Abstract
Bryonia laciniosa Linn. (Cucurbitaceae) seed is used in traditional medicine for a number of ailments including metabolic disorders. This investigation was carried out to investigate the anti-hyperglycemic and anti-hyperlipidemic potential of the ethanolic extract of seeds of B. laciniosa Linn. and its saponin fraction in streptozotocin-induced diabetic rats. The ethanolic extract (250 and 500 mg/kg; p.o.) and saponin fraction (100 and 200 mg/kg; p.o.) were administered to diabetic rats and standard drug insulin (5 IU/kg; i.p.) to the group serving as a positive control. Effects of the ethanolic extract and saponin fraction on various biochemical parameters were studied in diabetic rats. Data were statistically analysed by one-way ANOVA followed by Dunnett's t-test. Oral administration of the ethanolic extract and saponin fraction for 28 days to streptozotocin-induced diabetes rats significantly (P < 0.05) decreased the levels of blood glucose and improved the levels of plasma insulin. The levels of triglycerides, cholesterol, high density lipoprotein, low density lipoprotein, very low density lipoprotein, aspartate amino transferase and alanine amino transferase, urea, and creatinine were markedly altered in streptozotocin-induced diabetic rats. Oral administration of the ethanolic extract and saponin fraction restored all these biochemical parameters to near control levels. This study reveals the efficacy of B. laciniosa seed extract and its saponin fraction in the amelioration of diabetes and its associated complications.
Keywords: Bryonia laciniosa, oral glucose tolerance test, saponins
INTRODUCTION
The global prevalence of diabetes is appeared to be a major burden in developing countries. Despite the great efforts that have been made in understanding and management of diabetes, the disease and related complications are increasing unabated. Concurrently, phytochemicals identified from traditional medicinal plants are providing exciting opportunities for the development of new type of therapies.[1]
There are many compounds isolated from traditional medicinal plants with antidiabetic activity of which saponins are promising compounds with the potential to be developed into new drugs for anti-diabetic.[2,3] Many studies have demonstrated that saponins exert antioxidant action and alleviate the streptozotocin-induced toxicity and contribute to hypoglycemic and hypolipidemic effects.[4]
Bryonia laciniosa Linn. Syn (Cucurbitaceae) plant is commonly used as a hypoglycemic herb by the native people of the Porbandar region. An ayurvedic literature survey indicated the use of entire plant is a bitter tonic, hepatoprotective, anti-pyretic, laxative, and used to correct the metabolic abnormalities.[5–8] The occurrence of bitter principle bryonin, saponin, punicic acid, goniothalamine, and glucomannan has been reported in this plant.[9–12] The various pharmacological activates of the extract of B. laciniosa is already explored.[13–16] The anti-hyperglycemic and anti-hyperlipidemic activities of the B. laciniosa seeds are remain unclear. Hence the present study was planned to determine the antihyperglycemic and antihyperlipidemic activities of the B. laciniosa seed extract.
MATERIALS AND METHODS
Materials
Streptozotocin was purchased from Sisco Research Laboratory Pvt. Ltd, India. Insulin was commercially purchased from Novo Nordisk India Pvt. Ltd. All other standard chemicals were obtained from common commercial suppliers.
Collection of plant material and extraction
The fruits of B. laciniosa were collected in October to December from Anand and Jamnagar region of Gujarat, India. The plant was authenticated by comparison with voucher specimen no. VSM502 and ARM 2174 at the Prof. G.L. Shah Herbarium of S.P. University, Vallabh Vidyanagar, Anand, Gujarat, India. The seeds were separated from the fruits and washed with large quantity of water. The seeds were grinded mechanically to make powder. The powdered sample was extracted with ethanol by Soxhlet to give B. laciniosa ethanol extract (BLEE). Total saponins were prepared by the method described previously.[4] The alcohol extract was concentrated, suspended in distilled water, and then partitioned successively with n-butanol saturated with water. The n-butanol extract was combined and evaporated using a rotary evaporator at 60°C to yield B. laciniosa saponin fraction (BLSF).
Determination of total saponins
The total saponins content of each extract was determined approximately using the method described by Hiai et al..[17] Briefly, the extracts (50 μl) were mixed with the vanillin (8% w/v, 0.5 ml) and sulfuric acid (72% w/v, 5 ml). The mixture was incubated at 60°C for 10 min, cooled in an ice water bath for 15 min, and the absorbance read at 538 nm. Oleanolic acid was used as a reference standard, and the content of total saponins was expressed as oleanolic acid equivalents (OAE μg/mg extract).
Animals
All experiments were performed on Swiss albino mice of either sex weighing about 20–25 g and female albino Wistar rats of body weight 200–250 g. Animals were maintained under controlled conditions of humidity, temperature (22 ± 2°C), and 12 h light and dark cycle. The standard pelleted diet (Pranav Agro Industries limited, Vadodara) and water were given ad libitum. The animals experiments were conducted as per Institutional animal ethical committee (910/ac/05/CPCSEA) instructions.
Acute toxicity studies
The acute toxicity study was carried out in female Swiss albino mice using fixed dose producer described by the Organisation for Economic Cooperation and Development (OECD). The compound was tested for maximum recommended dose of 2000 mg/kg.[18] Based on the toxicity testing result the pharmacological experiment dose as fixed at 250 and 500 mg/kg for BLEE, and 100 and 200 mg/kg for BLSF.
Experimental induction of diabetes in rats
Hyperglycemia was induced in overnight fasted adult albino Wister rats weighing 200–250 g by a single intra-peritoneal injection of 50 mg/kg streptozotocin (dissolved in 0.1 M ice-cold citrate buffer, pH 4.5, immediately before use) in a volume of 1 ml/kg body weight. Hyperglycemia was confirmed by the elevated glucose level in plasma, determined at 48 h after injection. The rats found hyperglycemic were screened for the anti-hyperglycemic study.
Drug administration
The BLEE and BLSF suspended in 0.25% NaCMC and administered orally through orogastric tubes at the following doses of 250 and 500 mg/kg body wt for BLEE and 100 and 200 mg/kg body wt for BLSF.
Experimental design
Totally sixty-six rats (42 diabetic rats, 24 normal rats) were used for the experiment. Diabetes was induced in rats 5 days before starting the treatment. A study of effect of BLEE and BLSF on type 1 diabetic rats involved two sets of experiments. The groups of animals in two different sets were as follows:
Set 1: Effect of BLEE on type 1 diabetic rats
Group 1: Normal control rats were administered vehicle
Group 2: Normal rats were administered BLEE (500 mg/ kg)
Group 3: Diabetic control rats were administered vehicle
Group 4: Diabetic rats were administered BLEE (250 mg/ kg)
Group 5: Diabetic rats were administered BLEE (500 mg/ kg)
Group 6: Diabetic rats were administered Insulin (5 IU/kg)
Set 2: Effect of BLSF on type 1 diabetic rats
Group 1: Normal control rats were administered vehicle
Group 2: Normal rats were administered BLSF (0.2 g/kg)
Group 3: Diabetic control rats were administered vehicle
Group 4: Diabetic rats were administered BLSF (100 mg/ kg)
Group 5: Diabetic rats were administered BLSF (200 mg/ kg)
All the studies were carried out for a period of 28 days. During the study standard food and water were provided ad libitum. Changes in body weight and food intake were recorded. At the end of experimental period, the animals were deprived of food overnight. Blood were collected from retro-orbital plexus under light ether anesthesia for biochemical analyses.
Oral glucose tolerance test
At the end of the study, the oral glucose tolerance test was performed after an overnight fasting.[19] The animals were orally administered with 1.5 g/kg of glucose, and blood samples were collected from the retro orbital plexus under light ether anesthesia before, i.e. 0, 30, 60, and 120 min after oral glucose administration. Serum was analyzed for glucose by the GOD–POD method by using diagnostic reagent kits (Span diagnostics Limited, India). The results were expressed as integrated area under the curve (AUC) for glucose. This was calculated by the trapezoid rule [AUC = (C1+ C2)/2 × (t2 - t1)] and changes in glucose concentrations over 120 min during OGTT were expressed as AUCglucose (mg/dl 120 min).
Biochemical assays
Serum glucose, total cholesterol, triglycerides, high density lipoprotein (HDL-C), urea, creatinine, aspartate amino transferase (AST), and alanine amino transferase (ALT) levels were determined using standard kits of Span Diagnostics Ltd., India. Low-density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C) and atherogenic index (AI) were calculated.[20] Serum insulin was measured by radioimmuno assay (Rat Insulin RIA Kit, Linco Research, Inc., St. Charles, MO).
Statistical analysis
Data were statistically analysed by utilizing one-way ANOVA and expressed as mean ± SEM followed by Dunnett's t-test. A P < 0.05 was considered as significant.
RESULTS
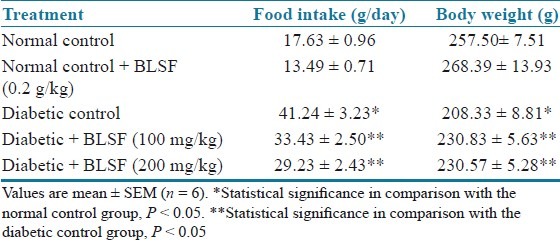
The total saponins content determination showed that BLEE and BLSF possess 6 and 15 μg/mg of OAE, respectively. In the study, the STZ treated diabetic rats developed uncontrolled type 1 diabetes mellitus. Diabetes was associated with reduced body weight and increased food intake when compared with the normal control rats. Final body weight (BW) and food intake of normal control and experimental groups are shown in Tables 1 and 2. However, treatment with BLEE and BLSF with all the doses appeared to protect the diabetic rats from body weight loss.
Table 1.
Effect of BLEE on food intake and body weight of STZ-induced diabetic rats
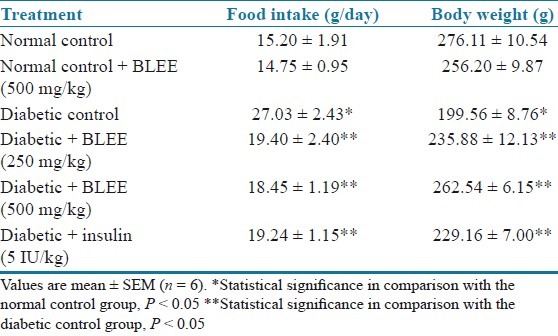
Table 2.
Effect of BLSF on food intake and body weight of STZ-induced diabetic rats
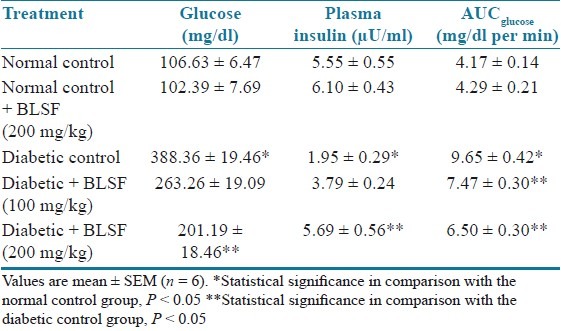
There was significant (P < 0.05) elevation in serum glucose levels associated with hypoinsulinemia in the diabetic animals as compared to normal control animals. Treatment with BLEE (250 and 500 mg/kg) and BLSF (100 and 200 mg/kg) significantly (P < 0.05) reduced glucose levels associated with an increase in serum insulin levels. STZ-induced diabetic animals showed significant (P < 0.05) increase in AUCglucose as compared to normal animals. Treatment with BLEE and BLSF at all the doses prevented the increase in AUCglucose at significant (P < 0.05) extent as compared to diabetic control animals [Tables 3 and 4].
Table 3.
Effect of BLEE on blood glucose, insulin, and AUCglucose of STZ-induced diabetic rats
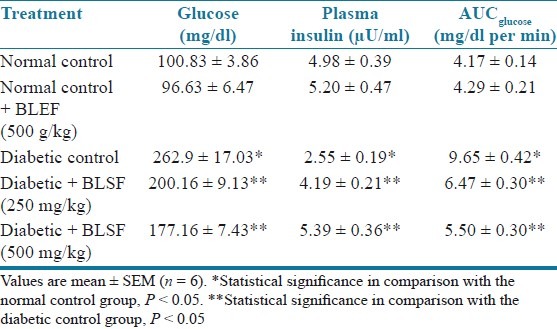
Table 4.
Effect of BLSF on blood glucose, plasma insulin, and AUCglucose of STZ- induced diabetic rats
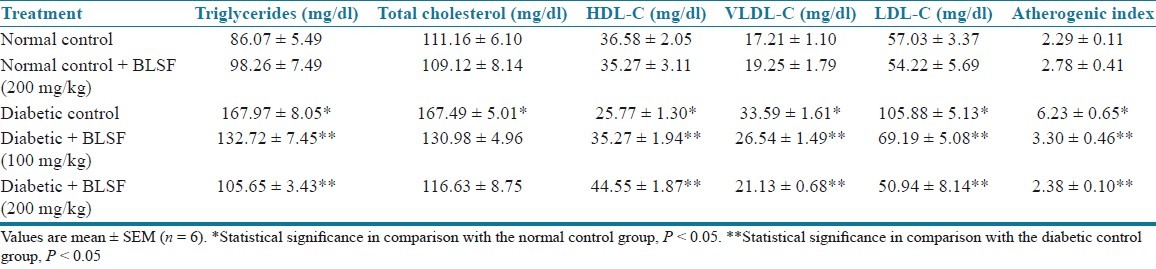
STZ in rats produced a significant (P < 0.05) increase in cholesterol, triglycerides, VLDL, and LDL while decrease in HDL levels as compared to nondiabetic control rats. Treatment of rats with BLEE (250 and 500 mg/kg) and BLSF (100 and 200 mg/kg) caused a significant (P < 0.05) decrease in cholesterol, triglycerides, VLDL, and LDL while increase in HDL levels of diabetic rats as compared to diabetic control rats [Tables 5 and 6].
Table 5.
Effect of BLEE on lipid profiles of STZ-induced diabetic rats
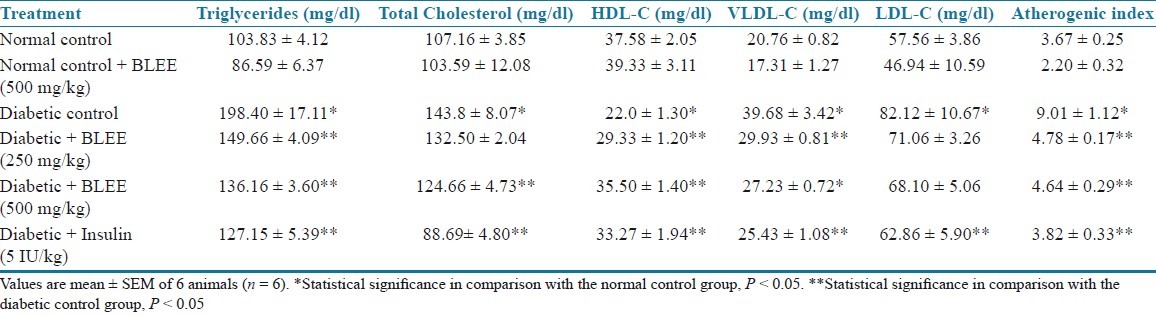
Table 6.
Effect of BLSF on blood glucose and lipid profiles of STZ-induced diabetic rats
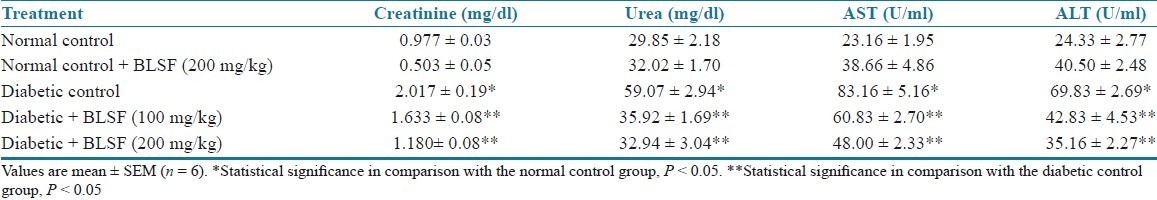
STZ-induced type 1 diabetic rats exhibited significantly (P < 0.05) higher AST and ALT levels as compared to nondiabetic control rats. Chronic treatment with BLEE (250 and 500 mg/kg) and BLSF (100 and 200 mg/kg) produced a significant (P < 0.05) decrease in AST and ALT levels of diabetic rats. STZ-induced type 1 diabetic rats exhibited significantly (P < 0.05) higher serum urea and serum creatinine levels as compared to nondiabetic control rats. Treatment with BLEE (250 and 500 mg/kg) and BLSF (100 and 200 mg/kg) decreased the elevated serum urea and creatinine levels of diabetic rats at significant extent (P < 0.05) [Tables 7 and 8].
Table 7.
Effect of BLEE on creatinine, urea, AST, and ALT of STZ-induced diabetic rats
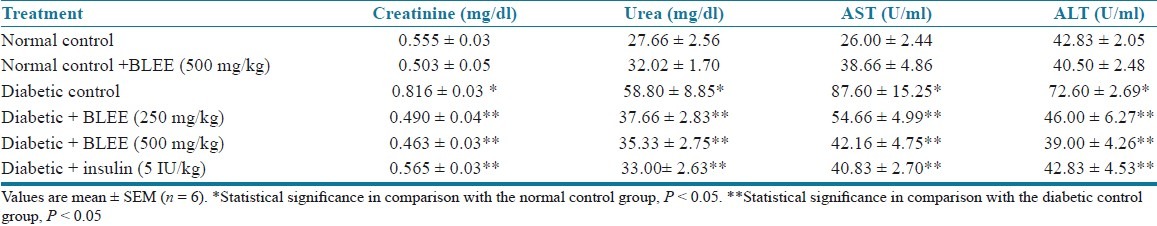
Table 8.
Effect of BLSF on creatinine, urea, AST, and ALT of STZ-induced diabetic rats
DISCUSSION
Within the context of present investigation, we evaluated the effect of BLEE and BLSF on various biochemical parameters in STZ-induced diabetic rats. STZ is an alkylating agent, causes β-cell necrosis through breaking of a DNA strand mediated by nitric oxide derived free radicals and induces “experimental diabetes” in many animal models.[21]
The destruction of β-cells during diabetes ultimately causes physico-metabolic abnormalities such as a decrease in body weight gain, and an increase in food.[20] A considerable reduction in the body weight change observed in the diabetic group of rats might be the result of protein wasting due to the unavailability of carbohydrates for energy metabolism and the loss or degradation of structural proteins.[22] The improvement in body weight gain in diabetic rats supplemented with BLEE and BLSF highlights the blood glucose homeostasis which in turn promotes the body weight gain.
Insulin deficiency resulted from STZ-mediated selective β-cells destruction ultimately results in increased production of glucose by the liver, and decreased utilization of glucose in peripheral tissues.[23] The elevated blood glucose level observed in the diabetic rats was significantly decreased in the BLEE- and BLSF-treated group of rats suggesting insulin stimulatory effects of B. laciniosa from the remnant β-cells. The reduced glucose levels suggested that BLEE and BLSF might exert insulin-like effect on peripheral tissues by either promoting glucose uptake metabolism or by inhibiting hepatic gluconeogenesis[24,25] or by interfering with the absorption of glucose into the muscle and adipose tissues,[26] by stimulating regeneration of pancreatic β-cells. The glucose levels of the normal rats administered with BLEE and BLSF were not significantly altered indicating its normo-glycemic effects.
An oral glucose tolerance test is a more sensitive index for early detection of abnormalities in glucose regulation than fasting glucose levels.[27] Impaired glucose tolerance reflects hepatic gluconeogenesis and reduced uptake of glucose from blood into skeletal muscle and adipose tissue following a meal.[28]
In STZ-induced diabetes, the increase in elevated glucose levels is usually associated with an increase in plasma cholesterol, triglycerides, LDL, and VLDL and decreases in HDL. The insulin deficient state turns into the activation of hormone-sensitive lipase (HSL) which is resulted to enhance the release of free fatty acids from adipose tissue.[29] Thus, excess fatty acids in the blood produced by the STZ-induced diabetes promote the conversion of excess fatty acids into phospholipids and cholesterol in the liver. These two substances, along with excess triglycerides formed in the liver, may be discharged into the blood in the form of lipoproteins.[29] Therefore, the marked hyperlipidemia that characterizes the diabetic state may be regarded as a consequence of the uninhibited actions of lipolytic hormones on fat depots. However, treatment with the BLEE and BLSF normalized the serum lipid status, which was presumably mediated by a control of lipid metabolism.
The increased activity of ALT in diabetes represents the hepatocellular damage which is usually accompanied by an increase in AST activity.[30] Moreover, the AST and ALT activity has been used as an indicator of liver function. The reversal of AST and ALT activity in BLEE- and BLSF-treated diabetic rats toward near normalcy is evidence of the prevention of cellular and tissue damage under diabetic conditions, which may further strengthen the optimized lipid metabolism in the liver of diabetic rats.
The elevated blood glucose levels in diabetes leads to several derangements in the protein metabolism in diabetic rats. This turns into the development of a negative nitrogen balance. Disturbance in the nitrogen balance elevates the level of urea and creatinine. Their levels reflect the renal dysfunction.[30] Treatment of diabetic rats with the BLEE and BLEF brought back their levels to the near normal level. These results indicate the efficacy of the Bryonial aciniosa to prevent kidney damage.
CONCLUSION
In conclusion, the B. laciniosa seed extract and its saponin-rich fraction alleviated hyperglycemia and hyperlipidemia. In addition, B. laciniosa could also ameliorate the impaired renal function and inhibit liver damage associated with streptozotocin diabetes suggesting that it might be useful for the prevention of diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tiwari AK, Rao JM. Diabetic mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr Sci. 2002;83:30–7. [Google Scholar]
- 2.Zhang LH, Xiao PG. Recent advances in studies of antihyperlipaemic and antihyperglycaemic compounds from Chinese traditional and herbal medicines. Phytother Res. 1993;7:217–26. [Google Scholar]
- 3.Xu R, Zhao W, Xu J, Shao B, Qin G. Studies on bioactive saponins from Chinese medicinal plants. Adv Exp Med Biol. 1996;404:371–82. doi: 10.1007/978-1-4899-1367-8_30. [DOI] [PubMed] [Google Scholar]
- 4.Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22:228–37. doi: 10.1002/ptr.2297. [DOI] [PubMed] [Google Scholar]
- 5.Kirtikar K, Basu B. Indian Medicinal Plants. 2nd ed. Allahabad: The Indian Press; 1987. p. 1549. [Google Scholar]
- 6.Gabrielian SE, Gevorgovich A. Bryonia, as novel plant adoptogen, for prevention and treatment of stress induced disorders. Promising Res Abstr. 1997:1–8. PRA- 5003. [Google Scholar]
- 7.Acharya D. Shivlingi: A common but important twine in Patalkot. Am Chron. 2007. [Last accessed on 2012 Jan 25]. Available from: http://www.americanchronicle.com/articles/view/40216 .
- 8.Nadkarni K. Indian Materia Medica. Mumbai: Popular Book Depot; 1927. p. 219. [Google Scholar]
- 9.Saxena N, Balyari N, Srivastva A. Pharmacological studies of novel pharmaceutical saponin molecules of seeds of Bryonialaciniosa. IUPAC. Internationational conference on biodiversity, natural product chemistry and medicinal applications, New Delhi. 2004 Jan 26-31;:368. [Google Scholar]
- 10.Gowrikumar G, Mani VV, Chandrasekhararao T, Kaimal TN, Lakshminarayana G. Diplocyclospalmatus L: A new seed source of punicic acid. Lipids. 1981;16:558–9. [Google Scholar]
- 11.Mosaddik MA, Ekramul Haque M, Abdur Rashid M. Goniothalamin from Bryonopsis laciniosa Linn (Cucurbiataceae) Biochem Syst Ecol. 2000;28:1039–40. doi: 10.1016/s0305-1978(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Malviya TA. Non-ionic glucomannan from the seeds of an indigenous medicinal plant: Bryonialacinosa. Carbohydr Polym. 2006;64:481–3. [Google Scholar]
- 13.Mosaddik MA, Haque ME. Cytotoxicity and antimicrobial activity of goniothalamin isolated from Bryonopsislaciniosa. Phytother Res. 2003;17:1155–7. doi: 10.1002/ptr.1303. [DOI] [PubMed] [Google Scholar]
- 14.Singh V, Malviya T, Tripathi DN, Naraian U. An Escherichia coli antimicrobial effect of arabinoglucomannan from fruit of Bryonialacinosa. Carbohydr Polym. 2009;75:534–7. [Google Scholar]
- 15.Gupta M, Mazumdar UK, Sivakumar T, Vamsi ML, Karki SS, Sambathkumar R, et al. Evaluation of anti-inflammatory activity of chloroform extract of Bryonialaciniosa in experimental animal models. Biol Pharm Bull. 2003;26:1342–4. doi: 10.1248/bpb.26.1342. [DOI] [PubMed] [Google Scholar]
- 16.Karageuzyan K, Vartanyan G, Agadjanov M. Restoration of disordered glucose-fatty acid cycle in alloxan-diabetic rats by trihydrixyoctadecdienoic acids from Bryonia alba- a native americal plant. Planta Med. 1998;64:417–22. doi: 10.1055/s-2006-957472. [DOI] [PubMed] [Google Scholar]
- 17.Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin sulphuric acid. Planta Med. 1976;29:116–22. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- 18.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–9. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olefsky JM. Insulin resistance and insulin action. An in vitro and in vivo respective. Diabetes. 1981;30:118–22. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Brodsky IG. Nutritional effects of dietary protein restriction in insulin dependent diabetes mellitus. J Nutr. 1998;128:37. doi: 10.1093/jn/128.2.337S. [DOI] [PubMed] [Google Scholar]
- 23.Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995;22:123–89. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 24.Ali L, Azad Khan AK, Mamun MI, Mosihuzzaman M, Nahar N, Nur-E-Alan M, et al. Studies on the hypoglycaemic effects of fruits pulp, seed and whole plant of Momordicacharantia on normal and diabetic model rats. Planta Med. 1993;59:408–12. doi: 10.1055/s-2006-959720. [DOI] [PubMed] [Google Scholar]
- 25.Gray AM, Abdel-Wahab YH, Flatt PR. The traditional plant treatment, Sabucusnigra (Elder), exhibits insulin-like and insulin releasing actions in vitro. J Nutr. 2000;130:15–20. doi: 10.1093/jn/130.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Kamanyi A, Djamen D, Nkeh B. Hypoglycaemic properties of the aqueous roots extract of Morindalucida (Rubiaceae) study in the mouse. Phytother Res. 1994;8:369–71. [Google Scholar]
- 27.Bolkent S, Yamardag R, Tabakogluoguz A, Ozsoy-Sacon O. Effects of Chord (Beta vulgaris L. var. cicla) extract on pancreatic B-cells in streptozotocin-diabetic rats: A morphologic and biochemical study. J Ethnopharmacol. 2000;73:251–9. doi: 10.1016/s0378-8741(00)00328-7. [DOI] [PubMed] [Google Scholar]
- 28.Alford FP, Martin FI, Pearson MJ. Significance and interpretation of mildly abnormal oral glucose tolerance test. Diabetologia. 1971;7:173–80. doi: 10.1007/BF01212550. [DOI] [PubMed] [Google Scholar]
- 29.Garber AJ. Attenuating CV risk factors in patients with diabetes: Clinical evidence to clinical practice. Diabetes Obes Metab. 2000;4:S5–12. doi: 10.1046/j.1462-8902.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 30.Mutalik S, Chetana M, Sulochana B, Devi PU, Udupa N. Effect of dianex, a herbal formulation on experimentally induced diabetes mellitus. Phytother Res. 2005;19:409–15. doi: 10.1002/ptr.1570. [DOI] [PubMed] [Google Scholar]


