Pulmonary vasculitis is characterized by inflammation and necrosis of the pulmonary blood vessels. Even though it can involve all parts of the pulmonary vasculature, pulmonary arteries, capillaries and pulmonary veins, it most commonly affects the pulmonary capillaries. Diffuse alveolar hemorrhage (DAH), characterized by the widespread extravasation of red blood cells into the pulmonary alveolar spaces, represents the most common clinical manifestation of pulmonary vasculitis. (Figure 1) DAH is typically attributable to disseminated injury of the pulmonary capillaries. It is associated with a disruption of the alveolar and capillary basement membranes facilitating the entry of red blood cells into the alveoli. Independent of the underlying cause, pulmonary capillaritis represents the most common histological finding if lung tissue biopsies are obtained in these patients.[1] (Figure 1) DAH can occur in the context of various systemic disorders or present in isolation. The etiologies of DAH can be broadly divided into immune – and non-immune mediated causes. As a group ANCA-associated vasculitis (AAV) represents the most common cause of pulmonary vasculitis. Even though all these diseases remain idiopathic, over the last few decades our understanding of their pathogenesis has improved significantly. In a recent effort to eliminate eponyms and establish more descriptive disease names reflective of the associated pathology and disease associations, a new nomenclature for AAV was proposed by an international expert panel.[2] Wegener’s granulomatosis was renamed into granulomatosis with polyangiitis (Wegener’s) GPA highlighting the granulomatous nature and vascular inflammation associated with the disease as well as its similarities to microscopic polyangiitis (MPA). To emphasize the contribution of eosinophilic inflammation and the resemblance to GPA, Churg-Strauss was re-named as eosinophilic granulomatosis with polyangiitis (Churg-Strauss) EGPA.[2]
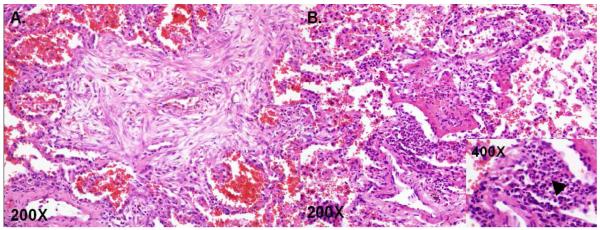
Figure 1.
Pathologic Findings of Diffuse Alveolar Hemorrhage. A. Organizing diffuse alveolar hemorrhage with hemosiderin laden macrophages with surrounding fibrosis, B. Pulmonary capillaritis, arrow head leukocytoclastic inflammation. (The Images are courtesy of Dr. Marie Christine Aubry, MD, Mayo Clinic, Rochester, MN)
Clinical Presentation and Diagnosis of DAH
The clinical presentation of DAH is highly variable. Patients can present within a spectrum ranging from asymptomatic radiographic abnormalities to severe life threatening respiratory failure. Even though the majority of patients experience a variable degree of hemoptysis, approximately one third of all patients with DAH are lacking this symptom. [3] Other common symptoms include dyspnea, cough, fever, and chest pain. Laboratory studies frequently demonstrate anemia and/or decreasing hemoglobin values as a marker of intra-pulmonary blood loss.[4, 5] In addition patients presenting with DAH frequently report signs and symptoms related to an etiologically related systemic disorder. Such abnormalities may include skin rashes, ocular, sinus, nasal or ear symptoms, airway obstruction, renal dysfunction due to glomerular inflammation characterized by an active urinary sediment, neurological symptoms such as mononeuritis multiplex, inflammatory arthritis, muscle weakness and many other symptoms. Patients presenting with DAH and concurrent glomerulonephritis are typically classified as pulmonary-renal syndrome. The vast majority of these cases is attributable to immune mediated causes most frequently AAV, systemic lupus erythematosus (SLE), or anti-glomerular basement membrane antibody (Anti-GBM) syndrome. A careful history including review of systems, review of exposures, and past medical history as well as a comprehensive physical examination are critically important for the characterization of any underlying systemic disease causing DAH.
Imaging studies, specifically high-resolution CT scan, provide additional information to support a diagnosis of DAH. (Figure 2) However, radiological findings are frequently non-specific and subject to change throughout the course of the disease. Typical patterns include focal or diffuse areas of ground glass opacification and/or consolidation as a consequence of alveolar filling. Upon cessation of the alveolar bleeding, most of the associated radiological abnormalities resolve within a few days to weeks. The resolution of this process is slower than that of the infiltrates related to pulmonary edema but faster than the disappearance of the inflammatory/infectious radiological changes observed in pneumonias. During the resolution of the acute hemorrhage “crazy-paving” with associated interlobular septal thickening may become more prominent.[6, 7] Additional radiological abnormalities may be attributable to many of the underlying systemic disorders or represent infectious complications related to systemic immunosuppression. Some of these findings include cavitating pulmonary nodules and masses and large airway inflammation and stenosis due to granulomatous inflammation in GPA, fibrosis and bronchiectasis in MPA, airway inflammation and inflammatory infiltrates in EGPA, and pleural effusions in SLE. (Figure 2)
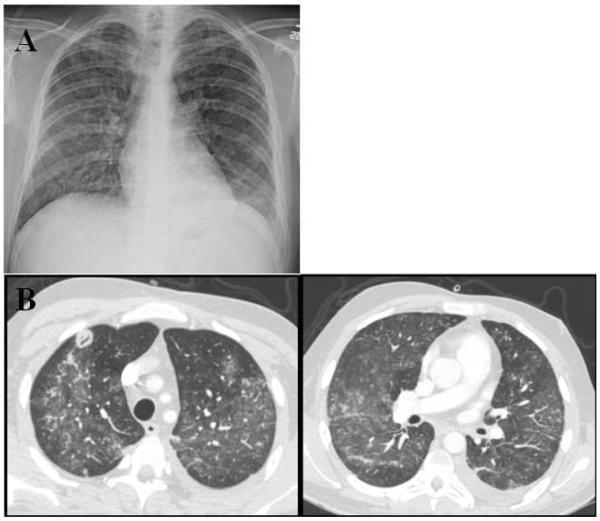
Figure 2.
Radiographic Findings of Diffuse Alveolar Hemorrhage. A. Chest x-ray with bilateral patchy infiltrate. B. CT images with bilateral ground glass infiltrates with associated necrotizing nodule in right upper lobe.
Due to the relatively non-specific nature of the clinical-radiological signs and symptoms of pulmonary vasculitis, additional laboratory tests and bronchoscopy with bronchoalveolar lavage (BAL) are frequently required to accurately diagnose and optimally manage patients with DAH. Laboratory studies typically include: complete blood count with differential, coagulation studies, serum creatinine and BUN, ANCA testing (by indirect immunofluorescence (c-ANCA and p-ANCA) and antigen specific ELISA (PR3- and MPO-ANCA)), anti-glomerular basement membrane (GBM) antibodies, anti-nuclear antibodies (ANA), anti-cyclic citrullinated peptide antibodies (Anti-CCP), rheumatoid factor (RF), anti-phospholipid antibodies, creatine kinase, urinalysis with urinary sediment and urine drug screen.
A transient, reversible increase in the diffusing capacity for carbone monoxide (DLCO) has been previously reported in patients with DAH.[8] This increase is attributed to the enhanced uptake of carbon monoxide by extra vascular blood. However, due to the acute onset, the often severe clinical manifestations of DAH and a lack of comparison baseline DLCO values, DLCO measurements are not routinely used to evaluate patients with suspected DAH.
The purpose of bronchoscopy with BAL is primarily to confirm the presence of intra-alveolar blood, exclude the large airways as a source of bleeding and rule out infection. Alveolar blood results in an increasingly hemorrhagic appearance of consecutive BAL aliquots.[4] BAL iron staining revealing the presence of greater than 20% hemosiderin laden macrophages (HLM) among all alveolar macrophages provides additional support for a diagnosis of DAH.[9] However, it is important to note that in cases of acute hemorrhage, increasingly bloody BAL returns may precede the appearance of HLM. In contrast HLM may be detectable in the BAL fluid weeks to months after the intra-alveolar red cells have disappeared. Unfortunately, the presence of HLM is not restricted to DAH and is also commonly seen in patients with diffuse alveolar damage.[10]
Since the majority of established diagnostic criteria for pulmonary vasculitis, e.g. the American College of Rheumatology criteria for GPA, do require the histological confirmation of vascular inflammation by tissue biopsy, tissue transbronchoscopic (TBBx) or surgical biopsies are often considered to establish a diagnosis in these patients. However, the diagnostic yield of TBBx remains suboptimal, the histological findings are frequently non-specific and the risks of the procedure commonly outweigh its benefits. Compared to TBBx, surgical lung biopsies have a higher diagnostic yield for pulmonary vasculitis. For example capillaritis was present in 17-43% of surgical lung biopsies in patients with GPA, however these procedures are also associated with a substantial risk for these patients.[11, 12] Consequently, recent efforts have focused on the development of diagnostic criteria that utilize clinical, radiological and serological information (e.g. ANCA for AAV) to establish a diagnosis.[13]
Differential Diagnosis of DAH
The etiology of DAH can be broadly divided into immune mediated and non-immune mediated causes. (Table 1) The list of diagnostic considerations is long and a careful history and physical examination represents a key component in the evaluation of these patients.
Table 1.
Differential Diagnosis of Diffuse Alveolar Hemorrhage
| Immune Mediated | Non-Immune Mediated |
|---|---|
| ANCA-Associated Vasculitis | Cardiac Disease |
| Granulomatosis with Polyangiitis (GPA) | Left ventricular dysfunction |
| Microscopic Polyangiitis (MPA) | Valvular disease |
| Eosinophilic Granulomatosis with Polyangiitis (EGPA) | |
| Infection | |
| Isolated Pulmonary Capillaritis | |
| Medications | |
| Anti-Glomerular Basement Membrane Antibody Syndrome | |
| Acute Respiratory Distress Syndrome | |
| Connective Tissue Disease | |
| Systemic Lupus Erythematosus | Idiopathic Pulmonary Hemosiderosis |
| Rheumatoid Arthritis | |
| Inflammatory Myopathies | Coagulopathy |
| Antiphospholipid antibody syndrome | Radiation Exposure |
| Henoch-Schönlein purpura/IgA Vasculitis | Occupational Exposure |
| Cryoglobulinemic Vasculitis | Crack Cocaine Inhalation |
| Behçet’s Disease | Bone Marrow Transplant1 |
| Lung Transplant Rejection | |
| Hypocomplementemic Urticarial Vasculitis (Anti-C1q Vasculitis) |
|
| Drug-Induced Vasculitis | |
| Bone Marrow Transplant1 |
In autopsy series of patients with bone marrow transplant with diffuse alveolar hemorrhage, there were complications of diffuse alveolar damage, rather than capillaritis, suggesting a non-immune mediated mechanism of diffuse alveolar hemorrhage.
In a 28-year retrospective cohort of patients presenting with DAH immune mediated causes including vasculitis, anti-GBM disease, and other connective tissue disease were identified in 36% of patients (35/97). Twenty-five patients had a diagnosis of vasculitis. Among the non-immune mediated etiologies systolic or diastolic cardiac dysfunction of the left ventricle and valvular heart disease accounted for 27% (26/97) of the cases.[3] Similarly, among critically ill patients with DAH vasculitis accounted for 19% (7/37) of all cases.[14] Since independent of the underlying etiology capillaritis represents the most common histological finding in DAH it is certainly possible that pulmonary vasculitis is responsible for up to 88% of these cases.[1]
Among DAH patients with pulmonary vasculitis AAV including GPA, MPA, and more rarely EGPA and isolated pulmonary capillaritis account for the majority of cases.[15] Although all AAV have been associated with pulmonary capillaritis and DAH, their frequency differs between the syndromes. The incidence of DAH ranges from 12-29% for MPA, 8-18% for GPA and 0-4% for EGPA.[16-20] Other less frequent causes of immune mediated pulmonary small vessel vasculitis include Henoch-Schönlein purpura, capillaritis including connective tissue disease (SLE, inflammatory myopathies and rheumatoid arthritis), antiphospholipid syndrome and anti-GBM-disease. DAH is also increasingly recognized as a pulmonary complication of bone marrow transplantation. However whether or not this represents an immune or non-immune mediated phenomenon remains controversial.
Acute Management of DAH
Acute management of DAH involves supportive care including ventilatory support ranging from oxygen supplementation to mechanical ventilation.[21] The coagulation cascade should be evaluated, and identified coagulation abnormalities should be corrected accordingly. Commonly accepted targets include a platelet count > 50,000/μl and an INR < 1.5. Depending on the cause of the coagulopathy platelet transfusions, vitamin K and fresh frozen plasma are used for correction.
It is crucial to identify and treat the underlying etiology of the DAH. Non-immune mediated causes are treated by addressing the cause e.g. heart failure management or discontinuation of any causative drugs. To quickly control the inflammatory activity in immune mediated DAH, prompt initiation of high dose methylprednisolone therapy is critical. Due to the high mortality associated with DAH, glucocorticoids are frequently started while diagnostic test results are pending.
If a non-immune mediated cause is not effectively controlled by supportive care or addressing the underlying etiology or in immune mediated cases that are refractory to aggressive initial immunosuppressive therapy and all other options are exhausted, consideration can be given to recombinant factor VIIa (rVIIa). Several recent case reports and small case series suggest that rVIIa may represent a treatment option for refractory cases. RVIIa is FDA approved for the prevention and management of hemorrhagic complications in patients with hemophilia A or B. However it has also been utilized for hemostasis in patients without hemophilia. Activated factor VIIa is thought to bind to activated platelets and activate factor X resulting in the generation of thrombin in a tissue factor independent fashion. In refractory cases of immune and non-immune mediated DAH, including cases due to pulmonary vasculitis, rVIIa has been successfully administered both systemically (intravenously) or bronchoscopically. The optimal dose and dosing intervals remain to be determined. Systemic administration usually involves the intravenous administration of 90-180 μg/kg as either a single dose or if needed repeated doses every 2-4 hours. Endobronchial therapy typically includes the bronchoscopic delivery of a total dose of 50 μg of activated factor VIIa diluted in 50 ml of normal saline. During the procedure 25 ml are instilled into each of the mainstem bronchi.[22-25] It needs to be noted that this represents an off label use of rVIIa. Thrombotic complications involving both arterial and venous events have been reported in some patients treated with rVIIa and patients should be monitored carefully. This risk is further increased in the known prothrombotic state of AAV. Therefore, this agent should be used with great caution in AAV.[26]
Another option includes the inhibition of fibrinolysis with the plasminogen inhibitor aminocaproic acid. The addition of aminocaproic acid to corticosteroids in patients with post-bone marrow transplant DAH was demonstrated to result in a lower 100-day disease related mortality rate as compared to corticosteroids alone.[27]
Advances in the Treatment of AAV
The treatment of AAV is typically stratified based on disease extent/severity and disease activity. The disease extent is classified as either non-severe (limited) or severe disease. Severe disease is defined as the presence of either life and/or organ threatening disease manifestations, which includes all cases of pulmonary vasculitis and DAH. Active disease typically requires the initiation of remission induction therapy. Once remission has been achieved (after 3-6 months of therapy) patients are transitioned to remission maintenance therapy. Currently a minimum of 18 months is the accepted duration of remission maintenance therapy. The optimal length is not yet known. However, it is being evaluated in a randomized clinical trial comparing 24 to 48 months.
In patients with severe disease, the selection of the appropriate remission induction and maintenance regimens appears to be independent of a clinical diagnosis of GPA or MPA. Recent clinical trials have stratified patients based on their ANCA-type rather than the specific underlying diagnosis. ANCA are classified based on their target antigens (either PR3 or MPO) and their staining pattern by indirect immunofluorescence on ethanol fixed neutrophils (cytoplasmic (c) or perinuclear (p)). The most common pattern is c-ANCA/PR3-ANCA (GPA), p-ANCA/MPO-ANCA (MPA and EGPA).
Remission Induction Therapy for Severe AAV
Historically, AAV was almost universally fatal, however over the past decades new treatment strategies resulted in a dramatic decrease in AAV morbidity and mortality. Disease severity typically dictates the aggressiveness of the employed treatment strategy. Traditionally combination therapy with high dose corticosteroids (intravenous high dose methylprednisolone 1000 mg/day for 3-5 days followed by oral prednisone 1mg/kg/day (maximum 80 mg/day) tapered over approximately 6-months) and oral cyclophosphamide (CYC) 2 mg/kg/day was utilized for almost all cases of severe GPA and MPA including cases of DAH. Approximately 75% of patients treated with Prednisone/CYC will achieve remission but disease relapse rates are as high as 50%. [28] Unfortunately, this regimen carries a substantial risk of severe treatment related toxicity. Treatment related complications include leukopenia and neutropenia due to bone marrow suppression, opportunistic infections, hemorrhagic cystitis, female and male infertility, bladder cancer and hematological malignancies.[29, 30] In order to decrease the occurrence of these side effects, intermittent intravenous pulse dose CYC has been compared to the daily oral administration of the drug. Despite a significant decrease in the cumulative CYC dose in patients treated intravenously, differences in the side effect profile were limited to fewer non-life threatening leukopenias in patients receiving intravenous CYC. There were no differences in remission induction.[31] However, the relapse rate was higher in the intravenous pulse CYC group, a finding that was recently confirmed during the long-term follow up of the study.[31, 32] Even though methotrexate (MTX) is effective for remission induction therapy in patients with non-severe (limited) GPA, this should not be used in the setting of DAH.[33]
Despite major advances in AAV remission induction, morbidity and mortality of patients with pulmonary vasculitis/DAH remains high and disease relapses occur frequently. Although the precise role of ANCA in the pathogenesis of AAV remains unclear, increasing clinical and experimental data suggest that these antibodies are at least modifying the autoimmune response. The presence of ANCA varies with the phenotype and the extent of the disease. Whereas ANCA is absent in up to 30-40% of patients with non-severe (limited) AAV, they are almost universally detectable in patients with pulmonary vasculitis/DAH. Consequently, several new therapeutic approaches have recently focused on the elimination of ANCA by depletion of B-lymphocytes as the precursors of antibody producing plasma cells or the direct removal of circulating antibodies.
Plasma Exchange (PLEX) for AAV
In an uncontrolled retrospective cohort study, 20 patients with AAV and DAH underwent PLEX in addition to standard immunosuppressive therapy. This approach resulted in excellent patient outcomes, DAH resolved in all cases and only 1/20 patients (5%) died, as compared to historic controls.[34] However, earlier other investigators had reported a high mortality (50%) among 14 patients with pulmonary-renal syndrome despite the use of PLEX in 12/14 patients.[34,35] Furthermore, in the MEPEX trial the addition of PLEX to immunosuppressive therapy was found to improve 12-months renal outcomes in AAV patients presenting with severe renal dysfunction (serum creatinine > 5.8 mg/dl).[49] Nevertheless, according to the recently reported long-term follow up data these benefits were not sustained. In accordance with the evidence based guidelines of the American Society for Apheresis we are currently using PLEX in AAV patients with DAH presenting with hypoxemic respiratory failure requiring either high-flow supplemental oxygen or mechanical ventilation.[36] PLEX is typically performed daily or on alternating days for 14 days. Each exchange involves 1-1.5 times the total plasma volume. The volume is replaced with albumin but FFP is used at the end of each treatment. Nevertheless the therapeutic indications of PLEX in AAV specifically for patients with DAH and glomerulonephritis remains controversial and is currently being investigated in an international randomized controlled clinical trial, the Plasma Exchange and Glucocorticoids for Treatment of AAV (PEXIVAS - NCT00987389).[37]
Rituximab (RTX) in AAV
RTX is a monoclonal chimeric antibody targeting CD20, a cell surface protein expressed on B-lymphocytes. These cells are the precursors for ANCA producing short-lived plasma cells. Antibody mediated modification and/or depletion of B-lymphocytes represents the proposed mechanism for this approach to decrease autoantibody production and control disease activity. Based on this rationale RTX was evaluated on a compassionate use basis for patients with refractory AAV. RTX is highly effective in patients with refractory AAV. [38-41] Successful treatment has been reported in more than 200 patients in at least 19 uncontrolled studies.[42] Therapeutic failures are uncommon and usually occur in patients with otherwise difficult to treat specific disease manifestations such as retroorbital pseudotumor. These very encouraging results led to two randomized controlled trials evaluating the use of RTX for remission induction therapy in patients with severe AAV.
The RTX in AAV (RAVE) trial compared combination therapy of glucocorticoids plus RTX or glucocorticoids plus CYC. After remission was achieved, patients in the CYC group were transitioned (after 3-6 months) to azathioprine (AZA) to complete 18 months remission maintenance therapy whereas RTX treated patients were observed in the absence of further therapy. The RAVE trial was a double blind, double dummy controlled trial with a primary endpoint of noninferiority. All patients received either 4 weekly infusions of 375 mg/m2 RTX or 2 mg/kg/day oral CYC. Based on the primary outcome measure (complete remission at 6 months: a Birmingham vasculitis assessment score (BVAS) = 0 in the absence of corticosteroid therapy), RTX was found to be non-inferior to CYC for remission induction therapy of severe AAV, including patients with pulmonary vasculitis and DAH. However it is important to note that patients with severe DAH causing respiratory failure requiring mechanical ventilation were excluded from participation in this study. Both medications demonstrated similar efficacy in the subgroup of patients with DAH. Rituximab was superior to cyclophosphamide for remission induction in patients who had a severe disease flare at the time of enrollment.[43] Somewhat surprisingly, relapse rates were similar between the two treatment groups at 18 months despite the absence of any active remission maintenance therapy in the RTX group.[44] Patients treated with RTX had fewer protocol defined adverse events. This difference was mainly due to an increased frequency of leukopenia in the CYC treated patients. Based on this data RTX became the first FDA approved drug for remission induction therapy in AAV.
Another study, the RTX versus CYC in ANCA-associated renal vasculitis study (RITUXVAS) enrolled 44 patients with newly diagnosed AAV (GPA and MPA) with renal involvement. Remission induction therapy included corticosteroids in combination with either RTX (4 weekly doses of 375 mg/m2) and two pulses of intravenous CYC (15 mg/kg with the first and the third RTX infusion) or monthly intravenous CYC pulses for 3-6 months (control patients received 15 mg/kg intravenous CYC every 2 weeks x 3 followed by every 3 weeks thereafter until stable remission, minimum 6, maximum 10 doses). Patients were randomized at a 3:1 ratio to experimental treatment (RTX plus two intravenous pulses of CYC) versus control treatment (intravenous pulse treatment with CYC for 6 months, followed by oral azathioprine for remission maintenance). Low dose corticosteroids (5 mg/day) were continued in both treatment arms through 18 months. Similar to the RAVE study there was no difference in remission induction and relapse rates between RTX and CYC.[45] Long-term data regarding the efficacy and safety of RTX is beginning to be available.[41]
Remission Maintenance Therapy for AAV
Remission induction therapy alone regardless of regimen is insufficient to prevent relapses and prolonged CYC treatment has been associated with unacceptable toxicities. Consequently a number of different remission maintenance regimens have been investigated. In a landmark randomized controlled trial maintenance therapy with AZA (2 mg/kg for 18 months) was demonstrated to be as effective as long-term CYC treatment.[46] MTX (25 mg weekly) has also been demonstrated to be effective for remission maintenance therapy of AAV and a recent randomized trial showed its equal efficiency compared to AZA.[47, 48]
Mycophenolate mofetil is another safe alternative for remission maintenance therapy. However in a recent randomized controlled trial it was found to be inferior to AZA. Consequently, it is mainly used as a second line option for patients who have contraindications to AZA or MTX or failed these first line remission maintenance options.[49, 50]
The intermittent administration of RTX without corticosteroids may represent another option for remission maintenance therapy of AAV. RTX will be investigated as such in an upcoming randomized controlled trial. However, recent data regarding the long-term use of RTX in patients with refractory AAV is very promising.[41]
Monitoring and Prophylaxis
In order to minimize treatment related morbidity, preemptive monitoring of the appropriate laboratory parameters should be conducted at regular intervals. Pneumocystis pneumonia prophylaxis should be prescribed to all patients on high doses of glucocorticoids, CYC, AZA, MTX, mycophenolate mofetil, or RTX. All patients on long-term glucocorticoid therapy should be offered prophylactic therapy for osteoporosis. Furthermore patients exposed to CYC should be monitored for the development of bladder cancer, and reproductive issues should be addressed in patients of childbearing age. All patients treated with MTX should receive folic acid supplementation.
Selected other Diseases Associated with Pulmonary Vasculitis and DAH
Primary Antiphospholipid Syndrome (APLS)
DAH represents a rare, frequently fatal non-thrombotic pulmonary complication of APLS. Pulmonary capillaritis without thrombosis has been demonstrated in lung biopsies from APLS patients presenting with DAH.[51] A proposed mechanism for the pathogenesis includes binding of antiphospholipid antibodies to endothelial cells promoting the increased expression of endothelial cell adhesion molecules, neutrophil binding and ultimately injury of alveolar capillaries and alveolar basement membrane.[51]
DAH in APLS is very difficult to treat. Historically, combination therapy of glucocorticoids and other immunosuppressants (CYC, AZA or mycophenolate mofetil) is combined with the intravenous administration of immunoglobulin G (IVIgG) and/or plasma exchange.[51, 52] However, a recent review of 17 consecutive cases of DAH in APLS demonstrated the limited success of aggressive standard immunosuppressive therapy in these patients. None of the patients treated with AZA or mycophenolate mofetil achieved remission and remission was only seen in a subgroup of patients treated with CYC, IVIgG, plasma exchange or RTX.[53] A recent case report also demonstrated a potential role for RTX for patients with primary APLS and DAH.[54] In addition to these challenging decisions regarding the appropriate immunosuppressive therapy the clinical management of these cases is typically complicated by the fact that many of these individuals are on therapeutic anticoagulation due to previous thrombotic complications. Since the DAH almost universally requires at least the temporary discontinuation of the anticoagulation these patients are at high risk for recurrent venous and/or arterial thrombosis.
Hematopoietic Stem Cell Transplant
DAH can complicate both allogeneic and autologous hematopoietic stem cell transplantation. It typically occurs early after stem cell transplantation.[55] The incidence of DAH is approximately 2%.[56, 57] The mortality is commonly > 50% in these patients. Outcomes are worse in allogeneic transplants and patients presenting with DAH > 30 days after their transplant.[58] While risk factors for DAH (older age and treatment regimens including intensive pre-transplant chemotherapy and total body irradiation) have been identified, the underlying pathogenesis remains poorly understood. Based on data from selected retrospective case series the current standard therapy frequently includes high dose corticosteroids implying an immune mediated nature of this disease.[55] A diagnosis of DAH in these typically immunocompromised patients is characteristically established based on clinical, radiologic and bronchoscopic data (respiratory decompensation in a patient with pulmonary infiltrates and BAL findings suggestive of DAH). Similar findings are also typically present in patients with diffuse alveolar damage with or without associated coagulopathy, both of which are frequently present after bone marrow transplant. Due to the associated risks, lung biopsies are usually not obtained in these patients. Interestingly, in a large autopsy series most of these cases demonstrated a histologic pattern of diffuse alveolar damage and capillaritis was notably absent in all cases. This information argues against the immune mediated nature of DAH in the context of bone marrow transplantation, and caution is warranted against the indiscriminate use of glucocorticoid therapy. This is especially important in a patient population with a high incidence of invasive fungal infections in whom further immunosuppressive therapy may result in worse clinical outcomes.
Conclusion
Pulmonary vasculitis most frequently manifests with DAH and represents its most common immune mediated cause. The acute management of these patients primarily focuses on respiratory support and the correction of abnormalities in the coagulation cascade. Simultaneously a careful history and physical examination in conjunction with a focused laboratory investigation (including serological testing for auto-antibodies) frequently facilitates targeted therapy by identifying the underlying systemic disease. AAV, specifically GPA and MPA, represent the most common cause of pulmonary vasculitis and immune mediated DAH. Due to their life threatening nature these cases are typically categorized as severe disease and treated accordingly. Based on the data from recent randomized controlled trials RTX represents an equally effective and likely less toxic alternative to CYC for remission induction therapy in these patients. The role of plasma exchange remains unclear and appropriate patients should be considered for participation in clinical trials.
Patients with pulmonary vasculitis benefit from a multidisciplinary team approach and expedited referral to an appropriate center with these resources should be considered for these patients. Further research is needed to continue to optimize the care of these challenging patients.
Synopsis*.
Diffuse alveolar hemorrhage is a clinical syndrome that can be a manifestation of multiple different etiologies. Identification of the underlying etiology is of utmost importance and dictates treatment. Pulmonary vasculitis including ANCA-associated vasculitis (AAV) is a common cause of diffuse alveolar hemorrhage. For AAV, treatment includes induction followed by maintenance therapy. Rituximab has an increasing role in the treatment for AAV.
Key Points.
Diffuse alveolar hemorrhage (DAH) represents the most common and potentially life threatening manifestation of pulmonary vasculitis.
Identification and treatment of the underlying cause of DAH are crucial for therapeutic success.
Pulmonary vasculitis, including AAV represents the most common immune mediated cause of DAH - Rituximab has an increasing role in the treatment of ANCA-associated vasculitis particularly in patients with severe relapsing or refractory disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Funding Sources/Conflicts of Interest Ulrich Specks - Research Grant from Genentech/BiogenIDEC
References
- 1.Travis WD, Colby TV, Lombard C, et al. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol. 1990;14(12):1112–25. doi: 10.1097/00000478-199012000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Gross WL, Guillevin L, et al. Granulomatosis with polyangiitis (wegener’s): An alternative name for wegener’s granulomatosis. Arthritis Rheum. 2011;63(4):863–4. doi: 10.1002/art.30286. [DOI] [PubMed] [Google Scholar]
- 3.de Prost N, Parrot A, Picard C, et al. Diffuse alveolar haemorrhage: Factors associated with in-hospital and long-term mortality. Eur Respir J. 2010;35(6):1303–11. doi: 10.1183/09031936.00075309. [DOI] [PubMed] [Google Scholar]
- 4.Cordier JF, Cottin V. Alveolar hemorrhage in vasculitis: Primary and secondary. Semin Respir Crit Care Med. 2011;32(3):310–21. doi: 10.1055/s-0031-1279827. [DOI] [PubMed] [Google Scholar]
- 5.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164–71. doi: 10.1378/chest.08-2084. [DOI] [PubMed] [Google Scholar]
- 6.Castaner E, Alguersuari A, Gallardo X, et al. When to suspect pulmonary vasculitis: Radiologic and clinical clues. Radiographics. 2010;30(1):33–53. doi: 10.1148/rg.301095103. [DOI] [PubMed] [Google Scholar]
- 7.Hansell DM. Small-vessel diseases of the lung: Ct-pathologic correlates. Radiology. 2002;225(3):639–53. doi: 10.1148/radiol.2253011490. [DOI] [PubMed] [Google Scholar]
- 8.Ewan PW, Jones HA, Rhodes CG, et al. Detection of intrapulmonary hemorrhage with carbon monoxide uptake. Application in goodpasture’s syndrome. N Engl J Med. 1976;295(25):1391–6. doi: 10.1056/NEJM197612162952502. [DOI] [PubMed] [Google Scholar]
- 9.De Lassence A, Fleury-Feith J, Escudier E, et al. Alveolar hemorrhage. Diagnostic criteria and results in 194 immunocompromised hosts. Am J Respir Crit Care Med. 1995;151(1):157–63. doi: 10.1164/ajrccm.151.1.7812547. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado F, Parambil JG, Yi ES, et al. Haemosiderin-laden macrophages in the bronchoalveolar lavage fluid of patients with diffuse alveolar damage. Eur Respir J. 2009;33(6):1361–6. doi: 10.1183/09031936.00119108. [DOI] [PubMed] [Google Scholar]
- 11.Mark EJ, Matsubara O, Tan-Liu NS, et al. The pulmonary biopsy in the early diagnosis of wegener’s (pathergic) granulomatosis: A study based on 35 open lung biopsies. Hum Pathol. 1988;19(9):1065–71. doi: 10.1016/s0046-8177(88)80088-1. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Hoffman GS, Leavitt RY, et al. Surgical pathology of the lung in wegener’s granulomatosis. Review of 87 open lung biopsies from 67 patients. The American journal of surgical pathology. 1991;15(4):315–33. doi: 10.1097/00000478-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the anca-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66(2):222–7. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabe C, Appenrodt B, Hoff C, et al. Severe respiratory failure due to diffuse alveolar hemorrhage: Clinical characteristics and outcome of intensive care. J Crit Care. 2010;25(2):230–5. doi: 10.1016/j.jcrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Papiris SA, Manali ED, Kalomenidis I, et al. Bench-to-bedside review: Pulmonary-renal syndromes--an update for the intensivist. Crit Care. 2007;11(3):213. doi: 10.1186/cc5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage CO, Winearls CG, Evans DJ, et al. Microscopic polyarteritis: Presentation, pathology and prognosis. Q J Med. 1985;56(220):467–83. [PubMed] [Google Scholar]
- 17.Serra A, Cameron JS, Turner DR, et al. Vasculitis affecting the kidney: Presentation, histopathology and long-term outcome. Q J Med. 1984;53(210):181–207. [PubMed] [Google Scholar]
- 18.Guillevin L, Durand-Gasselin B, Cevallos R, et al. Microscopic polyangiitis: Clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42(3):421–30. doi: 10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Guillevin L, Cohen P, Gayraud M, et al. Churg-strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore) 1999;78(1):26–37. doi: 10.1097/00005792-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Keogh KA, Specks U. Churg-strauss syndrome: Clinical presentation, antineutrophil cytoplasmic antibodies, and leukotriene receptor antagonists. Am J Med. 2003;115(4):284–90. doi: 10.1016/s0002-9343(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 21.Khan SA, Subla MR, Behl D, et al. Outcome of patients with small-vessel vasculitis admitted to a medical icu. Chest. 2007;131(4):972–6. doi: 10.1378/chest.06-2464. [DOI] [PubMed] [Google Scholar]
- 22.Henke D, Falk RJ, Gabriel DA. Successful treatment of diffuse alveolar hemorrhage with activated factor vii. Ann Intern Med. 2004;140(6):493–4. doi: 10.7326/0003-4819-140-6-200403160-00033. [DOI] [PubMed] [Google Scholar]
- 23.Heslet L, Nielsen JD, Levi M, et al. Successful pulmonary administration of activated recombinant factor vii in diffuse alveolar hemorrhage. Crit Care. 2006;10(6):R177. doi: 10.1186/cc5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastores SM, Papadopoulos E, Voigt L, et al. Diffuse alveolar hemorrhage after allogeneic hematopoietic stem-cell transplantation: Treatment with recombinant factor viia. Chest. 2003;124(6):2400–3. doi: 10.1016/s0012-3692(15)31709-8. [DOI] [PubMed] [Google Scholar]
- 25.Hicks K, Peng D, Gajewski JL. Treatment of diffuse alveolar hemorrhage after allogeneic bone marrow transplant with recombinant factor viia. Bone Marrow Transplant. 2002;30(12):975–8. doi: 10.1038/sj.bmt.1703731. [DOI] [PubMed] [Google Scholar]
- 26.Merkel PA, Lo GH, Holbrook JT, et al. Brief communication: High incidence of venous thrombotic events among patients with wegener granulomatosis: The wegener’s clinical occurrence of thrombosis (weclot) study. Ann Intern Med. 2005;142(8):620–6. doi: 10.7326/0003-4819-142-8-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 27.Wanko SO, Broadwater G, Folz RJ, et al. Diffuse alveolar hemorrhage: Retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant. 2006;12(9):949–53. doi: 10.1016/j.bbmt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med. 1992;116(6):488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 29.Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with wegener granulomatosis. Ann Intern Med. 1996;124(5):477–84. doi: 10.7326/0003-4819-124-5-199603010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Clowse ME, Copland SC, Hsieh TC, et al. Ovarian reserve diminished by oral cyclophosphamide therapy for granulomatosis with polyangiitis (wegener’s) Arthritis care & research. 2011;63(12):1777–81. doi: 10.1002/acr.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized trial. Ann Intern Med. 2009;150(10):670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 32.Harper L, Morgan MD, Walsh M, et al. Pulse versus daily oral cyclophosphamide for induction of remission in anca-associated vasculitis: Long-term follow-up. Ann Rheum Dis. 2012;71(6):955–60. doi: 10.1136/annrheumdis-2011-200477. [DOI] [PubMed] [Google Scholar]
- 33.De Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52(8):2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 34.Klemmer PJ, Chalermskulrat W, Reif MS, et al. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis. 2003;42(6):1149–53. doi: 10.1053/j.ajkd.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: A 4-year, single-center experience. Am J Kidney Dis. 2002;39(1):42–7. doi: 10.1053/ajkd.2002.29876. [DOI] [PubMed] [Google Scholar]
- 36.Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice--evidence-based approach from the apheresis applications committee of the american society for apheresis. J Clin Apher. 2010;25(3):83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed 5/26/2012];Plasma exchange and glucocorticoids for treatment of anti-neutrophil cytoplasm antibody (anca) - associated vasculitis. Available at: http://clinicaltrials.gov/ct2/results?term=pexivas.
- 38.Specks U, Fervenza FC, McDonald TJ, et al. Response of wegener’s granulomatosis to anti-cd20 chimeric monoclonal antibody therapy. Arthritis Rheum. 2001;44(12):2836–40. doi: 10.1002/1529-0131(200112)44:12<2836::aid-art471>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Keogh KA, Wylam ME, Stone JH, et al. Induction of remission by b lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52(1):262–8. doi: 10.1002/art.20718. [DOI] [PubMed] [Google Scholar]
- 40.Keogh KA, Ytterberg SR, Fervenza FC, et al. Rituximab for refractory wegener’s granulomatosis: Report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173(2):180–7. doi: 10.1164/rccm.200507-1144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartin-Ceba R, Golbin JM, Keogh KA, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (wegener’s): A single-center ten-year experience. Arthritis Rheum. 2012 doi: 10.1002/art.34584. [DOI] [PubMed] [Google Scholar]
- 42.Cartin-Ceba R, Fervenza FC, Specks U. Treatment of antineutrophil cytoplasmic antibody-associated vasculitis with rituximab. Curr Opin Rheumatol. 2012;24(1):15–23. doi: 10.1097/BOR.0b013e32834d5730. [DOI] [PubMed] [Google Scholar]
- 43.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for anca-associated vasculitis. N Engl J Med. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Specks U, Stone JH, Group. RR Grouplong-term efficacy and safety results of the rave trial [abstract] Clin Exp Immunol. 2011;164(Supp 1):65. [Google Scholar]
- 45.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in anca-associated renal vasculitis. N Engl J Med. 2010;363(3):211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 46.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349(1):36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 47.Pagnoux C, Mahr A, Hamidou MA, et al. Azathioprine or methotrexate maintenance for anca-associated vasculitis. N Engl J Med. 2008;359(26):2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 48.Langford CA, Talar-Williams C, Barron KS, et al. A staged approach to the treatment of wegener’s granulomatosis: Induction of remission with glucocorticoids and daily cyclophosphamide switching to methotrexate for remission maintenance. Arthritis Rheum. 1999;42(12):2666–73. doi: 10.1002/1529-0131(199912)42:12<2666::AID-ANR24>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 49.Langford CA, Talar-Williams C, Sneller MC. Mycophenolate mofetil for remission maintenance in the treatment of wegener’s granulomatosis. Arthritis Rheum. 2004;51(2):278–83. doi: 10.1002/art.20240. [DOI] [PubMed] [Google Scholar]
- 50.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2010;304(21):2381–8. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 51.Deane KD, West SG. Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: A case series and literature review. Semin Arthritis Rheum. 2005;35(3):154–65. doi: 10.1016/j.semarthrit.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Waterer GW, Latham B, Waring JA, et al. Pulmonary capillaritis associated with the antiphospholipid antibody syndrome and rapid response to plasmapheresis. Respirology. 1999;4(4):405–8. doi: 10.1046/j.1440-1843.1999.00212.x. [DOI] [PubMed] [Google Scholar]
- 53.Cartin-Ceba R, Peikert T, Ashrani A, et al. Diffuse alveolar hemorrhage caused by primary antiphospholipid syndrome [abstract]. European Respiratory Society Annual Congress; Vienna. 2012. [Google Scholar]
- 54.Scheiman Elazary A, Klahr PP, Hershko AY, et al. Rituximab induces resolution of recurrent diffuse alveolar hemorrhage in a patient with primary antiphospholipid antibody syndrome. Lupus. 2012;21(4):438–40. doi: 10.1177/0961203311422713. [DOI] [PubMed] [Google Scholar]
- 55.Afessa B, Tefferi A, Litzow MR, et al. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166(5):641–5. doi: 10.1164/rccm.200112-141cc. [DOI] [PubMed] [Google Scholar]
- 56.Majhail NS, Parks K, Defor TE, et al. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: Related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12(10):1038–46. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest. 2012;141(2):442–50. doi: 10.1378/chest.10-2889. [DOI] [PubMed] [Google Scholar]
- 58.Afessa B, Tefferi A, Litzow MR, et al. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166(10):1364–8. doi: 10.1164/rccm.200208-792OC. [DOI] [PubMed] [Google Scholar]