Abstract
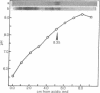
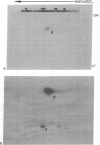
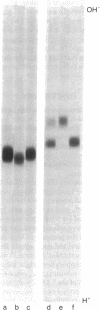
We have developed procedures for renaturing, in polyacrylamide isoelectric focusing gels, homomeric enzymes (i.e., enzymes with identical subunits) that have been denatured with sodium dodecyl sulfate or urea or both. The renatured enzymes can then be localized as discrete species by conventional histochemical staining. One of these enzymes, uridine diphosphoglucose pyrophosphorylase (UTP:α-D-glucose-1-phosphate uridylyltransferase, EC 2.7.7.9) of Dictyostelium discoideum, was studied in detail. Conditions have been established for renaturing and localizing this enzyme and for quantitating the amount of activity recovered. Up to 40% of the activity can be recovered after renaturation. This procedure is widely applicable because several enzymes, including alcohol dehydrogenase (EC 1.1.1.1) and lactate dehydrogenase (EC 1.1.1.27), can be localized. It is sensitive enough to resolve isozymes and enzyme variants that differ by a single charged amino acid. It can be used to localize enzymes in crude cell extracts that have been resolved in two-dimensional slab gels by sodium dodecyl sulfate electrophoresis and isoelectric focusing. These methods should allow detailed analysis of genes and their enzyme proteins that, though present in small amounts in eukaryotic cells, perform important metabolic or developmental functions.
Keywords: sodium dodecyl sulfate, urea, isoelectric focusing, two-dimensional electrophoresis, histochemical staining
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A. Renaturation of a multisubunit multiactivity enzyme complex: recovery of phage Qbeta RNA replicase, EF-Tu, and EF-Ts activities after denaturation in urea. Biochemistry. 1976 Jan 27;15(2):422–425. doi: 10.1021/bi00647a028. [DOI] [PubMed] [Google Scholar]
- Danno G. i. Isoelectric focusing of proteins separated by SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 Nov;83(1):189–193. doi: 10.1016/0003-2697(77)90525-5. [DOI] [PubMed] [Google Scholar]
- Dimond R. L., Farnsworth P. A., Loomis W. F. Isolation and characterization of mutations affecting UDPG pyrophosphorylase activity in Dictyostelium discoideum. Dev Biol. 1976 May;50(1):169–181. doi: 10.1016/0012-1606(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Franke J., Sussman M. Synthesis of uridine diphosphate glucose pyrophosphorylase during the development of Dictyostelium discoideum. J Biol Chem. 1971 Nov;246(21):6381–6388. [PubMed] [Google Scholar]
- Fyrberg E. A., Donady J. J. Actin Heterogeneity in primary embryonic culture cells from Drosophila melanogaster. Dev Biol. 1979 Feb;68(2):487–502. doi: 10.1016/0012-1606(79)90220-3. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gelsema W. J., de Ligny C. L., van der Veen N. G. Isoelectric points of proteins, determined by isoelectric focusing in the presence of urea and ethanol. J Chromatogr. 1979 Apr 1;171:171–181. doi: 10.1016/s0021-9673(01)95297-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller D. W., Elgin S. C. Isoelectric focusing of proteins exposed to sodium dodecyl sulfate. Anal Biochem. 1974 Jul;60(1):142–148. doi: 10.1016/0003-2697(74)90138-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Schwartz M. F., Jörnvall H. Structural analyses of mutant and wild-type alcohol dehydrogenases from drosophila melanogaster. Eur J Biochem. 1976 Sep;68(1):159–168. doi: 10.1111/j.1432-1033.1976.tb10774.x. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Singer B. S., Morrissett H., Gold L. An electrofocusing system for the analysis of proteins and their genetic variants. Anal Biochem. 1978 Mar;85(1):224–229. doi: 10.1016/0003-2697(78)90293-2. [DOI] [PubMed] [Google Scholar]
- Sofer W., Ursprung H. Drosophila alcohol dehydrogenase. Purification and partial characterization. J Biol Chem. 1968 Jun 10;243(11):3110–3115. [PubMed] [Google Scholar]
- Tuszynski G. P., Baker S. R., Fuhrer J. P., Buck C. A., Warren L. Glycopeptides derived from individual membrane glycoproteins from control and Rous sarcoma virus-transformed hamster fibroblasts. J Biol Chem. 1978 Sep 10;253(17):6092–6099. [PubMed] [Google Scholar]
- Tuszynski G. P., Buck C. A., Warren L. A two-dimensional polyacrylamide gel electrophoresis (PAGE) system using sodium dodecyl sulfate-PAGE in the first dimension. Anal Biochem. 1979 Mar;93(2):329–338. doi: 10.1016/s0003-2697(79)80159-1. [DOI] [PubMed] [Google Scholar]
- Vigue C., Sofer W. Adh-n5: a temperature-sensitive mutant at the Adh locus in Drosophila. Biochem Genet. 1974 May;11(5):387–396. doi: 10.1007/BF00486412. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]