Abstract
Escherichia coli DNA damage inducible protein DinG is a superfamily II DNA helicase and is closely related to human DNA helicase XPD. Here, we report that E. coli single-stranded DNA binding protein (SSB) is able to form a stable protein complex with DinG and to stimulate the DinG DNA helicase activity. An SSB mutant that retains the single-stranded DNA binding activity but fails to form a protein complex with DinG becomes a potent inhibitor for the DinG DNA helicase, suggesting that E. coli wild-type SSB stimulates the DinG DNA helicase via specific protein-protein interaction.
Keywords: DNA damage, DinG, XPD, SSB, DNA helicase, iron-sulfur protein
1. Introduction
Escherichia coli gene dinG (DNA damage inducible gene G) is a member of the regulon induced by DNA damaging agents [1]. Purified E. coli DinG has an ATP-dependent helicase activity that unwinds double-stranded DNA [2], DNA-RNA duplex, D-loops, and R-loops [3]. Although the physiological function of DinG has not been fully understood, recent studies suggested that DinG may act to remove R-loops or together with other DNA helicases Rep and UvrD to promote replication across highly transcribed regions in E. coli genome [4]. Structurally, E. coli DinG belongs to superfamily II DNA helicases with 5′ to 3′ direction [2], and is closely related to yeast DNA helicase Rad3 [5] and human DNA helicases XPD (Xeroderma pigmentosum factor D) [6,7], FANCJ/BACH1 (BRCA1-associated C-terminal helicase) [8], CHLR1 (a DNA helicase involving in sister chromatid cohesion) [9], and RTEL1 (a regulator of telomere length) [10]. Furthermore, like yeast Rad3 [5] and human XPD [11-14], E. coli DinG contains a [4Fe-4S] cluster that is essential for the DNA helicase activity [15]. While the redox property and physiological role of the iron-sulfur cluster in XPD/Rad3 still remain elusive [16,17], we previously reported that the [4Fe-4S] cluster in E. coli DinG is stable and the DNA helicase activity remains fully active after the protein is exposed to 100-fold excess of hydrogen peroxide [15]. On the other hand, reduction of the [4Fe-4S] cluster in DinG reversibly switches off the DNA helicase activity, suggesting that the helicase activity could be regulated by intracellular redox potentials via the [4Fe-4S] cluster [15].
Exposure to DNA damaging agents would dramatically increase the number of single-stranded DNA (ssDNA) ends. In response, cells utilize the specialized ssDNA binding proteins (SSB) to protect ssDNA ends from further damage or re-annealing [18-20]. Importantly, recent studies further showed that SSB not only binds ssDNA but also interacts with a diverse group of DNA processing enzymes (see review [21]). Since both SSB and DinG are highly induced when E. coli cells are subject to DNA damaging agents [18], it would be of interest to explore the possible regulation of the DinG DNA helicase activity by SSB. In this study, we report that E. coli SSB is able to form a stable protein complex with DinG and to stimulate the DinG DNA helicase activity. A possible mechanism underlying the SSB-mediated stimulation of the DinG DNA helicase activity will be discussed.
2. Materials and Methods
2.1 Protein preparation
A DNA fragment encoding the single-stranded DNA binding protein (SSB) was PCR-amplified from E. coli genomic DNA using two primers, SSB-1, 5′-GGAGACACGCATATGGCCAGCAGAG-3′, and SSB-2, 5′-ATTGTGCTAAGCACAAATCAGAACG-3′. The PCR product was digested with NdeI and BlpI, and ligated into an expression vector pET28b+. The cloned DNA fragment was confirmed by DNA sequencing and introduced into an E. coli strain BL21. Recombinant SSB was overproduced in the E. coli cells grown in LB media under aerobic conditions. Cell extracts were treated with DNase (10 units/mL) to remove DNA before protein was purified as previously described in [15]. The N-terminal his-tag in SSB was removed by digestion with thrombin overnight and protein was re-purified using Mono-Q column. Purified SSB contains three extra amino acid residues (Gly-Ser-His) in N-terminus and an intact C-terminus which is responsible for specific interaction with multiple DNA processing proteins [21]. SSB mutant F177C (Phe-177 to Cys) was constructed using the Quikchange mutagenesis kit (Stratagene), and confirmed by DNA sequencing. SSB mutant protein was purified as described for wild-type SSB. Purified wild-type SSB and SSB mutant F177C showed the same ssDNA binding activity, as reported previously by others [22]. Recombinant E. coli DNA helicase DinG was purified as described in [15]. The purity of purified proteins was analyzed using SDS-polyacrylamide electrophoresis. The protein concentration of purified SSB and DinG was estimated from the absorption peak at 280 nm using an extinction coefficient of 27.9 and 78.7 mM−1cm−1, respectively. The bacteriophage single-stranded DNA binding protein gp32 [19] was purchased from New England BioLab.
2.2 Protein-protein interaction analyses
A gel filtration column (Superdex™ 200 (10/300GL)) attached to the ÄKTA FPLC system (GE Healthcare Life Sciences) was used for the protein complex analyses. The column was calibrated using the standard gel filtration protein markers (Sigma). For each run, protein sample (500 μL) was loaded onto the column and eluted with buffer containing NaCl (500 mM) and Tris (20 mM, pH 8.0) at a flow rate of 0.5 mL/min inside a 4°C refrigerator. Eluted fractions (0.5 mL) were collected and aliquots were subject to the SDS polyacrylamide electrophoresis.
The protein-protein interactions were also analyzed using the protein co-precipitation approaches following the procedure described in [23]. Unlike most proteins, E. coli SSB precipitates at 150 g/liter ammonium sulfate. If a protein forms a complex with SSB, the protein will co-precipitate with SSB in the presence of 150 g/liter ammonium sulfate in solution [23].
2.3 DNA helicase activity assay
The DNA helicase activity of E. coli DinG was analyzed following the procedure described by Voloshin et al. [2] with slight modifications [15]. Briefly, an oligonucleotide (5′CCGTAACACTGAGTTTCGTCACCAGTACAAACTACAACGCCTGTAGCATTCCACA-3′) was labeled with 32P-γ-ATP using polynucleotide kinase (New England BioLab). The 32P-labeled oligonucleotide (0.2 μM) was annealed to M13mp18 ssDNA (0.1 μg/μL) (Fisher Scientific) in annealing buffer containing Tris (50 mM, pH 7.5), NaCl (50 mM) and MgCl2 (10 mM). The DNA solution was heated at 85°C for 5 min and cooled to room temperature over 3 hours. The annealed DNA duplex was purified using a gel filtration spin-column Chromaspin 400 (Clontech co.) pre-equilibrated with annealing buffer. The annealed substrate (at a final concentration of 2 nM) was incubated with indicated concentrations of DinG protein in 20 μL the reaction solution containing Tris (50 mM, pH 7.5), NaCl (100 mM), MgCl2 (5 mM), dithiothreitol (2 mM), glycerol (5%), and ATP (2 mM) at 30°C for 10 min. For each experiment, two controls in which the substrate was either denatured by heating at 85 °C for 5 min or incubated at 30°C for 10 min without any enzymes were included. The reactions were terminated by adding 4 μl stop solution (containing 6% SDS, 60 mM EDTA and 0.3% Bromophenol Blue). The reaction products were separated on 1% TAE agarose gel, transferred to nitrocellulose membranes, and exposed to x-ray films overnight for quantification of the reaction products.
3. Results and Discussion
3.1 E. coli DinG forms a stable protein complex with single-stranded DNA binding protein SSB
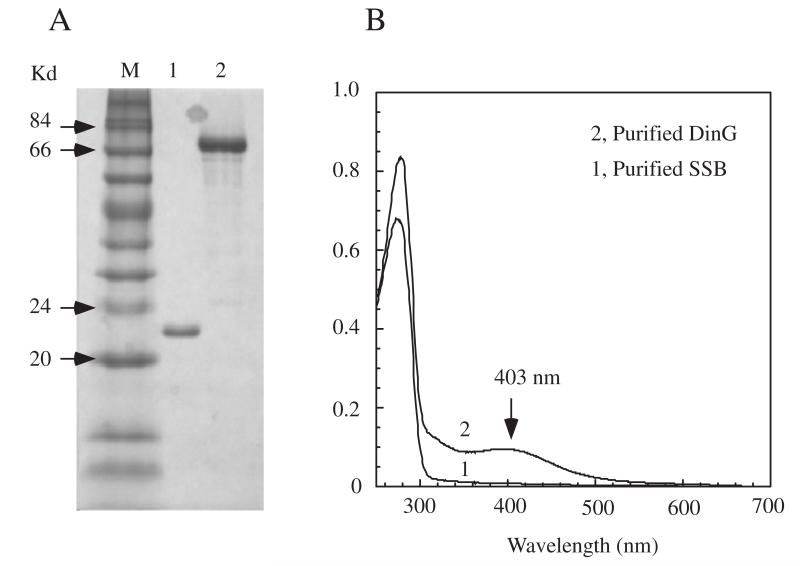
To explore the possible interaction between the DNA-damage inducible proteins DinG and SSB [18], we purified both proteins from E. coli cells as described in Materials and Methods. The SDS PAGE gel analysis showed that both proteins were purified to a single-band (Figure 1A). While purified DinG had an absorption peak at 403 nm of the [4Fe-4S] cluster [15], purified SSB only had the 280 nm protein absorption peak (Figure 1B).
Figure 1. Purification of E. coli DinG and SSB.
A), SDS-PAGE gel of purified E. coli DinG and SSB. Lane M, molecular weight markers; lane 1, purified SSB; lane 2, purified DinG. B), UV-vis absorption spectrum of purified E. coli SSB (spectrum 1) and DinG (spectrum 2). The proteins were dissolved in buffer containing NaCl (500 mM) and Tris (20 mM, pH 8.0). The contraction of SSB and DinG shown in B0 was 22 and 10 μM, respectively.
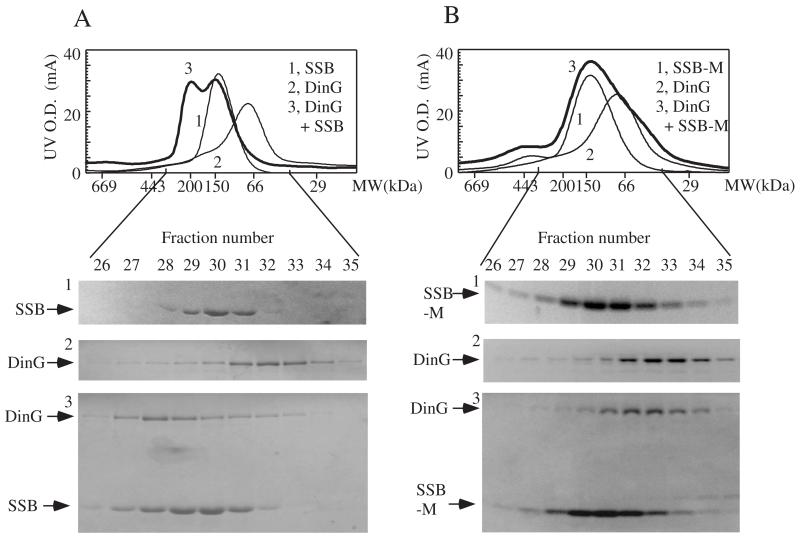
Figure 2A shows the gel filtration profiles of purified SSB and DinG. While purified E. coli SSB formed a tetramer with an apparent molecular weight of ~134 kDa, as reported previously [19,24], purified E. coli DinG existed as a monomer with an apparent molecular weight of ~78 kDa. However, when a mix of DinG and SSB was loaded onto the gel filtration column, a new elution peak with an apparent molecular weight of ~200 kDa appeared. The SDS-PAGE analyses of eluted fractions showed that the new elution peak contained both DinG and SSB (Figure 2A, bottom panel).
Figure 2. Gel filtration analyses of the SSB/DinG protein complex.
A), gel filtration profiles of SSB, DinG and a mix of SSB and DinG. Top panel, gel filtration profiles of SSB (80 μM) (trace 1), DinG (20 μM) (trace 2), and a mix of SSB (80 μM) and DinG (20 μM) (trace 3). The proteins were dissolved in buffer containing NaCl (500 mM) and Tris (20 mM, pH 8.0) and eluted from the gel filtration column using the same buffer. The molecular weights of the standard gel filtration protein markers were labeled on x-axis. Bottom panel, SDS gel photos of the fractions (26 to 35) eluted from the gel filtration column. The protein bands were indicated on the left side. B), gel filtration profiles of SSB mutant F177C, DinG and a mix of SSB mutant F177C and DinG. Top panel, gel filtration profiles of SSB mutant F177C (SSB-M) (80 μM) (trace 1), DinG (20 μM) (trace 2), and a mix of SSB-M (80 μM) and DinG (20 μM) (trace 3). The molecular weights of the standard gel filtration protein markers were labeled on X-axis. Bottom panel, SDS gel photos of the fractions (26 to 35) eluted from the gel filtration column. The protein bands were indicated on the left side. Data are representative of three independent experiments.
Because SSB and DinG are both the DNA binding proteins, any DNA contamination could contribute to formation of SSB/DinG complex. Using DNA indicator ethidium bromide, we were unable to detect any DNA in the protein samples. We also treated the protein samples with DNase before the gel filtration analyses, and found that the elution profiles were essentially identical when the protein samples were treated with or without DNase, further suggesting that formation of SSB/DinG complex does not depend on DNA.
E. coli SSB contains an N-terminal oligonucleotide/oligosaccharide binding domain serving as the ssDNA binding site and the C-terminal highly conserved end (Asp-Asp-Asp-Ile-Pro-Phe) involving in the protein-protein interaction with multiple DNA processing enzymes [21]. To examine whether the C-terminal end of SSB is involved in the protein-protein interaction with DinG, we constructed an E. coli SSB mutant in which the C-terminal end residue Phe-177 was replaced with Cys (F177C). Consistent with the previous report [22], we found that purified SSB mutant F177C formed a tetramer (Figure 2B) and retained the same DNA binding activity as wild-type SSB (data not shown). However, when a mix of SSB mutant F177C and DinG was loaded onto the gel filtration column, a broad elution profile corresponding to the combination of the peaks of SSB mutant F177C and DinG was observed (Figure 2B). The SDS-PAGE analyses of the eluted fractions confirmed that, unlike wild-type SSB, SSB mutant F177C failed to form a stable protein complex with DinG (Figure 2B, bottom panel).
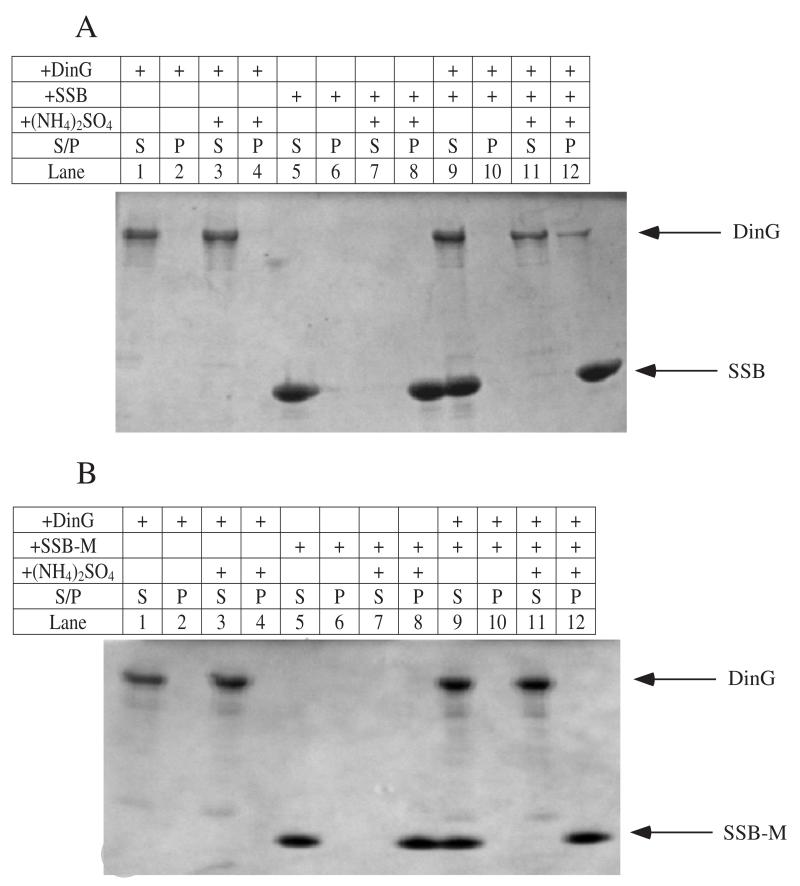
To further explore the protein-protein interaction between SSB and DinG, we adapted protein co-precipitation approaches following the procedures described in [23]. Unlike other proteins, SSB precipitates at a low concentration of ammonium sulfate in solution. Any protein that forms a stable protein complex with SSB would co-precipitate with SSB [23]. As shown in Figure 3A, wild-type SSB co-precipitated a significant amount of DinG in the presence of 150 g/liter ammonium sulfate. In contrast, SSB mutant F177C failed to co-precipitate any DinG under the same experimental conditions (Figure 3B). Thus, wild-type SSB, but not SSB mutant Y177C, is able to form a stable protein complex with DinG via specific protein-protein interaction.
Figure 3. Protein co-precipitation analyses of SSB and DinG.
E. coli DinG (20 μM), SSB (panel A) or SSB mutant F177C (pane B) (80 μM) in buffer containing Tris (10 mM, pH 7.2) NaCl (150 mM), and glycerol (10% (v/v)) was incubated with ammonium sulfate (150 g/liter) in various solutions as indicated by + symbols. After incubation, samples were centrifuged. Pellet (P) and supernatant (S) fractions were loaded on the SDS polyacrylamide gel. The results are representative of three independent experiments.
3.2 E. coli SSB enhances the DinG DNA helicase activity
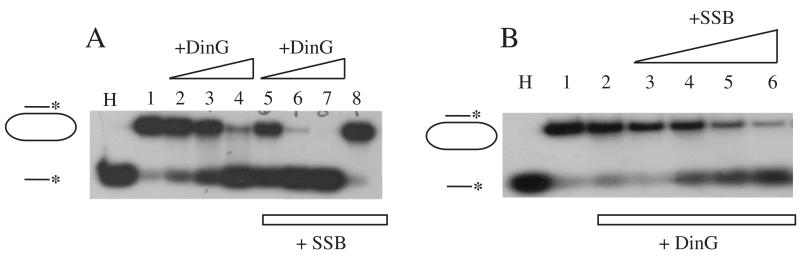
Formation of SSB/DinG complex led to an idea that SSB may modulate the DinG DNA helicase activity via protein-protein interaction. Using the previously established DNA helicase activity assay [2], we explored the effect of SSB on the DinG DNA helicase activity. Figure 4A shows that addition of SSB indeed stimulated the DinG DNA helicase activity by at least two folds. We also analyzed the DinG DNA helicase activity in the presence of a fixed concentration of DinG and increasing concentrations of SSB, and found that as the SSB concentration was gradually increased, the DinG DNA helicase activity was progressively increased (Figure 4B). A 5-10 fold excess of SSB required for stimulating the DinG DNA helicase activity (Figure 4B) could be due to the substrate ssDNA M13 plasmid which may titrate out SSB in the reaction solution. Nevertheless, the results clearly suggest that E. coli SSB is able to stimulate the DinG DNA helicase activity under the experimental conditions.
Figure 4. E. coli SSB stimulates the DinG DNA helicase activity.
A), purified DinG was incubated with the 32P-radioactive-labeled substrate and ATP with or without SSB at 30°C for 15 min. Lane H, the sample was heated at 85°C for 5 min. Lane 1, no DinG. Lanes 2 to 4, with 25, 50 and 100 nM DinG. Lanes 5 to 7, with 1 μM SSB and 25, 50 and 100 nM DinG). Lane 8, no DinG and 1 μM SSB only. B), purified DinG was incubated with the 32P-radioactive-labeled substrate (2 nM), ATP (2 mM) and SSB at 30°C for 15 min. Lane H, the sample was heated at 85°C for 5 min. Lane 1, no DinG or SSB. Lanes 2 to 6, with 50 nM DinG and 0, 50, 100, 250, 500 nM SSB, respectively. Similar results were obtained from three independent experiments.
3.3 SSB mutant F177C is a potent inhibitor for the DinG DNA helicase
As a single-stranded DNA binding protein, SSB may regulate the DinG DNA helicase activity by binding to ssDNA, a substrate/product of the DNA helicase. If a protein that binds ssDNA could stimulate the DinG DNA helicase activity, we expect that SSB mutant F177C which retains the same ssDNA binding activity as wild-type SSB should also stimulate the DinG DNA helicase activity.
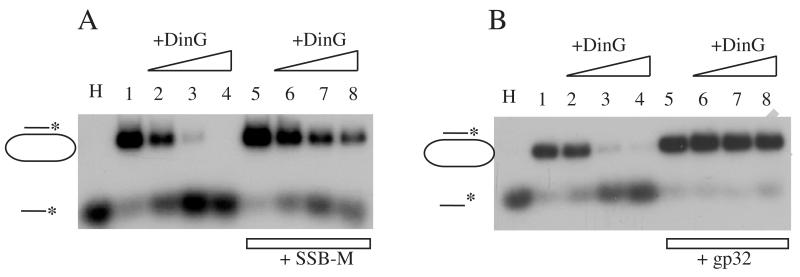
To our surprise, unlike wild-type SSB, SSB mutant F177C not only failed to enhance the DinG DNA helicase activity but inhibited the DinG DNA helicase activity (Figure 5A). To further explore whether other ssDNA binding proteins could inhibit the DinG DNA helicase activity, we used bacteriophage protein gp32, a structurally unrelated ssDNA binding protein [19] and found that gp32 had an even stronger inhibitory effect on the DinG DNA helicase activity (Figure 5B). Thus, the specific protein-protein interaction between wild-type SSB and DinG is likely responsible for stimulation of the DinG DNA helicase activity by SSB. On the other hand, the ssDNA binding activity of SSB appears to inhibit the DinG DNA helicase activity.
Figure 5. SSB mutant F177C inhibits the DinG DNA helicase activity.
A), purified DinG was incubated with the 32P-radioactive-labeled substrate (2 nM) and ATP (2 mM) with or without SSB mutant F177C at 30°C for 15 min. Lane H, the sample was heated at 85°C for 5 min. Lane 1, no DinG. Lanes 2 to 4, with 50, 100 and 200 nM DinG. Lanes 5 to 8, with 1 μM SSB mutant F177C and 0, 50, 100, and 200 nM DinG. B), purified DinG was incubated with the 32P-radioactive-labeled substrate (2 nM) and ATP (2 mM) with or without bacteriophage protein gp32 at 30°C for 15 min. Lane H, the sample was heated at 85°C for 5 min. Lane 1, no DinG. Lanes 2 to 4, with 50, 100 and 200 nM DinG. Lanes 5 to 8, with 500 nM gp32 and 0, 50, 100, and 200 nM DinG. Data are representative of three independent experiments.
The observation that wild-type SSB and SSB mutant F177C have an opposite effect on the DinG DNA helicase activity demonstrates the crucial role of the C-terminal end F-177 in SSB. It has been reported that mutation of F177C in SSB severely impairs the E. coli cell’s viability [22], and F177 may directly interact with multiple DNA processing enzymes [21]. Here we show that SSB mutant F177C, which retains the ssDNA binding activity as wild-type SSB [22], fails to form a stable SSB/DinG/complex. We envision that formation of SSB/DinG complex may subtly alter the structure of both proteins: for DinG, binding of SSB may lead to an enhanced DNA helicase activity; for SSB, binding of DinG may weaken the ssDNA binding activity. As a consequence, specific protein-protein interaction between SSB and DinG stimulates the DinG DNA helicase activity. In contrast, SSB mutant F177C does not form a stable protein complex with DinG, thus fails to stimulate the DinG DNA helicase activity. Instead, the ssDNA binding activity of SSB mutant F177C effectively blocks the access of DinG to substrate ssDNA and inhibits the DinG DNA helicase activity. In line with this idea, we found that while wild-type SSB can enhance the endogenous ATPase activity of DinG, SSB mutant F177C effectively inhibits the ATPase activity of DinG (unpublished data). Nevertheless, additional experiments are required to illustrate molecular details of the SSB-mediated activation of the DinG DNA helicase activity.
The known proteins that interact with E. coli SSB include the primase for DNA replication DnaG [25], exonuclease I [26], the DNA helicase RecQ [23,24], uracil DNA glycosylase [27], the χ subunit of DNA polymerase III [28], DNA polymerase V [29], topoisomerase III [30], the replication re-start protein DNA helicase PriA [31], DNA helicase RecG [32], recombination mediator RecO [33,34], and the maintenance of genome stability protein A [35]. In a number of of the SSB-binding proteins, a hydrophobic pocket and basic residues have been identified for accommodation of the C-terminal end Phe-177 and Asp residues of SSB [21,24,33,36]. In Gram-positive Bacillus subtilis, SSB has also been shown to recruit DNA helicases PriA and RecG and recombination mediator RecO, and to re-start the arrested chromosomal replication forks [37]. In archaea, the single-stranded DNA binding protein RPA (Replication Protein A) has been shown to interact with DNA helicase XPD [38,39] and RNA polymerase [40]. In eukaryotes, RPA interacts with DNA polymerase α [41] and DNA helicase FANCJ/BACH1 [42,43], and is likely responsible for coordinating repair of double-stranded DNA breaks [44]. In this context, we propose that E. coli DinG is a new member of the DNA processing protein family that can be regulated by SSB. When cells are subject to DNA damaging agents, DinG together with other DNA repair proteins including SSB are highly induced [1,18], and SSB in turn stimulates the activity of the DinG DNA helicase and other DNA repair enzymes to promote efficient repair of DNA damage.
Highlights.
-
►
Specific interaction between E. coli SSB and DNA helicase DinG was explored.
-
►
SSB forms a protein complex with DinG and stimulates the DNA helicase activity.
-
►
A mutant SSB that fails to interact with DinG inhibits the DNA helicase activity.
Acknowledgements
This work was supported by the National Cancer Institute of the National Institutes of Health under award number RO1CA107494. A.C. was supported by the HHMI summer research fellowship at LSU.
Abbreviations
- DinG
E. coli protein encoded by the DNA damage inducible gene G
- SSB
E. coli single-stranded DNA binding protein
- XPD
human Xeroderma pigmentosum factor D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Structured summary of protein interactions:
SSB and SSB bind by molecular sieving (View interaction)
DinG and SSB bind by molecular sieving (View interaction)
dinG and SSB bind by cosedimentation in solution (View interaction)
References
- [1].Lewis LK, Jenkins ME, Mount DW. Isolation of DNA damage-inducible promoters in Escherichia coli: regulation of polB (dinA), dinG, and dinH by LexA repressor. J Bacteriol. 1992;174:3377–85. doi: 10.1128/jb.174.10.3377-3385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Voloshin ON, Vanevski F, Khil PP, Camerini-Otero RD. Characterization of the DNA damage-inducible helicase DinG from Escherichia coli. J Biol Chem. 2003;278:28284–93. doi: 10.1074/jbc.M301188200. [DOI] [PubMed] [Google Scholar]
- [3].Voloshin ON, Camerini-Otero RD. The DinG protein from Escherichia coli is a structure-specific helicase. J Biol Chem. 2007;282:18437–47. doi: 10.1074/jbc.M700376200. [DOI] [PubMed] [Google Scholar]
- [4].Boubakri H, de Septenville AL, Viguera E, Michel B. The helicases DinG, Rep and UvrD cooperate to promote replication across transcription units in vivo. EMBO J. 2010;29:145–57. doi: 10.1038/emboj.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rudolf J, Makrantoni V, Ingledew WJ, Stark MJR, White MF. The DNA Repair Helicases XPD and FancJ Have Essential Iron-Sulfur Domains. Molecular Cell. 2006;23:801–808. doi: 10.1016/j.molcel.2006.07.019. [DOI] [PubMed] [Google Scholar]
- [6].Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, three diseases. Genes Dev. 2001;15:15–23. doi: 10.1101/gad.859501. [DOI] [PubMed] [Google Scholar]
- [7].Coin F, Oksenych V, Egly JM. Distinct roles for the XPB/p52 and XPD/p44 subcomplexes of TFIIH in damaged DNA opening during nucleotide excision repair. Mol Cell. 2007;26:245–56. doi: 10.1016/j.molcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [8].Gupta R, Sharma S, Sommers JA, Jin Z, Cantor SB, Brosh RM., Jr Analysis of the DNA substrate specificity of the human BACH1 helicase associated with breast cancer. J Biol Chem. 2005;280:25450–60. doi: 10.1074/jbc.M501995200. [DOI] [PubMed] [Google Scholar]
- [9].Wu Y, Sommers JA, Khan I, de Winter JP, Brosh RM., Jr Biochemical characterization of Warsaw breakage syndrome helicase. J Biol Chem. 2012;287:1007–21. doi: 10.1074/jbc.M111.276022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uringa EJ, Youds JL, Lisaingo K, Lansdorp PM, Boulton SJ. RTEL1: an essential helicase for telomere maintenance and the regulation of homologous recombination. Nucleic Acids Res. 2011;39:1647–1655. doi: 10.1093/nar/gkq1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pugh RA, Honda M, Leesley H, Thomas A, Lin Y, Nilges MJ, Cann IK, Spies M. The iron-containing domain is essential in Rad3 helicases for coupling of ATP hydrolysis to DNA translocation and for targeting the helicase to the single-stranded DNA-double-stranded DNA junction. J Biol Chem. 2008;283:1732–43. doi: 10.1074/jbc.M707064200. [DOI] [PubMed] [Google Scholar]
- [12].Liu H, et al. Structure of the DNA repair helicase XPD. Cell. 2008;133:801–12. doi: 10.1016/j.cell.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fan L, Fuss JO, Cheng QJ, Arvai AS, Hammel M, Roberts VA, Cooper PK, Tainer JA. XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations. Cell. 2008;133:789–800. doi: 10.1016/j.cell.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wolski SC, Kuper J, Hanzelmann P, Truglio JJ, Croteau DL, Van Houten B, Kisker C. Crystal structure of the FeS cluster-containing nucleotide excision repair helicase XPD. PLoS Biol. 2008;6:e149. doi: 10.1371/journal.pbio.0060149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ren B, Duan X, Ding H. Redox control of the DNA damage-inducible protein DinG helicase activity via its iron-sulfur cluster. J Biol Chem. 2009;284:4829–4835. doi: 10.1074/jbc.M807943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].White MF. Structure, function and evolution of the XPD family of iron-sulfur-containing 5′-->3′ DNA helicases. Biochem Soc Trans. 2009;37:547–51. doi: 10.1042/BST0370547. [DOI] [PubMed] [Google Scholar]
- [17].White MF, Dillingham MS. Iron-sulphur clusters in nucleic acid processing enzymes. Current Opinion in Structural Biology. 2012;22:94–100. doi: 10.1016/j.sbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- [18].Fernandez De Henestrosa AR, Ogi T, Aoyagi S, Chafin D, Hayes JJ, Ohmori H, Woodgate R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol Microbiol. 2000;35:1560–72. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- [19].Chase JW, Williams KR. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–36. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- [20].Lohman TM, Ferrari ME. Escherichia coli single-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527–70. doi: 10.1146/annurev.bi.63.070194.002523. [DOI] [PubMed] [Google Scholar]
- [21].Shereda RD, Kozlov AG, Lohman TM, Cox MM, Keck JL. SSB as an organizer/mobilizer of genome maintenance complexes. Crit Rev Biochem Mol Biol. 2008;43:289–318. doi: 10.1080/10409230802341296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Genschel J, Curth U, Urbanke C. Interaction of E-coli single-stranded DNA binding protein (SSB) with exonuclease I. The carboxy-terminus of SSB is the recognition site for the nuclease. Biological Chemistry. 2000;381:183–192. doi: 10.1515/BC.2000.025. [DOI] [PubMed] [Google Scholar]
- [23].Shereda RD, Bernstein DA, Keck JL. A Central Role for SSB in Escherichia coli RecQ DNA Helicase Function. Journal of Biological Chemistry. 2007;282:19247–19258. doi: 10.1074/jbc.M608011200. [DOI] [PubMed] [Google Scholar]
- [24].Shereda RD, Reiter NJ, Butcher SE, Keck JL. Identification of the SSB binding site on E. coli RecQ reveals a conserved surface for binding SSB’s C terminus. J Mol Biol. 2009;386:612–25. doi: 10.1016/j.jmb.2008.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yuzhakov A, Kelman Z, O’Donnell M. Trading places on DNA--a three-point switch underlies primer handoff from primase to the replicative DNA polymerase. Cell. 1999;96:153–63. doi: 10.1016/s0092-8674(00)80968-x. [DOI] [PubMed] [Google Scholar]
- [26].Lu D, Myers AR, George NP, Keck JL. Mechanism of Exonuclease I stimulation by the single-stranded DNA-binding protein. Nucleic Acids Research. 2011;39:6536–6545. doi: 10.1093/nar/gkr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Handa P, Acharya N, Varshney U. Chimeras between single-stranded DNA-binding proteins from Escherichia coli and Mycobacterium tuberculosis reveal that their C-terminal domains interact with uracil DNA glycosylases. J Biol Chem. 2001;276:16992–7. doi: 10.1074/jbc.M100393200. [DOI] [PubMed] [Google Scholar]
- [28].Witte G, Urbanke C, Curth U. DNA polymerase III chi subunit ties single-stranded DNA binding protein to the bacterial replication machinery. Nucleic Acids Res. 2003;31:4434–40. doi: 10.1093/nar/gkg498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arad G, Hendel A, Urbanke C, Curth U, Livneh Z. Single-stranded DNA-binding protein recruits DNA polymerase V to primer termini on RecA-coated DNA. J Biol Chem. 2008;283:8274–82. doi: 10.1074/jbc.M710290200. [DOI] [PubMed] [Google Scholar]
- [30].Suski C, Marians KJ. Resolution of converging replication forks by RecQ and topoisomerase III. Mol Cell. 2008;30:779–89. doi: 10.1016/j.molcel.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cadman CJ, McGlynn P. PriA helicase and SSB interact physically and functionally. Nucleic Acids Res. 2004;32:6378–87. doi: 10.1093/nar/gkh980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Buss JA, Kimura Y, Bianco PR. RecG interacts directly with SSB: implications for stalled replication fork regression. Nucleic Acids Res. 2008;36:7029–42. doi: 10.1093/nar/gkn795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ryzhikov M, Koroleva O, Postnov D, Tran A, Korolev S. Mechanism of RecO recruitment to DNA by single-stranded DNA binding protein. Nucleic Acids Research. 2011;39:6305–6314. doi: 10.1093/nar/gkr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Inoue J, Nagae T, Mishima M, Ito Y, Shibata T, Mikawa T. A Mechanism for Single-stranded DNA-binding Protein (SSB) Displacement from Single-stranded DNA upon SSB-RecO Interaction. Journal of Biological Chemistry. 2011;286:6720–6732. doi: 10.1074/jbc.M110.164210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Page AN, George NP, Marceau AH, Cox MM, Keck JL. Structure and Biochemical Activities of Escherichia coli MgsA. Journal of Biological Chemistry. 2011;286:12075–12085. doi: 10.1074/jbc.M110.210187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu D, Keck JL. Structural basis of Escherichia coli single-stranded DNA-binding protein stimulation of exonuclease I. Proc Natl Acad Sci U S A. 2008;105:9169–74. doi: 10.1073/pnas.0800741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lecointe F, et al. Anticipating chromosomal replication fork arrest: SSB targets repair DNA helicases to active forks. EMBO J. 2007;26:4239–51. doi: 10.1038/sj.emboj.7601848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pugh RA, Lin Y, Eller C, Leesley H, Cann IK, Spies M. Ferroplasma acidarmanus RPA2 facilitates efficient unwinding of forked DNA substrates by monomers of FacXPD helicase. J Mol Biol. 2008;383:982–98. doi: 10.1016/j.jmb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [39].Honda M, Park J, Pugh RA, Ha T, Spies M. Single-Molecule Analysis Reveals Differential Effect of ssDNA-Binding Proteins on DNA Translocation by XPD Helicase. Molecular Cell. 2009;35:694–703. doi: 10.1016/j.molcel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Richard DJ, Bell SD, White MF. Physical and functional interaction of the archaeal single-stranded DNA-binding protein SSB with RNA polymerase. Nucleic. 2004 doi: 10.1093/nar/gkh259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Braun KA, Lao Y, He Z, Ingles CJ, Wold MS. Role of protein-protein interactions in the function of replication protein A (RPA): RPA modulates the activity of DNA polymerase alpha by multiple mechanisms. Biochemistry. 1997;36:8443–54. doi: 10.1021/bi970473r. [DOI] [PubMed] [Google Scholar]
- [42].Suhasini AN, Sommers JA, Mason AC, Voloshin ON, Camerini-Otero RD, Wold MS, Brosh RM., Jr FANCJ helicase uniquely senses oxidative base damage in either strand of duplex DNA and is stimulated by replication protein A to unwind the damaged DNA substrate in a strand-specific manner. J Biol Chem. 2009;284:18458–70. doi: 10.1074/jbc.M109.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wu Y, Shin-ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol Cell Biol. 2008;28:4116–28. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yan H, Toczylowski T, McCane J, Chen C, Liao S. Replication protein A promotes 5′→3′ end processing during homology-dependent DNA double-strand break repair. The Journal of Cell Biology. 2011;192:251–261. doi: 10.1083/jcb.201005110. [DOI] [PMC free article] [PubMed] [Google Scholar]