41 (45%) | 22 (22%) | <0.001 |
| Exercise Three Times Per Week or More | 323 (77%) | 101 (72%) | 69 (78%) | 73 (79%) | 80 (81%) | 0.34 |
| Current Smoker | 22 (5%) | 7 (5%) | 5 (6%) | 3 (3%) | 7 (7%) | 0.70 |
| Diabetes Mellitus | 33 (8%) | 3 (7%) | 7 (8%) | 13 (14%) | 10 (10%) | <0.01 |
| Hypertension | 241 (57%) | 61 (43%) | 54 (61%) | 58 (63%) | 68 (69%) | <0.001 |
| Current Use Any Anti-Hypertensive Med | 118 (28%) | 26 (19%) | 27 (31%) | 29 (31%) | 36 (36%) | 0.012 |
| Current Use Any Statin | 93 (22%) | 18 (13%) | 19 (22%) | 25 (27%) | 31 (31%) | <0.01 |
| Current Use Any Lipid Lowering Med | 158 (38%) | 43 (31%) | 30 (34%) | 41 (45%) | 44 (44%) | 0.06 |
| Current Use Calcium Supplement | 178 (42%) | 75 (53%) | 37 (42%) | 35 (38%) | 31 (31%) | <0.01 |
| Current Use Vitamin D Supplement | 96 (23%) | 36 (26%) | 21 (24%) | 22 (24%) | 17 (17%) | 0.48 |
Values for continuous variables are mean (SD) or amedian (25–75%ile) for skewed variables and for categorical variables are number of participants (percentages); P value for Kruskal-Wallis test for continuous variables and Pearson chi-square for categorical variables; SI Conversion Factors: To convert serum creatinine in mg/dL to μmol/L, multiply by 88.4; 25(OH)D to nanomoles per liter, multiply by 2.496, 1,25(OH)2D to picomoles per liter, multiply by 2.6, intact parathyroid hormone to nanograms per liter, multiply by 1.0
At the time of first CAC measurement 2000–2001, one-third of these older participants had no or minimal CAC (≤10) and nearly one-quarter had severe CAC (≥400). As expected, CAC severity increased with age and greater CAC was more common in men. Serum 25(OH)D, 1,25(OH)2D, and intact parathyroid hormone levels did not differ significantly by CAC. Vitamin D supplement use was not associated with CAC, and calcium supplement use was less common in those with higher CAC. Anti-hypertensive medications were used by one-quarter of participants, mainly diuretics (n=47), calcium channel blockers (n=46), beta-blockers (n=39), and angiotensin converting enzyme-inhibitors (n=27).
In separate unadjusted models using ordinal logistic regressions, there were no statistically significant associations between decrease in eGFR or increase in ACR and CAC severity (Table 2). When eGFR and ACR were modeled jointly (mutually adjusting for eGFR and ACR) and in interaction (eGFR*ACR) models, neither the main nor interaction terms was significantly associated with CAC severity (Table 2). These null findings persisted in models adjusting for traditional CVD risk factors (age, sex, body mass index, systolic blood pressure, fasting glucose, high density lipoprotein cholesterol, and triglycerides) (Table 2), and in a multivariable model including all of the above and additional CVD and kidney covariates biologically implicated in vascular calcium (smoking, low density lipoprotein cholesterol, 25(OH)D, 1,25(OH)2D, intact parathyroid hormone, lipid-lowering medications, anti-hypertensive medications, calcium and vitamin D supplements) (data not shown). There was no effect modification of CAC severity by age or sex (data not shown; P’s for interaction >0.16) and sex-stratified analyses showed no important difference (data not shown). Repeating analyses using logistic regression for CAC ≥101 versus <100 or linear regression using continuous CAC also revealed no statistically significant associations (data not shown).
Table 2.
Odds for Increase in Coronary Artery Calcium Score in 2001–2002 Per 1-Standard Deviation Decline Kidney Function in 1997–1999 (Ordinal Logistic Regression)
| Model | Sample Size |
|
|||
|---|---|---|---|---|---|
| Estimated Glomerular Filtration Ratea (n=421) | Urine Albumin/Creatinine Ratiob (n=362) | ||||
|
| |||||
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | ||
| 1 | 421 | 0.95 (0.80, 1.12) | 0.52 | - | |
| 2 | 421 | 1.01 (0.85, 1.20) | 0.91 | - | |
| 3 | 420 | 0.99 (0.82, 1.19) | 0.90 | - | |
| 1 | 362 | - | 1.04 (0.85, 1.27) | 0.68 | |
| 2 | 362 | - | 1.07 (0.86, 1.33) | 0.55 | |
| 3 | 361 | - | 1.00 (0.81, 1.23) | 0.98 | |
| 1 | 362 | 0.93 (0.77, 1.23) | 0.48 | 1.05 (0.86, 1.28) | 0.66 |
| 2 | 362 | 1.03 (0.85, 1.25) | 0.77 | 1.07 (0.86, 1.33) | 0.56 |
| 3 | 361 | 0.99 (0.81, 1.22) | 0.94 | 1.00 (0.81, 1.24) | 1.00 |
| 1 | 362 | 0.94 (0.78, 1.14) | 0.56 | 0.99 (0.80, 1.24) | 0.95 |
| 2 | 362 | 1.02 (0.84, 1.24) | 0.85 | 1.11 (0.89, 1.39) | 0.37 |
| 3 | 361 | 1.01 (0.81, 1.22) | 0.91 | 0.99 (0.80, 1.27) | 0.91 |
Model 1: Unadjusted
Model 2: Age, sex adjusted
Model 3: Age, sex, body mass index, systolic blood pressure, fasting plasma glucose, high density lipoprotein cholesterol, triglycerides
Urine albumin/creatinine ratio (ACR); confidence interval (CI); estimated glomerular filtration rate (eGFR)
Odds Ratio for 1-standard deviation decrease in eGFR
Odds Ratio for 1-standard deviation increase in ACR
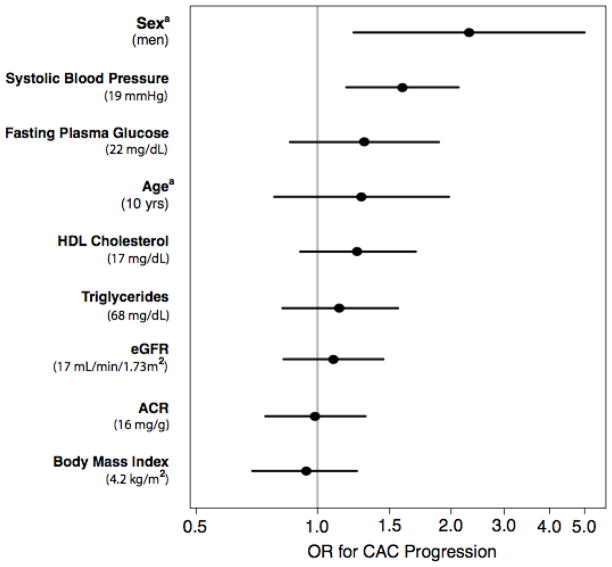
To aid interpretation of the null results observed in this study, Figure 2A compares the cross-sectional associations of eGFR and ACR with CAC severity to that of other CVD covariates in separate models adjusting for age and sex. Results are presented per population 1-SD increase for continuous variables (except age which is per 10 years), and for male sex. As expected, age and sex were both strongly associated with CAC severity; systolic blood pressure and fasting plasma glucose were weakly associated with CAC. Point estimates for eGFR, ACR, body mass index triglycerides and high density lipoprotein cholesterol were near one and not significant. Importantly, the confidence intervals around the associations of eGFR and ACR with CAC were narrow and similar to those of other covariates, suggesting that any missed association would be small.
FIGURE 2.
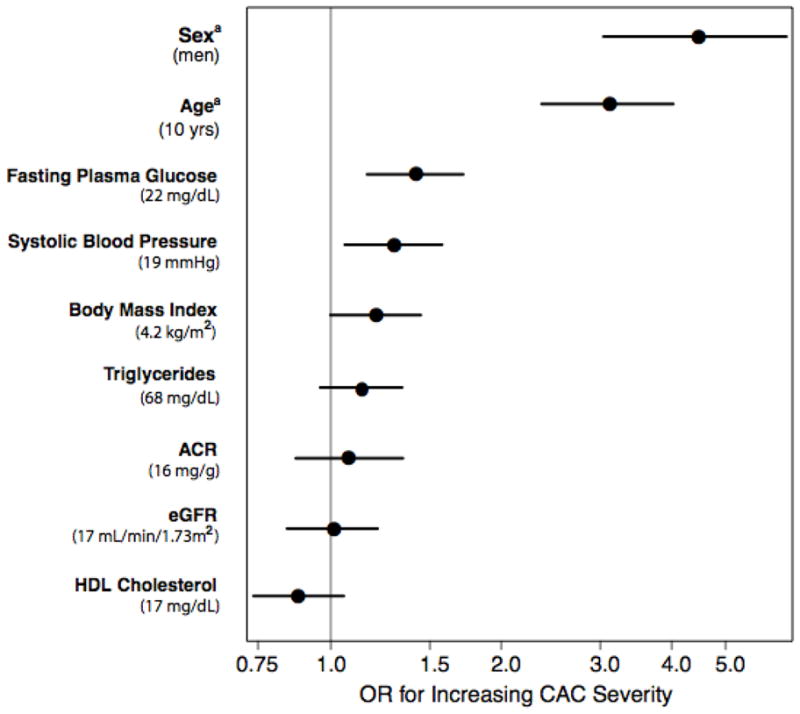
FIGURE 2A. Odds ratio estimates for the association of eGFR and ACR and CVD risk factors with increasing CAC severity at baseline. Each factor is adjusted for age and sex. Odds ratios for continuous variables are presented for 1-SD intervals, except aage, which is presented for 10-year intervals and asex, which is for men. The values shown in the middle of the bar are the OR estimates; the bars represent the 95% CIs
FIGURE 2B. Odds ratio estimates for the association of eGFR and ACR and CVD risk factors with CAC progression. Each factor is adjusted for age, sex and time interval. Odds ratios for continuous variables are presented for 1-SD intervals, except aage, which is presented for 10-year intervals and asex, which is for men. The values shown in the middle of the bar are the OR estimates; the bars represent the 95% CI
Compared to 338 participants who returned for a repeat CAC scan a mean of 4.5 (0.5) years after the first scan, 84 participants who did not were older (71 vs. 66 years, P<0.001) at baseline and had higher systolic blood pressure (135 vs. 129 mmHg, P=0.02), but did not differ significantly by baseline eGFR (75 vs. 79 mL/min/1.73m2) or median ACR (9.8 vs. 10.2 mg/g).
Table 3 summarizes the differences in baseline characteristics among 157 (46%) participants with and 181 (54%) without CAC progression. Similar to baseline CAC, CAC progression was more common in men than women but those with CAC progression were not significantly older than those without.
Table 3.
Baseline Characteristics in 1997–1999 of Participants Who Returned for Second Scan in 2005–2006, by Coronary Artery Calcium Progressiona Status
|
|
||||
|---|---|---|---|---|
| Progression | ||||
|
| ||||
| Characteristic | All (n=338) | No (n=181) | Yes (n=157) | P value |
| Age (years) | 66 (7) | 66 (7) | 67 (6) | 0.13 |
| Systolic Blood Pressure (mmHg) | 129 (18) | 126 (16) | 133 (19) | <0.001 |
| Body Mass Index (kg/m2) | 26 (4) | 26 (4) | 27 (4) | 0.45 |
| Fasting Plasma Glucose (mg/dL) | 103 (21) | 101 (13) | 106 (28) | 0.03 |
| Low Density Lipoprotein (mg/dL) | 123 (30) | 121 (29) | 126 (32) | 0.19 |
| High Density Lipoprotein (mg/dL) | 59 (17) | 60 (17) | 58 (16) | 0.51 |
| Triglycerides (mg/dL) | 130 (71) | 128 (70) | 132 (65) | 0.59 |
| Serum Calcium (mg/dL) | 9 (0.3) | 9 (0.3) | 9 (0.3) | 0.96 |
| 25(OH)D (ng/mL) | 44 (15) | 43 (14) | 45 (15) | 0.15 |
| 1,25(OH)2D (pg/mL) | 34 (18) | 34 (15) | 35 (22) | 0.53 |
| Intact Parathyroid Hormone (pg/mL) | 49 (24) | 50 (28) | 47 (19) | 0.21 |
| Serum Creatinine (mg/dL) | 0.9 (0.4) | 0.9 (0.2) | 1.0 (0.6) | 0.08 |
| Glomerular Filtration Rate (mL/min/1.73m2) | 79 (17) | 79 (17) | 78 (16) | 0.54 |
| Urine Albumin/Creatinine (mg/g)b | 9.8 (5.7–17.8) | 10 (6–18) | 10 (7–15) | 0.93 |
|
| ||||
| Female | 182 (54%) | 112 (62%) | 70 (45%) | <0.01 |
| Exercise Three Times Per Week or More | 258 (76%) | 139 (77%) | 119 (76%) | 0.83 |
| Current Smoker at Baseline | 18 (5%) | 9 (5%) | 9 (6%) | 0.76 |
| Diabetes Mellitus | 21 (6%) | 8 (4%) | 13 (8%) | 0.13 |
| Hypertension | 191 (57%) | 87 (48%) | 104 (66%) | 0.01 |
| Baseline Use Any Anti-Hypertensive Med | 94 (28%) | 44 (24%) | 50 (32%) | 0.12 |
| Baseline Use Any Statin | 77 (23%) | 34 (19%) | 43 (28%) | 0.06 |
| Current Use of Any Statin at 2nd Scan | 68 (20%) | 29 (16%) | 39 (25%) | 0.04 |
| Baseline Use Any Lipid Lowering Med | 130 (39%) | 63 (35%) | 67 (43%) | 0.14 |
| Current Use of Any Lipid Lowering Med at 2nd Scan | 68 (20%) | 29 (16%) | 39 (25%) | 0.04 |
| Baseline Use Calcium Supplement | 144 (43%) | 82 (46%) | 62 (40%) | 0.26 |
| Baseline Use Vitamin D Supplement | 77 (24%) | 45 (25%) | 32 (20%) | 0.33 |
CAC progression defined as difference between square root of baseline and square root of follow-up CAC volume ≥ 2.5 mm3
Values for continuous variables are mean (SD) or bmedian (25–75%ile) for skewed variables and for categorical variables are number of participants (percentages); P value for Kruskal-Wallis test for continuous variables and Pearson chi-square for categorical variables; SI Conversion Factors: To convert serum creatinine in mg/dL to μmol/L, multiply by 88.4; 25(OH)D to nanomoles per liter, multiply by 2.496, 1,25(OH)2D to picomoles per liter, multiply by 2.6, intact parathyroid hormone to nanograms per liter, multiply by 1.0
In independent unadjusted models using logistic regression, there were no statistically significant associations between decrease in eGFR or increase in ACR and CAC progression (Table 4). When eGFR and ACR were modeled jointly and in interaction models, neither the main (Table 4) nor the interaction term (data not shown) was significantly associated with CAC progression. These null findings persisted in models adjusting for: 1) time interval between the first and second scan, 2) traditional CVD risk factors (age, sex, body mass index, systolic blood pressure, fasting glucose, high density lipoprotein cholesterol, and triglycerides) (Table 4), and 3) a multivariable model including all of the above and additional CVD and kidney covariates biologically implicated in vascular calcium (smoking, low density lipoprotein cholesterol, 25(OH)D, 1,25(OH)2D, intact parathyroid hormone, lipid-lowering medications, anti-hypertensive medications, calcium and vitamin D supplements) (data not shown). There was no effect modification of CAC progression by age or sex (data not shown; P’s for interaction >0.20); sex-stratified analyses showed no important difference (data not shown). There was no association between eGFR or ACR and absolute or relative change in CAC, or in CAC initiators or progressors (data not shown). Repeating analyses excluding those with hypertension or diabetes at the 1997–1999 visit did not materially change results (data not shown). A sensitivity analysis was performed assigning the 21 participants who had died between the baseline and follow-up CAC visits to the highest CAC progression category; this did not materially change results (data not shown).
Table 4.
Odds for Increase in Coronary Artery Calcium Progression From 2001–2002 to 2005–2006 Per 1-Standard Deviation Decline Kidney Function in 1997–1999 (Logistic Regression)
| Model | Sample Size |
|
|||
|---|---|---|---|---|---|
| Estimated Glomerular Filtration Ratea (n=338) | Urine Albumin/Creatinine Ratiob (n=292) | ||||
|
| |||||
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | ||
| 1 | 338 | 1.07 (0.86, 1.33) | 0.54 | - | |
| 2 | 337 | 1.06 (0.85, 1.33) | 0.57 | - | |
| 3 | 336 | 1.08 (0.86, 1.38) | 0.50 | - | |
| 1 | 292 | - | 0.99 (0.78, 1.26) | 0.93 | |
| 2 | 291 | - | 1.06 (0.82, 1.37) | 0.67 | |
| 3 | 290 | - | 0.98 (0.75, 1.29) | 0.91 | |
| 1 | 292 | 1.05 (0.83, 1.33) | 0.69 | 0.99 (0.78, 1.26) | 0.95 |
| 2 | 291 | 1.05 (0.82, 1.35) | 0.68 | 1.06 (0.82, 1.38) | 0.64 |
| 3 | 290 | 1.10 (0.83, 1.43) | 0.53 | 0.99 (0.75, 1.30) | 0.94 |
| 1 | 292 | 1.06 (0.83, 1.35) | 0.65 | 1.01 (0.79, 1.30) | 0.92 |
| 2 | 291 | 1.08 (0.83, 1.40) | 0.58 | 1.12 (0.85, 1.48) | 0.40 |
| 3 | 290 | 1.14 (0.86, 1.49) | 0.36 | 1.07 (0.79, 1.45) | 0.63 |
Model 1: Unadjusted
Model 2: Age, time interval, sex adjusted
Model 3: Age, time interval, sex, body mass index, systolic blood pressure, fasting plasma glucose, high density lipoprotein cholesterol, triglycerides
Odds Ratio for 1-standard deviation decrease in eGFR
Odds Ratio for 1-standard deviation increase in ACR
Urine albumin/creatinine ratio (ACR); confidence interval (CI); estimated glomerular filtration rate (eGFR)
Figure 2B compares the longitudinal associations of eGFR and ACR with CAC progression to that of other CVD covariates in separate models adjusting for baseline age, sex, and time interval. Results are presented per population 1-SD increase for continuous variables (except age which is per 10 years), and for male sex. Only systolic blood pressure and male sex were positively associated with CAC progression. Point estimates for eGFR, ACR and body mass index were near one and not significant.
DISCUSSION
In this longitudinal study of older, community-dwelling men and women without known coronary heart disease, neither eGFR, nor ACR, nor both together, was a significant predictor of CAC severity or CAC progression in unadjusted analyses or after adjusting for CVD risk factors, and for serum levels of vitamin D and intact parathyroid hormone. The point estimates of the odds ratios were very close to one with narrow confidence intervals, which did not extend into a region considered clinically relevant.23
Prior studies in community-dwelling participants investigating the associations between eGFR and CAC,4, 5, 6, 7, 12, 10, 11 and ACR and CAC,7, 8, 9, 10, 11, 12 have had contradictory results. Few have focused on participants age 60 and older 4, 5, 9, 12 and only three examined CAC progression.6, 8, 12
Results from the three prior studies of kidney function and CAC progression in community-dwelling populations are contradictory. Tuttle and colleagues studied 838 participants (mean age 48, mean eGFR 103 mL/min/1.73m2) from the Spokane Heart Study over a 6-year follow-up with repeat scans every 2 years. Similar to our study, in cross-sectional analyses, they found no difference in eGFR in those with or without CAC at baseline. However, in contrast to our findings, in longitudinal analyses, they noted an inverse association between eGFR (per 10 mL/min/1.73m2) and CAC prevalence (β= −0.065, P=0.02) and incidence (β= −0.065, P=0.03) in multivariable models adjusted for CVD risk factors. They reported no measure of albuminuria and did not include vitamin D or parathyroid hormone as covariates.6 Compared to the Spokane cohort, Rancho Bernardo participants were older (mean 67 vs. 48 years) and had lower eGFR (mean 78 vs. 103 mL/min/1.73m2), suggesting that while eGFR may be a more important risk factor for CAC in middle-age, its importance may be diminished in otherwise healthy older adults with other competing risk factors.
Also contrasting with our findings, is a longitudinal analysis of 5666 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) (mean age 62, mean eGFR 87 mL/min/1.73m2, free of clinical CVD at baseline) which reported positive associations between microalbuminuria and prevalent CAC, incident CAC, and CAC progression over 2.4 year follow-up. At baseline, participants with microalbuminuria (ACR>30 mg/g) were more likely to have CAC>0 and had higher Agatston scores than those without microalbuminuria. Microalbuminuria was also associated with a greater relative risk (RR) for incident CAC (RR [95% CI] 1.76 [1.19, 2.61]) at follow-up among those without baseline CAC (initiators) and with greater CAC progression in those with baseline CAC (progressors), independent of CVD risk factors. This study did not report an association between eGFR and CAC, but stated that adjusting for eGFR in the multivariable model did not change the association between ACR and CAC.8
The only prior longitudinal study to report the association between both eGFR and ACR and CAC progression in community-dwelling adults was a subset analysis from the Multi-Ethnic Study of Atherosclerosis, which selected for participants with early CKD (defined as an eGFR<60 mL/min/1.73m2) but no clinical CVD. Kestenbaum and colleagues studied 562 participants (mean age 69, mean eGFR 52 mL/min/1.73m2, mean ACR 7 mg/g), who completed two scans for CAC a median of 2 years apart. Congruent with our findings, they found no association between eGFR or ACR and CAC progression in models adjusted for CVD risk factors, despite selecting for a population with eGFR in a range previously demonstrated to be associated with increased CVD risk; they also found no association between cystatin C and CAC progression.12 Rancho Bernardo participants were similar in age and ACR to participants from this cohort, but had higher mean eGFR (78 vs. 52 mL/min/1.73m2), as expected, given that they were not selected for diminished eGFR.
Strengths of the present study include the broad range of CAC, not available in young cohorts, and the large number of plausible covariates available for study. On the other hand, older age is a limitation in that it may introduce survival bias. It is possible that persons with more severe kidney disease died or were too ill to return to the follow-up visit, possibly attenuating a weak association, however, there was no significant difference in eGFR or ACR among those who did and did not return for follow-up CAC assessment. Furthermore, because those with known coronary heart disease were excluded from the study by design, less healthy participants were excluded from the baseline CAC visit. To further exclude survival bias as an explanation, a sensitivity analysis was performed assigning the 21 participants who had died between the baseline and follow-up CAC visits to the highest CAC progression category (in the event that these participants had greater CAC progression); this did not materially change results. Excluding those with hypertension or diabetes at the 1997–99 visit also did not change results. All members of our cohort had health insurance and were relatively healthy. Another limitation is that the Rancho Bernardo Study participants are almost entirely Caucasian, middle-class, and live in sunny southern California; few have vitamin D deficiency (25[OH]D <20 ng/mL).24 These results may not be generalizable to other ethnic groups or geographic locations. A gold standard measure of eGFR, such as inulin or radioisotope clearance, was not performed in this, or other epidemiologic studies. Some may question whether this study was adequately powered to identify an association between eGFR or ACR and CAC. 191 (45%) participants had moderate or severe CAC (≥101); 51 (12%) had an eGFR<60 mL/min/1.73m2 and 181 (50%) had an ACR>10 mg/g--levels that have been associated with CVD mortality in a large meta-analysis.25 As shown in Figure 2, there was sufficient power to identify associations for established risk factors (age and sex) and point estimates and confidence intervals for ACR and eGFR were similar to other covariates which are not considered to be clinically relevant risk factors; both strengthen confidence in the null results. Of course negative associations are always more difficult to evaluate. If a true association was missed, it is likely to be small.
If the increased CVD mortality seen in older patients with early CKD is not mirrored by excess vascular calcium or disorders of calcium, vitamin D or intact parathyroid hormone,26 then other potential mechanisms should be explored. Increased circulating levels of pro-inflammatory cytokines and oxidative stress have been implicated in endothelial dysfunction in CKD and may contribute to CVD risk.21 Kidney dysfunction may lead to dysregulation in other mediators such as fibroblast growth factor-23,27 or extracellular calcium-regulatory proteins such as fetuin-A and uncarboxylated matrix Gla protein,28 which have been implicated in CVD mortality. In a cross-sectional analysis from our cohort, fetuin-A levels were inversely associated with CAC severity.29 These pathways warrant further study.
Acknowledgments
Grants and Financial Support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK31801); the National Institute on Aging (AG07181); and National Institutes of Health and the National Institute on Aging (R01AG028507). MC is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1R01 DK081473-01A, 1R01DK078112-01A2). GAL is supported by American Heart Association (0930073N). JHI is supported by the National Heart, Lung, and Blood Institute (1R01HL096851).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 2.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Ix JH, Katz R, Kestenbaum B, Fried LF, Kramer H, Stehman-Breen C, Shlipak MG. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. doi: 10.1681/ASN.2007070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox CS, Larson MG, Keyes MJ, Levy D, Clouse ME, Culleton B, O’Donnell CJ. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: the Framingham Heart Study. Kidney Int. 2004;66:2017–2021. doi: 10.1111/j.1523-1755.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4:1968–1973. doi: 10.2215/CJN.01250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer H, Jacobs DR, Jr, Bild D, Post W, Saad MF, Detrano R, Tracy R, Cooper R, Liu K. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 8.DeFilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, Budoff MJ, Blumenthal RS, Nasir K. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3:595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 10.Parikh NI, Hwang SJ, Larson MG, Hoffmann U, Levy D, Meigs JB, O’Donnell CJ, Fox CS. Indexes of kidney function and coronary artery and abdominal aortic calcium (from the Framingham Offspring Study) Am J Cardiol. 2008;102:440–443. doi: 10.1016/j.amjcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho I, Min HS, Chun EJ, Park SK, Choi Y, Blumenthal RS, Rivera JJ, Nasir K, Kim YJ, Sohn DW, Oh BH, Park YB, Chang HJ. Coronary atherosclerosis detected by coronary CT angiography in asymptomatic subjects with early chronic kidney disease. Atherosclerosis. 2010;208:406–411. doi: 10.1016/j.atherosclerosis.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–998. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 14.Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Vol. 1999 Geneva: World Health Organization; 1999. [Google Scholar]
- 15.NIH UDoH, Education and Welfare. Lipid Research Clinics Program Manual of Laboratory Operations. 2. Vol. 1. US Govt Printing Office; 1974. pp. 75–628. [Google Scholar]
- 16.Friedewald WTLR, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1976;18:499–502. [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Rumberger JA, Sheedy PF, 2nd, Breen JF, Fitzpatrick LA, Schwartz RS. Electron beam computed tomography and coronary artery disease: scanning for coronary artery calcification. Mayo Clin Proc. 1996;71:369–377. doi: 10.4065/71.4.369. [DOI] [PubMed] [Google Scholar]
- 20.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 21.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46:1171–1178. [PubMed] [Google Scholar]
- 23.Man-Son-Hing M, Laupacis A, O’Rourke K, Molnar FJ, Mahon J, Chan KB, Wells G. Determination of the clinical importance of study results. J Gen Intern Med. 2002;17:469–476. doi: 10.1046/j.1525-1497.2002.11111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Muhlen DG, Greendale GA, Garland CF, Wan L, Barrett-Connor E. Vitamin D, parathyroid hormone levels and bone mineral density in community-dwelling older women: The Rancho Bernardo Study. Osteoporos Int. 2005 doi: 10.1007/s00198-005-1910-8. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jassal SK, Chonchol M, von Muhlen D, Smits G, Barrett-Connor E. Vitamin D, Parathyroid Hormone, and Cardiovascular Mortality in Older Adults: the Rancho Bernardo study. Am J Med. 2010;123:1114–1120. doi: 10.1016/j.amjmed.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moe SM, Reslerova M, Ketteler M, O’Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD) Kidney Int. 2005;67:2295–2304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 29.Ix JH, Barrett-Connor E, Wassel CL, Cummins K, Bergstrom J, Daniels LB, Laughlin GA. The associations of fetuin-A with subclinical cardiovascular disease in community-dwelling persons: the Rancho Bernardo Study. J Am Coll Cardiol. 2011;58:2372–2379. doi: 10.1016/j.jacc.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]