Abstract
Mercury is one of the noxious heavy metal environmental toxicants and is a cause of concern for human exposure. Mangiferin (MGN), a glucosylxanthone found in Mangifera indica, reported to have a wide range of pharmacological properties. The objective of this study was to evaluate the cytoprotective potential of MGN, against mercury chloride (HgCl2) induced toxicity in HepG2 cell line. The cytoprotective effect of MGN on HgCl2 induced toxicity was assessed by colony formation assay, while antiapoptotic effect by fluorescence microscopy, flow cytometric DNA analysis, and DNA fragmentation pattern assays. Further, the cytoprotective effect of MGN against HgCl2 toxicity was assessed by using biochemical parameters like reduced glutathione (GSH), glutathione-S-transferase (GST), superoxide dismutase (SOD), catalase (CAT) by spectrophotometrically, mitochondrial membrane potential by flowcytometry and the changes in reactive oxygen species levels by DCFH-DA spectrofluoremetric analysis. A significant increase in the surviving fraction was observed with 50 µM of MGN administered two hours prior to various concentrations of HgCl2. Further, pretreatment of MGN significantly decreased the percentage of HgCl2 induced apoptotic cells. Similarly, the levels of ROS generated by the HgCl2 treatment were inhibited significantly (P < 0.01) by MGN. MGN also significantly (P < 0.01) inhibited the HgCl2 induced decrease in GSH, GST, SOD, and CAT levels at all the post incubation intervals. Our study demonstrated the cytoprotective potential of MGN, which may be attributed to quenching of the ROS generated in the cells due to oxidative stress induced by HgCl2, restoration of mitochondrial membrane potential and normalization of cellular antioxidant levels.
Keywords: apoptosis, cytotoxicity, mangiferin, mercury chloride and reactive oxygen species
INTRODUCTION
Metals are widely distributed in the environment and their toxicity is very well established. Mercury is one of the noxious heavy metal environmental toxicants because of its bioaccumulative properties (WHO, 1990). The major sources of mercury include alkali and metal processing, incineration of coal, mining, and use of mercury derivatives and atmospheric depositions (Wiener et al., 2003). Human beings are exposed to mercury and mercury compounds through occupational and environmental setting, primarily via food (Bolger and Schwetz, 2002) and gets accumulated in many tissues and systems. They induce acute and chronic pathogenic alterations especially neurological problems. One of the major mechanisms behind mercury toxicity has been attributed to oxidative stress. A plethora of published data supply evidence that metals are capable of reacting with nuclear proteins and DNA causing oxidative worsening of these biological macromolecules (Valko et al., 2005).
Another mechanism by which mercury damages DNA molecule is by the generation of reactive oxygen species (ROS) such as hydrogen peroxides or oxygen free radicals (Cantoni et al., 1984). When there is an imbalance between free radical production and radical scavenging capacity of antioxidant system, vicious effects of free radicals begin (Battin and Brumaghim, 2009). Mercury induced oxidative stress creates an important contribution to molecular mechanism for liver injury (Valko et al., 2005; Flora et al., 2008). In view of the omnipresence of mercury and unavoidability of exposure, it is important to develop an effective strategy to prevent mercury toxicity. Although, several dietary factors such as nutritional supplements, antioxidants, vitamins, and essential elements are known for their beneficial role in mercury induced cellular toxicity, assisting the body’s natural processes of detoxification and elimination, their practical applicability is limited due to toxicity at their effective doses (Blanusa et al., 2005; Flora et al., 2008). Because of this low acceptability of the chemical protectors, the search has been shifted to the plant products and their role in modulation of heavy metal induced toxicity. Plant-derived agents such as polyphenols, flavonoids and xanthones have been known to have protective roles against heavy metal toxicity (Flora, 2002).
As phenolic compounds, xanthones are described for their antioxidant properties, free radical scavenging, and metal chelating effects (Ghosal et al., 1996). One of the most widely studied xanthone is Mangiferin (MGN) which is predominantly found in the bark, fruits, roots, and leaves of Mangifera indica Linn and various other medicinal plants (Martin and Qian, 2008). MGN has been found to exhibit a wide range of pharmacological effects (Sato et al., 1992; Guha et al., 1996; Ichiki et al., 1998; Garrido et al., 2004). Our earlier in vitro (Satish Rao et al., 2009) and in vivo (Kasi et al., 2010, accepted for publication) reports showed the protective efficacies of MGN against cadmium chloride (CdCl2) induced oxidative stress. With this background, the present study has been conducted to evaluate the efficacy of MGN in mitigating the effects of the HgCl2 induced effects in HepG2 cells.
MATERIALS AND METHODS
Chemicals
MGN, Eagle’s minimum essential medium (MEM), trypsin (0.1%), trypan blue (0.1%), rhodamine 123, ethylene diamine tetraacetic acid (EDTA), fetal calf serum (FCS), 2′, 7′-dichlorofluorescein diacetate (DCFH-DA), 5,5′-dithiobis 2-nitrobenzoic acid (DTNB), TBA (2-Thiobarbituric acid), 1-chloro-2,4-dinitrobenzene (CDNB), GSH [reduced glutathione (GSH)], sodium dodecyl sulfate (SDS), ethidium bromide (EtBr), Tris-HCl, ascorbic acid and acridine orange (AO) were purchased from Sigma Chemical Co. (St. Louis, MO). HgCl2 was purchased from Merck Specialities Pvt. Ltd, India. Dimethyl sulfoxide (DMSO), disodium hydrogen phosphate, dipotassium hydrogen phosphate, potassium dihydrogen phosphate, sodium carbonate and all other chemicals were purchased from Qualigens Fine Chemicals (A Division of GlaxoSmithKline Pharmaceuticals), Mumbai, India.
Cell Line and Culture
HepG2 (human hepatocellular carcinoma) cells were procured from National center for cell sciences (Pune, India). Cells were grown in 25-cm2 flasks (Falcon, Becton Dickinson, USA) with loosened caps, containing MEM supplemented with 10% FCS, 1% L-glutamine and 50 µg/mL gentamycin sulfate at 37°C in a humidified 5% CO2 incubator (NuAire incubator, Plymouth, MN), in a humidified atmosphere with 5% CO2.
Preparation of HgCl2/MGN Solutions
HgCl2 was dissolved in double distilled water (DDW) to get a stock of 1 mM. The stock was further diluted with MEM to obtain the desired concentrations. MGN was dissolved in 0.02% DMSO and further diluted with media to give 1 mM concentration immediately before use. In this study, the different concentrations of MGN/HgCl2 used to assess the cytoprotective potential of MGN were selected on the basis of our earlier studies (unpublished observation).
Clonogenic Survival Assay
This assay was performed according to the method of Puck and Marcus (1955). A fixed number (5 × 105) of exponentially growing cells were inoculated into several individual 25-cm2 culture flasks and allowed to grow, these cultures were then divided into Group I (HgCl2 alone), the cultures of this group were exposed to different concentrations of HgCl2 (1–10 µM) for 3 h. Group II (MGN + HgCl2), the cultures of this group were treated with 50 µg/mL of MGN for two hours before exposure to different concentrations of HgCl2 (1–10 µM) for 3 h. The cells from above groups were trypsinized and the single cell suspensions were counted using a hemocytometer and plated into 25 cm2 petri-dishes (Nunc, Denmark) containing 5 mL growth medium in triplicates for each concentration in each group. The cells were allowed to grow for 14 days. At the end of 14th day, the media was removed and the petri-dishes were washed with PBS and stained with crystal violet (1%). Colonies containing 50 cells or more were considered to be viable colony. The experiments were repeated three times and the survival curves were plotted as surviving fraction against radiation/HgCl2 alone or as combination treatments.
The Plating Efficiency (PE) and the Surviving Faction (SF) were calculated as follows:
Reactive Oxygen Species
The protective effect of MGN on HgCl2 induced ROS in HepG2 cells was detected by fluorometric assay using intracellular oxidation of DCFH-DA as described earlier by Bai and Cederbaum (2003). Briefly, the exponentially growing HepG2 cells (5 × 105) were divided into group I (HgCl2 alone) the cultures of this group were treated with 20 µM of HgCl2 alone for 3 h. Group II (MGN + HgCl2) cultures treated with 50 µM of MGN for 2 h before being treated with 20 µM concentrations of HgCl2 for 3 h and the cells were allowed to grow for 60, 90, and 180 min. After the treatment, cells were incubated with 5 µM DCFH-DA in MEM for 30 min at 37°C in dark. The cells were then washed in PBS, harvested, and processed for estimation of intracellular ROS levels by using fluorescence spectrophotometer (RF-5301PC, Shimadzu) at 488 nm for excitation and at 525 nm for emission. The results were expressed as arbitrary units of the fluorescence intensity.
Determination of Mitochondrial Membrane Potential (Ψm)
The alterations in mitochondrial membrane potential produced by HgCl2 and its stabilization by using MGN were carried out according to the method described earlier by Scaduto and Grotyohann (1999). Rhodamine 123, a cell-permeable cationic dye that preferentially enters into mitochondria based on highly negative mitochondrial membrane potential. Depolarization of mitochondrial membrane potential results in the loss of Rhodamine 123 from the mitochondria and a decrease in intracellular fluorescence. Briefly, the exponentially growing HepG2 cells (5 × 105) were divided into group I (HgCl2 alone)-cells were treated with different concentrations of HgCl2 (5, 10, and 20 µM) for 3 h. Group II (MGN + HgCl2)-cells were treated with 50 µM of MGN for 2 h before being treated with different concentrations of HgCl2 (5, 10, and 20 µM) for 3 h and the cells were allowed to grow for further 2 h. At the end of the treatment period, the media containing MGN/HgCl2 was removed and fresh medium (MEM without serum and FBS) was added. Rhodamine-123 (5 µg/mL) was added to the media and incubated for 30 min in the dark at 37°C. The cells were harvested and suspended in PBS. The mitochondrial membrane potential was measured by FACSCalibur™ flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA), and data analyzed using WinMDI software Version 2.9 (CA, USA).
Cell Death by Apoptosis
Exponentially growing cells (106) were seeded in 25 cm2 culture T-flasks and allowed to attach and grow overnight. After 24 h, cells were treated with different concentrations of MGN (25, 50, and 75 µM) for 2 h followed by treatment with 20 µM of HgCl2 for 3 h and the cells were allowed to grow. Twenty four hours after post incubation, media was removed and cells were dislodged by trypsin EDTA (0.1%) treatment and the cells were processed for microscopic analysis, flow cytometry, and DNA fragmentation assays.
Fluorescence Microscopic Analysis of Apoptotic Cells
The morphological changes that occur in a cell during apoptosis/ necrosis were also analyzed by the differential uptake of fluorescent DNA binding ethidium bromide and acridine orange (AO/EtBr) stains as described by Renvoize and coworkers (1998). Briefly, both adherent and floating cells were collected and stained with a mixture of AO (50 µg/mL) and EtBr (10 µg/mL). The stained cells were visualized under a fluorescent microscope (Olympus BX51, Olympus Microscopes, Japan) using 40X magnification. Altogether 200 cells were analyzed and differentiated as live, apoptotic, and necrotic cells as described earlier (Aithal et al., 2009). The Apoptotic Index (AI) and the percentage of necrotic cells were expressed as a fraction of total number of cells.
Analysis of Sub-G1 Cell Population by Flow Cytometry
Flow cytometry was performed in order to determine the apoptotic sub-G1 hypodiploid cells according to the protocol as described by Nicoletti and coworkers (1991). After the various treatments, cells from the above groups were harvested by trypsinization and fixed in cold 70% ethanol overnight at 4°C. Further, cells were washed twice with PBS and treated with RNAse (100 µg/mL) for 1 h at 37°C. Then, 5 µL of propidium iodide (1 mg/mL) was added in dark on ice, and cells were further incubated with the dye for 20 min. Using the CellQuest software the cells (104) were analyzed after appropriate gating in a FACSCalibur™ flow cytometer using WinMDI, version 2.9 software.
Detection of DNA Fragmentation by Agarose Electrophoresis
The formation of ladder pattern from the DNA fragmentation indicating apoptosis was performed according to the protocol described by Giri et al. (2003) with minor modifications. After the various treatments, the floating and adherent cells from above groups were treated with lysis buffer (0.02 M EDTA, 0.05 M Tris HCl, 1% Nonidet P-40) overnight at 37°C. The lysed cells were then centrifuged at 1000 rpm for 10 min and the supernatant was collected in microfuge tubes. To this, RNAse A (100 µg/mL) and SDS (final concentration 1%) were added and kept at 56°C for overnight, followed by proteinase K (100 µg/mL) treatment at 56°C for 8 h. DNA was precipitated by adding almost half the volume of 10 M ammonium acetate and 2.5 volumes of 100% ethanol and the samples were stored overnight at −20°C for “ethanol precipitation.” The DNA was recovered by centrifugation at 13,000 rpm for 15 min at 4°C. The pellet was bench dried and the DNA was dissolved in Tris-EDTA (TE) buffer, pH 8.0. Agarose gel electrophoresis was carried out on 1.5% agarose gel at 60 V for 90 min. DNA fragments were visualized on the gel stained with ethidium bromide under UV light (UVITEC, Cambridge, UK).
Biochemical Assays
A fixed number of cells (106) growing in 25-cm2 culture flasks were treated with 50 µg/mL of MGN for 2 h followed by treatment with different concentrations of HgCl2 (20, 30, and 40 µM) for 3 h and the cells were allowed to grow for further 24, 48, and 72 h. The cells were then lysed in a lysis buffer appropriate for the requirements of each assay, as described below. Total protein contents were estimated by the modified method of Lowry et al. (1951). The results were expressed as enzyme activity per mg protein compared with corresponding control cultures.
GSH Estimation
The cells were lysed at 4°C for 2 h using 5% w/v metaphosphoric acid (chilled) to extract the cellular GSH. The suspension was then centrifuged at 13,000 rpm for 5 min and GSH content was measured by the method of Moron et al. (1979). Briefly, proteins were precipitated by 25% TCA, centrifuged, and the supernatant was collected. The supernatant was mixed with 0.2 M sodium phosphate buffer pH 8.0 and 0.06 mM DTNB and incubated for 10 min at room temperature. The absorbance was read at 412 nm and the GSH concentration was calculated from the standard curve.
Gluthione-S-Transferase Activity
Gluthione-S-Transferase (GST) activity was determined according to the procedure of Habig et al. (1974). Briefly, the reaction mixture containing 850 µL phosphate buffer and 50 µL CDNB was incubated for 10 min at 37°C. Then 50 µL of cell lysate with 50 µL of GSH is added just before taking the reading. The absorbance was recorded against blank at 340 nm and the specific activity of GST was expressed as µmol of reduced CDNB conjugate formed per minute per mg protein.
Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was assayed according to Misra and Fridovich (1972). This method is based on the ability of SOD to inhibit the auto-oxidation of epinephrine at alkaline pH (10.2) to pink-colored adrenochrome, which is markedly inhibited by the presence of SOD. Epinephrine was added to the reaction mixture, containing cell lysate and the increase in the absorbance which is proportional to the rate of auto-oxidation of epinephrine to adrenochrome was read immediately at 480 nm using a spectrophotometer.
Catalase Activity
The catalase activity was estimated by catalytic reduction of hydrogen peroxide using the method of Aebi (1984). Briefly, the reaction mixture contained 12 µL 3% (v/v) H2O2 and 100 µL of cell lysate in 50 mm phosphate buffer (pH 7.0) at a final volume of 1.0 mL. The samples were incubated for 2 min at 37°C. The decomposition of hydrogen peroxide was monitored by recording the absorbance against the blank at 240 nm.
Statistical Analysis
The statistical significance between the treatments was evaluated by Student’s “t” test for the biochemical studies and One-way ANOVA test was used to compare the results whenever more than two experimental groups were compared. All the data are expressed as Mean ± SEM (standard error of the mean).
RESULTS
Clonogenic Survival Assay
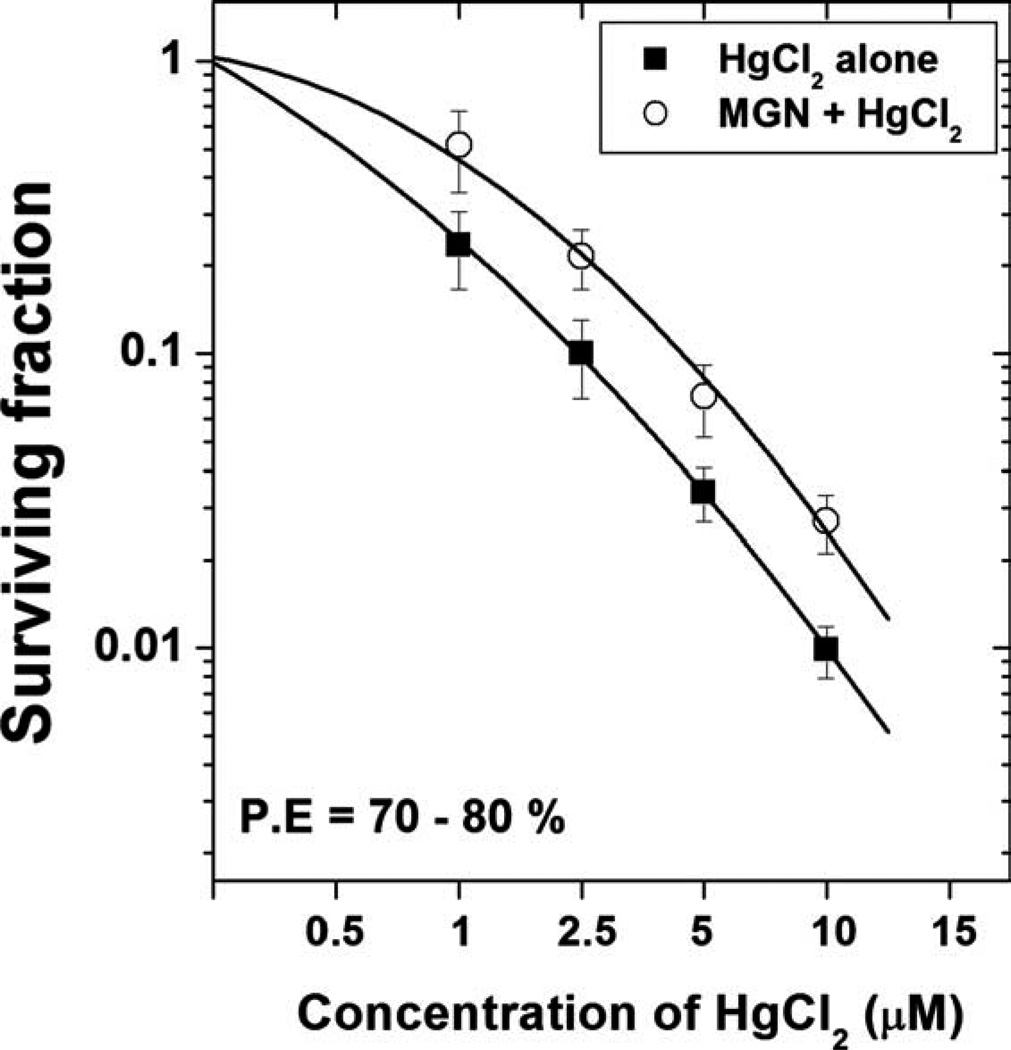
To investigate the cytoprotective potential of MGN to inhibit HgCl2 induced toxicity on HepG2 cells, clonogenic survival assay was performed. In the present study, treatment of HepG2 cells with different concentrations of HgCl2 for 3-h duration resulted in a concentration dependent decrease in cell survival as indicated by the decline of surviving fraction. Pretreatment with the MGN at a dose of 50 µM for 2 h before exposure to different concentrations of HgCl2 resulted increased cell survival when compared with HgCl2 alone treatment. The protective effect of MGN was seen at all concentrations of mercuric chloride used (Fig. 1).
Fig. 1.
Effect of mangiferin on the cell survival of HepG2 cells treated with different concentrations of mercuric chloride. (Cells were treated with 50 µM mangiferin for 2 h followed by treatment with different concentrations of mercuric chloride for 3 h).
Intracellular ROS Estimation
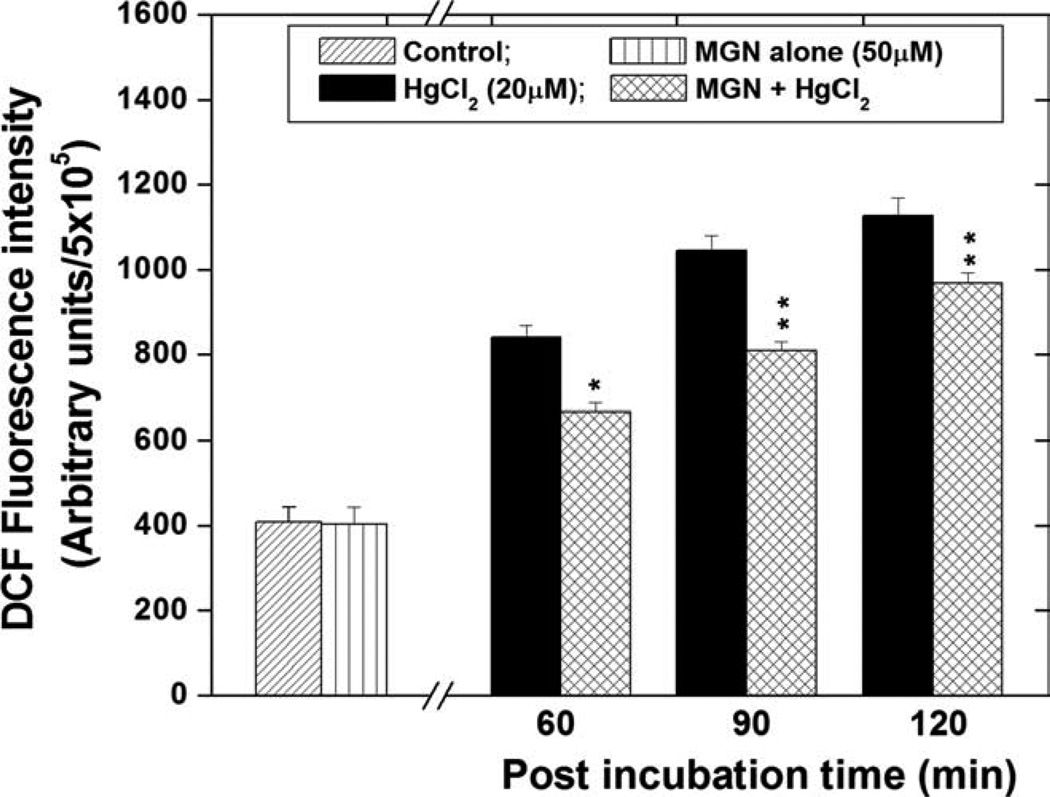
To determine the role of ROS production in HgCl2 toxicity, DCFH-DA assay was performed. HgCl2 (20 µM) treatment resulted in a significant (P < 0.01), time-dependent increase in ROS generation in HepG2 cells beginning at 60 min (the earliest time point measured), as compared with untreated cells (Fig. 2). At 90 and 120 min, HgCl2-induced cellular ROS formation was increased by 2.55 and 2.75 folds, as compared with control cells. Treatment with the best effective dose (50 µM) of MGN alone for 2 h did not induce any ROS generation in HepG2 cells. However, MGN significantly (P < 0.01) inhibited the HgCl2 induced ROS generation at all post incubation time periods when compared with the respective HgCl2 alone groups.
Fig. 2.
Effect of mangiferin on ROS generation in HepG2 cells treated with 20 µM of HgCl2 and post incubation for different time periods. The significant levels *P < 0.05, **P < 0.01, and No symbol = Nonsignificant, when compared with respective HgCl2 alone group. Each experiment was performed at least three times and the data are expressed as mean ± SEM.
Determination of Mitochondrial Membrane Potential (Ψm)
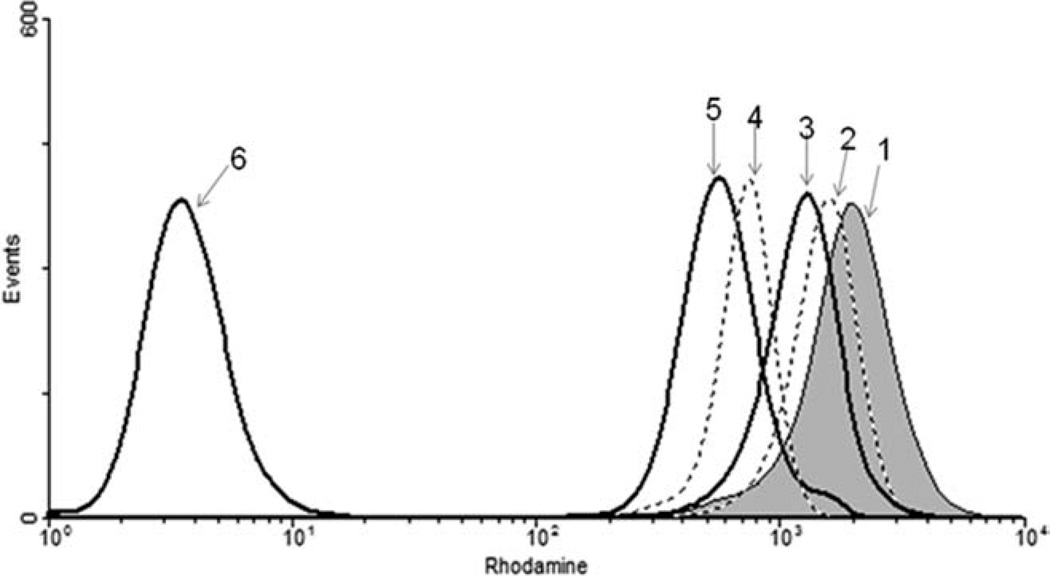
Rhodamine 123 is a lipophilic cationic dye and thus enters the mitochondria and gets retained within the mitochondria due to its binding to the inner mitochondrial membrane in proportion to the mitochondrial membrane potential. Figure 3 shows HgCl2 induced reduction in the mitochondrial membrane potential with a shift in the peaks to the left. A marker was set to identify the maximum number of cells falling within the control (normal cells with fluorescent dye) and the number of cells in the treated groups was determined by the shift in their peaks with respect to the control. A dose-dependent increase in the percentage of cells with decreasing mitochondrial membrane potential was observed. On pretreatment of cells with MGN, the membrane potential was seen to rise up almost to the level of control as seen by the shift in the peaks towards the right (Fig. 3). Thus MGN normalized the HgCl2 induced decrease in the mitochondrial potential.
Fig. 3.
Normalization of mitochondrial membrane potential by MGN in HgCl2 treated HepG2 cells. (1) Control; (2) MGN (50 µM) + HgCl2 (10 µM); (3) HgCl2 (10 µM) alone; (4) MGN (50 µM) + HgCl2 (20 µM); (5) HgCl2 (20 µM) alone; (6) Cells without dye.
Effect of MGN on HgCl2 Induced Apoptosis
DNA Fragmentation Assay
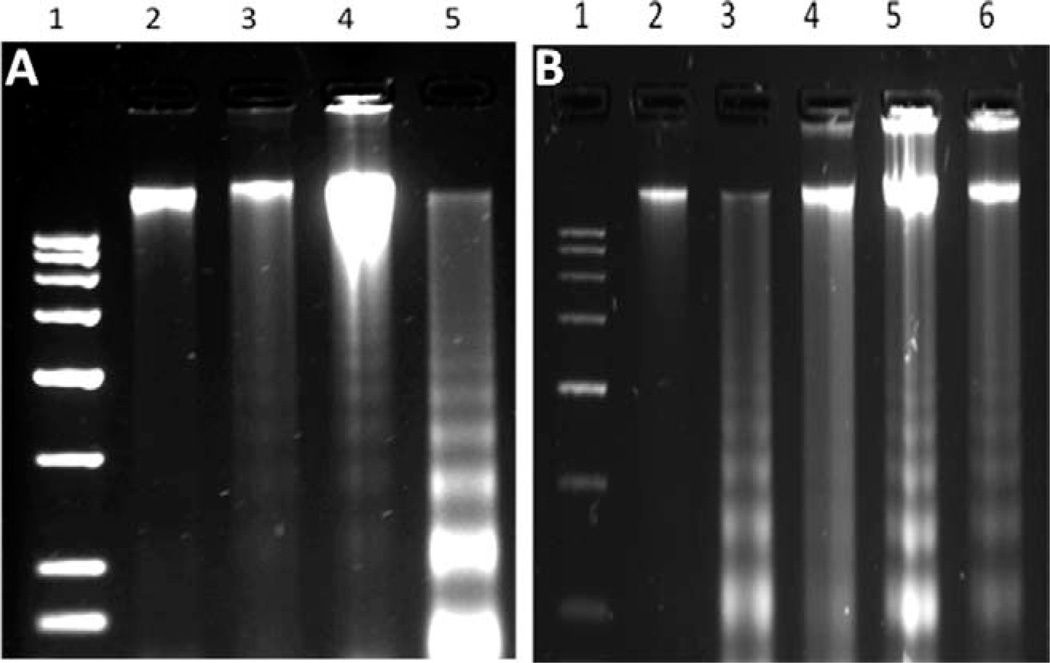
To evaluate HgCl2 induced apoptosis by gel electrophoresis, nucleosomal DNA fragmentation assay was performed. The results of DNA fragmentation assay is shown in Figure 4(A). The results indicated a clearly visible ladder pattern in 25 µM HgCl2 treated group when compared with HgCl2 alone treated groups [Fig. 4(A), Lane 5]. To assess the protective effect of MGN on HgCl2 induced apoptosis, cells were treated with various doses of MGN (25 µM, 50 µM, and 75 µM) prior to an optimal dose of HgCl2 (25 µM). The higher concentrations of MGN (50 µM and 75 µM) were ineffective in inhibiting HgCl2 induced apoptosis and at 25 µM concentration inhibition of apoptosis was clearly evident [Fig. 4(B), Lane 4]. The nontoxic nature of optimal concentration (25 µM) of MGN was indicated in our earlier studies using HepG2 cells demonstrated both in MTT and apoptosis assays (Satish Rao et al., 2009).
Fig. 4.
(A) Detection of DNA fragmentation in HgCl2 treated cells by agar gel electrophoresis. Lane 1, 3kb marker; Lane 2, Control; Lane 3, HgCl2 (20 µM); Lane 4, HgCl2 (22.5 µM); Lane 5, HgCl2 (25 µM). (B) Effect of mangiferin on HgCl2 induced DNA fragmentation. (Lane 1, 3 kb marker; Lane 2, Control; Lane 3, HgCl2 alone (25 µM); Lane 4, MGN (25 µM) + HgCl2; Lane 5, MGN (50 µM) + HgCl2; Lane 6, MGN (75 µM) + HgCl2).
Morphological Analysis of Apoptosis
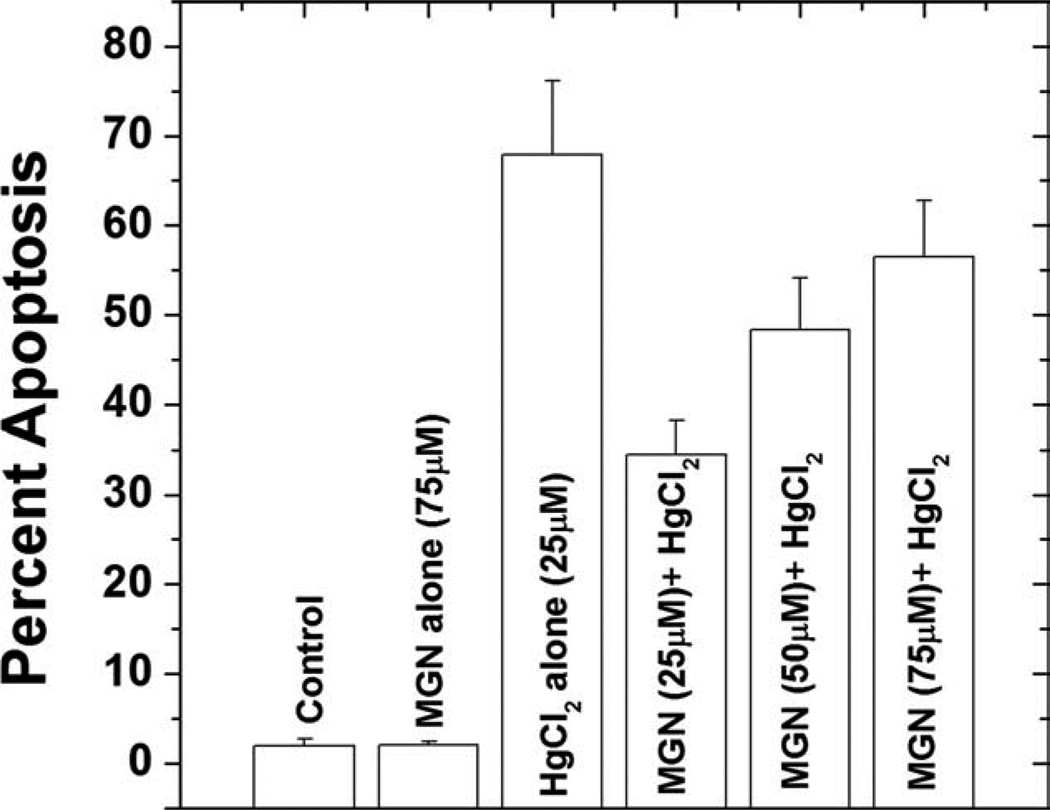
The morphological changes in HepG2 cells after various treatments was observed by dual staining with AO/EtBr and the apoptotic index was obtained as the percentage of the apoptotic cells. The microscopic analysis demonstrated that the control cells possessed intact green nuclei, while the HgCl2 (25 µM) treated cells with green fragmented nuclei (AI = 58.5), characteristic feature of early apoptosis (cells with green cytoplasm and green fragmented nuclear bodies) or late apoptotic stages (cells with orange cytoplasm and orange stained fragmented nuclear bodies). However, when the cells were pretreated with 25 µM MGN resulted in a significant (P < 0.001) decrease in the nuclear fragmentation (AI = 27.0) when compared with HgCl2 alone treated groups (Table I). Although, MGN at 50 µM (AI = 39.5) significantly inhibited the induction of HgCl2 induced apoptosis, it was ineffective at a higher dose of 75 µM (Table I).
TABLE I.
Apoptotic index assessed by ethidium bromide/acridine orange staining in HepG2 cells treated with different concentrations of MGN before treatment with HgCl2
| Treatment | (%) Apoptotic Index |
|---|---|
| Control | 2.50 ± 0.51 |
| HgCl2 (25 µM) | 58.5 ± 2.54* |
| 25 µM MGN + HgCl2 (25 µM) | 27.0 ± 1.86** |
| 50 µM MGN + HgCl2 (25 µM) | 39.5 ± 2.15** |
| 75 µM MGN + HgCl2 (25 µM) | 54.0 ± 2.61 |
P < 0.001 compared with control,
P < 0.001 compared with only HgCl2 group,
SEM, standard error of mean; MGN, mangiferin.
Flow Cytometric Analysis
Further, to confirm the apoptotic cell death observed by DNA fragmentation, we analyzed the changes in DNA content by flow cytometry. The univariate cell cycle analysis after staining the cells with PI indicated that the proportion of cells in the sub-G1 region increased from 1.90% in control untreated cell to 67.84% with HgCl2 treatment and a maximum decline 34.48% was observed at 25 µM of MGN pretreated group than that of others (Fig. 5).
Fig. 5.
Protective effect of mangiferin against HgCl2 induced sub-G1 cell population at 24 h of post treatment in HepG2 cells assessed by flow cytometry.
Biochemical Assays
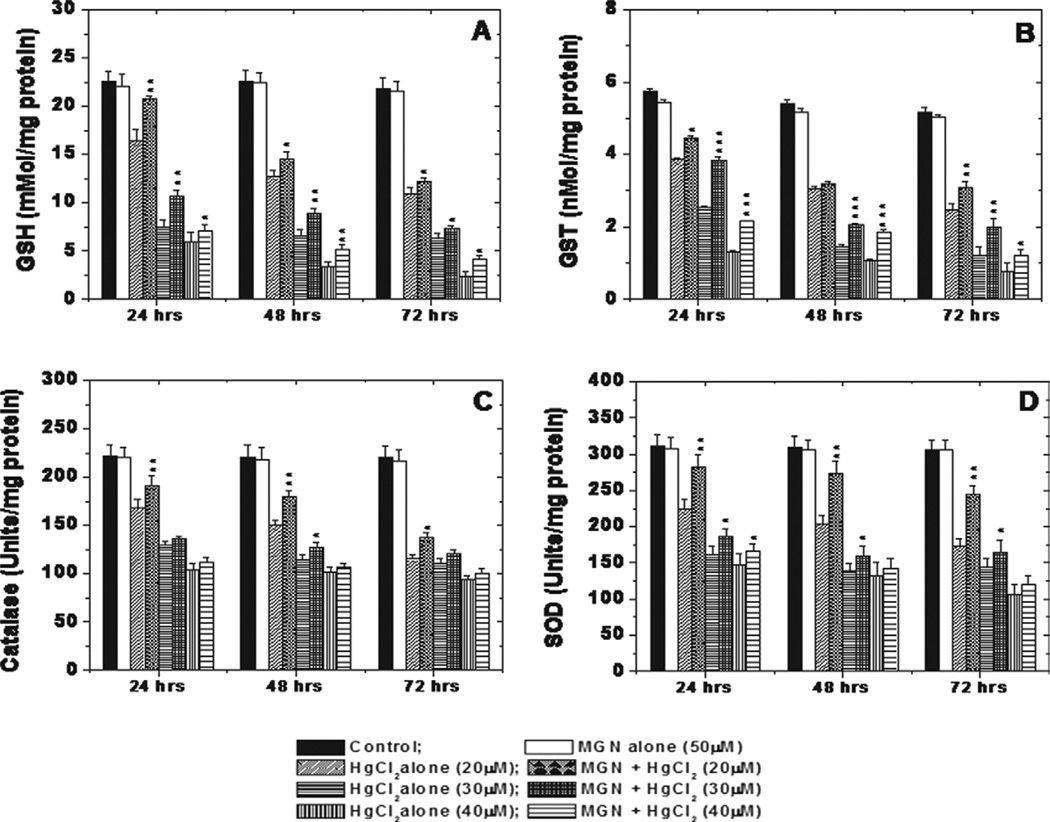
To assess the cytoprotective effect of MGN against HgCl2 induced toxicity, we analyzed the major antioxidants, GSH, GST, SOD, and catalase in HepG2 cells. Treatment with 50 µM of MGN alone did not alter the GSH, GST, catalase and SOD levels in HepG2 cells when compared with untreated control, while treatment of cells with different concentrations of HgCl2 aloneshowed a significant (P < 0.01) decrease in cellular GSH, GST, SOD, and catalase levels HgCl2 dose when compared with untreated at 24, 48, and 72 h of post-treatment time intervals. The depletion in antioxidant levels was in a dose and time-dependent manner. Treatment of cells with MGN (50 µM) 2 h prior to HgCl2 treatment significantly (P < 0.01) increased the GSH, GST, SOD, and catalase levels when compared with those of HgCl2 alone groups (Fig. 6).
Fig. 6.
Graphs showing toxic effect of HgCl2 and protective effect of MGN on GSH (A), GST (B), SOD (C) and CAT (D) activities in HepG2 cells after 24, 48 and 72 h of post incubation. The significant levels *P < 0.05; **P < 0.01; ***P < 0.001 and No symbol = Non significant, when compared with respective HgCl2 alone group. Each experiment was performed at least three times and the data are expressed as average enzyme units per mg of protein.
DISCUSSION
Mercury, although naturally occurring element in the earth’s crust because of its indiscriminate use as metallic, inorganic and organic forms in industry, agriculture etc., known to pose serious threats to human health owing to its toxic and hazardous nature. Moreover, because of its profuseness in the environment, mercury enters the human body directly or through food chain causing health problems such as pneumonitis, bronchitis, nephrotoxicity, mental retardation, cerebral palsy, seizures, and ultimately death (Ratcliffe et al., 1996; Clarkson et al., 2003). Apart from this, mercury exposure, especially in the organic form as methyl mercury through contaminated food has been largely associated with neurotoxicity in the central and peripheral nervous system of children (Myers and Davidson, 1998; Castoldi et al., 2001). Besides some of the chemical metal chelating agents, natural products, dietary constituents and minerals such as selenium, zinc etc., have shown their potential to attenuate heavy metal induced toxicity both in vivo and in vitro (Blanusa et al., 2005; Flora et al., 2008; Satish Rao et al., 2009).
The natural products in their crude forms as well as isolated constituents such as polyphenols, flavonoids, and xanthones have various pharmacological properties like antidepressant, antimicrobial, cardiotonic, diuretic, and antiviral activities (Pinto et al., 2005). Many of these agents also form part of our daily dietary intake and known to lower the toxic effects of many environmental pollutants (Flora, 2002; Furst, 2002). MGN, derived primarily from Mangifera indica is a naturally occurring glucosylxanthone known to possess iron-chelating and radical scavenging properties owing to which it may have its potential as a dietary supplement. MGN was found to protect hepatocytes, lymphocytes, neutrophils, and macrophages from oxidative stress (Muruganandan et al., 2005). Here we report for the first time the cytoprotective potential of MGN against HgCl2 induced cytotoxicity and apoptosis against HepG2 cells growing in vitro.
The use of hepatocyte culture as a model cells for pharmacological and toxicological studies has been well established as these cells were well-characterized. We used HepG2 cells (from human hepatoma), which has been considered as an excellent model to investigate xenobiotic induced mitochondrial toxicity, membrane damage, genotoxicity as a whole cell death (Sahu 2003). Further, this cell line is a suitable model for toxicological studies as HepG2 cell line possesses differentiated parenchymal functions of normal hepatocytes, particularly the expression of P450 isoenzymes (Medina-Díaz and Elizondo, 2005).
The most interesting observation is that MGN is non toxic per se against HepG2 cells even at the highest concentration (75 µM) used in this study and rendered cytoprotective potential. The cytotoxic effect of HgCl2 is well established phenomenon and therefore in our earlier study the cells treated with HgCl2 at all the concentrations as well as for various durations exhibited significant cell killing effect assessed by MTT assay, with an IC50 value of 27 µM (unpublished observation). These findings were substantiated by the results of clonogenic survival assay in the present study, a dose-dependent decline in the surviving fraction of the cells was observed on treatment with the increasing doses of the HgCl2. MGN pretreatment led to a significant increase in the surviving fraction. MGN with its antioxidant potential scavenged the HgCl2 induced free radicals thereby protecting the cells from oxidative stress and alleviated/attenuated the cellular damage. This could be one of the plausible mechanism by which the surviving fraction of HepG2 cells increased with MGN pretreatment. In an earlier study using MTT assay as well as clonogenic survival asssy we demonstrated the beneficial effect of MGN pretreatment against CdCl2-induced cytotoxicity (Satish Rao et al., 2009) and now the results of this study with clonogenic survival assay clearly indicated the cytoprotective potential of MGN against another heavy metal i.e., HgCl2. Earlier studies demonstrated the protective potential of MGN against carbon tetrachloride induced hepatotoxicity and t-butyl-hydroperoxide induced cytotoxicity in HepG2 cells (Rodeiro et al., 2008). Similarly, our earlier investigations convincingly demonstrated the cytoprotective effect of MGN in vitro on HepG2 cells and in vivo against cadmium induced toxicity (Satish Rao et al., 2009; Kasi et al., 2010).
In our recent study the modulating effect of MGN on CdCl2 induced ROS generation was convincingly demonstrated using DCFH-DA assay and also with HgCl2 alone (paper communicated). These results are similar to what was seen by Belyaeva et al. (2008) where HgCl2 showed increased ROS production in AS-30D hepatoma cells only at low (10 µM) concentrations and short incubation times of 1 h and 3 h, whereas a pronounced decrease of free radical generation ensued at 50 µM, both within 60 min and after 2 h. This was explained by an almost complete blockage of cell respiration at that Hg2+ concentration. MGN due to its radical scavenging property reduced the ROS levels significantly on pretreatment when compared with the only HgCl2 treated group of cells.
The dose-dependent reduction in the mitochondrial membrane potential by HgCl2 was clearly evident and it suggested the involvement of mitochondria in cytotoxicity. This could be due to the fact that mercury causes inhibition of respiration, uncoupling of oxidative phosphorylation and increases mitochondrial membrane permeability. Also, these effects have been ascribed to blockage of essential thiol groups in membrane proteins and depletion of membrane-bound magnesium (Bogucka and Wojtczak, 1979). Interestingly, pretreatment of MGN helped to restore the membrane potential to normal levels in each of the HgCl2 treated groups. Similarly, in an earlier in vivo study of myocardial infracted rats (induced by isoproterenol) prior administration of MGN helped to overcome the mitochondrial alterations with the inhibition of oxidative process during energy metabolism and thereby protecting the TCA cycle enzyme activities to nearly normal values. Thus the protective effect of MGN can be attributed to its reducing effect on oxidative damage and activation of mitochondrial energy metabolism (Prabhu et al., 2006).
Earlier reports have shown that the HgCl2 resulted in the destabilization of mitochondrial permiablity transition, resulting in calcium imbalance and the release of cytochrome-c ultimately leading to apoptotic death in liver cells via caspase cascade (Andreu et al., 2005). Induction of apoptosis by HgCl2 in HepG2 cells was evaluated by gel electrophoresis, flow cytometry, and microscopic methods they give specific information’s such as fluorescence microscopy using ethidium bromide and acridine orange dual staining technique is highly useful to differentiate apoptotic and necrotic cells after various treatments. Whereas, flow cytometric DNA analysis determines the apoptotic sub-G1 (hypodiploid) cells which gives a quantitative measure of total apoptotic cells. Further, DNA ladder assay is considered as a gold standard assay for apoptosis analysis as this gives qualitative information. MGN (25 µM) in combination with HgCl2 showed a significant decrease in apoptotic index, reduction in apoptotic cells (sub-G1 cells) and lesser intensity of ladder formation when compared with higher concentrations. Similar concentration dependent effect was also reported for agents such as flavanoids and polyphenolic compounds and this was attributed the antioxidant nature at lower concentration and prooxidant effect at their higher concentrations. Their prooxidant activity may deplete the nuclear antioxidant defense and lead to oxidative DNA damage, which may be responsible for their mutagenicity at their higher doses. (Sahu et al., 1996; Shih et al., 2004). Although, the exact molecular mechanism of inhibition of HgCl2 induced apoptosis needs to be explored further, the role of mitochondrial damage and oxidative stress cannot be ruled out.
HgCl2 toxicity has been ascribed to the depletion of cellular glutathione (GSH) content (Lash and Zalups, 1996; Becker and Soliman, 2009). In our study, the GSH content was seen to decrease in a concentration and time-dependent manner on treatment of HepG2 cells with HgCl2. This could be due to the binding of the reduced sulfhydryl groups on GSH to mercury ions as explained by many others (Cookson and Pentreath, 1996; Lee et al., 2001). Hg2+ on being absorbed into the cell has been shown to covalently binding to two GSH molecules causing its excretion from the cells and thereby further aggravating the toxicity of mercuric chloride (Lash and Zalups, 1996).
Further, glutathione-S-transferase has a pivotal role in eliminating or neutralizing environmental xenobiotics by conjugating them with the thiol groups of cellular GSH. In this study, increasing doses of HgCl2 led to a decline in cellular GST levels in a dose-dependent manner. These results are similar to that obtained by (El-Demerdash, 2001). MGN due to its antioxidant activity increased the GST levels as well as its activity thereby facilitating rapid elimination of a xenobiotic such as mercuric chloride from the HepG2 cells. Catalase catalyzes the conversion of the H2O2 generated in the cells normally or under conditions of oxidative stress into water and oxygen thereby protecting the cells against damage caused by peroxide radicals. HgCl2 was shown to decrease the cellular catalase levels with increasing doses (Hussain et al., 1999). As a result, inorganic-Hg has been suggested to increase H2O2 production by impairing the efficiency of oxidative phosphorylation and the electron transport chain at the ubiquinone-cytochrome b5 step (Chavez and Holguin, 1988). The cumulative effect of these activities could possibly lead to cell death at high HgCl2 concentrations. MGN helped to elevate the catalase levels on pretreatment with it. Further, SOD dismutase’s the O2− radicals generated as a result of oxidative stress to yield water and oxygen. In this study, SOD levels were found to decrease on treatment of HepG2 cells with mercuric chloride as also observed in an earlier study (Mahboob et al., 2001). However, pretreatment with MGN significantly increased the SOD levels.
Antioxidant property which normalized the levels of cellular defense enzymes has been mainly ascribed to the catechol moiety possessed by MGN. This moiety is also present in numerous flavonoids and other polyphenols which conferred antioxidative properties to them (Sato et al., 1992). During protection against free radical production, the catechols are oxidized, generating products like semiquinone radicals and quinones. These compounds can arylate protein thiol groups and are therefore considered to be toxic (Andreu et al., 2005).
This study demonstrated that MGN, an active component of Mangifera indica has the potential to modulate cytotoxicity caused by HgCl2 and thus protects HepG2 cells. The cytoprotective potential of MGN may be attributed to quenching of the ROS generated in the cells due to oxidative stress induced by HgCl2, restoration of mitochondrial membrane potential and normalization of cellular antioxidant levels. Therefore, a plant-derived dietary compound, MGN may have its potential in counteracting the toxicity caused by environmental heavy metal pollutants such as HgCl2 and others. Further, as MGN by itself is nontoxic at lower concentrations which makes it a safe dietary component for human consumption.
Acknowledgments
The authors are thankful to Dr. K. Satyamoorthy, The Director, Manipal Life Sciences Centre, Manipal University, Manipal for his help and encouragement during this study and for all the facilities offered by TIFAC-CORE in Pharmacogenomics, MLSC.
Contract grant sponsor: ITREOH program, University of Alabama at Birmingham and Manipal University, Manipal
REFERENCES
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Aithal BK, Kumar MR, Rao BN, Udupa N, Rao BS. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol Int. 2009;33:1039–1049. doi: 10.1016/j.cellbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Andreu GL, Delgado R, Velho JA, Curti C, Vercesi AE. Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium-induced permeability transition. Arch Biochem Biophys. 2005;439:184–193. doi: 10.1016/j.abb.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Bai J, Cederbaum AI. Catalase protects HepG2 cells from apoptosis induced by DNA-damaging agents by accelerating the degradation of p53. J Biol Chem. 2003;278:4660–4667. doi: 10.1074/jbc.M206273200. [DOI] [PubMed] [Google Scholar]
- Battin EE, Brumaghim JL. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys. 2009;55:1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- Becker A, Soliman KF. The role of intracellular glutathione in inorganic mercury-induced toxicity in neuroblastoma cells. Neurochem Res. 2009;34:1677–1684. doi: 10.1007/s11064-009-9962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva EA, Dymkowska D, Wieckowski MR, Wojtczak L. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol. 2008;231:34–42. doi: 10.1016/j.taap.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Blanusa M, Varnai VM, Piasek M, Kostial K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr Med Chem. 2005;12:2771–2794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- Bogucka K, Wojtczak L. On the mechanism of mercurial-induced permeability of the mitochondrial membrane to K+ FEBS Lett. 1979;100:301–304. doi: 10.1016/0014-5793(79)80356-7. [DOI] [PubMed] [Google Scholar]
- Bolger PM, Schwetz BA. Mercury and health. N Engl J Med. 2002;347:1735–1736. doi: 10.1056/NEJMp020139. [DOI] [PubMed] [Google Scholar]
- Cantoni O, Christie NT, Swann A, Drath DB, Costa M. Mechanism of HgCl2 cytotoxicity in cultured mammalian cells. Mol Pharmacol. 1984;26:360–368. [PubMed] [Google Scholar]
- Castoldi AF, Coccini T, Ceccatelli S, Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull. 2001;55:197–203. doi: 10.1016/s0361-9230(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Chavez E, Holguin JA. Mitochondrial calcium release as induced by Hg2+ J Biol Chem. 1988;263:3582–3587. [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—Current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Pentreath VW. Protective roles of glutathione in the toxicity of mercury and cadmium compounds to C6 glioma cells. Toxicol In Vitro. 1996;10:257–264. doi: 10.1016/0887-2333(96)00012-4. [DOI] [PubMed] [Google Scholar]
- El-Demerdash FM. Effects of selenium and mercury on the enzymatic activities and lipid peroxidation in brain, liver, and blood of rats. J Environ Sci Health B. 2001;36:489–499. doi: 10.1081/PFC-100104191. [DOI] [PubMed] [Google Scholar]
- Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J Med Res. 2008;128:501–523. [PubMed] [Google Scholar]
- Flora SJS. Nutritional components modify metal absorption, toxic response and chelation therapy. J Nutr Environ Med. 2002;12:53–67. [Google Scholar]
- Furst A. Can nutrition affect chemical toxicity? Int J Toxicol. 2002;21:419–424. doi: 10.1080/10915810290096649. [DOI] [PubMed] [Google Scholar]
- Garrido G, Gonzalez D, Lemus Y, Garcia D, Lodeiro L, Quintero G, Delporte C, Nunez-Selles AJ, Delgado R. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG) Pharmacol Res. 2004;50:143–149. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Rao G, Saravanan V, Misra N, Rana D. A plausible chemical mechanism of the bioactivities of mangiferin. Indian J Chem. 1996;35:561–566. [Google Scholar]
- Giri K, Ghosh U, Bhattacharyya NP, Basak S. Caspase 8 mediated apoptotic cell death induced by beta-sheet forming polyalanine peptides. FEBS Lett. 2003;555:380–384. doi: 10.1016/s0014-5793(03)01294-8. [DOI] [PubMed] [Google Scholar]
- Guha S, Ghosal S, Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy. 1996;42:443–451. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hussain S, Atkinson A, Thompson SJ, Khan AT. Accumulation of mercury and its effect on antioxidant enzymes in brain, liver, and kidneys of mice. J Environ Sci Health B. 1999;34:645–660. doi: 10.1080/03601239909373219. [DOI] [PubMed] [Google Scholar]
- Ichiki H, Miura T, Kubo M, Ishihara E, Komatsu Y, Tanigawa K, Okada M. New antidiabetic compounds, mangiferin and its glucoside. Biol Pharm Bull. 1998;21:1389–1390. doi: 10.1248/bpb.21.1389. [DOI] [PubMed] [Google Scholar]
- Kasi Viswanadh E, Satish Rao BS, Nageshwar Rao B. Antigenotoxic effect of Mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with Cadmium chloride. Hum Exp Toxicol. 2010;29:409–418. doi: 10.1177/0960327110361752. [DOI] [PubMed] [Google Scholar]
- Lash LH, Zalups RK. Alterations in renal cellular glutathione metabolism after in vivo administration of a subtoxic dose of mercuric chloride. J Biochem Toxicol. 1996;11:1–9. doi: 10.1002/(SICI)1522-7146(1996)11:1<1::AID-JBT1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Lee YW, Ha MS, Kim YK. Role of reactive oxygen species and glutathione in inorganic mercury-induced injury in human glioma cells. Neurochem Res. 2001;26:1187–1193. doi: 10.1023/a:1013955020515. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mahboob M, Shireen KF, Atkinson A, Khan AT. Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury. J Environ Sci Health B. 2001;36:687–697. doi: 10.1081/PFC-100106195. [DOI] [PubMed] [Google Scholar]
- Martin M, Qian H. Major mango polyphenols and their potential significance to human health. Compr Rev Food Sci Food Saf. 2008;7:309–319. doi: 10.1111/j.1541-4337.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- Medina-Díaz IM, Elizondo G. Transcriptional induction of CYP3A4 by o, p -DDT in HepG2 cells. Toxicol Lett. 2005;157:41–47. doi: 10.1016/j.toxlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Lal J, Gupta PK. Immunotherapeutic effects of mangiferin mediated by the inhibition of oxidative stress to activated lymphocytes, neutrophils and macrophages. Toxicology. 2005;215(1–2):57–68. doi: 10.1016/j.tox.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW. Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect. 1998;106:841–847. doi: 10.1289/ehp.98106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Pinto MM, Sousa ME, Nascimento MS. Xanthone derivatives: New insights in biological activities. Curr Med Chem. 2005;12:2517–2538. doi: 10.2174/092986705774370691. [DOI] [PubMed] [Google Scholar]
- Prabhu S, Jainu M, Sabitha KE, Shyamala Devi CS. Effect of mangiferin on mitochondrial energy production in experimentally induced myocardial infarcted rats. Vascul Pharmacol. 2006;44:519–525. doi: 10.1016/j.vph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Puck TT, Marcus PI. A rapid method for viable cell titration and clone production with hela cells in tissue culture: The use of X-irradiated cells to supply conditioning factors. Proc Natl Acad Sci USA. 1955;41:432–437. doi: 10.1073/pnas.41.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe HE, Swanson GM, Fischer LJ. Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J Toxicol Environ Health. 1996;49:221–270. doi: 10.1080/713851079. [DOI] [PubMed] [Google Scholar]
- Renvoize C, Biola A, Pallardy M, Breard J. Apoptosis: Identification of dying cells. Cell Biol Toxicol. 1998;14:111–120. doi: 10.1023/a:1007429904664. [DOI] [PubMed] [Google Scholar]
- Rodeiro I, Donato MT, Martinez I, Hernandez I, Garrido G, Gonzalez-Lavaut JA, Menendez R, Laguna A, Castell JV, Gomez-Lechon MJ. Potential hepatoprotective effects of new Cuban natural products in rat hepatocytes culture. Toxicol In Vitro. 2008;22:1242–1249. doi: 10.1016/j.tiv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Sahu SC. Hepatocyte culture as an in vitro model for evaluating the hepatotoxicity of food-borne toxicants and microbial pathogens: A review. Toxicol Mech Methods. 2003;13:111–119. doi: 10.1080/15376510309839. [DOI] [PubMed] [Google Scholar]
- Sahu SC, Gray GC. Pro-oxidant activity of flavonoids: Effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cancer Lett. 1996;104:193–196. doi: 10.1016/0304-3835(96)04251-6. [DOI] [PubMed] [Google Scholar]
- Satish Rao BS, Sreedevi MV, Nageshwar Rao B. Cytoprotective and antigenotoxic potential of Mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem Toxicol. 2009;47:592–600. doi: 10.1016/j.fct.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Sato T, Kawamoto A, Tamura A, Tatsumi Y, Fujii T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid) Chem Pharm Bull (Tokyo) 1992;40:721–724. doi: 10.1248/cpb.40.721. [DOI] [PubMed] [Google Scholar]
- Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76(Part 1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih CM, Lin H, Liang YC, Lee WS, Bi WF, Juan SH. Concentration-dependent differential effects of quercetin on rat aortic smooth muscle cells. Pharmacol. 2004;496(1–3):41–48. doi: 10.1016/j.ejphar.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- WHO. Geneva, Switzerland: Methylmercury: World Health Organization, International Program on Chemical Safety (IPCS); 1990. Environmental Health Criteria 101. [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of Ecotoxicology. Chapter 16. Boca Raton, Florida: CRC Press; 2003. [Google Scholar]