Abstract
The early host response during pulmonary nocardiosis is highly dependent on neutrophils and the successful clearance of bacteria in tissue. The data presented in this study showed that IL-17 mediated the neutrophil response following intranasal inoculation with Nocardia asteroides strain GUH-2. Flow cytometry revealed that neutrophil levels in C57BL/6 mice were increased by day 1 post inoculation and remained elevated until day 3, during which time the majority of bacterial clearance occurred. Intracellular cytokine staining for IL-17 showed a 3.5- to 5-fold increase in IL-17 producing T-lymphocytes that were predominately comprised by CD4−CD8− γδ T-lymphocytes. The importance of IL-17 and γδ T-cells was determined by the in vivo administration of antibody, capable of blocking IL-17 binding or TCR δ, respectively. Neutralization of either IL-17 or γδ T-cells in Nocardia treated mice resulted in attenuated neutrophil infiltration. Paralleling this impaired neutrophil recruitment, nearly a 10-fold increase in bacterial burden was observed in both anti-IL-17 and anti-TCR δ treated animals. Together, these data indicate a protective role for IL-17 and suggest that IL-17 producing γδ T-lymphocytes contribute to neutrophil infiltration during pulmonary nocardiosis.
Keywords: INTERLEUKIN-17, NEUTROPHIL, T-LYMPHOCYTES, NOCARDIOSIS
1. Introduction
Species of the genus Nocardia are increasingly recognized worldwide as a primary etiology of pulmonary disease in both normal and immunocompromised humans [1–3]. Nocardia consist of aerobic, branching filamentous Gram-positive, and weakly acid-fast bacteria, which are ubiquitous in the soil [2]. These bacteria commonly infect their host by inhalation. As a result, most clinical reports of human nocardiosis are associated with pulmonary disease, of which members of the Nocardia asteroides complex are the leading causative agents [4]. Within human and other mammalian lungs, these organisms may induce an acute, necrotizing pneumonia commonly presenting with cavitation, a feature that contributes to misdiagnosis due to its association with other chronic suppurative lung diseases and malignancies [2].
A murine model of pulmonary nocardiosis has been developed [5]. The animal model mimics most of the pathogenic features observed in human pulmonary nocardiosis. Within hours of intranasal inoculation with N. asteroides strain GUH-2, bacteria invade pulmonary epithelium and induce an extensive inflammatory response characterized by neutrophil recruitment, which results in a fulminant necrotizing pneumonia [6]. Host defense against N. asteroides is dependent on both innate leukocytes and non-conventional T-lymphocytes. It has been reported that in the absence of neutrophils, the host experiences unimpeded nocardial growth, thus increasing susceptibility to infection [7, 8]. Furthermore, the importance of non-conventional T-lymphocytes during pulmonary nocardiosis becomes apparent when γδ T-cell deficient mice are infected with GUH-2. Nocardia infected γδ T-cell deficient mice experience dysfunctional neutrophil infiltration, resulting increased susceptibility and defective clearance of N. asteroides in the lungs [7]. Based on these observations, it appears that protective immunity against N. asteroides GUH-2 and nocardial clearance from tissue is highly dependent on a robust neutrophil response, which may be mediated by γδ T-lymphocytes.
Lymphocytes expressing γδ T-cell receptors are more similar to innate than adaptive immune effector cells [9]. Unlike their more conventional counterparts (αβ T-cells), γδ T-cells do not require antigen processing to be activated and they exert their effector functions rapidly after directly recognizing antigen. It is within this context that γδ T-cells are recognized as immunoregulatory T-lymphocytes, with roles associated with both immunosurveillance and maintenance of immunological balance [10]. This concept is supported by mounting evidence showing that γδ T-cells contribute to protective immunity by the induction and regulation of the neutrophil response [11–15].
It has been reported that γδ T-cell expression of CXC chemokines such as MIP-1β and keratinocyte-derived chemokine (KC) is upregulated during neutrophil-mediated tissue damage [16]. More recently, there have been several reports that have established a role for IL-17 produced by γδ T-cells in the induction of CXC chemokines, granulocyte colony stimulating factor (G-CSF), and adhesion molecules [17–20] that augment neutrophil infiltration and protective innate immunity against both extracellular and intracellular bacterial pathogens [13, 14, 21, 22]. The importance of γδ T-cells as critical sources of IL-17 is further supported by reports demonstrating that expression of IL-17 is reduced in γδ T-deficient mice that are infected with either Mycobacterium tuberculosis [21] or Salmonella enterica [23].
In the experimental model of pulmonary nocardial infection, Moore et al. have shown that neutrophil recruitment and nocardial clearance correlated with the upregulation of CXC chemokines (KC and MIP-2) [8]. To date, there are no data to support that γδ T-cells in the lungs directly produce these chemokines during pulmonary nocardiosis. However, CXC chemokines are regulated by G-SCF, which in turn is induced by IL-17 produced by neutrophil-regulatory T-lymphocytes such as γδ T-cells [24]. Thus, the presence and upregulation of CXC chemokines in mice following infection with N. asteroides may be the downstream phenotype associated with immunoregulation by IL-17 produced by γδ T-cells. Because the nature of neutrophil induction by γδ T-cells during pulmonary nocardiosis remains undefined, this study was undertaken to investigate the role of IL-17 and specifically, the role of IL-17 producing γδ T-cells in the murine host following challenge with GUH-2. Using flow cytometry in conjunction with intracellular IL-17 staining, the temporal staging and cellular source of IL-17 during the early host response is evaluated. In addition, by neutralizing either IL-17 or γδ T-lymphocytes in vivo, this study evaluates the contribution of this cytokine and/or cells bearing TCR δ for neutrophil infiltration and nocardial clearance.
2. Materials and Methods
2.1. Animals
Six- to eight-week old C57BL6/J female mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Animals were maintained on a prescribed NIH diet and provided water ad libitum. All mice were housed in pathogen-free conditioned animal rooms at the University of California, Davis and were cared for by the Center for Laboratory Animal Science (CLAS) following standard and approved protocols. CLAS monitored sentinel mice for infectious agents, and none were reported during these studies.
2.2. Pulmonary infection with N. asteroides
Mice were intranasally inoculated with N. asteroides strain GUH-2 as described previously [25]. Briefly, GUH-2 was grown to mid-logarithmic phase in brain heart infusion (BHI, Difco) broth. The culture was adjusted with fresh BHI broth to yield a bacterial inoculum of 6 × 107 CFU/ml (approximately OD580nm= 0.60). Mice were anesthetized with nembutal (50 mg/kg of body weight), 50 l of the nocardial suspension were pipetted onto the anterior nares, and the mice were permitted to aspirate the inoculum. Sham-inoculated control animals were administered 50 µl of BHI broth.
2.3. GUH-2 count in pulmonary tissue
To establish the kinetics of nocardial clearance from immunocompetent murine hosts, lungs were excised at 3 hours, 1, 3, 5, and 7 days post challenge. Pulmonary tissue was homogenized in 3 ml of Hank’s balanced salt solution, serially diluted, and plated onto BHI agar (Difco). Nocardial load at each time point was enumerated from plates incubated at 37 °C for 3 days. Plate counts from 6 animals per time point were averaged. This method was also used to quantitate nocardial burden following in vivo antibody neutralization of either IL-17 or TCR δ. Lung homogenates from infected mice that were co-treated with nonspecific IgG or co-treated with antibody that targeted either IL-17 or TCR δ were plated.
2.4. In vivo neutralization of IL-17 or γδ T-lymphocytes
To neutralize IL-17 in C57BL6 mice, animals were intraperitoneally administered 50 µg of anti-murine IL-17 antibody suspended in 100 µl PBS (clone 50104, R & D Systems) at −1, 0, and +1 days post challenge. Alternatively, for the neutralization of γδ T-lymphocytes, mice were intraperitoneally administered 400 µg of anti-murine TCR δ antibody (clone UC7-13D5) in 100 µl PBS at −1, 0, and +1 days post infection. Monoclonal antibody that targeted TCR δ was derived from hybridoma (CRL-1989, ATCC) culture filtrate purified through a HiTrap Protein G HP column (GE Healthcare Life Sciences). Control animals were intraperitoneally injected with a low endotoxin, azide-free isotype cocktail that consisted of 50 µg of rat IgG and 200 µg Armenian hamster IgG (BD Biosciences), according to the schedule described above.
2.5. Isolation of pulmonary leukocytes
Pulmonary leukocytes used to immunophenotype the host response following challenge with N. asteroides were isolated from animals, as previously described [25]. Briefly, animals were euthanized at specific time points post nocardial exposure and lungs from mice were intracardially perfused with cold phosphate-buffered saline (PBS) at pH 7.4. The perfused lungs were minced into a fine slurry in RPMI 1640 (Difco) and resuspended in 5 ml of digestion medium, which consisted of RPMI, 10% fetal calf serum (FCS), 10 U of DNase I and 20 U of collagenase type VIII per ml, and 50 µM β-mercaptoethanol and incubated for 2 hours at 37 °C. Undisrupted tissue in the digestion medium was teased with a 20-gauge needle and filtered through a 40-µm nylon cell strainer. The cell filtrate was washed and centrifuged at 200 × g for 10 min. This pellet was then resuspended in RPMI with 10 % FCS, carefully overlaid onto a 40 % to 70 % Percoll gradient, and centrifuged for 30 min at 200 × g. Pulmonary murine leukocytes were collected from the interface of the 40 to 70 % Percoll gradient.
2.6. Flow cytometric analysis
Lung cells from tissue were isolated at 1, 3, 5, 7, and 14 days post challenge, as described above. Erythrocytes were lysed by the addition of BD PharmLyse™ solution (BD Biosciences) to cells. Samples of 1 × 106 cells from each animal were placed into fresh tubes containing 2 µg of anti-CD16/32 Fc block (BD Pharmingen) in PBS and incubated for 20 minutes at 4 °C to reduce nonspecific binding. Following the blocking step, cells were stained with combinations of monoclonal antibodies for 20 minutes at 4 °C. For intracellular cytokine staining, 1 × 106 lung cells were immediately cultured ex vivo in RPMI 1640 containing 10% FCS, 2 mM L-glutamine, 50 µM β-mercaptoethanol, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, and 10 µg/ml each of brefeldin A and monensin for 4 hours at 37°C with 5% CO2. After the incubation period, cells were surface labeled and treated with the BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit (BD Biosciences), according to the manufacturer’s suggested protocol. Anti-murine IL-17 monoclonal antibody was added to cells and stained for 20 minutes at 4°C. All flow cytometric analysis was performed with the BD LSR II cytometer (BD Biosciences) and data were analyzed using FlowJo software (Tree Star Inc).
2.7. Statistical analysis
Means and standard errors for all assays were calculated using Prism Software version 5.0 (GraphPad Inc). Statistical analysis of means between control and N. asteroides GUH-2 animals was performed using a two-tailed unpaired Student’s t test. Statistical significance of means from the neutralization studies was analyzed by a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Statistical significance was defined as P < 0.05.
3. Results
3.1. Clearance of N. asteroides GUH-2 occurred within 7 days post challenge
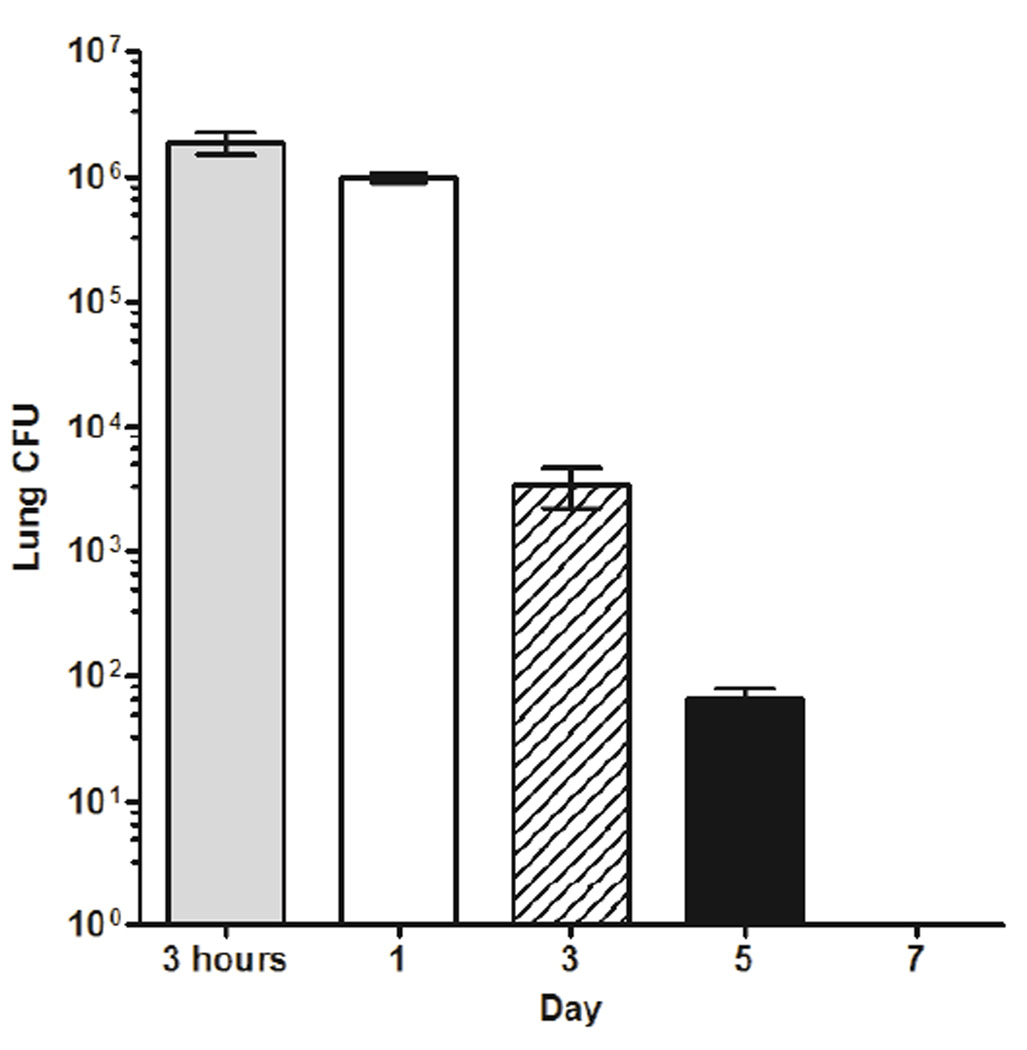
The kinetics of bacterial clearance in normal hosts was established by plating homogenized pulmonary tissue from Nocardia inoculated animals at 3 hrs, 1, 3, 5, and 7 days (Fig. 1). Plate counts from the 3-hour time point showed that the effective dosage delivered intranasally was approximately 1.875 × 106 CFU/animal. Within 3 hours to 24 hours, 46 ± 4.16 % of bacteria were removed by the host, and by 3 days post challenge, 98.2 ± 0.65 % of the original inoculum was eliminated. By 7 days, the lungs of infected animals were bacteria free.
Figure 1.
Nocardial clearance from pulmonary tissue occurred within 7 days. Plate counts of whole lung homogenates from N. asteroides GUH-2 treated mice at 1, 3, 5, and 7 days post challenge are graphed. At 3 hours post inoculation, the effective dose administered was 1.875×106 CFU/animal and at 7 days, animals were bacteria-free. Each point represents the mean ± standard error at each day sampled (n = 6). Presented data are representative of three separate experiments.
3.2. Neutrophils and T-lymphocytes were increased during clearance of N. asteroids from pulmonary tissue
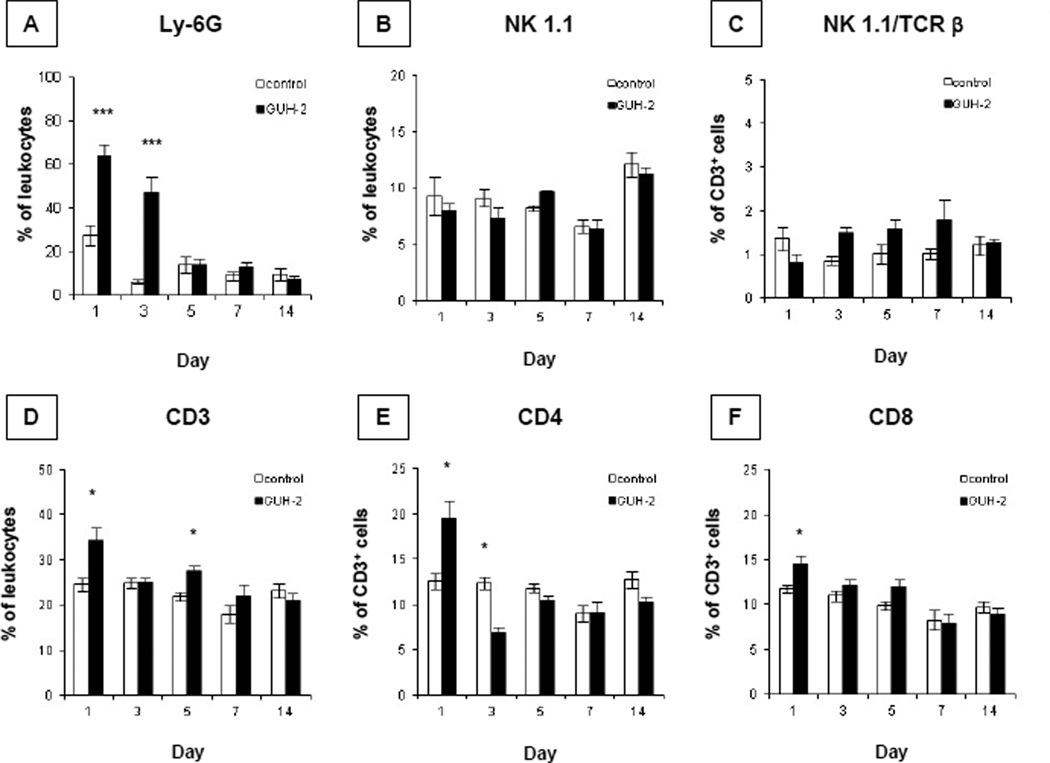
The overall host response against GUH-2 was immunophenotyped by flow cytometric analysis. To assess the neutrophil response, leukocytes from GUH-2 treated mice, as well as leukocytes from control animals (sham-inoculated with BHI broth) were stained with antibody against the neutrophil marker Ly-6G (Gr-1). Over 100,000 total events were collected and from a gated population displaying forward/side scatter profile characteristic of granular cells, neutrophils were identified by positive-labeling with α-Ly-6G antibody and negative-labeling with α-I-AB MHC class II molecules. Levels of neutrophils were expressed as a percentage of leukocytes. As early as day 1 post exposure, granulocytes were significantly increased in mice challenged with GUH-2 (Fig. 2A). When sampled at day 1, the level of polymorphonuclear cells from infected animals was 63.98 ± 5.34 % of gated cells, representing a 2.32-fold increase when compared to sham-inoculated control mice. By 3 days post challenge, the level of neutrophils (47.07 ± 7.42 % of gated cells) remained significantly elevated in experimental animals and was 7.5-fold higher than controls. Because there was no statistical difference between mean levels of neutrophils in experimental mice at day 1 and 3, it appeared that the peak of the neutrophil response occurred within 1–3 days post nocardial exposure. By 5 days post inoculation, the levels of neutrophils in GUH-2 treated animals had returned to basal levels, which were comparable to controls. Throughout the remainder of this study, neutrophils remained quiescent.
Figure 2.
The early host response following intranasal challenge with N. asteroids GUH-2 is marked by increased frequency of neutrophils and T-lymphocytes. At 1, 3, 5, 7 and 14 days post inoculation, flow cytometric analysis on lung cells was performed to measure proportion of neutrophils (A) stained with anti-Ly-6G (clone RB6-8C5). Natural killer cells (B) stained with anti-NK-1.1 (clone PK136), and natural killer T-lymphocytes (C) stained with anti-NK-1.1 and anti-TCR β (clone H57-597) were evaluated. T-lymphocyte frequencies increase in Nocardia treated animals at early time points, shown by increases of overall T-lymphocytes (D) stained with anti-CD3ε (clone 145-2C11) and subsets stained with anti-CD4 (E, clone RM4-5) or anti-CD8α (F, clone 53–6.7). The levels of cell types are expressed as a percentage of leukocytes or CD3-positive cells. Each bar represents the mean ± standard error of GUH-2 treated and sham-inoculated control mice at each time point (n = 6). The means of infected mice and controls at each time point were analyzed by a two-tailed unpaired Student’s t test. * signifies significance at P < 0.05 and *** signifies significance at P < 0.001. Presented data are representative of three separate experiments.
Flow cytometric analysis also revealed an increase in T-lymphocytes, as well as subsets of T-cells, during early onset of bacterial clearance (Fig. 2D, E, and F). Over 100,000 total events were collected and from a population displaying forward/side scatter profile characteristic of mononuclear cells, T-lymphocytes and subsets were identified by positive-labeling with α-CD3, α-TCR β, α-NK-1.1, α-CD4, and α-CD8. Overall T-lymphocyte levels, as well as NK1.1/TCR β, CD4, and CD8 subset frequencies, were expressed as percentages of CD3-positive cells. At day 1 post exposure, the overall proportion of T-lymphocytes from challenged animals increased. In Nocardia treated animals, T-lymphocytes represented 34.32 ± 2.84 % of the gated population. When compared to controls, experimental animals experienced a 1.4 fold increase in total T-cells at this time point (Fig. 2D). In addition, an overall increase in T-lymphocytes was paralleled by increases in CD4 (Fig. 2E) and CD8 subsets (Fig. 2F) in GUH-2 treated mice. No significant changes in the levels of CD3ε, CD4, or CD8-positive cells were detected at latter time points, except for a slight increase in overall T-cells at day 5 and a slight decrease in CD4 cells from infected animals at day 3. From these results, the expansion of T-cells (including CD4- or CD8-positive subsets) appeared to coincide with the clearance of GUH-2.
In contrast, flow cytometric analysis revealed that frequencies of other innate cells such as natural killer (NK) cell and NK T-cell levels remained unchanged during pulmonary nocardiosis. Lung leukocytes positively-labeled with anti-NK-1.1 alone were depicted as a percentage of total leukocytes. Meanwhile, NK 1.1- and anti-TCR β-positive cells were depicted as a percentage of CD3+ cells. No significant changes in either NK cells (Fig. 2B) or NK T-cells (Fig. 2C) were observed throughout the course of this study. Thus, it appears the early increase in overall T-lymphocytes following intranasal inoculation of mice with GUH-2 was not attributed to an increase in NK T-cells.
3.3. γδ T-lymphocytes are the predominant IL-17 producing cells in response to N. asteroides GUH-2
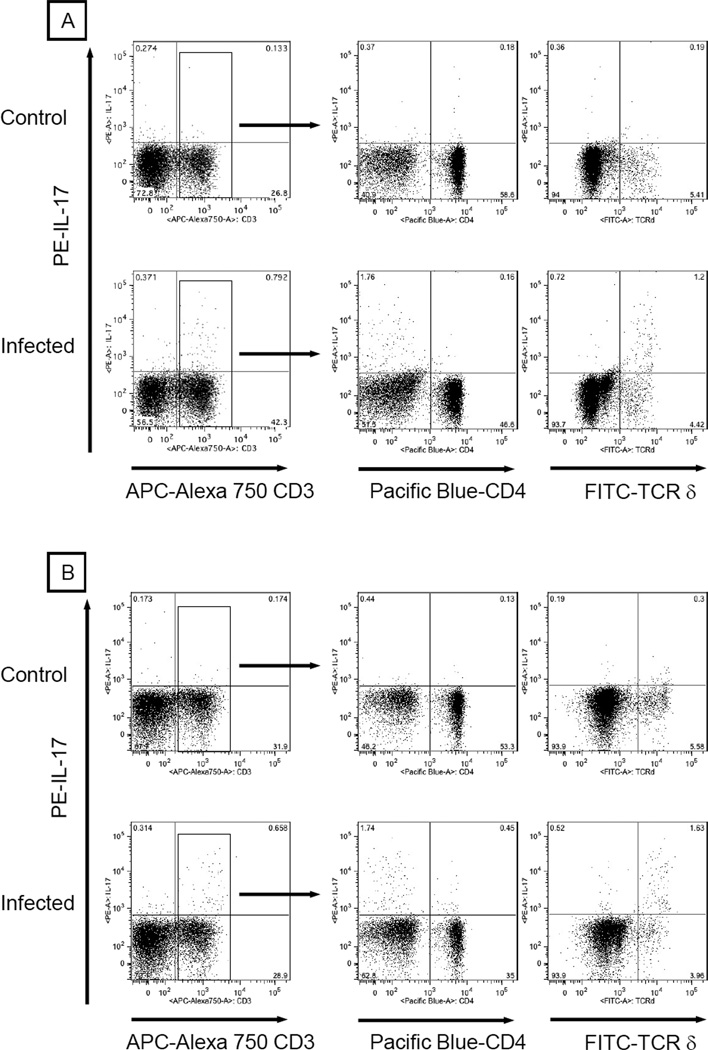
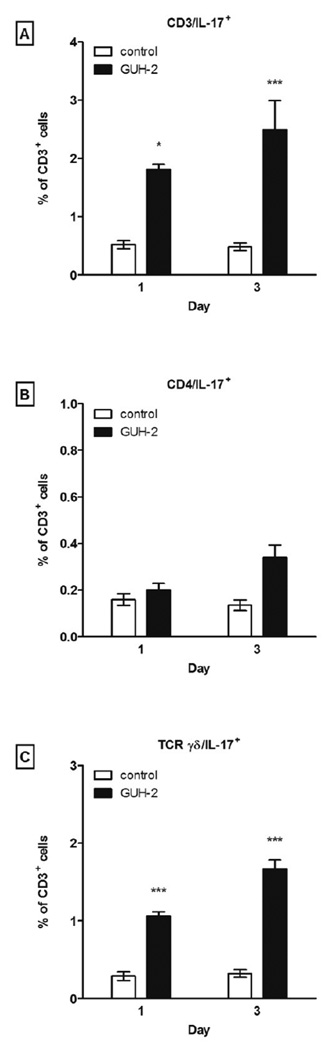
It has been shown that IL-17 induces the production of granulocyte colony G-CSF, which then initiates production of CXC chemokines (KC/CXCL1 and MIP-2/CXCL2) that influence neutrophil trafficking and protective immunity [24, 26, 27]. Furthermore, other studies have reported a wide range of IL-17 producing T-lymphocyte subsets, including CD4+, CD8+, CD4−CD8− αβ, and γδ T-lymphocytes. Therefore, intracellular cytokine staining was performed to identify the possible cell types that produce IL-17 during neutrophil recruitment and bacterial clearance following challenge with N. asteroides. At days 1 and 3 post challenge, the mean levels of IL-17 producing T-lymphocytes in Nocardia inoculated animals were 1.81 ± 0.092 % and 2.5 ± 0.5 % of total T-cells, representing 3.5- and 5.1-fold increases over control animals, respectively (Fig. 3, 4A). The observed IL-17+ lymphocyte increase was not due to CD4+ (Fig. 3, 4B) or CD8+ (data not shown) T-cells. However, at days 1 and 3 the overall increase of IL-17 producing lymphocytes in infected animals was paralleled by 3.7- and 5.2-fold increases in IL-17 producing γδ T-cells, respectively (Fig 3, 4C). Intracellular cytokine staining showed that γδ T-cells were the major IL-17 producing in response to GUH-2. Also, the inclusion of anti-CD4, CD8, TCR β, and TCR δ in the same staining panel revealed that all IL-17+ γδ T-cells were CD4−CD8− and a minor source of IL-17 producing lymphocytes was comprised of CD4−CD8− TCR β+ cells (data not shown)
Figure 3.
γδ T-lymphocytes were the major producers of IL-17 in response to N. asteroides GUH-2 in vivo. At 1 and 3 days post inoculation, lung cells from infected C57BL/6 mice (n = 6/ time point) were harvested and stained with antibodies that recognized CD3, CD4, TCR δ (clone GL3), and intracellular IL-17 (clone TC11-18H10). Lung cells from wild-type mice administered BHI broth served as controls. Flow cytometric analysis was performed on cells initially gated based on forward and side scatter profile characteristic of mononuclear cells. Because the predominant source of IL-17 was CD3-positive cells (left panels), phenotyping of IL-17 producing cells within the CD3-positive population was emphasized. Except for the left panels (% of “mononuclear” cells), IL-17 producing cells bearing CD4 or TCR δ are expressed as a percentage of CD3-positive cells (middle and right panels). Presented data are representative of three separate experiments.
Figure 4.
Bar graph summary of IL-17 expression by T-lymphocytes (A), CD4 cells (B), and γδ T-cells (C). Each bar represents the mean levels of IL-17 production by CD3, CD4, and TCR γδ cells within the CD3 gate ± standard error for each time point. The means of GUH-2 treated mice and controls at each time point were analyzed with a two-tailed unpaired Student’s t test. * signifies significance at P < 0.05 and *** signifies significance at P < 0.001. Presented data are representative of three separate experiments.
3.4. Neutrophil infiltration was impaired and IL-17 following neutralization of either IL-17 or γδ T-lymphocytes
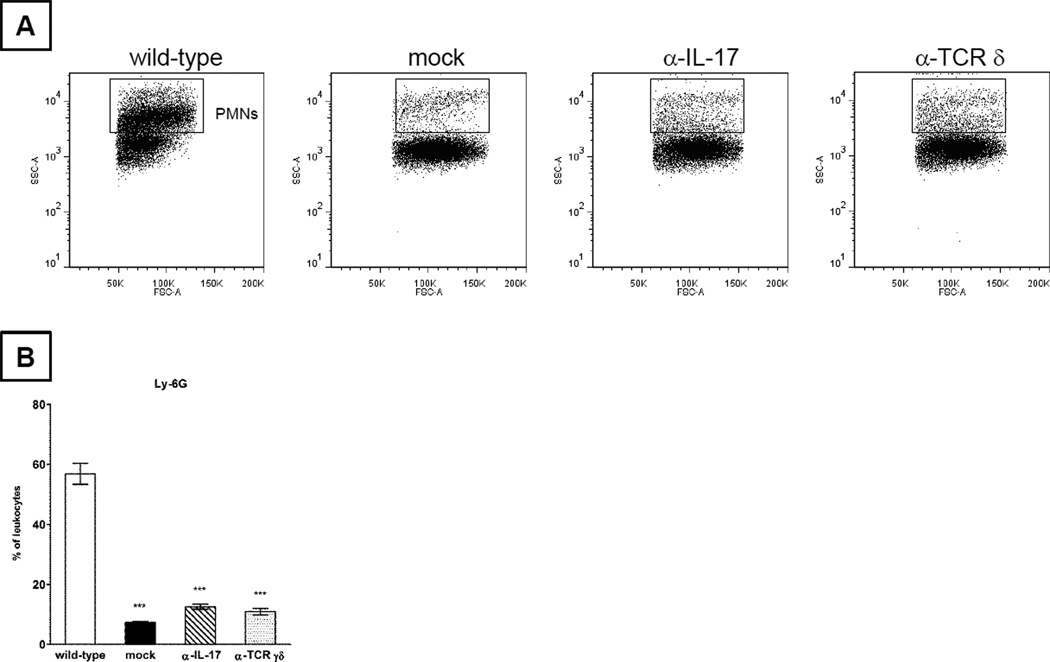
The importance of IL-17 during pulmonary nocardiosis was studied further by antibody neutralization of either IL-17 or γδ T-lymphocytes in vivo. The kinetics of neutrophil recruitment and IL-17 production were monitored. At 2 days post infection, the levels of neutrophils were significantly blunted in infected animals that were concomitantly administered either anti-IL-17 or anti-TCR δ antibody (Fig. 5A, B). When compared to GUH-2 treated animals that received non-specific IgG antibody (wild-type), anti-IL-17 treated mice experienced a 4.5-fold decrease in neutrophils (Fig. 5B). Anti-TCR δ treated animals also resulted in fewer pulmonary neutrophils (5.2-fold less than wild-type controls, Fig. 5B).
Figure 5.
Pulmonary neutrophils were significantly decreased in N. asteroides GUH-2 inoculated C57BL/6 mice that were also treated with anti-IL-17 or anti-TCR δ. At 2 days post challenge, lung cells from sham-inoculated mice co-treated with non-specific IgG (mock) and GUH-2 infected mice co-treated with nonspecific IgG (wild-type), anti-IL-17 (α-IL-17), or anti-TCR δ (α-TCR γδ) were stained with antibodies that targeted Ly-6G. Fig. 5 (A) depicts representative dot plots of decreased neutrophils (based on light scatter properties). The relative neutrophil levels for each group are summarized by bar graphs (B) after staining with the Ly-6G antibody and excluding MHC II-positive cells within the gate shown above. Comparative analysis of means for each treatment was performed by using one way ANOVA followed by Tukey’s multiple comparison test. *** signifies significance at P < 0.001 when compared to the wild-type control group. Presented data are representative of two separate experiments.
3.5. IL-17 producing cells were reduced following neutralization of either IL-17 or γδ T-lymphocytes
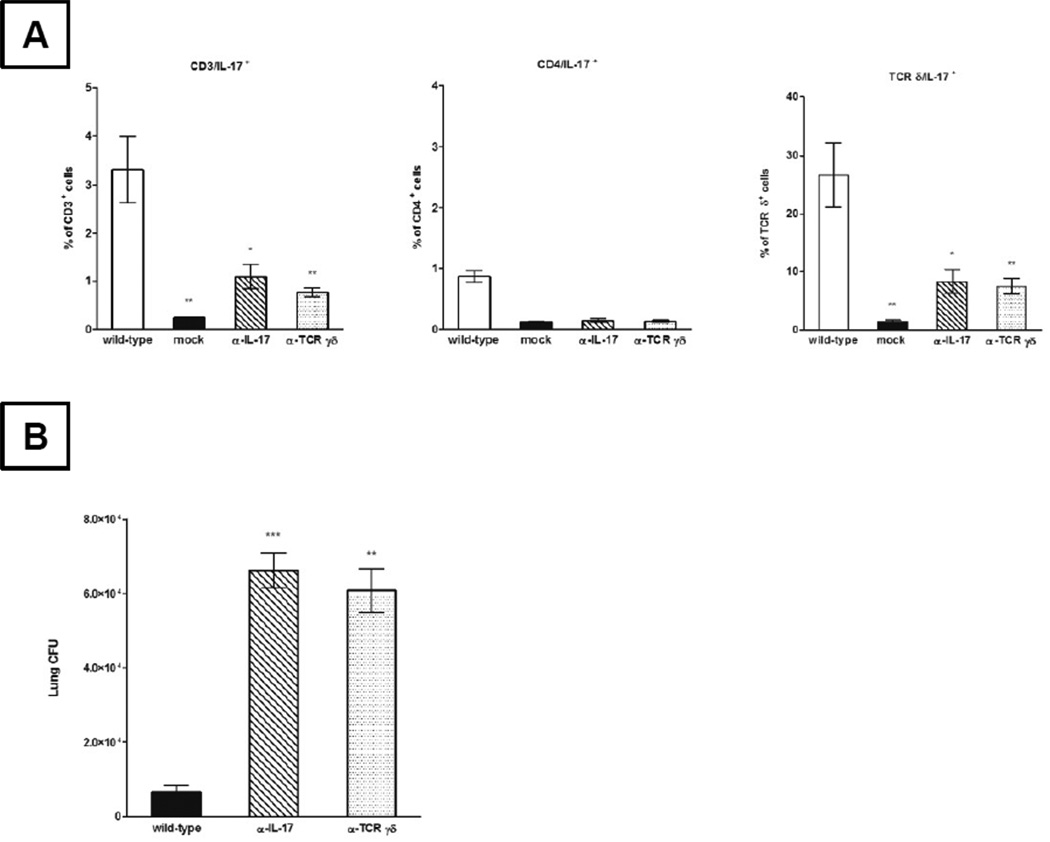
As shown in Fig. 6A, antibody neutralization with α-IL-17 significantly reduced the frequency of IL-17+ T-lymphocytes (Fig. 6A left panel) and the IL-17+ γδ T-cell subset (Fig. 6A right panel; by approximately 3- and 3.2-fold) when compared to GUH-2 treated wild-type control animals, respectively. Similar results were achieved by the neutralization of γδ T-lymphocytes. When compared to control animals, infected mice that were concomitantly treated with α-TCR δ antibody had 4.3- and 3.6-fold fewer IL-17+ T-lymphocytes (Fig 6A left panel) and the IL-17+ γδ T-cells (Fig. 6A right panel), respectively. Although it appeared that neutralization with α-TCR α was slightly more effective in attenuating the IL-17 response than α-IL-17 treatment, no statistically significant difference was observed between treatments with respect to either overall IL-17 producing T- or γδ T-lymphocytes. The results from the neutralization of γδ T-cells with α-TCR δ demonstrated that γδ T-cells were an important source of IL-17 in Nocardia infected animals. When combined with the impaired neutrophil infiltration results from antibody treated animals (described above), these data suggested that IL-17 and IL-17 producing γδ T-lymphocytes play a role in neutrophil recruitment during pulmonary nocardiosis.
Figure 6.
For intracellular IL-17 analysis following antibody treatment, cells were initially gated based on forward and side scatter profile (characteristic of lymphocytes), followed by a CD3-positive enrichment gate. Shown in panel (A) are bar graphs that represent the mean levels of IL-17 producing CD3, CD4, and TCR γδ cells ± standard error for each treatment (n = 5/ treatment). IL-17 or TCR γδ inactivation increased lung bacterial burden in C57BL/6 mice following intranasal inoculation with N. asteroides GUH-2 (B). Comparative analysis of means for each treatment was performed by using a one way ANOVA followed by Tukey’s multiple comparison test. * signifies significance at P < 0.05, ** signifies significance at P < 0.01, and *** signifies significance at P < 0.001 when compared to the wild-type control group. Presented data are representative of two separate experiments.
3.6. Nocardial clearance was impaired following neutralization of either IL-17 or γδ T-lymphocytes
Having established that the majority of nocardial clearance by immunocompetent mice occurred within the first 3 days post inoculation, bacterial burden was assessed infected mice that were co-administered either α-IL-17 or α-TCR δ. At 2 days post challenge, plate counts of lung homogenates from normal hosts were significantly reduced when compared to their IL-17 or γδ T-cell neutralized counterparts (Fig. 6B). Nocardial counts from IL-17 neutralized hosts were approximately 10-fold higher (66,200 versus 6,500 CFU/lungs) and counts from γδ T-cell neutralized animals were 9.3-fold higher (60,722 CFU/ lungs). Thus, neutralization of protective immunity mediated by IL-17 and γδ T-cells markedly affected nocardial clearance.
4. Discussion
The host immune response during pulmonary nocardiosis is orchestrated by different types of leukocytes from both arms of the innate and adaptive immunity [2]. From previous results generated in the murine model of pulmonary nocardiosis, it is well established that bacterial clearance in infected tissue and host survival are highly dependent on protective immunity conferred by neutrophils and γδ T-lymphocytes [7, 8]. It is believed that neutrophils directly contribute to nocardial clearance by utilizing oxygen-independent mechanisms to inhibit bacterial growth following phagocytosis [28, 29]. On the other hand, anecdotal evidence suggests that γδ T-lymphocytes are involved with neutrophil infiltration during pulmonary nocardiosis [7], but it remains unknown as to how this subset influences the neutrophil response. Moore et al. have identified (KC/CXCL1 and MIP-2/CXCL2) chemokines as critical immunoregulatory molecules for neutrophil trafficking into the lungs of Nocardia infected mice [8], while others have shown that γδ T-cells can produce CXC chemokines in response to pulmonary injury [16]. However, using real-time RT-PCR, we have yet to find upregulation of CXC chemokines from sorted γδ T-cells that are isolated from infected mice (unpublished data). Thus, it appears that if γδ T-cells directly induce neutrophil influx during pulmonary nocardiosis, they may act by producing alternative immunoregulatory molecules.
Interleukin-17 has been implicated in protective immunity against a variety of both extracellular and intracellular bacterial pathogens such as Klebsiella pneumoniae, Escherichia coli, Mycobacterium bovis BCG and M. tuberculosis [13, 14, 21, 27, 30]. Against some of these bacteria, IL-17 produced by γδ T-cells plays a critical role in the induction of neutrophil infiltration and bacterial clearance [13, 14, 21]. Furthermore, the interplay between IL-17 and CXC chemokines can be further explained by a neurostat feedback loop, such that IL-17 produced by neutrophil-regulatory T-cells including γδ T-cells induces the production of G-CSF, which in turn initiates CXC chemokine production that directly promotes neutrophil trafficking and protective immunity [24, 26, 27]. In this current study, we addressed whether the induction of neutrophil infiltration by γδ T-lymphocytes is mediated by IL-17.
To this end, plate counts of lungs from Nocardia treated animals and flow cytometric analysis were utilized initially to correlate temporal staging of bacterial clearance with neutrophil infiltration. Our results revealed that over 98% of nocardial cells were cleared in the lungs within 3 days post inoculation. Coinciding with this period of bacterial clearance, the host response against N. asteroides GUH-2 was dominated by an influx of neutrophils. As early as 1 day post challenge, the neutrophil level was significantly elevated and remained elevated until day 3. The neutrophil frequencies that are observed at these time points correlated with previous reports of increased myeloperoxidase activity and neutrophil counts in mice that are infected with N. asteroides [8]. Therefore, even in the absence of absolute neutrophil counts, it is likely that the Nocardia clearance that is reported in this study coincides with an increase in neutrophils within infected lungs.
When neutrophils accumulated in lungs and nocardial clearance occurred, a concomitant increase in overall T-lymphocytes was also observed at day 1 post challenge. Paralleling the overall T-lymphocyte increase, CD4+ and CD8+ subsets were also elevated. In contrast, no significant increase in γδ T-cells was detected during the first day post inoculation, but beginning at day 3 and peaking by 5 days post inoculation the host response was marked by a resurgence in overall T-lymphocytes, which was attributed to an accumulation of γδ T-cells in lungs [25]. Other innate cells such as NK cells (including NK T-cells) also remained at basal levels throughout this study. Because all γδ T-cells in the lung through day 14 were CD4−CD8− (data not shown) and no change in the levels of NK T-cells (which might also express either CD4 or CD8 receptors) was observed, we concluded that the early increase in overall T-lymphocytes was attributed to CD4+ and CD8+ subsets, presumably originating from the αβ T-lymphocyte population. Based on their increased presence in pulmonary tissue when neutrophil trafficking and bacterial clearance occurred, CD4+ and CD8+ αβ T-lymphocytes were initially targeted as potential sources of IL-17 during early stages of pulmonary nocardiosis.
Cellular sources of IL-17 that are described in the literature include neutrophils, CD4+ and CD8+ T-cells, CD4−CD8− αβ T-cells, CD4+NK-1.1− iNK T-cells, and γδ T-cells [24, 30–34]. Intracellular cytokine staining in conjunction with surface marker labeling revealed that IL-17 was indeed produced by leukocytes during a period of neutrophil influx and nocardial clearance in lungs, but IL-17 was not produced by neutrophils or NK T-cells (defined by NK-1.1 and TCR β markers). Within three days post inoculation, we identified T-lymphocytes (CD3+ cells) as the major source of IL-17 in infected mice. Although the results from the immunophenotypic analysis of the early host response suggested that CD4+ and CD8+ cells may also represent highly probable sources of IL-17, intracellular cytokine staining showed that IL-17 was not significantly produced by these subsets. Moreover, the majority of IL-17 producing T-lymphocytes consisted of CD4−CD8− γδ T-cells, and the remaining population of IL-17 producing T-lymphocytes was comprised of CD4−CD8−TCR β+ cells. Although we speculate that the CD4−CD8−TCR β+ cells originate from the αβ T-lymphocyte population, the staining panels used during this study do not provide sufficient resolution to completely rule out other invariant NK T-cell subsets, which may include NK-1.1-negative, CD4 single positive, CD4/CD8-double negative, and TCR β-positive cells [35].
The biological functions of γδ T-lymphocytes are slowly emerging, and this is partly due to the intrinsic limitations of the B6.129P2-Tcrdtm1Mom (γδ T-cell deficient) mouse used to study γδ T-cells. This mouse strain appears to have developed compensatory mechanisms in the absence of γδ T-cells, such that functions associated with the γδ T-cells may be substituted by opportunistic αβ T-cells that occupy microenvironments that are restricted to γδ T-cells [36]. In addition, recent reports have surfaced and demonstrate that IL-17 expression is only blunted, but not completely abrogated in γδ T-cell deficient mice [23, 37]. Thus, to study IL-17 producing γδ T-lymphocytes and to interrogate their influence on the early host response, we decided to implement antibody neutralization in lieu of γδ T-cell deficient mice.
The importance of IL-17 and IL-17 producing γδ T-lymphocytes with respect to neutrophil infiltration and nocardial clearance was addressed by administration of either α-IL-17 or α-TCR δ in vivo. Consequently, neutralization of IL-17 and/or γδ T-cells led to alterations in protective immunity. Animals treated with either α-IL-17 or α-TCR δ exhibited comparable reductions in neutrophil recruitment and comparable increases in nocardial burden in pulmonary tissue. Interestingly, in mice that were administered α-IL-17, we observed similar reductions in overall IL-17 producing T-lymphocytes (including comparable decreases in IL-17 producing γδ T-cells) to mice that were treated with α-TCR δ. This is an unexpected result, for neutralization by IL-17 monoclonal antibody is predicated on inactivating the biological function of IL-17 at the protein level, not interfering with IL-17 production at the effector cell level. Because IFN-γ is also produced during the early host response against GUH-2 in lungs [38] and IFN-γ acts to suppress the development of IL-17 producing T-lymphocytes [39, 40], a plausible explanation is that antibody neutralization of IL-17 decreases the concentration of extracellular IL-17 in tissue and shifts the cytokine milieu in favor of IFN-γ, resulting in the suppression of IL-17 production by γδ T-cells.
Although researchers have used α-TCR δ as a means to deplete γδ T-cells and to study γδ T-cell responses in the mouse [13, 41], a recent study by Koenecke et al. [42] questions whether in vivo administration of TCR δ monoclonal antibodies results in the absolute depletion of γδ T-cells in animals. Using TCR delta locus histone 2B enhanced GFP knock-in mice, Koenecke et al. have shown that following engagement with α-TCR δ monoclonal antibodies (clone GL3 or UC7-13D5), γδ T-cell receptors are localized intracellularly and masked. As a result, γδ T-cells may appear to be depleted in the animal when assessed by flow cytometry. In light of this observation, it is likely that some γδ T-cells in our TCR δ treated animals may escape depletion and remain in the lungs. Confounding an incomplete depletion is that a resident pulmonary subset of γδ T-lymphocytes may undergo cellular proliferation during early stages of pulmonary nocardiosis (unpublished data), resulting in some restoration of function. This may explain the presence of IL-17 producing γδ T-cells in animals that are treated with anti-TCR δ antibody.
Nevertheless, the data of this current study showed that even without absolute depletion of γδ T-lymphocytes in tissue, administration of α-TCR antibody functionally blunted γδ T-cells, resulting in blunted IL-17 production and an impaired neutrophil response in the GUH-2 infected host. Although the antigens recognized by γδ TCR are yet to be clearly elucidated, it appears that the induction of the IL-17 response in γδ T-cells during pulmonary nocardiosis may require antigen/TCR engagement. In summary, our data indicate that IL-17 is produced locally in lung tissue as part of the early host response to a bacterial challenge with N. asteroides strain GUH-2. Presently, it is unknown whether IL-17 γδ T-lymphocytes directly impact CXC chemokines, which also strongly influence neutrophil infiltration and host susceptibility to Nocardia infection. However, from the data presented in this study, it is tempting to speculate that IL-17 producing γδ T-cells contribute to the induction of neutrophil recruitment and resolution of N. asteroides GUH-2. To what degree and how indispensible are IL-17 producing γδ T-lymphocytes to host protection during pulmonary nocardiosis requires further investigation.
Acknowledgment
This work was supported in part by the public health service grants HL69426 “Mechanisms of Homeostasis and Repair in the Lung” from the National Heart, Lung and Blood Institute (NHLB/NIH), NIH Training Grant NIHT32 RR07038 “Animal Models of Infectious Diseases”, and “Interdisciplinary Training in Comparative Lung Biology and Medicine” 5T32HL007013-33. In addition, this investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR-12088 from the National Center for Research Resources, National Institutes of Health. We also wish to thank Dr. Andreas Bäumler and Dr. Charles Bevins for kindly providing reagents and editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlaberg R, Huard RC, Della-Latta P. Nocardia cyriacigeorgica an emerging pathogen in the United States. J. Clin. Microbiol. 2008;46:265–273. doi: 10.1128/JCM.00937-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin. Microbiol. Rev. 1994;7:213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yildiz O, Doganay M. Actinomycoses and Nocardia pulmonary infections. Curr. Opin. Pulm. Med. 2006;12:228–234. doi: 10.1097/01.mcp.0000219273.57933.48. [DOI] [PubMed] [Google Scholar]
- 4.Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J. Clin. Microbiol. 2003;41:4497–4501. doi: 10.1128/JCM.41.10.4497-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaman BL, Goldstein E, Gershwin ME, Maslan S, Lippert W. Lung response to congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides . Infect. Immun. 1978;22:867–877. doi: 10.1128/iai.22.3.867-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaman BL. Differential binding of Nocardia asteroides in the murine lung and brain suggests multiple ligands on the nocardial surface. Infect. Immun. 1996;64:4859–4862. doi: 10.1128/iai.64.11.4859-4862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, Stovall MY, Van Winkle LS, Beaman BL, Ferrick DA. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J. Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 8.Moore TA, Newstead MW, Strieter RM, Mehrad B, Beaman BL, Standiford TJ. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J. Immunol. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- 9.Chien YH, Bonneville M. Gamma delta T cell receptors. Cell. Mol. Life Sci. 2006;63:2089–2094. doi: 10.1007/s00018-006-6020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 11.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J. Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 13.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 14.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 15.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O'Brien R L, Ikuta K, Kaku M, Fujita J, Kawakami K. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes. Infect. 2007;9:251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J. Leukoc. Biol. 2004;76:545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 17.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 19.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournie JJ, Dieli F. Differentiation, phenotype, and function of interleukin-17- producing human V{gamma}9V{delta}2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 20.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 22.Markel G, Bar-Haim E, Zahavy E, Cohen H, Cohen O, Shafferman A, Velan B. The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis . PLoS One. 2010;5:e11176. doi: 10.1371/journal.pone.0011176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godinez I, Raffatellu M, Chu H, Paixao TA, Haneda T, Santos RL, Bevins CL, Tsolis RM, Baumler AJ. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect. Immun. 2009;77:387–398. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Tam S, King DP, Beaman BL. Increase of gammadelta T lymphocytes in murine lungs occurs during recovery from pulmonary infection by Nocardia asteroides . Infect. Immun. 2001;69:6165–6171. doi: 10.1128/IAI.69.10.6165-6171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J. Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 27.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 28.Filice GA. Inhibition of Nocardia asteroides by neutrophils. J. Infect. Dis. 1985;151:47–56. doi: 10.1093/infdis/151.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Filice GA. Resistance of Nocardia asteroides to oxygen-dependent killing by neutrophils. J. Infect. Dis. 1983;148:861–867. doi: 10.1093/infdis/148.5.861. [DOI] [PubMed] [Google Scholar]
- 30.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 32.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umemura M, Kawabe T, Shudo K, Kidoya H, Fukui M, Asano M, Iwakura Y, Matsuzaki G, Imamura R, Suda T. Involvement of IL-17 in Fas ligand- induced inflammation. Int. Immunol. 2004;16:1099–1108. doi: 10.1093/intimm/dxh111. [DOI] [PubMed] [Google Scholar]
- 34.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 35.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 36.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte- responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 37.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 38.Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides . J. Leukoc. Biol. 2002;72:373–381. [PubMed] [Google Scholar]
- 39.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17- producing CD4 T cells during mycobacterial infection. J. Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 40.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Wang YM, Wang Y, Hu M, Zhang GY, Knight JF, Harris DC, Alexander SI. Depletion of gammadelta T cells exacerbates murine adriamycin nephropathy. J. Am. Soc. Nephrol. 2007;18:1180–1189. doi: 10.1681/ASN.2006060622. [DOI] [PubMed] [Google Scholar]
- 42.Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates "invisible" gammadelta T cells. Eur. J. Immunol. 2009;39:372–379. doi: 10.1002/eji.200838741. [DOI] [PubMed] [Google Scholar]