Abstract
Iron-sulfur (Fe-S) clusters are inorganic cofactors required for a variety of biological processes. In vivo biogenesis of Fe-S clusters proceeds via complex pathways involving multiple protein complexes. In the Suf Fe-S cluster biogenesis system, SufB may be a scaffold for nascent Fe-S cluster assembly whereas SufA is proposed to act as either a scaffold or an Fe-S cluster carrier from the scaffold to target apo-proteins. However, SufB can form multiple stable complexes with other Suf proteins, such as SufB2C2 and SufBC2D and the specific functions of these complexes in Fe-S cluster assembly are not clear. Here we compare the ability of the SufB2C2 and SufBC2D complexes as well as SufA to promote in vitro maturation of the [2Fe-2S] ferredoxin (Fdx). We found that SufB2C2 was most proficient as a scaffold for de novo assembly of holo-Fdx using sulfide and iron as freely available building blocks while SufA was best at direct transfer of a pre-formed Fe-S cluster to Fdx. Furthermore, cluster transfer from [4Fe-4S] SufB2C2 or SufBC2D to Fdx will proceed through a SufA intermediate to Fdx is SufA is present. Finally, addition of ATP repressed cluster transfer from [4Fe-4S] SufB2C2 to Fdx and from SufBC2D to [2Fe-2S] SufA or Fdx. These studies indicate that SufB2C2 can serve as a terminal scaffold to load the SufA Fe-S cluster carrier for in vitro maturation of [2Fe-2S] enzymes like Fdx. This work is the first to systematically compare the cluster transfer rates of a scaffold (SufB) to the transfer rates of a carrier (SufA) under the same conditions to the same target enzyme and is also the first to reconstitute the full transfer pathway (from scaffold to carrier to target enzyme) in a single reaction.
1. Introduction
Iron-sulfur (Fe-S) clusters are inorganic cofactors [1] used for fundamental biological processes such as photosynthesis, respiration, and nitrogen fixation. In vivo formation of Fe-S clusters requires dedicated biosynthesis pathways consisting of one or more multi-protein complexes that carry out stepwise assembly of the cofactor. The three most well-characterized Fe-S cluster biosynthesis pathways in bacteria are the Isc system (which is also partially conserved in eukaryotic mitochondria) [2–4], the Nif system [5], and the Suf system (which is partially conserved in eukaryotic chloroplasts) [6–10]. These three pathways share some common mechanistic features. During Fe-S cluster assembly by the three pathways, sulfur is mobilized from free cysteine via the activity of a cysteine desulfurase enzyme, such as IscS, NifS, or SufS. The product of the cysteine desulfurase reaction is a persulfide species bound to an active site cysteine residue within the enzyme. This mobilized sulfur atom is transferred to a scaffold protein (IscU, NifU, or SufB) as a persulfide and is reduced to sulfide at some point during the assembly of the full cluster. The scaffold protein is the site where the nascent and labile Fe-S cluster is first assembled prior to insertion into a target metalloprotein. In vivo iron donation is not as clearly defined but could involve a metallochaperone such as frataxin or other pathways [11, 12].
The transfer of the nascent Fe-S cluster from a scaffold protein to a target apo-protein is a key step in Fe-S cluster biogenesis. The correct Fe-S cluster must find the correct target metalloprotein while ignoring potential binding sites in other proteins or small metabolites and while avoiding destruction by oxidation or chelation. Fe-S cluster scaffold proteins can transfer intact clusters to target proteins in vitro. However, there are accessory Fe-S cluster carrier proteins (IscA and SufA in the Isc and Suf systems) that can also carry out cluster transfer and may be important for Fe-S cluster metabolism in vivo [13]. Presumably these carrier proteins are not used as sites for de novo cluster assembly but instead bind and traffic intact clusters. Fe-S cluster transfer from IscU and IscA to other proteins such as Fdx and BioB is well-characterized [14–18]. Similarly, Fe-S cluster transfer from the NifU scaffold to the subunits of nitrogenase has also been investigated [19]. Less is known about Fe-S cluster transfer among the Suf proteins.
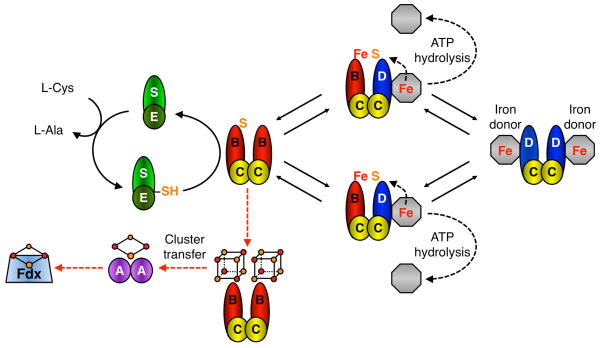
SufB is the site of nascent cluster assembly in the Suf pathway (Scheme 1) [20]. SufB receives persulfide from the SufS cysteine desulfurase enzyme via the sulfur transfer protein SufE [20]. In vivo iron delivery to SufB is not yet clearly delineated but requires two accessory proteins, the SufC ATPase and SufD [21]. SufB interacts tightly with SufC and SufD leading to the formation of a stable SufBC2D complex [7, 22]. Recently it was also shown that SufB can form a stable SufB2C2 complex in vivo [21]. In vitro reconstituted SufB can form a [4Fe-4S]2+ cluster when alone or as part of the SufBC2D complex [20, 23]. SufB can also accommodate a [2Fe-2S] cluster if the [4Fe-4S] cluster is exposed to oxygen [20]. SufB purified after in vivo co-expression with SufCDSE is present as both SufB2C2 and SufBC2D and contains a mixture of linear [3Fe-4S] and [4Fe-4S] clusters [21]. Sequential cluster assembly on SufB, SufB2C2, or SufBC2D has not been as carefully characterized as the IscU scaffold and it is not clear if these multiple cluster types formed as intermediates during cluster assembly on SufB, are breakdown products from the [4Fe-4S] form of SufB, or perhaps are both.
Scheme 1. Putative model of Suf-mediated Fe-S cluster assembly.
SufS releases sulfur from L-cysteine as a persulfide intermediate that is passed on to SufE and then SufB as part of the SufB2C2 complex [20]. The SufC2D2 complex may interact with an unidentified iron donor/iron source (grey octagon). SufB2C2 and SufC2D2 complexes interact to form two SufBC2D complexes where ATP hydrolysis by SufC is used to release iron from a donor or otherwise mobilize iron for cluster assembly (black dashed arrows) [21]. The complexes then dissociate to perform another round of sulfur and iron acquisition. SufD and SufC ATPase activity are required for in vivo iron acquisition although they may also play other roles in cluster assembly. Once a maximum of 2 × [4Fe-4S] clusters have formed, the terminal SufB2C2 scaffold may exit the cycle and, via an ATP-independent step, transfer clusters to Fe-S carrier proteins like SufA for further cluster trafficking to target enzymes like Fdx (red dashed arrows). All multi-protein complexes shown in Scheme 1 are known to form in vivo and/or in vitro (except for the putative interaction with the as yet unidentified iron donor) [6, 7, 22, 31, 45, 46].
It was previously established that SufBC2D enhances Fe-S cluster assembly on SufA during in vitro Fe-S cluster assembly using free iron and SufS, SufE, and L-cysteine as the sulfur source, suggesting that SufB acts as an Fe-S cluster scaffold for the Suf pathway, possibly in conjunction with SufC and SufD. Those same studies showed that SufBC2D can transfer Fe-S clusters to SufA but SufA cannot transfer clusters to SufBC2D [24], even though SufA can transfer clusters to downstream apo-proteins such as Fdx, AcnA, and BioB [25, 26]. It was also recently shown that SufBC2D can transfer clusters to AcnB without any requirement for SufA [23].
The previous results raise several questions as to the relative roles of the SufB2C2 and SufBC2D complexes and SufA in Fe-S cluster assembly and trafficking. We recently proposed a hypothetical model that attempts to integrate the published data for the Suf system to provide a possible pathway for Fe-S cluster assembly (Scheme 1) [21]. As a start to rigorously testing this proposed model, here we determined if SufB2C2, SufBC2D, or SufA can act as a scaffold to stimulate cluster assembly in the native E. coli target Fe-S metalloprotein ferredoxin (Fdx). We also investigated transfer of Fe-S clusters from the various Fe-S Suf proteins to apo-Fdx. Finally, we determined if ATP hydrolysis by SufC affects Fe-S cluster transfer to SufA or Fdx.
2. Experimental Procedures
2.1 Strains, plasmids, and growth conditions
IscFdx (hereafter referred to as Fdx) was amplified from MG1655 chromosomal DNA using the primers 5′-CCGTGGACGAGGTTTCATATGCCAAAG-3′ and 5′-CCCATACTAAGCTTTGTTAATGCTCACG-3′. PCR products were digested with NdeI and HindIII and cloned into the corresponding sites of pET21a (Novagen), generating plasmid pET21a-Fdx. The nucleotide sequence of the plasmid insert was confirmed by DNA sequencing. E. coli BL21(DE3) containing the pET21a-Fdx expression vector was grown in LB at 37°C. Iso-propyl-1-thio-β-D-galactopyranoside (IPTG) was added at 500 μM final concentration for 3 h to induce Fdx expression. The plasmid pGSO164 [22] containing the entire suf operon under the control of an arabinose-inducible promoter was used to over-express SufABCDSE in the Top10 strain of E. coli. The cells were grown in LB at 37°C and L-Arabinose was added to 0.2% final concentration by weight for 3 h to induce the expression of SufABCDSE. The plasmid pET21a-SufA was used to overexpress SufA in E. coli BL21(DE3) strain [24]. Cells were grown at 37°C and IPTG was added at 500 μM final concentration for 6 h to induce SufA expression. Recombinant His6-SufB2C2 was co-expressed with SufSE using expression vector pFWO469 in E. coli strain BL21(DE3). Cultures were grown in LB at 37°C and induced with 100 μM of IPTG when OD600 was 0.5 – 0.6 followed by overnight induction at 18°C. After induction, cells were harvested by centrifugation and cell pellets were frozen at −80°C.
2.2 Protein purification
The SufBC2D complex was purified as described previously [20], using Phenyl FF, Q-sepharose, and Superdex 200 gel chromatography resins in sequence. SufA was also purified as described previously [24]. His6-SufB2C2 was purified aerobically using a Ni2+-NTA column as described previously [21]. Fdx was purified by freeze-thaw method as follows: the cell pellet was thawed on ice and resuspended in buffer containing 25 mM Tris-Cl, pH 7.5, 100 mM NaCl, 10 mM β-mercaptoethanol (β-ME), and 1 mM phenylmethanesulphonylfluoride (PMSF) (Sigma). The pellet was refrozen at −80°C for 1 h. The freeze-thaw cycle was repeated two more times. The freeze-thaw extract was centrifuged at 20,000 × g for 25 min and the clear lysate was loaded onto a Q-sepharose anion exchange column equilibrated with buffer containing 25 mM Tris-Cl, pH 7.5, and 10 mM β-ME. After washing, the protein was eluted with a linear gradient of 25 mM Tris-Cl, pH 7.5, 1 M NaCl, and 10 mM β-ME. The colorless fractions containing apo-Fdx were collected separately from the colored fractions of holo-Fdx (which were used for later comparison to in vitro reconstituted Fdx). Apo-Fdx fractions were concentrated to 3 ml and loaded onto HiLoad16/60 Superdex 75 gel filtration column equilibrated with 25 mM Tris-Cl, pH 7.5, 150 mM NaCl, and 10 mM β-ME. Fractions containing Fdx were concentrated and frozen at −80°C until further use.
2.3 De novo Fe-S assembly on Fdx in the presence of SufB2C2, SufBC2D, and SufA
The pure SufA, SufBC2D, SufB2C2 and Fdx proteins used in this study always had variable amounts of Fe in their as-purified forms and were converted to their fully apo forms following published procedures [27]. Briefly, the apoproteins were obtained by incubating the proteins with EDTA and potassium ferricyanide (molar ratios 1:50:20) on ice for 5–10 min followed by desalting. In order to see the effect of scaffold proteins on cluster assembly on Fdx, SufB2C2, SufBC2D, or SufA (200 μM) were added to the reconstitution buffer containing Fdx (200 μM) in 25 mM Tris-Cl, pH 7.5, 100 mM NaCl, 5 mM dithiothreitol (DTT), with 1 mM of ferrous ammonium sulfate (FAS). The reconstitution was initiated by the addition of 1 mM of sodium sulfide (Na2S) and the circular dichroism (CD) spectra were recorded at different intervals of time (5 min–18 h) at 23°C in 0.2 cm path length cuvettes using a JASCO-815 spectrometer. As a control, reconstitution on Fdx alone was also performed under the same conditions. The amount of holo-Fdx formed during reconstitution was calculated by comparing the change in ellipticity at 434 nm to that of holo-Fdx containing one equivalent of [2Fe-2S]2+ cluster (as-purified from E. coli and confirmed by iron and sulfide analysis, Fig. S2A). To separate the proteins for additional analysis, 200 μM Fdx was incubated with 200 μM of apo-His6-SufB2C2, apo-His6-SufBC2D, or apo-His6-SufA in the presence of 5-fold molar excess of FAS and Na2S. After 20 min de novo assembly, the reaction mixtures were loaded onto a 1 ml HiTrap Ni2+-NTA column. Fdx was removed from the column by washing with 3 ml of binding buffer (25 mM Tris-Cl, pH 7.4, 0.5 M NaCl, 5 mM imidazole, and 2 mM DTT). His6-SufB2C2, His6-SufBC2D, or His6-SufA was eluted with 3 ml of elution buffer (25 mM Tris-Cl, pH 7.4, 0.5 M NaCl, 500 mM imidazole, and 2 mM DTT). All wash and elution fractions were collected and concentrated separately using Nanosep centrifugal devices. All steps were carried out anaerobically in an anaerobic glove box (Coy). Equal volumes of elution fractions (concentrated to the same final volume) were separated by SDS-PAGE. Both wash and elution fractions also were analyzed by UV-Visible absorption and CD spectroscopy.
2.4 Fe-S cluster transfer to Fdx
Reconstitution on His6-SufB2C2, SufBC2D, or SufA (300–350 μM) was performed in an anaerobic glove box (Coy) in reconstitution buffer (25 mM Tris-Cl, pH 7.5, 100 mM NaCl) with 10-fold excess of L-cysteine and ferrous ammonium sulfate (FAS) and 1.2–1.4 μM of SufS and SufE. After 1.5 – 2 h, the proteins were purified by anaerobic anion exchange chromatography using a Hitrap QFF 1 ml column. The cluster containing fractions of His6-SufB2C2, SufBC2D, or SufA were concentrated on Nanosep 30K or 10K centrifugal devices. The reconstituted holo-SufBC2D had 2.1 Fe/complex and 2.2 S/complex while SufB2C2 had 6 Fe/complex and 5.6 S/complex. This difference in cluster content arises from the presence of one SufB in SufBC2D but two SufB subunits in SufB2C2. Each complex had similar amounts of Fe and S per SufB subunit. Reconstituted SufA had 1.5 Fe/monomer and 1.3 S/monomer. Representative UV-visible absorption spectra of reconstituted Suf proteins are shown in Fig. S1.
Fe-S cluster transfer was monitored anaerobically at different time intervals (10 min– 18h) at 23°C in 0.2 cm path length cuvettes using a JASCO-815 spectrometer. All transfer reactions were carried out in buffer containing 25 mM Tris-Cl, pH 7.5, 150 mM NaCl, and 2 mM DTT. Fe-S cluster transfer to apo-Fdx was from the holo forms of His6-SufB2C2, SufBC2D, or SufA added in slight molar excess (300 μM) to provide 1.14–1.6 equivalents of Fe-S cluster for apo-Fdx (200 μM). The transfer was also repeated from holo-His6-SufB2C2 or holo-SufBC2D (300 μM) to apo-Fdx (200 μM) in the presence of apo-SufA (200 μM). Despite slightly different levels of starting cluster content, the protein concentration of the cluster donors was kept constant at 300 μM. Transfer from holo-SufBC2D to Fdx was also monitored in the presence of 60 μM EDTA added after the addition of Fdx to holo-SufBC2D. De novo reconstitution of Fdx in the presence of 60 μM EDTA was carried out using amounts of FAS and Na2S equivalent to Fe and S content in holo-SufBC2D. To monitor the effect of ATP on cluster transfer the reactions were repeated under the same conditions except for the presence of 2 mM ATP in a mix with 40 mM MgCl2 and 150 mM KCl, which was added last to the reaction mixture. For all assays, Fe content was determined colorimetrically using the ferrozine assay [28]. Acid-labile sulfide was determined by previously reported methods [29].
3. Results
3.1 Circular dichroism of holoproteins
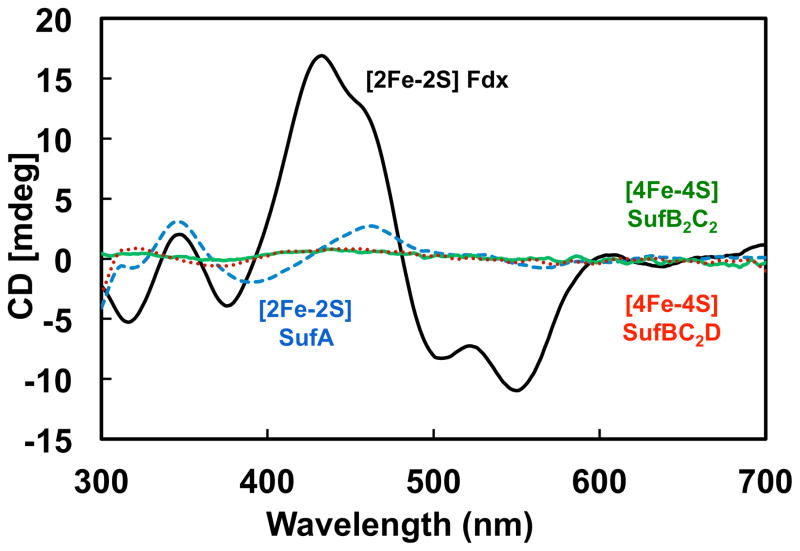
Real time monitoring of Fe-S cluster assembly or transfer in a mixture of Fe-S proteins requires one to distinguish between the apo- and holo-forms of each protein. While UV-visible absorption spectroscopy is a facile method for rudimentary cluster analysis, circular dichroism (CD) spectroscopy can provide more information about certain cluster types and their protein environments during cluster maturation. Both holo-SufA and holo-Fdx primarily bind cysteine-ligated [2Fe-2S]2+ clusters and have distinctive CD spectra in the visible region. As published previously [24], the CD spectrum of holo-SufA has distinct positive peaks at 345 nm and 460 nm and a negative peak at 390 nm (Fig. 1). However, the CD spectrum of holo-Fdx overall is more intense than holo-SufA and has a sharp peak at 434 nm as well as a smaller shoulder ~460 nm and minima at 378, 500, and 550 nm (Fig. 1) consistent with previous reports [30]. In contrast to SufA and Fdx, the CD spectrum of [4Fe-4S]2+ His6-SufB2C2 or SufBC2D has only low-intensity, broad features ~400–500 nm (Fig. 1) consistent with previous reports [24]. These differences among the spectra allow us to partially distinguish Fe-S cluster binding among the SufA, His6-SufB2C2, SufBC2D, and Fdx proteins in real-time using CD spectroscopy.
Fig. 1. Circular dichroism spectra of holo-SufB2C2, holo-SufBC2D, holo-SufA, and holo-Fdx.
CD spectra of 300 μM holo-SufB2C2 (green line), holo-SufBC2D (red dotted line), or holo-SufA (blue dashed line), and 200 μM of as-purified holo-Fdx (black line).
3.2 De novo assembly of clusters on Fdx in the presence of the Suf proteins
Fe-S apo-proteins can spontaneously form Fe-S clusters in vitro when supplied with iron and sulfide under reducing conditions in an anaerobic atmosphere. However, Fe-S scaffold proteins enhance Fe-S cluster assembly in target apo-proteins even if sodium sulfide and ferrous ammonium sulfate (FAS) are used as easily accessible cluster building blocks [16]. Due to its distinctive CD spectrum, Fdx was chosen as a target protein to test if His6-SufB2C2, SufBC2D, or SufA are able to enhance cluster assembly in an apo-protein native to E. coli.
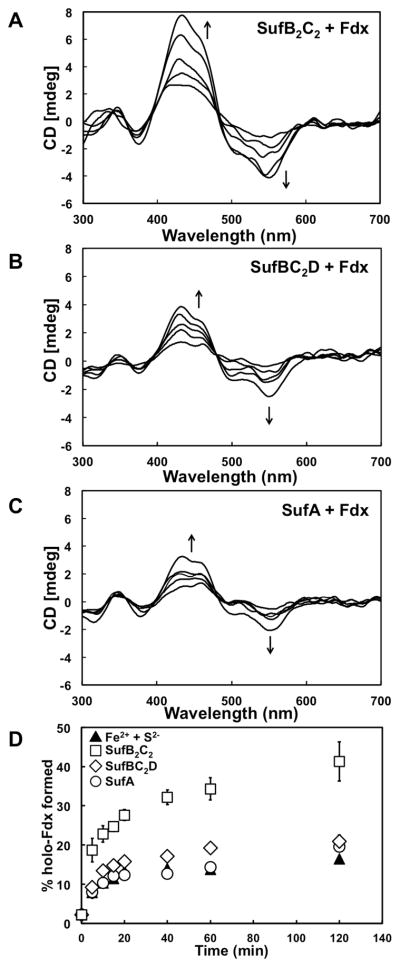
To determine if His6-SufB2C2 enhances Fe-S cluster formation as a scaffold, apo-Fdx and apo-His6-SufB2C2 were incubated anaerobically in a 1:1 molar ratio in the presence of 5-fold molar excess of FAS and Na2S. For comparison, Fdx was reconstituted in the absence of His6-SufB2C2 under similar conditions (data not shown). The assembly of a [2Fe-2S]2+ cluster on Fdx was monitored at 5 min intervals by CD spectroscopy (Fig. 2A). The CD spectra show the appearance of features similar to holo-Fdx, indicating that cluster assembly is occurring on Fdx (Fig. 2A). Over the time, the presence of His6-SufB2C2 significantly enhanced the amount of [2Fe-2S]2+ Fdx formed by over 3-fold as compared to a control assembly reaction containing only Fdx (Fig. 2D). If the de novo assembly reaction was allowed to proceed overnight (18 h), 46% of the apo-Fdx was converted to [2Fe-2S]2+ Fdx if His6-SufB2C2 was present but only 21% [2Fe-2S]2+ Fdx formed in the control assembly reaction.
Fig. 2. De novo Fe-S cluster assembly on Fdx in the presence of SufB2C2, SufBC2D, or SufA.
A, 1 mM FAS and Na2S were added to a solution containing 200 μM apo-Fdx with 200 μM (A) apo-SufB2C2, (B) apo-SufBC2D, or (C) apo-SufA and CD spectra were recorded at 5 min intervals for 2 h with a final scan at 18 h. For clarity only spectra obtained at 5, 10, 20, 60, and 120 min are shown. D, Comparison of cluster formation on Fdx in the presence of apo-SufB2C2 (squares), apo-SufBC2D (diamonds), apo-SufA (circles), or just Na2S and FAS (filled triangles). Holo-Fdx formation was calculated by comparing the changes in ellipticity at 434 nm with the 434 nm ellipticity of 100% [2Fe-2S] Fdx prepared separately. Error bars for multiple experiments are shown (but may be obscured by some sybmols).
Apo-SufBC2D and apo-SufA were also tested for their ability to function as a scaffold and enhance cluster assembly on Fdx (Fig. 2B – 2D). Fig. 2 shows that neither SufBC2D nor SufA significantly enhance de novo cluster formation on Fdx. Over time, the presence of SufBC2D mildly increased the amount of [2Fe-2S]2+ Fdx formed by approximately 50% as compared to a control assembly reaction containing only Fdx (Fig. 2D). If the de novo assembly reaction was allowed to proceed overnight (18 h), 33% of the apo-Fdx was converted to [2Fe-2S]2+ Fdx if SufBC2D was present compared to 21% in the control assembly reaction. The amount of cluster formed on Fdx in the presence of SufA is quantitatively the same as that formed with just Fdx alone (Fig. 2C and 2D). If the de novo assembly reaction was allowed to proceed overnight (18 h), 19% of the apo-Fdx was converted to [2Fe-2S]2+ Fdx if SufA was present as compared to 21% [2Fe-2S]2+ Fdx formed in the control assembly reaction. Therefore SufA does not function as a scaffold for de novo assembly of Fe-S cluster on Fdx using FAS and Na2S.
Since SufB and SufA can accommodate multiple cluster types, it is possible that novel Fe-S cluster types on His6-SufB2C2, SufBC2D, and/or SufA during the de novo assembly reactions lead to new CD features that resemble [2Fe-2S] Fdx. To confirm that [2Fe-2S] Fdx is formed during the de novo assembly reaction, Fdx was separated from His6-SufB2C2, His6-SufBC2D, or His6-SufA after 20 min of de novo assembly and analyzed independently (Fig. S3). Fdx could be efficiently separated from His6-SufB2C2, His6-SufBC2D, or His6-SufA using chromatography, as assessed by SDS-PAGE analysis of the elution fractions, and no stable co-complexes between His6-SufB2C2, His6-SufBC2D, or His6-SufA and Fdx were observed (data not shown). The UV-visible absorption and CD spectroscopy features of holo-Fdx assembled in the presence of His6-SufB2C2, His6-SufBC2D, or His6-SufA are essentially identical to those of holo-Fdx purified from E. coli (Figs. S2A and S3) indicating that holo-Fdx is formed. Based on UV-visible absorption spectroscopy, His6-SufB2C2, His6-SufBC2D, and His6-SufA also contained Fe-S clusters after the assembly reaction although their absorption intensities were lower than Fdx. The CD spectrum of His6-SufA also did not show any prominent features (Fig. S3F), further demonstrating that little [2Fe-2S] cluster was on His6-SufA after the de novo assembly reaction. In conclusion, these results show that [2Fe-2S] Fdx is formed during the de novo assembly reactions and validate the use of CD spectroscopy to monitor this process.
Previously it was shown that the IscU scaffold from E. coli functions as a catalyst to enhance Fe-S cluster assembly on Fdx [16]. IscU can carry out multiple turnovers of cluster assembly and transfer to Fdx (with a kcat of 0.21 min−1) when provided with excess Na2S and FAS. The reaction kinetics of His6-SufB2C2, SufBC2D, or SufA mediated cluster assembly on Fdx were investigated by varying the amount of apo-Fdx from 10–200 μM while using a constant catalytic amount of apo-His6-SufB2C2, apo-SufBC2D, or apo-SufA (10 μM). By monitoring the change in ellipticity at 434 nm over time, the initial velocity of the reaction was determined. In stark contrast to previous results with IscU, none of the Suf proteins were effective catalysts when present at sub-stoichiometric amounts (Fig. S5). Indeed there was actually a lower rate of cluster formation on Fdx in the presence of SufA as compared to Fdx alone, especially at higher Fdx concentrations (Fig. S5C). The lack of catalytic action by the Suf proteins (including His6-SufB2C2) is probably due to a strict requirement for the proper iron donor and/or other associated proteins in order for the Suf pathway to turn over effectively. Here we are essentially analyzing only a small piece of the complex network of protein-protein interactions necessary for Suf-mediated Fe-S cluster assembly and transfer, as will be discussed below (Scheme 1).
3.3 Direct cluster transfer from Suf proteins to apo-Fdx
Based on the results above, His6-SufB2C2 can enhance de novo cluster assembly on Fdx while SufBC2D and SufA have little impact on Fdx formation. While these results suggest His6-SufB2C2 is a scaffold complex, both SufB and SufA can transfer pre-assembled clusters to target proteins. To compare the Fe-S cluster transfer abilities of His6-SufB2C2, SufBC2D, and SufA, we investigated direct transfer of pre-assembled Fe-S clusters from holo-Suf proteins to apo-Fdx. His6-SufB2C2, SufBC2D, or SufA were separately reconstituted with Fe-S clusters and purified as described in Experimental Procedures. Holo-Suf proteins were then added in a 1.5-fold molar excess to apo-Fdx. The CD spectra of the cluster transfer mixtures were recorded at 10 min intervals for a period of 60 min. The appearance of CD features specific for holo-Fdx was tracked to monitor cluster transfer from the Suf proteins to Fdx.
During the transfer of cluster from holo-His6-SufB2C2 or holo-SufBC2D to apo-Fdx, holo-Fdx CD features were observable within the first 10 min (Fig. 3A and 3B). Cluster transfer from holo-His6-SufB2C2 and holo-SufBC2D to Fdx was faster than de novo holo-Fdx maturation using iron and sulfide in solution (Fig. 3D) and the final 18 h yield of holo-Fdx formed using holo-His6-SufB2C2 or holo-SufBC2D as the cluster donor was approximately 80 – 100% whereas the 18 h yield observed for de novo reconstitution on Fdx in the absence of SufBC2D is only ~21%. To ensure that maturation of holo-Fdx does not indirectly occur due to degradation of the SufB cluster and subsequent rebinding of free iron and sulfide in solution by Fdx, the same transfer was repeated in the presence of the divalent metal chelator EDTA (Fig. S2B). While EDTA could inhibit de novo cluster reconstitution of Fdx using free iron and sulfide, it had no appreciable effect on Fe-S cluster transfer from SufB to Fdx (Fig. S2B). Thus cluster maturation of Fdx using holo-SufB occurs via direct cluster transfer to Fdx.
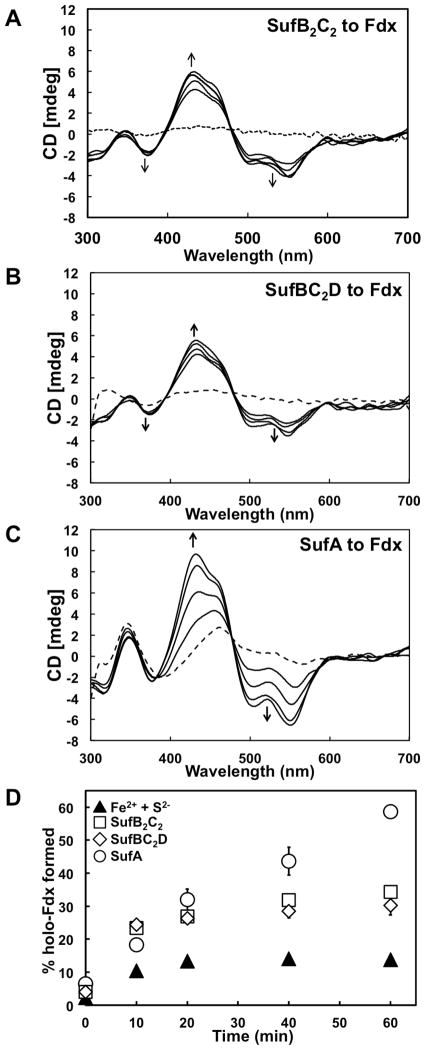
Fig. 3. Fe-S cluster transfer from holo-SufB2C2, holo-SufBC2D, or holo-SufA to apo-Fdx.
A, CD spectra of a mixtures containing 300 μM (A) holo-SufB2C2, (B) holo-SufBC2D, or (C) holo-SufA and 200 μM of apo-Fdx recorded at 10, 20, 40 and 60 min time intervals. The dashed lines show the CD spectrum of holo-Suf proteins at 0 min time point. D, Comparison of holo-Fdx formed over time during transfer from holo-SufB2C2 (squares), holo-SufBC2D (diamonds), or holo-SufA (circles) to de novo reconstitution on Fdx with an equivalent amount of Na2S and FAS in the absence of Suf proteins (filled triangles). Error bars for multiple experiments are shown (but may be obscured by some sybmols).
Cluster transfer from holo-SufA to apo-Fdx was also monitored over time by CD spectroscopy (Fig. 3C). Since both holo-SufA and holo-Fdx exhibit partially overlapping CD features, the resulting transfer CD spectra look slightly different than those obtained using holo-SufBC2D for cluster transfer. However, Fdx has a distinct high intensity peak at 434 nm whereas SufA has a 460 nm peak with much lower intensity (see Fig. 1). Initially the peak at 460 nm from holo-SufA is observed with no distinct peak at 434 nm. Over time the maximum positive intensity shifts from the 460 nm peak towards a 434 nm peak, indicating the formation of holo-Fdx (Fig. 3C). Fe-S cluster transfer from holo-SufA to apo-Fdx appears to occur more rapidly than transfer from holo-His6-SufB2C2 or holo-SufBC2D (Fig. 3D). The final 18 h yield of holo-Fdx formed after cluster transfer from holo-SufA was ~77%, slightly lower but still comparable to the yield using holo-His6-SufB2C2 or holo-SufBC2D and greater than that obtained from de novo reconstitution on Fdx alone (~21%).
To confirm that the final spectra observed for the transfer reaction only arises from [2Fe-2S] Fdx and does not include an altered form of holo-SufA, Fdx was separated from the donor at an intermediate time point (20 min) of the transfer reaction and analyzed independently (Fig. S6). Fdx separated from His6-SufA after 20 min of the transfer reaction and isolated holo-Fdx showed nearly identical UV-visible absorption and CD features (Fig. S6 compared to Figs. S2A and S3). Nearly 100% of the total iron initially present in holo-His6-SufA was recovered in either His6-SufA or Fdx after the separation. Only 20% of the total iron was still in His6-SufA following the separation; the remaining 80% of total iron was bound to Fdx. The intensity of the CD spectrum for separated Fdx was in good agreement with the measured iron content of the Fdx sample.
Levels of holo-Fdx formation during cluster transfer show that while holo-His6-SufB2C2 or holo-SufBC2D can carry out the cluster transfer step in vitro, SufA is more efficient at the Fe-S cluster transfer step than either SufB complex (Fig 3D). These results suggest that SufA is more proficient than SufB as an Fe-S cluster carrier protein for downstream maturation of target [2Fe-2S] metalloproteins.
3.4 SufA is an intermediate for cluster transfer from SufB to apo-Fdx
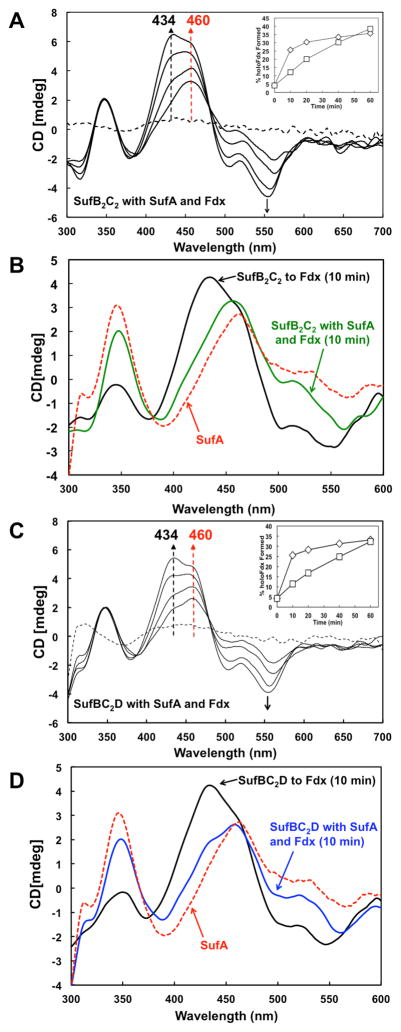
Previous studies showed that SufA interacts with SufB, efficiently receives Fe-S cluster from holo-SufBC2D, and that the presence of SufB (as part of SufBC2D) enhances de novo cluster maturation in SufA [24]. SufA also can transfer pre-assembled clusters to Fe-S target proteins such as Fdx or AcnA [25]. Since results from this study also show that holo-His6-SufB2C2 or holo-SufBC2D can transfer cluster to Fdx, we tested if the addition of apo-SufA would affect the cluster transfer from holo-His6-SufB2C2 or holo-SufBC2D to apo-Fdx. Apo-SufA was included in equimolar amounts to apo-Fdx in the transfer reaction mixture containing holo-His6-SufB2C2 or holo-SufBC2D. The CD spectra of the reaction mixture were recorded at 10 min time intervals (Fig. 4).
Fig. 4. Fe-S cluster transfer from holo-SufB2C2 or holo-SufBC2D to apo-Fdx in the presence of apo-SufA.
A, CD spectra of 300 μM of holo-SufB2C2 with 200 μM each of apo-Fdx and apo-SufA were recorded over time. Inset, Comparison of holo-Fdx formed during transfer from holo-SufB2C2 alone (diamonds) or from holo-SufB2C2 with apo-SufA (squares). B, 10 min time point CD spectra of cluster transfer to apo-Fdx from holo-SufB2C2 alone (black line) or from holo-SufB2C2 with apo-SufA (green line). 300 μM Holo-SufA alone (red dashed line) is shown for comparison. C, CD spectra of 300 μM of holo-SufBC2D with 200 μM each of apo-Fdx and apo-SufA were recorded over time. Inset, Comparison of holo-Fdx formed during transfer from holo-SufBC2D alone (diamonds) or from holo-SufBC2D with apo-SufA (squares). D, 10 min time point CD spectra of cluster transfer to apo-Fdx from holo-SufBC2D alone (black line) or from holo-SufBC2D with apo-SufA (blue line). 300 μM holo-SufA alone (red dashed line) is shown for comparison.
During Fe-S cluster transfer from holo-His6-SufB2C2 or holo-SufBC2D to apo-Fdx in the presence of apo-SufA there is an early appearance of a 460 nm peak with no distinct 434 nm peak (Fig. 4A and 4C). Over time, the peak at 460 nm becomes overshadowed by the appearance of an intense 434 nm peak as seen previously for cluster transfer directly from holo-His6-SufB2C2, holo-SufBC2D, or holo-SufA to apo-Fdx (Fig. 3). When the spectra measured at the first time point (10 min) of the cluster transfer reactions are overlaid, it is clear that cluster transfer from holo-His6-SufB2C2 or holo-SufBC2D to holo-SufA occurs prior to appreciable holo-Fdx formation (Fig. 4B and 4D). If apo-SufA is present, the CD spectrum at 10 min looks nearly identical to the CD spectrum of [2Fe-2S] SufA alone (Fig. 4B and 4D) [24]. In particular, the two pronounced peaks of similar intensity at 345 nm and 460 nm are indicative of holo-SufA. In contrast, at 10 min there is little to no peak at 434 nm, showing that holo-Fdx has not yet begun to mature to a significant level (Fig. 4B and 4D). As the transfer reaction progresses beyond 10 min, there is an increase in holo-Fdx specific CD features, such as the intense peak at 434 nm (Fig. 4A and 4C). If apo-SufA is not present during the transfer, by 10 min holo-His6-SufB2C2 or holo-SufBC2D generate only holo-Fdx with a strong 434 nm peak (Fig. 4B and 4D). Maturation of Fdx during the first 20 min of cluster transfer from holo-His6-SufB2C2 or holo-SufBC2D is slightly slower in the presence of apo-SufA than if SufA is omitted but the amount of holo-Fdx formed catches up after approximately 30 min (Fig. 4A and 4C, insets). Though the presence of SufA slows the early maturation of holo-Fdx, SufA does not limit overall cluster acquisition by apo-Fdx since the final 18 h yield of holo-Fdx formed by transfer from holo-His6-SufB2C2 or holo-SufBC2D in the presence of SufA was 90 – 96%. The holo-Fdx yield is comparable to the yields observed in the earlier one-step cluster transfer reactions from holo-His6-SufB2C2 or holo-SufBC2D. Since the early appearance of holo-SufA does not restrict the final yield of holo-Fdx, this argues that SufA acts as a Fe-S cluster transfer intermediate from holo-SufBC2D to Fdx and is not simply competing with Fdx for cluster acquisition from holo-SufBC2D.
3.5 Effect of ATP on cluster transfer to apo-Fdx
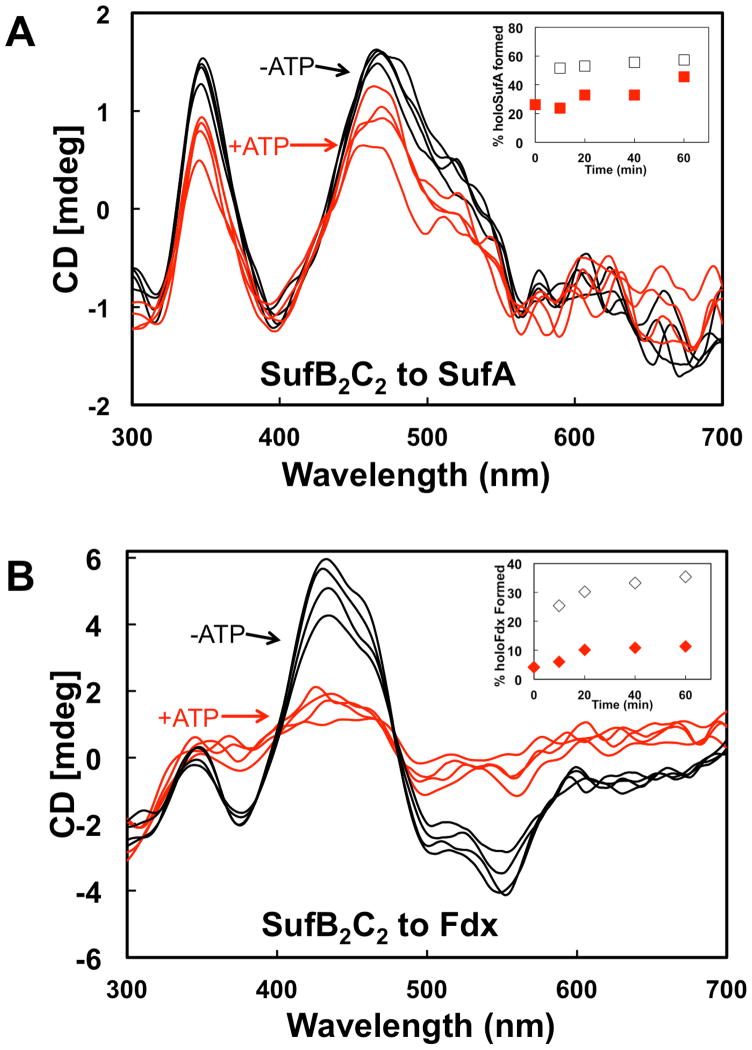
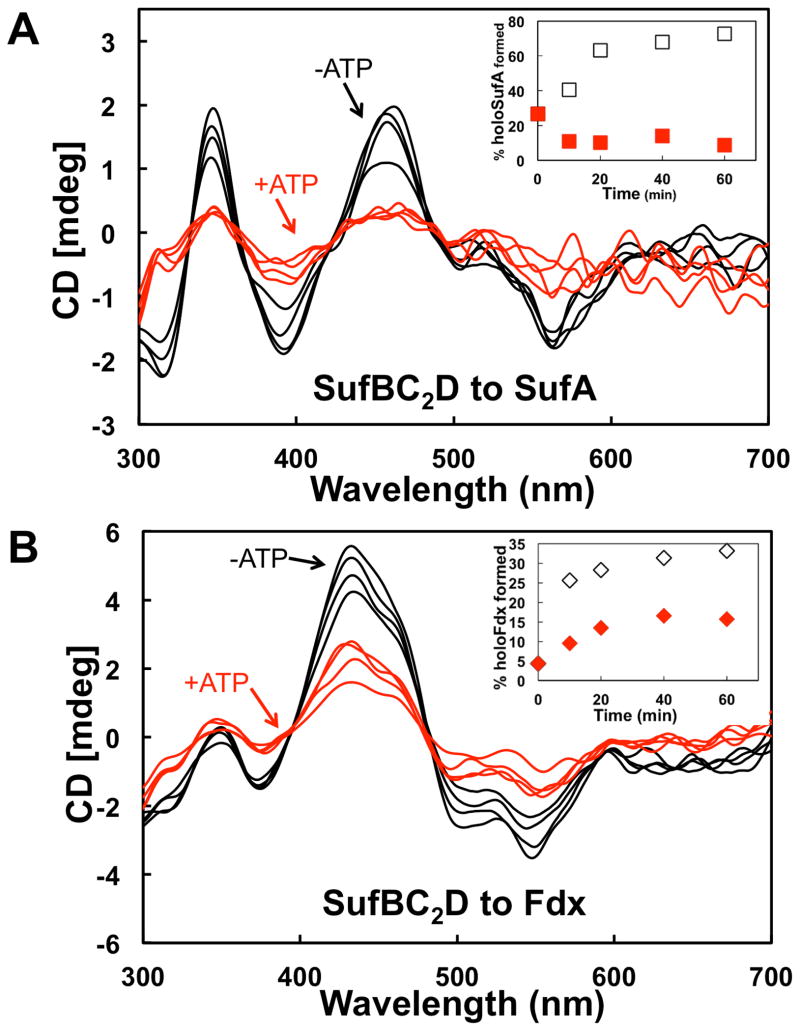
SufC has ATPase activity that is enhanced by interaction with SufB and SufD [31]. Recent studies suggest that SufC ATPase activity might be required for in vivo Fe acquisition [21]. However, in vitro studies of the A. vinelandii Isc system show that the ATPase activity of the HscA-HscB chaperone system mediates cluster transfer from [2Fe-2S] IscU to apo-Fdx [18]. It is possible that SufC ATPase activity could be utilized for cluster transfer from holo-His6-SufB2C2 or holo-SufBC2D as well as for in vivo iron acquisition by apo-SufBC2D. We tested the effect of ATP on cluster transfer from holo-His6-SufB2C2 or holo-SufBC2D to apo-SufA or apo-Fdx. Cluster transfer from holo-His6-SufB2C2 to SufA was somewhat slowed by the addition of ATP although SufA maturation by 60 minutes was nearly the same in both conditions (Fig. 5A, inset). In contrast, cluster transfer from holo-His6-SufB2C2 to Fdx was significantly reduced by Mg-ATP addition (Fig. 5B). These results show that addition of ATP inhibits [2Fe-2S] cluster transfer from holo-His6-SufB2C2 to Fdx but only slightly slows transfer from holo-His6-SufB2C2 to SufA. Fe-S cluster transfer from holo-SufBC2D to SufA was inhibited approximately 10-fold in the presence of Mg-ATP (Fig. 6A). Fe-S cluster transfer from holo-SufBC2D to Fdx also was slowed upon addition of Mg-ATP but only by approximately 50% compared to without ATP (Fig. 6B). In summary, SufB-mediated Fe-S cluster transfer to the [2Fe-2S] proteins SufA and Fdx is partially or fully inhibited by the addition of Mg-ATP, although the holo-His6-SufB2C2 to SufA transfer is least affected by ATP.
Fig. 5. Fe-S cluster transfer from holo-SufB2C2 in the presence of Mg-ATP.
CD spectra of cluster transfer from holo-SufB2C2 to (A) SufA or (B) Fdx at 10 min intervals with 2 mM Mg-ATP (red traces) or without Mg-ATP (black traces). Insets, Comparison of Fe-S SufA (A inset) or Fdx (B inset) formed over time during cluster transfer from SufB2C2 with added Mg-ATP (red symbols) or without Mg-ATP (open symbols). % holo-proteins were calculated from intensity of CD features at 460 nm (SufA) or 434 nm (Fdx). Transfer reactions were conducted as described in Experimental Procedures.
Fig. 6. Fe-S cluster transfer from holo-SufBC2D in the presence of Mg-ATP.
CD spectra of cluster transfer from holo-SufBC2D to (A) SufA or (B) Fdx at 10 min intervals with 2 mM Mg-ATP (red traces) or without Mg-ATP (black traces). Insets, Comparison of Fe-S SufA (A inset) or Fdx (B inset) formed over time during cluster transfer from SufBC2D with added Mg-ATP (red symbols) or without Mg-ATP (open symbols). % holo-proteins were calculated from intensity of CD features at 460 nm (SufA) or 434 nm (Fdx). Transfer reactions were conducted as described in Experimental Procedures.
4. Discussion
Recent studies demonstrated that SufBC2D can transfer Fe-S clusters to [4Fe-4S] targets (AcnB) or [2Fe-2S] targets (SufA) in vitro [23, 24]. While a detailed time-course of sequential cluster assembly on SufB2C2 and SufBC2D has not been published, SufB primarily reconstitutes with a [4Fe-4S]2+ cluster but can accommodate a [2Fe-2S] cluster and may also bind a linear [3Fe-4S] cluster [20, 21, 23]. Thus it appears that SufB has the ability to exist in multiple cluster-bound states. Similarly, it has been shown that SufA can form both [2Fe-2S]2+ and [4Fe-4S]2+ clusters in vitro (although the [2Fe-2S]2+ form appears to predominate in vivo) and that holo-SufA can transfer Fe-S clusters to target proteins such as AcnA or Fdx [25, 26]. Furthermore, it seems likely that several multi-protein complexes form during the Suf Fe-S cluster assembly, including SufB2C2 and SufBC2D, and that transient interactions also occur between the Suf proteins, potential iron donors, Fe-S carriers, and target metalloenzymes (Scheme 1).
Stoichiometric SufB2C2 enhanced de novo assembly of [2Fe-2S] Fdx but was unable to carry out multiple turnovers under these in vitro conditions (Fig. 2). In contrast to the IscU scaffold, sub-stoichiometric SufB2C2 cannot efficiently catalyze multiple cycles of de novo cluster assembly and cluster transfer to Fdx when using FAS and Na2S as building blocks (Fig. S5). In vivo, SufS and SufE are necessary to mobilize sulfur from free cysteine and then transfer the resulting persulfide to SufB [8, 20, 22, 32]. We also conducted the de novo cluster assembly reactions using SufS, SufE, and cysteine for sulfur delivery (Fig. S4). SufS-SufE and L-cysteine were able to “donate” sulfur to Fdx alone leading to a low level of Fdx maturation, although this was likely due to the non-specific release of sulfide via DTT-mediated reduction of the SufS and/or SufE persulfide intermediates rather than true sulfur transfer. Using SufS-SufE with L-cysteine as the sulfur donor actually slowed the overall rate of [2Fe-2S] Fdx formation in the presence of SufB complexes as compared to using free Na2S. The slower cluster maturation may be due to the additional time required to release sulfur from the L-cysteine substrate and transfer persulfide from SufS to SufE to SufB for de novo cluster assembly. The presence of SufS-SufE also did not promote multiple turnovers of cluster assembly on SufB, indicating that our in vitro assay still lacks one or more critical component to drive Suf catalysis. Despite the slower rates of cluster maturation in all samples using SufS-SufE as sulfur donors, addition of His6-SufB2C2 still enhanced [2Fe-2S] Fdx maturation compared to SufBC2D, SufA, or the control (Fig. S4), providing further support for its role as the terminal scaffold. It is likely that both the in vivo sulfur donor (SufS-SufE) and an unknown iron donor must be present for SufB2C2 and/or SufBC2D to turn over. Without the in vivo iron donor it may be difficult to fully reconstitute the entire Suf cluster assembly system in vitro. However, these results highlight important differences between IscU and SufB. In particular, SufB cluster assembly appears to be more tightly regulated than IscU cluster assembly. Presumably this regulation is necessary to protect iron, persulfide, and the nascent Fe-S cluster during stress conditions when the E. coli Suf pathway is induced.
4.1 SufA is a Fe-S carrier for target [2Fe-2S] metalloproteins
In contrast to SufB2C2, SufA did not function as a scaffold to enhance de novo assembly of [2Fe-2S] Fdx (Fig. 2). However, cluster transfer from pre-assembled [2Fe-2S] SufA to Fdx was more efficient than cluster transfer from [4Fe-4S] SufB2C2 and SufBC2D to Fdx (Fig. 3). The differential activity of SufA in the de novo assembly reaction compared to the transfer reaction indicates that the rate-limiting step in de novo cluster maturation using SufA is the de novo assembly of intact cluster on SufA itself. SufA cluster assembly seems to be slower than de novo assembly on Fdx such that assembly on SufA does not enhance Fdx maturation. It has also been shown that apo-SufA has significant iron-binding ability in vitro which may complicate the de novo assembly reaction [33]. Why is SufA better at cluster transfer to Fdx than the SufB-containing complexes? Since SufA is primarily a [2Fe-2S]2+ protein as purified or as reconstituted, it would not need to undergo a cluster conversion step prior to transfer to Fdx, which could explain its relative transfer efficiency as compared to [4Fe-4S] SufB2C2 and SufBC2D in the cluster transfer reaction [25]. Alternatively, the structure of SufA may promote more rapid release of the [2Fe-2S] cluster as compared to SufB2C2 and SufBC2D. Further studies are necessary to fully explain the differences in transfer efficiency.
Previous studies on the Isc system showed specific Fe-S cluster transfer from IscU to IscA without appreciable cluster transfer from IscA to IscU [14]. Similarly, in the Suf system, cluster transfer proceeds from SufB to SufA but not in the reverse direction [24]. These results suggest a unidirectional pathway of cluster transfer from scaffold proteins (IscU, SufB) to carrier proteins (IscA, SufA). However, it was not clear from these earlier studies if the presence of a target apoprotein would alter this linear cluster transfer pathway from SufB to SufA. Here we have found that Fe-S cluster transfer from SufB2C2 and SufBC2D to apo-Fdx will proceed through a holo-SufA intermediate if apo-SufA is present during transfer (Fig. 4). Formation of the [2Fe-2S] SufA intermediate slightly decreases the overall rate of cluster transfer but does not alter the final yield of [2Fe-2S] Fdx. Although the presence of SufA does not enhance the rate of cluster transfer from SufB to the target proteins in our in vitro conditions, it is possible this carrier protein provides other mechanistic advantages for cluster transfer to specific target proteins under physiological conditions. For example, Fe-S carriers may provide additional downstream specificity for different target proteins in vivo [13]. Fe-S carriers may also provide additional protection for the cluster that would not occur during direct transfer from scaffolds to target proteins [34, 39, 41] but without necessarily enhancing the speed of transfer.
Genetic and biochemical studies also have suggested that the IscU and SufB scaffolds likely interact with multiple Fe-S carriers in E. coli, including NfuA and the monothiol glutaredoxin GrxD, as well as the A-type carrier proteins like SufA, IscA, and ErpA [13, 34–40]. Together these carrier proteins likely constitute a labile pool of intact Fe-S clusters that can then be rapidly distributed to multiple Fe-S metalloproteins as required by cellular metabolism and environmental conditions. In this scenario the main function of scaffold proteins would be to maintain the labile pool of Fe-S clusters on carrier proteins (as opposed to directly loading target proteins). Nascent Fe-S clusters on scaffold proteins like IscU and SufB are notoriously labile, probably due to the various conformations the scaffold protein must utilize for iron and sulfur acquisition as well as inter-cluster conversion. Rapid transfer of the Fe-S cluster to a pool of Fe-S carrier proteins may be required in vivo to limit cluster degradation on the more exposed scaffold proteins after de novo cluster assembly.
4.2 ATP represses cluster transfer from [4Fe-4S] SufBC2D to [2Fe-2S] target metalloproteins
In the Isc system, ATP hydrolysis by the HscA chaperone plays a crucial role in transferring the cluster from holo-IscU to apo-proteins [42]. ATP hydrolysis by HscA/B enhances cluster transfer up to 20-fold from [2Fe-2S] IscU to Fdx [18, 43] but does not enhance cluster transfer from [4Fe-4S] IscU to AcnA [17]. We found that ATP actually diminishes cluster transfer from [4Fe-4S] His6-SufB2C2 to [2Fe-2S] Fdx and from [4Fe-4S] SufBC2D to [2Fe-2S] SufA or [2Fe-2S] Fdx (Figs. 5 and 6). Since ATP is typically present at 3 – 4 mM in E. coli, ATP inhibition of cluster transfer from [4Fe-4S] SufBC2D to SufA (as well as Fdx) likely occurs in vivo. Previous results suggested a role for both SufD and SufC ATP hydrolysis in the iron acquisition step of cluster assembly in vivo (Scheme 1) [21]. If so, ATP hydrolysis could be coupled to insertion of iron into intermediate cluster forms on SufBC2D during stepwise assembly. In that case, it is logical that ATP binding or hydrolysis by SufBC2D would additionally inhibit cluster transfer to limit premature release of a partially formed cluster from SufBC2D during stepwise assembly.
Since a number of studies have shown that multiple complexes containing SufC can occur, including SufBC2D, SufB2C2, and SufC2D2 (Scheme 1) [21, 44], it is possible that the role of the SufC ATPase varies depending on the specific complex with which it is associated. In fact we observed that Fe-S cluster transfer from SufB2C2 to SufA was only weakly affected by the presence of Mg-ATP whereas cluster transfer from SufB2C2 to Fdx was much more inhibited (Fig. 5B). This result suggests that ATP binding to SufC in SufB2C2 effects cluster transfer differently than ATP binding to SufC in SufBC2D, at least when SufA is the cluster acceptor. Since ATP is likely present throughout the cluster assembly and transfer process, ATP binding to SufB2C2 may limit cluster transfer to downstream target apoproteins but allow transfer to SufA or other A-type carriers in vivo. Another important caveat is that SufB can bind multiple cluster types including [2Fe-2S] (formed upon oxygen exposure), linear [3Fe-4S], and cuboidal [4Fe-4S] clusters [20, 21, 23]. ATP hydrolysis by SufC did not enhance cluster transfer from [4Fe-4S] SufB to [2Fe-2S] target proteins but ATP hydrolysis may have different effects if [2Fe-2S] SufB is used as the donor for cluster transfer to Fdx or if a [4Fe-4S] target protein is used. Studies are underway to test the effect of ATP on these other cluster transfer combinations.
These studies suggest that SufA is an Fe-S cluster carrier protein and does not function as a Fe-S cluster scaffold for target proteins. In contrast, SufB2C2 is able to stimulate de novo assembly of Fe-S clusters in Fdx by building Fe-S clusters from iron and sulfide. [4Fe-4S] SufB is not as efficient as [2Fe-2S] SufA at cluster transfer to [2Fe-2S] Fdx and SufB will interact with SufA first to preferentially transfer cluster to SufA if multiple [2Fe-2S] acceptor proteins are present, providing further evidence that SufB is a Fe-S cluster scaffold complex that distributes clusters to carrier proteins like SufA. The results also demonstrate that SufB2C2 is better than SufBC2D at enhancing de novo cluster assembly in the target protein Fdx, consistent with its proposed function as the terminal scaffold in the Suf pathway (Scheme 1).
Supplementary Material
Highlights.
SufB2C2 acts as a scaffold to enhance Fdx cluster formation
SufA acts as an intermediate during Fe-S cluster transfer
ATP suppresses cluster transfer from SufBC2D to SufA
SufA does not function as a de novo cluster scaffold
Acknowledgments
The authors would like to thank Dr. Caryn E. Outten for thoughtful commentary and critical reading of the manuscript. This work was supported by National Institutes of Health grant GM81706 and a Cottrell Scholar Award from the Research Corporation for Science Advancement (to F.W.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beinert H. J Biol Inorg Chem. 2000;5:2–15. doi: 10.1007/s007750050002. [DOI] [PubMed] [Google Scholar]
- 2.Kispal G, Csere P, Prohl C, Lill R. EMBO J. 1999;18:3981–3989. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schilke B, Voisine C, Beinert H, Craig E. Proc Natl Acad Sci U S A. 1999;96:10206–10211. doi: 10.1073/pnas.96.18.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng L, Cash VL, Flint DH, Dean DR. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson MR, Cash VL, Weiss MC, Laird NF, Newton WE, Dean DR. Mol Gen Genet. 1989;219:49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- 6.Nachin L, El Hassouni M, Loiseau L, Expert D, Barras F. Mol Microbiol. 2001;39:960–972. doi: 10.1046/j.1365-2958.2001.02288.x. [DOI] [PubMed] [Google Scholar]
- 7.Nachin L, Loiseau L, Expert D, Barras F. EMBO J. 2003;22:427–437. doi: 10.1093/emboj/cdg061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outten FW, Djaman O, Storz G. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 9.Patzer SI, Hantke K. J Bacteriol. 1999;181:3307–3309. doi: 10.1128/jb.181.10.3307-3309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi Y, Tokumoto U. J Biol Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 12.Xu XM, Moller SG. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3259. [DOI] [PubMed] [Google Scholar]
- 13.Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. PLoS Genet. 2009;5:e1000497. doi: 10.1371/journal.pgen.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ollagnier-de-Choudens S, Sanakis Y, Fontecave M. J Biol Inorg Chem. 2004;9:828–838. doi: 10.1007/s00775-004-0581-9. [DOI] [PubMed] [Google Scholar]
- 15.Ollagnier-de-Choudens S, Mattioli T, Takahashi Y, Fontecave M. J Biol Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- 16.Bonomi F, Iametti S, Ta D, Vickery LE. J Biol Chem. 2005;280:29513–29518. doi: 10.1074/jbc.M504344200. [DOI] [PubMed] [Google Scholar]
- 17.Unciuleac MC, Chandramouli K, Naik S, Mayer S, Huynh BH, Johnson MK, Dean DR. Biochemistry. 2007;46:6812–6821. doi: 10.1021/bi6026665. [DOI] [PubMed] [Google Scholar]
- 18.Chandramouli K, Johnson MK. Biochemistry. 2006;45:11087–11095. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AD, Jameson GN, Dos Santos PC, Agar JN, Naik S, Krebs C, Frazzon J, Dean DR, Huynh BH, Johnson MK. Biochemistry. 2005;44:12955–12969. doi: 10.1021/bi051257i. [DOI] [PubMed] [Google Scholar]
- 20.Layer G, Gaddam SA, Ayala-Castro CN, Ollagnier-de Choudens S, Lascoux D, Fontecave M, Outten FW. J Biol Chem. 2007;282:13342–13350. doi: 10.1074/jbc.M608555200. [DOI] [PubMed] [Google Scholar]
- 21.Saini A, Mapolelo DT, Chahal HK, Johnson MK, Outten FW. Biochemistry. 2010;49:9402–9412. doi: 10.1021/bi1011546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Outten FW, Wood MJ, Munoz FM, Storz G. J Biol Chem. 2003;278:45713–45719. doi: 10.1074/jbc.M308004200. [DOI] [PubMed] [Google Scholar]
- 23.Wollers S, Layer G, Garcia-Serres R, Signor L, Clemancey M, Latour JM, Fontecave M, Ollagnier de Choudens S. J Biol Chem. 2010;285:23331–23341. doi: 10.1074/jbc.M110.127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chahal HK, Dai Y, Saini A, Ayala-Castro C, Outten FW. Biochemistry. 2009;48:10644–10653. doi: 10.1021/bi901518y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta V, Sendra M, Naik SG, Chahal HK, Huynh BH, Outten FW, Fontecave M, Ollagnier de Choudens S. J Am Chem Soc. 2009;131:6149–6153. doi: 10.1021/ja807551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ollagnier-de Choudens S, Nachin L, Sanakis Y, Loiseau L, Barras F, Fontecave M. J Biol Chem. 2003;278:17993–18001. doi: 10.1074/jbc.M300285200. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy MC, Beinert H. J Biol Chem. 1988;263:8194–8198. [PubMed] [Google Scholar]
- 28.Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Anal Biochem. 2004;331:370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Beinert H. Anal Biochem. 1983;131:373–378. doi: 10.1016/0003-2697(83)90186-0. [DOI] [PubMed] [Google Scholar]
- 30.Ta DT, Vickery LE. J Biol Chem. 1992;267:11120–11125. [PubMed] [Google Scholar]
- 31.Petrovic A, Davis CT, Rangachari K, Clough B, Wilson RJ, Eccleston JF. Protein Sci. 2008;17:1264–1274. doi: 10.1110/ps.034652.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loiseau L, Ollagnier-de-Choudens S, Nachin L, Fontecave M, Barras F. J Biol Chem. 2003;278:38352–38359. doi: 10.1074/jbc.M305953200. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Huang H, Tan G, Si F, Liu M, Landry AP, Lu J, Ding H. Biochem J. 2010;432:429–436. doi: 10.1042/BJ20101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angelini S, Gerez C, Ollagnier-de Choudens S, Sanakis Y, Fontecave M, Barras F, Py B. J Biol Chem. 2008;283:14084–14091. doi: 10.1074/jbc.M709405200. [DOI] [PubMed] [Google Scholar]
- 35.Bandyopadhyay S, Naik SG, O’Carroll IP, Huynh BH, Dean DR, Johnson MK, Dos Santos PC. J Biol Chem. 2008;283:14092–14099. doi: 10.1074/jbc.M709161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butland G, Babu M, Diaz-Mejia JJ, Bohdana F, Phanse S, Gold B, Yang W, Li J, Gagarinova AG, Pogoutse O, Mori H, Wanner BL, Lo H, Wasniewski J, Christopolous C, Ali M, Venn P, Safavi-Naini A, Sourour N, Caron S, Choi JY, Laigle L, Nazarians-Armavil A, Deshpande A, Joe S, Datsenko KA, Yamamoto N, Andrews BJ, Boone C, Ding H, Sheikh B, Moreno-Hagelseib G, Greenblatt JF, Emili A. Nat Methods. 2008;5:789–795. doi: 10.1038/nmeth.1239. [DOI] [PubMed] [Google Scholar]
- 37.Loiseau L, Gerez C, Bekker M, Ollagnier-de Choudens S, Py B, Sanakis Y, Teixeira de Mattos J, Fontecave M, Barras F. Proc Natl Acad Sci U S A. 2007;104:13626–13631. doi: 10.1073/pnas.0705829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Yang J, Tan G, Ding H. Biochem J. 2008;409:535–543. doi: 10.1042/BJ20071166. [DOI] [PubMed] [Google Scholar]
- 39.Tan G, Lu J, Bitoun JP, Huang H, Ding H. Biochem J. 2009;420:463–472. doi: 10.1042/BJ20090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeung N, Gold B, Liu NL, Prathapam R, Sterling HJ, Willams ER, Butland G. Biochemistry. 2011;50:8957–8969. doi: 10.1021/bi2008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson DC, Unciuleac MC, Dean DR. J Bacteriol. 2006;188:7551–7561. doi: 10.1128/JB.00596-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vickery LE, Cupp-Vickery JR. Crit Rev Biochem Mol Biol. 2007;42:95–111. doi: 10.1080/10409230701322298. [DOI] [PubMed] [Google Scholar]
- 43.Bonomi F, Iametti S, Morleo A, Ta D, Vickery LE. Biochemistry. 2008;47:12795–12801. doi: 10.1021/bi801565j. [DOI] [PubMed] [Google Scholar]
- 44.Wada K, Sumi N, Nagai R, Iwasaki K, Sato T, Suzuki K, Hasegawa Y, Kitaoka S, Minami Y, Outten FW, Takahashi Y, Fukuyama K. J Mol Biol. 2009;387:245–258. doi: 10.1016/j.jmb.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangachari K, Davis CT, Eccleston JF, Hirst EM, Saldanha JW, Strath M, Wilson RJ. FEBS Lett. 2002;514:225–228. doi: 10.1016/s0014-5793(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 46.Wada K, Sumi N, Nagai R, Iwasaki K, Sato T, Suzuki K, Hasegawa Y, Kitaoka S, Minami Y, Outten FW, Takahashi Y, Fukuyama K. J Mol Biol. 2009;387:245–258. doi: 10.1016/j.jmb.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.