Abstract
Background
TOMM40 (translocase of the outer mitochondrial membrane pore subunit) is in linkage disequilibrium with apolipoprotein E (APOE). APOE e4 is linked to long (L; 21–29 T residues) poly-T variants within intron 6 of TOMM40 while APOE e3 can be associated with either with a short (S; <21 T residues) or very long (VL; >29 T residues) variant. To assess the possible contribution of TOMM40 to Alzheimer’s disease (AD) onset, we compared the effects of TOMM40 and APOE genotype on preclinical longitudinal memory decline.
Methods
An APOE e4 enriched cohort of 639 cognitively normal individuals age 21–97 years of known TOMM40 genotype underwent longitudinal neuropsychological testing every two years. We estimated the longitudinal effect of age on memory using statistical models that simultaneously modeled cross sectional and longitudinal effects of age on the auditory verbal learning test long term memory score (AVLT) by APOE, TOMM40, and the interaction between the two.
Results
There were significant effects overall for both TOMM40 (p=0.04 linear effect, p=0.03 quadratic effect) and APOE (p=0.06 linear effect, p=0.008 quadratic effect) with no significant interaction (p=0.63). These differences were age-dependent: there was a significant TOMM40 effect prior to age 60 (p=0.009) characterized by flattened test-retest improvement (VL/VL subgroup only) but no significant APOE effect; and a significant APOE effect after age 60 (p=0.006) characterized by accelerated memory decline (e4 carriers) but no significant TOMM40 effect.
Conclusion
Both TOMM40 and APOE significantly influence age-related memory performance, but appear to do so independently of each other.
Keywords: TOMM40, APOE, preclinical Alzheimer’s disease, cognitive aging, age-related memory loss, mitochondria, very long term memory, test-retest effects
1. Introduction
Mitochondrial alterations have long been suspected of contributing to the pathophysiology of, or even possibly causing Alzheimer’s disease (AD) as well as other neurodegenerative illnesses1. Oxidative stress2, vascular endothelial dysfunction3, hypoxia4, glucose deprivation5, and mitochondrial dysfunction6 all promote amyloidogenesis. The TOMM40 gene encodes the translocase of the outer mitochondrial membrane pore subunit through which cytoplasmic proteins pass7. Apolipoprotein (apoe) e4 and e3 isoforms interact with the outer mitochondrial membrane leading to mitochondrial dynamic dysfunction8. Other aggregating proteins, including amyloid precursor protein accumulates in these pores also contributing to mitochondrial dysfunction9.
The apolipoprotein E (APOE) e4 allele influences risk and age of onset of AD in a gene-dose dependent fashion10, and the apoe e4 isoform itself is toxic to mitochondria8,11. Deep sequencing of the APOE-TOMM40 linkage disequilibrium region recently revealed a variable–length, deoxythymidine homopolymer (poly-T) within intron 6 of the TOMM40 gene that sorts nonrandomly with APOE alleles, and that Roses and colleagues have suggested may have an effect on AD risk and age of onset12–14. Currently, the length of the poly-T homopolymer is categorized as short (14–20 repeats), long (21–29 repeats) or very long (more than 29 repeats). Among Caucasians, APOE e4 is usually linked to long (L) variants while APOE e3 is primarily associated with either with a short (S) or very long (VL) poly-T variant of TOMM4013. Associations within non-Caucasian populations, as well as the TOMM40 associations with the APOE e2 allele are still under study.
Correlations between age of symptomatic AD onset in a clinical series of thirty-four APOE e3/4 heterozygotes as well as in an autopsy series of 22 APOE e3/3 individuals have showed that those with a “long-long” TOMM40 genotype (in which L and VL variants were grouped together) had a younger age of onset than those with an SL or SS genotype14. Using a cohort genetically enriched with APOE e4 carriers, we were able to show that age-related memory decline accelerates preclinically in APOE e4 carriers beginning around age 55–60 years15. If TOMM40 further influences AD age of onset either in conjunction with or instead of APOE, then it should also alter preclinical cognitive aging patterns in a similar way. We therefore performed TOMM40 genotyping of our APOE e4 enriched cohort to compare the age-related trajectories of preclinical memory change among TOMM40 genotype subgroups.
2. Methods
2.1. Study population
From January 1, 1994 through August 6, 2007 cognitively normal residents of Maricopa County age 21 years and older were recruited through local media ads, underwent APOE genotyping and longitudinal neuropsychological assessment15. Demographic, family, and medical history data were obtained on each individual undergoing APOE genotyping, and identity was coded by a study assistant. Ancestral origin was self-reported. Genetic determination of APOE allelic status was performed using a polymerase chain reaction (PCR) based assay.
All identified e4 homozygotes (HMZ) were matched by age, gender, and education to one e3/4 heterozygote (HTZ) and two non-carriers. We identified more HTZ and non-carriers than HMZ, (especially those persons over age 70 years reflecting the greater number of e4 HMZ developing MCI and AD by this age) who were also eligible for enrollment. Each participant had screening tests to establish their neuropsychiatrically normal state including a neurological examination, the Folstein Mini-Mental Status Exam (MMSE), the Hamilton Depression (Ham-D) Rating Scale, the Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-IIIR. We excluded anyone with potentially confounding medical, neurologic, or psychiatric problems. None met the published criteria for mild cognitive impairment (MCI)16, AD17, other forms of dementia or major depressive disorder18. Entry criteria for all participants included a score of at least 27 on the MMSE (and scoring at least 1 out of 3 on the recall subtest), a score of 10 or less on the Ham-D rating scale at the time of their first visit, and no indication of loss of function on the FAQ and IADL.
We also excluded from the analysis anyone who subsequently met published criteria for MCI, AD, or any other form of dementia during follow-up, and thus excluded 16 participants (four non-carriers, four heterozygous persons and 8 homozygous persons).
2.2. TOMM40
Genotyping of the genetic variants was performed by a sequencing vendor (Polymorphic DNA Technologies, Alameda CA, http://www.polymorphicdna.com). Four polymorphisms were analyzed for each genomic sample: rs8106922, rs429358, rs7412, and rs10524523. Polymorphisms rs429358 and rs7412 define the APOE genotype, and rs8106922, within TOMM40, is a key polymorphism identified in previous studies (phylogenetic and genome-wide association) as associated with risk of AD. The fourth polymorphism, rs10524523, is a homopolymer length polymorphism (poly-T) located in an intronic region of TOMM40. In the human reference sequence, the number of T residues in the homopolymer, “N”, is 35, and the specific variation described by rs10524523 is a 19 base pair deletion, making the homopolymer T16 (N=16 T residues) the variant allele. In the Duke/Polymorphic haplotyping work, other alleles of this homopolymer have been observed, with values of N ranging from 14 to 46 residues. For each genomic sample, Polymorphic simultaneously PCR amplified each polymorphism and then performed bidirectional direct Sanger sequencing of the DNA templates on an ABI 3730xl sequencing platform and sequence data analysis. The TOMM40 poly-T lengths for each chromosome were converted to genotypes using the following standards3: short (S) poly-T (14–20 T residues), long (L) poly-T (21–29 T residues), and very long (>29 residues).
2.3. Neuropsychological Testing
As previously described15, we selected a single measure of long-term memory loss, the Long Term Memory (LTM; Trial 7) score of the Auditory Verbal Learning Test (AVLT) (score range from worst to best is 0–15)19 as the primary endpoint. The AVLT was administered to participants as part of a standardized battery of neuropsychological tests at baseline and then at intervals of one to two years. (Duration of participation is until death, onset of MCI or dementia, or the participant’s decision to stop.)
2.4. Statistics
To isolate the effect of longitudinal cognitive change, we used a statistical method to separate baseline from change in performance over time20. To assess the linear and quadratic effect of TOMM40 (S carriers versus L/L, L/VL, or VL/VL variant) and APOE (e4 noncarriers versus carriers) and the linear interaction between TOMM40 and APOE on cognitive change, a previously described mixed model21 was modified to include a term for the quadratic effect of a binary risk factor. To compare the cognitive change between TOMM40 S/S and VL/VL variants within the APOE e3/3 group and to compare cognitive change between APOE e4/4 subjects with the TOMM40 L/L variant to the TOMM40 VL/VL group, a previously described mixed model21 was applied without modification. Finally, to assess early (before age 60) and late (after age 60) effects of TOMM40 (S carriers versus L/L, L/VL, or VL/VL variants) and APOE (e4 noncarriers versus carriers), the following piecewise linear model for Yij (the jth response for the ith individual) was developed:
where APOEi is the APOE status for the ith individual (1=e4 carrier; 0=e4 noncarrier); TOMM40i is the TOMM40 status for the ith individual (1= L/L, L/VL, or VL/VL variant; 0=S carrier); Ageij is the age of the ith individual at the time of the jth response; Ageij,60 is the maximum of Ageij minus 60 or zero; and b1i is an individual specific random effect allowing each subject to have a different intercept. This model allows for comparison of the constant annual change (i.e., slope) before age 60 and after age 60 between the TOMM40 groups and between the APOE groups. Age 60 was selected because it was approximately equal to the median entry age. Modeling was carried out using SAS PROC MIXED (SAS Version 9, SAS Institute, Cary, NC). Baseline characteristics and followup were compared among groups by using the two-sample t-test/analysis of variance (ANOVA) F-test or Pearson chi-square test.
3. Results
639 individuals between ages 21 and 97 had the following TOMM40 genotypes: S/S (n=88), L/L (n=54), VL/VL (n=110), S/L (n=107), S/VL (n=193), and L/VL (n=87). Table 1 summarizes the demographic data. They did not differ in years of education (15.6 +/− 2.5, p=0.99), gender (69.6% women, p=0.22), racial background (99.4% Caucasian, p=0.68), proportion with more than one followup visit (81.2%, p=0.76), or duration of followup (6.1 +/−3.1 years, p=0.27), but family history of dementia in a first degree relative was highest in the L/L subgroup (81.1%; p<0.001) reflecting the known linkage disequilibrium with APOE e4.
Table 1.
| TOMM40S/S | TOMM40 S/L | TOMM40 S/VL | TOMM40 L/L | TOMM40 L/VL | TOMM40 VL/VL | p | |
|---|---|---|---|---|---|---|---|
| N | 88 | 107 | 193 | 54 | 87 | 110 | |
| Mean Entry Age (standard deviation) | 57.8 (13.6) | 57.3 (12.9) | 59.3 (12.9) | 56.1 (9.9) | 55.3 (12.1) | 60.9 (11.2) | 0.02 |
| Mean Years Education (standard deviation) | 15.6 (2.3) | 15.7 (2.5) | 15.6 (2.5) | 15.8 (2.3) | 15.5 (2.6) | 15.6 (2.7) | 0.99 |
| #Female (%) | 67 (76.1) | 72 (67.3) | 127 (65.8) | 42 (77.8) | 65 (74.7) | 72 (65.5) | 0.22* |
| % FDR | 52.9 | 69.8 | 47.6 | 81.1 | 79.1 | 57.3 | <0.001* |
| %Caucasian | 100 | 100 | 98.1 | 100 | 100 | 100 | 0.68* |
| % >1 epoch | 81.8 | 84.1 | 78.8 | 85.2 | 78.2 | 82.7 | 0.76* |
FDR=first degree relative with dementia. Unpaired t-tests except (*) chi square.
Combined TOMM40 and APOE genotype subgroups
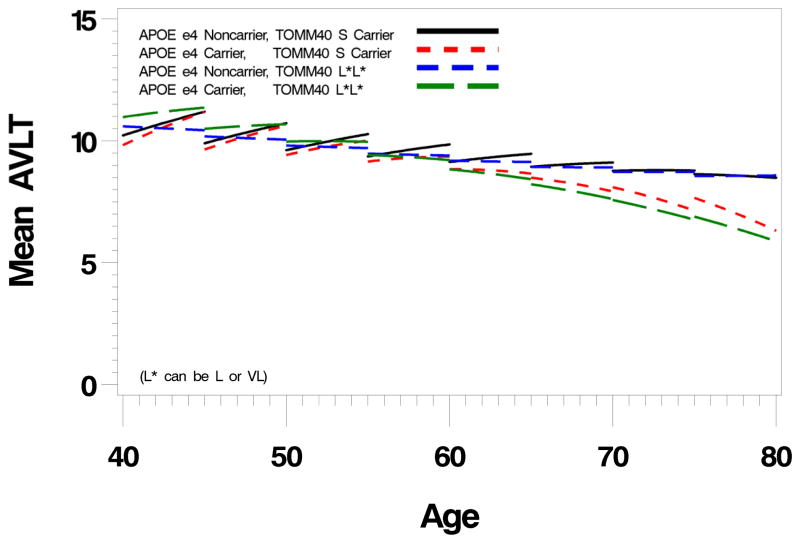
To first gain an overall perspective of TOMM40 and APOE effects, we condensed the large number of potential genotype combinations into four subgroups. We grouped S carriers together (n=388) and L and VL variants (L/L, L/VL, VL/VL genotypes) together (n=251) to generate two TOMM40 groups: S carriers and L*L* (where L* can be L or VL). We also grouped APOE subgroups into e4 carriers and noncarriers, and thus created four subgroups: S/e4+, S/e4−, L*L*/e4+, L*L* e4−. Their cognitive aging trajectories are shown in figure 1. There were significant effects for both TOMM40 (p=0.04 linear effect, p=0.03 quadratic effect) and APOE (p=0.06 linear effect, p=0.008 quadratic effect) with no significant interaction (p=0.63).
Figure 1.
Longitudinal trajectories (divided into five year intervals) of Auditory Verbal Learning Test Long Term Memory scores in four TOMM40-APOE defined subgroups (see text) including L*/L*/e4+, L*/L*/e4−, S/e4+, and S/e4−. There are noninteracting significant TOMM40 and APOE effects with a flattening of the normal test-retest effect in the L*/L* subgroups prior to age 60, and accelerated decline in the e4+ subgroups after age 60. (L* is either the L or VL TOMM40 allele)
Separate TOMM40 and APOE genotype subgroups
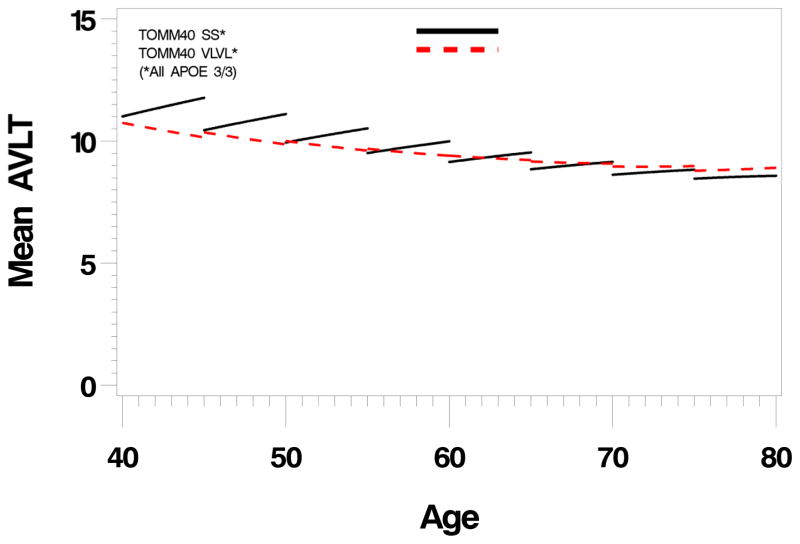
To better understand specific TOMM40 and APOE genotype effects, we next looked at specific genotype combinations that contained adequate numbers of individuals. Table 2 shows the distribution of APOE genotypes with TOMM40 genotypes and demonstrates the association of the S and VL TOMM40 variants with the e3 allele, and the L TOMM40 variant with the APOE e4 allele. When only individuals with the APOE e3/3 genotype are considered, there is a significant difference in longitudinal memory trajectories between TOMM40 S/S and VL/VL variants (p=0.04, linear effect, figure 2). The VL/VL group fails to show a normal test-retest effect from the earliest ages that is preserved in the S/S group. When VL/VL, S/VL, and SS groups were compared there was no evident VL gene dose effect of the test-retest flattening (linear trend, p=0.33). To compare the effect of L/L with VL/VL, we compared APOE e4/4 HMZ with the TOMM40 L/L genotype (the only group with a sufficient number of L/L) with the entire sample of VL/VL (none were also HMZ) (supplementary figure 1) and found that the L/L group, like the S/S group retained a normal test-retest pattern, but unlike the e3/3 subgroup, showed a striking accelerated decline in memory performance (p=0.009, quadratic effect). Because of tight linkage between e4 and L we cannot disentangle their respective effects on this decline, but more pertinently, unlike the VL/VL group, there is no loss of the normal test-retest effect.
Table 2.
Crosstabulation of TOMM40 and APOE Genotypes
| TOMM40-S/S | TOMM40-S/L | TOMM40-S/VL | TOMM40-L/L | TOMM40-L/VL | TOMM40-VL/VL | Total | |
|---|---|---|---|---|---|---|---|
| APOE-2/3 | 13 | 1 | 15 | 1 | 0 | 12 | 42 |
| APOE-3/3 | 69 | 10 | 159 | 2 | 4 | 92 | 336 |
| APOE-3/4 | 5 | 88 | 19 | 5 | 75 | 6 | 198 |
| APOE-4/4 | 1 | 8 | 0 | 46 | 8 | 0 | 63 |
| Total | 88 | 107 | 193 | 54 | 87 | 110 | 639 |
Figure 2.
Longitudinal trajectories (divided into five year intervals) of Auditory Verbal Learning Test Long Term Memory scores in people with the APOE e3/3 genotype subdivided into TOMM40 S/S and VL/VL subgroups. There is a significant difference in the velocity of decline (p=0.04) between the TOMM40 subgroups, that appears to reflect falttening of the test-retest effect in the VL/VL subgroup compared to the S/S subgroup.
These results suggested that there was an early effect (before age 60) attributable to TOMM40 VL characterized by a flattened test-retest effect that may persist lifelong, and a late (after age 60) effect attributable to APOE e4 characterized by accelerated memory decline. To test this early TOMM40 vs late APOE hypothesis, we compared TOMM40 and APOE effects before and after age 60 years (the center point of our age distribution). This was formally tested by creating linear models before and after age 60 again using the following 4 subgroups: S/e4+, S/e4−, L*L*/e4+, L*L* e4− (supplementary figure 2). As suspected there was a significant TOMM40 effect prior to age 60 (p=0.009) but no significant APOE effect; and a significant APOE effect after age 60 (p=0.006) but no significant TOMM40 effect.
4. Discussion
APOE e4 carriers and e4 HMZ in particular are at greater risk for AD and have an earlier age of onset than e4 noncarriers. This association has been replicated many times since the original reports in the early 1990’s10. Yet, the strong linkage disequilibrium patterns between specific alleles of APOE and TOMM40 have led Roses himself and others to question whether TOMM40 underlies or contributes to the apparent APOE effect, a suspicion that has received preliminary support from several small clinical series.
Although we found that the e4-L combination was associated with accelerated memory decline after age 60, the roles of APOE e4 and TOMM40 L could not be separated due to strong linkage disequilibrium (table 2). Among those with the APOE e3/3 genotype, the TOMM40 S/S subgroup showed a normal test-retest effect (illustrated by the positive slope of the 5 year interval changes in test performance at the younger age intervals), as did the TOMM40L/L subgroup (all of who also carried the APOE e4/4 genotype). The VL/VL subgroup, however, was associated with significant reduction of this test-retest effect. This “non-effect” of the VL allele on accelerated memory decline accords well with the recent clinical series by Cruchaga et al who found an association between age of AD onset and the S but not the VL TOMM40 allele, though we were unable to identify accelerated memory decline in association with the S allele as their study suggested22.
The exact relationship of TOMM40 to AD pathogenesis is not yet clear, but the greatest differences between TOMM40 variants were found prior to age 60 with attenuated decline after age 60 among the S/S and VL/VL genotypes. Notably, this effect, as well as the previously demonstrated onset of preclinical memory decline in APOE e4 carriers15 is occurring at a younger age than the Alzheimer’s Disease Neuroimaging Initiative cohort on which a current popular model of AD pathogenesis is based23. The posterior cingulate is a brain region that is consistently observed to be hypometabolic with FDG-PET in patients with AD and in asymptomatic APOE e4 carriers, including young adults24. In expired young APOE e4 carriers Valla et al have shown that posterior cingulate cortex neurons have lower mitochondrial cytochrome oxidase activity than noncarriers despite the absence of soluble or fibrillar abeta amyloid or tau pathology suggesting this may be a very early change that precedes and promotes BACE1 activity and amyloidogenesis25. Whether TOMM40 variants might help to account for the observed metabolic differences in young adults warrants further study since it is a mitochondria-based alteration with functional correlations in this age range.
Some might question whether a potential limitation of our study is that it was not population based, but instead genetically enriched for APOE e4. Because our intent was to study the behavior of genetic subgroups rather than determine incidence or prevalence rates of symptomatic disease, and because of the pattern of linkage disequilibrium between APOE and TOMM40 alleles, our genetic enrichment strategy proved to be a strength that afforded us sufficient power to assess, to the extent possible, the major APOE-TOMM40 combinations. In the absence of random community based sampling we could risk recruiting individuals concerned about their own cognitive health perhaps due to early stage AD in some. To address this we eliminated anyone who developed clinically symptomatic MCI or dementia at any point. Further, 80% had at least two epochs of testing with mean followup duration of 6 years that further reduced the likelihood that individuals with incipient symptoms were enrolled.
Another potential limitation of our study was that we did not reproduce an extensive analysis of the haplotype structure of this region. Roses et al. presented an extensive analysis of the haplotype structure of the TOMM40-APOE region based on molecular or phased-separated haplotypes13. This analysis defined the relationship between APOE alleles and rs10524523 lengths. An analysis of 300 phase-separated haplotypes showed that the APOE e3 allele is linked to either an S or VL allele of 523 98% of the time and that the APOE e4 allele is linked to the L allele of 523 87% of the time. It is possible to infer the haplotype structure of APOE and 523 with an accuracy of between 97% and 99%. Since these results were obtained in Caucasians and the present study is nearly 100% comprised of Caucasian subjects, the same haplotypic relationship is likely to exist between the APOE alleles and 523 alleles in our study.
How TOMM40 will ultimately fit into the pathophysiologic mechanism of AD is not yet clear, and important basic insights still await evaluation in further sufficiently large clinical cohorts. Our results show that differences in a mitochondrial protein can correlate with differences in cognition and cognitive aging that are distinct from those of apoe, and that such effects may be most evident in cohorts younger than those on which current disease models are based.
Supplementary Material
Acknowledgments
Funded by P30AG19610, R01AG6031581, Deane Drug Discovery Institute (Duke University), and the Arizona Alzheimer Research Consortium. The authors wish to thank Ms. Sandra Yee-Benedetto, Mr. Bruce Henslin, Ms. Marci Zomok, and Ms. Jessie Jacobsen for expert technical assistance.
Footnotes
Conflicts
Dr. Roses is president of 3 companies filed as S Corporations in North Carolina: Cabernet Pharmaceuticals, Shiraz Pharmaceuticals, and Zinfandel Pharmaceuticals. Dr. Saunders is married to Dr. Roses. Drs. Caselli, Dueck, Huentelman, Lutz, and Reiman have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 2.Karuppagounder SS, Xu H, Shi Q, et al. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiol Aging. 2009;30:1587–1600. doi: 10.1016/j.neurobiolaging.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auston SA, Santhanam AV, Katusic ZS. Endothelial nitric acid modulates expression and processing of amyloid precursor protein. Circ Res. 2010;107:1498–1502. doi: 10.1161/CIRCRESAHA.110.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell RD, Deane R, Chow N, et al. SRF and myocardin regulate LRP-mediated amyloid-B clearance in brain vascular cells. Nature Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor T, Sadleir KR, Maus E, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes angiogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dragicevic N, Mamcarz M, Zhu Y, et al. Mitochondrial amyloid-B levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J Alz Dis. 2010;20 (suppl 2):S535–S550. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- 7.Humphries AD, Streimann IC, Stojanovski D, et al. Dissection of the mitochondrial import and assembly pathway for human Tom 40. J Biol Chem. 2005;280:11535–11543. doi: 10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- 8.Chang S, ran Ma T, Miranda RD, Balestra ME, Mahley RE, Huang Y. Lipid and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandtheerthavarda HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26 (35):9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 11.Chen HK, Ji ZS, Dodson SE, et al. Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. J Biol Chem. 2011;286:5215–21. doi: 10.1074/jbc.M110.151084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 13.Roses AD, Lutz MW, Amrine-Madsen H, et al. A TOMM40 variable length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics Journal. 2009:1–10. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutz MW, Crenshaw DG, Saunders AM, Roses AD. Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimers Dement. 2010;6:125–31. doi: 10.1016/j.jalz.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurol. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington D.C: American Psychiatric Association; 1994. [Google Scholar]
- 19.Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires; 1964. [Google Scholar]
- 20.Ware JH, Dockery DW, Louis TA, XU X, Ferris BG, Speizer FE. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiology. 1990;132:685–700. doi: 10.1093/oxfordjournals.aje.a115710. [DOI] [PubMed] [Google Scholar]
- 21.Caselli RJ, Dueck AC, Locke DE, et al. Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruchaga C, Mowotny P, Kauwe JSK, et al. Association and expression analyses with singly-nucleotide polymorphisms in TMM40 in Alzheimer Disease. Arch Neurol. 2011;68:1013–1019. doi: 10.1001/archneurol.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiman EM, Chen KW, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE e4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22:307–313.h. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.