Abstract
Injected liquid silicone continues to be employed by unscrupulous practitioners in many parts of the world for the purpose of breast augmentation. Complications vary; however, inflammation, foreign body reaction, and granuloma formation often lead to painful and disfigured breasts. Furthermore, migrations of silicone to remote tissues cause additional problems. We present a review of cases and propose an updated algorithm for the diagnosis and management silicone mastitis. We describe two representative cases of mastitis cause by injected liquid silicone. Patients uniformly developed inflammation and granuloma formation causing painful and disfigured breasts. Each patient required bilateral mastectomy and breast reconstruction. Although injection of liquid silicone has been condemned by the legitimate medical community for the purpose of breast augmentation, it continues to be illicitly performed and there exists a sizable patient population suffering from the complications of this procedure. Accurate identification requires a high index of suspicion in patients presenting with firm and painful breasts. An aggressive management strategy is recommended in the setting of silicone mastitis due to the risk of obscuring malignancy.
KEY WORDS: Foreign body, granuloma, mastitis, silicone
INTRODUCTION
In 1960, the Dow Corning Corporation developed the first injectable liquid silicone that was sterile, pure, and of constant viscosity for medical application. It was marketed under the name of Dermagen and its initial purpose was for waterproofing the skin of burn patients.[1] Previously, liquid silicone was available only for industrial applications such as waterproofing, lubrication, and electrical insulation. However, since the end of World War II, prostitutes in Japan had been injecting industrial-grade silicone for the purposes of breast augmentation. In the 1960s, injection of medical-grade liquid silicone became popular in the US, although the material was never approved by the Food and Drug Administration (FDA) for such a purpose. By 1966, many complications appeared, prompting the FDA to designate liquid silicone as a “new drug” and limit its use to a study of patients getting treatment for severe facial deformities by a group of ten physicians. After only a few years, Dow Corning discontinued the project and abandoned efforts to gain FDA approval, claiming that they could not prevent misuse of the product. In the ensuing years, hundreds of thousands of patients were injected with liquid silicone despite known complications. In 1992, the FDA finally banned the injection of all liquid silicone products by physicians. However, there are many unscrupulous physicians and lay practitioners that still continue to use liquid silicone for cosmetic breast augmentation, especially outside the United States.[1–5]
The long-term sequelae of injected silicone for breast augmentation result from the devastating effects of inflammation, foreign body reaction, and granuloma formation within the breast.[1–11] Depending on the areas of injection and migratory pattern of the silicone, skin inflammation and muscle infiltration can also occur.[1,3–5] Although there is no clear increase risk of breast cancer associated with injected silicone, inflammatory changes hamper detection of malignancy by interfering with breast examination and imaging.[7–9] In this report, we describe two patients who presented with silicone mastitis, discuss their evaluation and surgical treatment, and present an algorithm for management of the complications of injected silicone in the breast.
CASE REPORTS
Case 1
A forty-three year old Hispanic woman presented complaining of pain, erythema, and firmness of both breasts. These symptoms initially started four years prior to presentation, and gradually worsened to the point where she was in constant pain. She admitted to a history of serial injections of silicone to both breasts in the Dominican Republic for the purpose of cosmetic augmentation. She was otherwise healthy and denied any other surgery. On exam, the breast skin was erythematous, hard, and tender [Figure 1]. Furthermore, there were numerous firm masses palpable in both breasts. There was no axillary adenopathy present. Nipple sensation was intact, although skin sensation was decreased. MRI was consistent with silicone deposition in the breast parenchyma and skin. Silicone mastitis was confirmed on tissue biopsy. The patient was treated with bilateral simple mastectomy, removing all tissues involved with silicone including skin and portions of the pectoralis major muscles [Figure 2]. The breast were immediately reconstructed using bilateral muscle-sparing free transverse rectus abdominus flaps [Figure 3]. Proline mesh was used to reinforce the abdominal wall at the time of reconstruction. Pathology results confirmed granulomatous tissue without malignancy. There were no postoperative complications and the patient was discharged after five days in the hospital. The patient recovered without incident, but was unfortunately lost to follow-up after four months.
Figure 1.
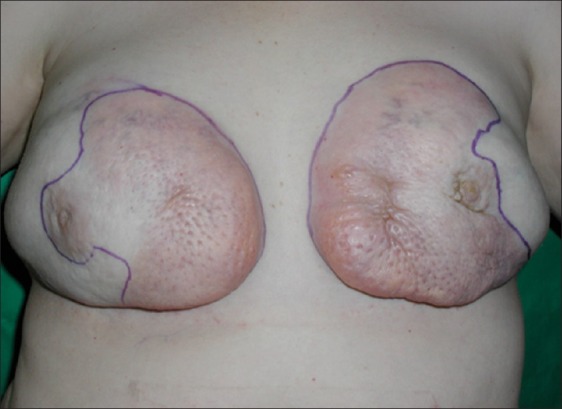
Forty-three year old woman with silicone mastitis presents with hard, painful, and disfigured breasts
Figure 2.
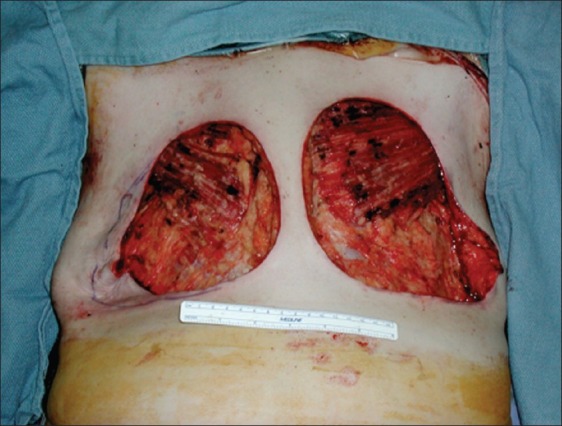
Bilateral simple mastectomy with removal of all tissues involved with silicone including skin and portions of the pectoralis major muscles
Figure 3.
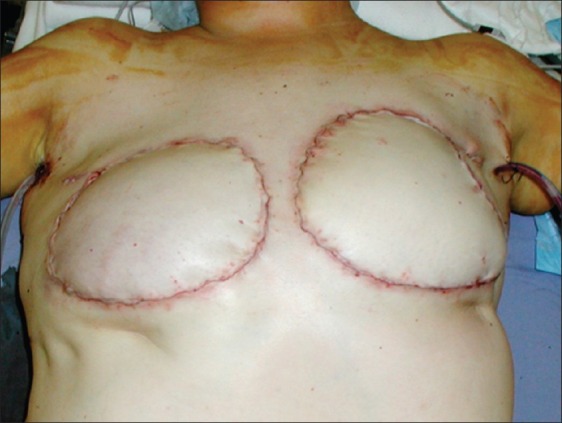
Immediate reconstruction with bilateral free transverse rectus abdominus myocutaneous flaps
Case 2
A forty-six year old Asian woman presented with a history of silicone injections to both breasts performed in the Philippines in twenty-five years earlier. She initially underwent bilateral subtotal mastectomy with implant reconstruction twelve year prior to presentation. However, she experienced recurrent pain, hardness, hypertrophic scars, breast asymmetry, and poor cosmesis secondary to retained silicone in the breast tissue [Figure 4]. On exam, the breast skin was firm and there were multiple areas of hyperpigmentation. The diagnosis of silicone mastitis was confirmed with MRI and tissue biopsy. The patient was treated by removal of the previous implants, completion simple mastectomy including involved skin, and placement of bilateral subpectoral tissue expanders. She underwent serial tissue expansion in the office and finally had placement of breast implants four months later. Another operation involved nipple reconstruction. The patient completed reconstruction with areolar tattooing in the office. She did well and was very pleased with the final results.
Figure 4.
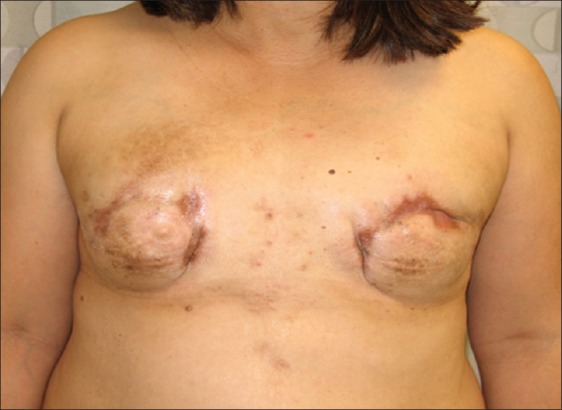
Forty-six year old woman with incomplete treatment of silicone mastitis. Retained silicone results in persistent formation of granulomatous tissue
DISCUSSION
Although the injection of liquid silicone into breasts as a method for augmentation was generally abandoned in the 1970's, illicit silicone injections are still performed today. The combination of a desire for larger breasts, a false sense that silicone injections are simple and safe, and a relatively cheap price will continue to influence uneducated patients to undergo this risky procedure. A high-index of suspicion is required when evaluating patients presenting with painful and firm breasts and a history of silicone injection should always be sought. Breast examination and imaging for breast cancer screening is obscured secondary to the inflammatory effects of injected silicone.[7–10] Magnetic resonance imaging is the most useful imaging modality for the diagnosis and evaluation of silicone-injected breasts and associated liquid silicone migration. The typical MRI findings of silicone particles are well documented- hyperintensity on T2-weighted images, intermediate signal intensity on nonfat-supressed T1-weighted images, and usually low signal intensity on fat-suppressed T1-weighted images without definite enhanc ement.[12,13] An absence of breast lesion enhancement in post-contrast MRI studies is believed to reliably exclude malignancy.[14]
The goal of treatment is the complete removal of silicone from the breast. In fact, incomplete removal of silicone often results in continued symptoms.[2–5] Fortunately, there is no clear link between the inflammatory changes secondary to silicone deposition in breast tissue and an increased risk of breast cancer. Anecdotal reports, however, suggest that the difficulty in examination and imaging contributes to a delay in the diagnosis.[7–10] If the patient has a family or personal history of an increased risk of breast cancer, a low threshold for bilateral mastectomy with sentinel node biopsy is recommended.[7–10] There are only a handful of articles focusing on the strategy for management of silicone mastitis.[1–5] Clinical evaluation should elicit a history of injected silicone [Figure 5]. Infection should always be treated immediately and prior to definitive surgery.[6,11] Magnetic resonance imaging is recommended to confirm silicone deposition and to serve as a baseline for future breast screening. Image-guided biopsy is performed to exclude malignancy and to confirm silicone mastitis.[1–5] We propose a low threshold for bilateral mastectomy, including resection of involved breast skin, in order to minimize the occurance of retained silicone. Breast reconstruction is best performd immediately and reconstructive options include use of implants or autologous tissue. If breast skin is involved, autologous tissue reconstruction is usually required. However, tissue expansion may be utilized in some patients with smaller skin defects.
Figure 5.
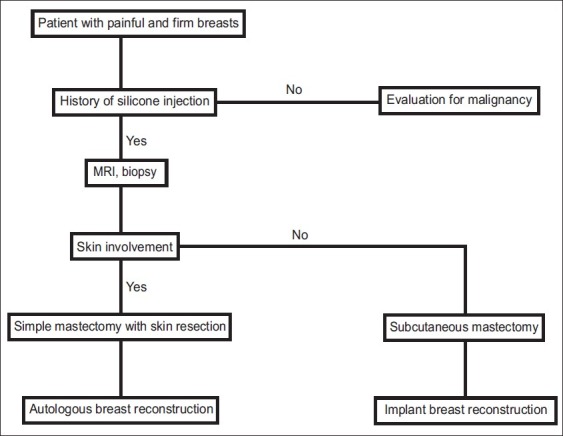
Algorithm for the evaluation and treatment of silicone mastitis
CONCLUSION
The diagnosis of silicone mastitis requires a high index of suspicion. Patients presenting with signs of chronic inflammation in the breasts should always elicit a history of silicone injection. An agressive management strategy is recommended due to the increased challenges in detecting breast cancer in this setting. We advocate resection of all tissues involved with silicone in order to avoid persistent symptoms and future complications. In our experience, immediate breast reconstruction results in the best aesthetic outcomes and the most satisfied patients.
Footnotes
Disclosures: None of the authors have any commercial associations or financial interests in any of the products, devices, or drugs mentioned in this manuscript.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Peters W, Fornasier V. Complications from injectable materials used for breast augmentation. Can J Plast Surg. 2009;17:89–96. doi: 10.1177/229255030901700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symmers WS. Silicone mastitis in “topless” waitresses and some other varieties of foreign-body mastitis. Br Med J. 1968;3:19–22. doi: 10.1136/bmj.3.5609.8-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wustrack KO, Zarem HA. Surgical management of silicone mastitis. Plast Reconstr Surg. 1979;63:224–9. doi: 10.1097/00006534-197902000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Chen TH. Silicone injection granulomas of the breast: Treatment by subcutaneous mastectomy and immediate subpectoral breast implant. Br J Plast Surg. 1995;48:71–6. doi: 10.1016/0007-1226(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz-Monasterio F, Trigos I. Management of patients with complications from injections of foreign materials into the breasts. Plast Reconstr Surg. 1972;50:42–7. doi: 10.1097/00006534-197207000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Giron GL, Tartter PI. Image of the month. Silicone mastitis with abscess. Arch Surg. 2004;139:341–2. doi: 10.1001/archsurg.139.3.341. Erratum in: Arch Surg 2004;139:608. [DOI] [PubMed] [Google Scholar]
- 7.Luo SK, Chen GP, Sun ZS, Cheng NX. Our strategy in complication management of segmentation mammaplasty with polyacrylamide hydrogel injection in 235 patients. J Plast Reconstr Aesthet Surg. 2010;10 doi: 10.1016/j.bjps.2010.10.004. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Timberlake GA, Looney GR. Adenocarcinoma of the breast associated with silicone injections. J Surg Oncol. 1986;32:176–9. doi: 10.1002/jso.2930320205. [DOI] [PubMed] [Google Scholar]
- 9.Lewis CM. Inflammatory carcinoma of the breast following silicone injections. Plast Reconstr Surg. 1980;66:134–6. doi: 10.1097/00006534-198007000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Ko C, Ahn Cy, Markowitz BL. Injected liquid silicone, chronic mastitis, and undetected breast cancer. Ann Plast Surg. 1995;34:176–9. doi: 10.1097/00000637-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Perry RR, Jaques DP, Lesar MS, d’Avis JC, Peterson HD. Mycobacterium avium infection in a silicone-injected breast. Plast Reconstr Surg. 1985;75:104–6. doi: 10.1097/00006534-198501000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Caskey CI, Berg WA, Hamper UM, Sheth S, Chang BW, Anderson ND. Imaging spectrum of extracapsular silicone; correlation of US, MRI imaging, mammographic, and histopathologic findings. Radiographics. 1999;19:S39–51. doi: 10.1148/radiographics.19.suppl_1.g99oc11s39. [DOI] [PubMed] [Google Scholar]
- 13.Peng HL, Wu CC, Choi WM, Hui MS, Lu TN, Chen LK. Breast cancer detection using magnetic resonance imaging in breasts injected with liquid silicone. Plast Reconstr Surg. 1999;104:2116–20. doi: 10.1097/00006534-199912000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Orel SG, Schnall MD, LiVolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic-pathologic correlation. Radiology. 1994;190:485–93. doi: 10.1148/radiology.190.2.8284404. [DOI] [PubMed] [Google Scholar]


