Summary
The fraction of exhaled nitric oxide (FeNO), a measure of airway inflammation, shows promise as a noninvasive tool to guide asthma management, but there is a paucity of longitudinal data about seasonal variation and environmental predictors of FeNO in children. The objective of this project was to evaluate how environmental factors affect FeNO concentrations over a 12-month study period among children with doctor diagnosed asthma. We conducted a prospective cohort study of 225 tobacco-smoke exposed children age 6 to 12 years with doctor-diagnosed asthma including measures of FeNO, medication use, settled indoor allergens (dust mite, cat, dog, and cockroach), and tobacco smoke exposure. Baseline geometric mean FeNO was 12.4 ppb (range 1.9 to 60.9 ppb). In multivariable analyses, higher baseline FeNO levels, atopy, and fall season were associated with increased FeNO levels, measured 6 and 12 months after study initiation, whereas inhaled steroid use, summer season, and increasing nicotine exposure were associated with lower FeNO levels. In secondary analyses of allergen sensitization, only sensitization to dust mite and cat were associated with increased FeNO levels. Our data demonstrate that FeNO levels over a year long period reflected baseline FeNO levels, allergen sensitization, season, and inhaled steroid use in children with asthma. These results indicate that FeNO levels are responsive to common environmental triggers as well as therapy for asthma in children. Clinicians and researchers may need to consider an individual’s baseline FeNO levels to manage children with asthma.
Keywords: allergen, sensitization, tobacco smoke, inhaled corticosteroid
INTRODUCTION
Asthma, the most common chronic and disabling disease of childhood, is one of the leading reasons for clinic visits, emergency room visits, and hospitalizations.1–3 In primary care settings, the main tools used to guide asthma management are asthma symptoms, physical examination, and peak expiratory flow meters, but these are inadequate measures of disease status and airway inflammation.4–6 Moreover, investigators have begun to question whether current asthma classifications provide a complete picture of the severity in children.7,8 To enhance the medical management of asthma, physicians need a convenient and accurate test to measure airway inflammation, asthma control, and response to treatment. An ideal measure of airway inflammation would reflect pharmacologic therapy and respond to changes in an individual’s exposure to environmental triggers.
The fraction of nitric oxide in exhaled air (FeNO) has been proposed as a biomarker for gauging an individual’s response to pharmacologic treatment because it reflects airway inflammation, and it decreases with steroid therapy.9–13 In a recent randomized trial, Smith et al found that maintenance doses of inhaled corticosteroid could be significantly reduced by using FeNO to guide treatment in chronic asthma in adults.14 Thus, FeNO, is increasingly recognized as a supplementary tool to optimize pharmacologic therapy.
FeNO may also enhance our ability to integrate the control of environmental triggers into asthma management. Environmental interventions are an important component of asthma management, and investigators have found that exposures to some environmental triggers increase FeNO levels. 15–18 Sensitized individuals with asthma who are exposed to indoor allergens have higher FeNO levels.15–18 In contrast, smoking has been associated with decreased FeNO levels in adults.19,20 The association of environmental tobacco smoke exposure and FeNO in children, however, is mixed, with no relationship in some studies and an inverse association in others.21,22 Environmental exposures and asthma symptoms can vary by season, and these exposures affect asthma control.23 One study, demonstrated that mean FeNO levels were higher in the winter than the spring, but only winter and spring FeNO levels were examined.24 Taken together, these studies suggest that FeNO may be used as a biomarker to identify children with ongoing exposure to environmental triggers.16–18,25
While FeNO shows promise as a tool to optimize pharmacologic therapy and to identify children with exposure to environmental triggers, questions remain. First, many existing studies that found associations of environmental exposures with FeNO levels were cross sectional; few evaluated temporal relationships.18 Second, most prior studies did not examine seasonal variation in FeNO. Third, few investigators used biomarkers to evaluate the effects of a combination of environmental exposures on FeNO in children with asthma. The utility of FeNO for integrating control of environmental triggers into asthma management thus remains unclear.
The objectives of this analysis were to evaluate seasonal variability in FeNO levels among children who had doctor diagnosed asthma and to determine how environmental triggers affect FeNO levels, accounting for indoor exposures and medical management.
MATERIALS & METHODS
We used data from the cohort of children enrolled in the Cincinnati Asthma Prevention (CAP) study for this analysis. The CAP study is described in detail elsewhere.25,26 Briefly, the CAP study was a randomized controlled trial with the primary goal to evaluate the health effects of use of HEPA-CPZ (High Efficiency Particulate Air-Carbon, Permanganate, Zeolite) air cleaners in the homes of 225 children (age 6–12) who had physician diagnosed asthma and were exposed to tobacco smoke. Children with cystic fibrosis and neuromuscular disorders were excluded. Children and their families provided written informed consent prior to enrollment in this year long study. This project and the CAP study were approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
FeNO Measurement
We employed trained research assistants to collect exhaled air for FeNO analysis. Air was collected in Mylar balloons from each participant using a previously validated offline technique at the baseline, 6 month, and 12 month home visits.27,28 Briefly, children breathed through a bacterial attached to the mouthpiece of a collecting tube which had a charcoal filter on the inspiratory end of the tube to remove nitric oxide from the inhaled ambient air. The child exhaled slowly into the collecting tube that by design maintained a flow of approximately 50 to 79 mL/sec. The first part of each breath was not collected; after two seconds of exhalation, a valve was closed to direct the remainder of the exhalate into the balloon. This procedure was repeated to partially fill the balloon. We assessed sample stability by assaying balloon samples serially (1–21 days) and used these data to adjust the FeNO levels that were not assayed within 24 hours (E Table 1). The adjusted FeNO level was our primary outcome variable. We confirmed our multivariable models using the original FeNO level and the time to sample analysis variables. We used the Model 280i nitric Oxide Analyzer (NOA) from Sievers Instruments in accordance with the manufacturer’s instructions for all nitric oxide analyses.29
Environmental Exposure Measures
We collected measures of the home environment during the study. We employed trained environmental technicians to collect environmental samples during three home visits over the study period (baseline, six months, and twelve months). The technicians also measured the volume and condition of the housing unit and assessed the type of heating and air-conditioning during the home visits.
Settled Dust Allergens
The technician collected a dust sample from the midpoint or largest area of the floor in the child’s bedroom using a standardized HVS-3 dust collection method during the baseline home visit.30 We then analyzed dust samples for levels of dust mite (Der f 1), dog (Can f 1), cat (Fel d 1), and cockroach (Bla g 1) allergen using monoclonal ELISA technique.31–33
Indoor Airborne Particulates
We measured airborne particulate concentration (particles per ft3) at the beginning and end of each home visit using a GT-321 particulate monitor (Met One Instruments, Grants Pass, OR).
Allergen Sensitization
A trained pediatric phlebotomist obtained serum samples by venipuncture at the baseline home visit. We evaluated these samples using the ImmunoCap test (Pharmacia Diagnostics, Portage, MI) to determine allergen specific IgE levels (dust mite, dog, cat, and cockroach).34 We considered children sensitized to an allergen if their allergen specific IgE level was Class I or higher (≥0.35 kU/L).
Tobacco Smoke Exposure Measures
We measured tobacco exposure using a survey, biomarkers of internal dose, and a nicotine dosimeter. At each home visit, we surveyed guardians about the number of cigarettes smoked per day in and around the home. We also measured hair and serum cotinine, biomarkers of exposure to tobacco smoke, using established techniques.35–37 Additionally, we measured tobacco smoke exposure in the home using nicotine dosimeters that were located on the back of the HEPA machines in the main activity room at the baseline and 6-month visits, and retrieved at the 6 and 12-month visits respectively. The dosimeters were analyzed for nicotine level using a standardized protocol (limit of detection of 0.01 µg/ filter).38–40 We examined both nicotine µg/ filter and µg/m3 in our analyses.
Additional Covariates
We conducted surveys about asthma history, asthma therapy, and other characteristics of the children and their families to use as potential covariates. We surveyed the participant’s guardian about the child’s asthma medication use, demographic information, and housing characteristic information (such as volume, presence of carpeting, and heating type). We also explored seasonal variation by evaluating the calendar defined season of FeNO collection in our models.
Data Analysis
The distributions of the FeNO data, cotinine data, and nicotine data were log-normal, so geometric means and 95% confidence intervals (95% CI) were the primary descriptors of central tendency and dispersion. We made comparisons between groups using t-tests and chi-square tests. Changes in exposures, medication use, and FeNO over time were modeled with repeated measures analysis. For all analyses, we employed the standard two sided 5% level to determine statistical significance.
To determine the relationship between environmental factors and FeNO, the changes in FeNO levels were modeled as a repeated measures design using a mixed-effects linear model (Proc Mixed in SAS). This approach accounts for the correlation of measurements within individual subject and permits modeling of the correlation structure. We considered the six and twelve month FeNO data longitudinally while adjusting for baseline visit FeNO values (hereafter called baseline). First we conducted separate exploratory (bivariate-type) analyses to evaluate the relationship of each environmental factor (e.g., allergen sensitization and exposure, tobacco smoke exposure, and medication use) or covariate with FeNO. Next, we developed a full multivariable model guided by the results from the exploratory analysis. Although the intervention in the CAP study (HEPA filters) did not affect FeNO levels, to account for the design effects of intervention group assignment and time (6 or 12 month study time point), a group and a time variable were included in all models.
We conducted secondary analyses to attempt to distinguish the contribution of sensitization to each of the four individual allergens separately. In these analyses, we included the covariates from the full multivariable model. Sensitization to each allergen was treated as dichotomous and replaced sensitization to any allergen in the full model. Additionally, we evaluated the joint contribution of sensitization to each of the four allergens by adding all four into the full multivariable model (with sensitization to any allergen removed). SAS Version 9.1 (SAS Institute, Inc., Cary, NC) was used for all data analyses.
RESULTS
Complete data (including baseline allergen sensitization status, medication use, season of FeNO collection, and FeNO) was available for 88% of participants (198 of 225) at baseline (Table 1), 72% of participants (162 of 225) at the 6 month visit, and 78% of participants (175 of 225) at the 12 month visit. There were no statistically significant differences between children with complete data and those missing data. There was an even distribution of participants across months of enrollment (E-table 2&3).
Table 1.
Baseline Demographics, Characteristics, Exposures, and Geometric Mean FeNO levels of Study Participants
| N (%) | |
|---|---|
| Number of subject | 198 (88) |
| Male | 126 (63.6) |
| Female | 72 (36.4) |
| Age (years) (Mean ± Std dev) | 9.4 ± 1.8 |
| 6–8.5 | 71 (35.9) |
| 8.5–10.5 | 64 (32.3) |
| 10.5–13 | 63 (31.8) |
| Race | |
| African American | 111 (56.1) |
| Caucasian | 82 (41.4) |
| Other | 5 (2.5) |
| Income | |
| <$20,000 | 89 (46.8) |
| $20–40,000 | 61 (32.1) |
| >$40,000 | 40 (21.2) |
| Season at Baseline FeNO collection | |
| Winter | 53 (26.8) |
| Spring | 56 (28.3) |
| Summer | 52 (26.2) |
| Fall | 37 (18.7) |
| Dust Mite Allergen Sensitized | |
| Yes | 91 (46.0) |
| No | 107 (54.0) |
| Cat Allergen Sensitized | |
| Yes | 77 (38.9) |
| No | 122 (61.1) |
| Dog Allergen Sensitized | |
| Yes | 34 (17.2) |
| No | 164 (82.8) |
| Cockroach Allergen Sensitized | |
| Yes | 61 (30.8) |
| No | 137 (69.2) |
| Sensitized to Any Allergen | |
| Yes | 130 (65.7) |
| No | 68 (34.3) |
| Settled Allergen Levels (Mean, 95% CI) | |
| Dust Mite (µg/g) | 7.0 (2.8,10.2) |
| Cat (µg/g) | 15.2 (7.9,22.5) |
| Dog (µg/g) | 20.2 (8.4,31.9) |
| Cockroach (Units/g) | 0.1 (0.05, 0.2) |
There were also no statistically significant changes over the study period in reported medication use, FeNO, or exposure to tobacco smoke over the study period as measured by biomarkers and air nicotine dosimeters; however, the reported number of cigarettes smoked in the home decreased significantly (Table 2).
Table 2.
Medication use, Tobacco Smoke Exposure, and FeNO of Study Participants at Each Visit
| Baseline Visit |
6 Month Visit |
12 Month Visit |
p-Value (time trend)* |
|
|---|---|---|---|---|
| Reported use of inhaled corticosteroid, N (%) |
50(25.3) | 40 (24.7) | 38 (21.7) | 0.31 |
| Reported an oral corticosteroid burst the last 3 months, N (%) |
63 (32) | 38 (23.5) | 46 (26.3) | 0.15 |
| Serum Cotinine ng/ml Geometric Mean (95% CI) |
1.23 (1.04, 1.47) |
1.04 (0.84, 1.290) |
1.17 (0.94, 1.46) |
0.33 |
| Hair Cotinine ng/mg Geometric Mean (95% CI) |
0.14 (0.11, 0.16) |
0.11 (0.09, 0.14) |
0.15 (0.12, 0.18) |
0.33 |
| Cigarettes smoked in the home (Mean ± Std dev) |
16.9 ± 12.2 | 14.9 ± 13 | 13.2 ± 11.1 | <0.001 |
| Cigarettes smoked in the home per home volume (Mean ± Std dev) |
0.08 ± 0.07 | 0.07 ± 0.06 | 0.07 ± 0.06 | <0.001 |
| Air Nicotine (µg) Geometric Mean (95% CI) |
NA | 2.77 (2.02, 3.8) |
3.13 (2.33, 4.21) |
0.94 |
| Air Nicotine (µg per m3) Geometric Mean (95% CI) |
NA | 0.44 (0.32, 1.67) |
0.5 (0.37, 0.67) |
0.82 |
| FeNO (ppb) Geometric Mean (95% CI) |
12.4 (11.2, 13.6) |
12.3 (11.1, 13.6) |
13.4 (12.1, 14.8) |
0.15 |
There was no statistically significant association of group assignment or group* time with any of these variables.
Candidate Predictors of FeNO
Increasing sensitization to any allergen (p=0.001), dust mite sensitization (p=0.02), cat sensitization (p<0.001), dog sensitization (p=0.004), increasing baseline FeNO (p<0.001), and fall season (highest level in the fall and lowest in the summer, p=0.02) were associated with higher FeNO levels. There was no statistically significant relationship of intervention group assignment with FeNO levels over the study time period (p=0.35).
Age, race, gender, income, cockroach allergen sensitization, settled dust allergens, airborne particulate concentrations, reported tobacco smoke exposure, serum cotinine, hair cotinine, and recent upper respiratory infection were not associated with FeNO levels. The log of air nicotine level µg/filter (p=0.131) and the log of air nicotine level µg per m3 (p=0.105) were not associated with FeNO levels but met criteria for consideration for inclusion in the full multivariable model.
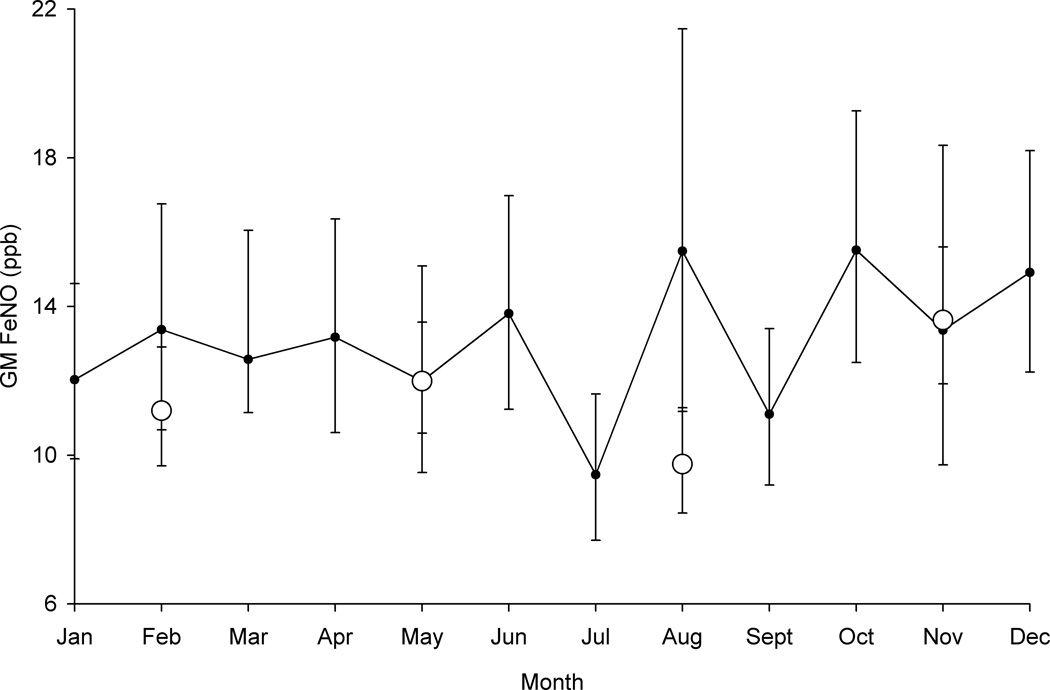
FeNO varied by season with the highest levels in the fall followed by spring, winter, and summer. Compared with the fall, FeNO levels were significantly lower in the summer (p=0.002), and marginally lower in spring (p=0.054) and in winter (p=0.12). To attempt to further differentiate seasonal effects, we created a variable for month of FeNO collection. The relationship of the month variable with FeNO levels approached statistical significance (Fig 1, p=0.052).
Fig 1. Geometric Mean FeNO by Month, Adjusted for Baseline FeNO, Group Assignment, and Time (study time point) of Collection (p=0.052).
Solid Circle = Month; Open Circle = Season (winter: December 20 – March 19, spring: March 20 – June 20, summer: June 21 – September 21, and fall: September 22 – December 19)
Full Multivariable Models of Predictors of FeNO
In multivariable analysis, baseline FeNO, sensitization to any allergen, season, reported use of an inhaled steroid, and nicotine exposure (log of nicotine per m3) were predictors of FeNO measured 6 and 12 months after study initiation, adjusting for baseline age, intervention group assignment, and time (Table 3). Being sensitized to any allergen, measurement season (highest in the fall and lowest in the summer), and higher baseline FeNO were associated with an increased FeNO, whereas inhaled steroid use and increasing nicotine exposure was associated with a decreased FeNO. The adjusted geometric mean FeNO in allergen sensitized children was 13.7 ppb (95% CI 12.4, 15.2) compared to 10 ppb (95% CI 8.6, 11.6) in non-sensitized children. The adjusted geometric mean FeNO in children using inhaled steroids was 10.8 ppb (95% CI 9.2, 12.6) compared to 12.8 ppb (95% CI 11.7, 14.0) in children not using inhaled steroids.
Table 3.
Full Multivariable Model of Predictors of FeNO, adjusted for baseline FeNO, intervention group assignment, and Time (study time point)
| Variable | Adjusted Parameter Estimate |
Standard Error |
P value |
|---|---|---|---|
| Baseline Age | 0.024 | 0.022 | 0.269 |
| Group | |||
| Placebo filter | −0.069 | 0.076 | 0.3675 |
| HEPA filter | Ref. | -- | |
| Time (study time point) | 0.016 | 0.0087 | 0.066 |
| Baseline FeNO | 0.3298 | 0.061 | <0.0001 |
| Sensitized to Any allergen | |||
| No | −0.3155 | 0.09 | 0.0006 |
| Yes | Ref. | -- | |
| Season | (0.002) | ||
| Winter | −0.1564 | 0.0901 | 0.0849 |
| Spring | −0.1270 | 0.0731 | 0.0846 |
| Summer | −0.3562 | 0.0912 | 0.0001 |
| Fall | Ref. | -- | Ref. |
| Uses Inhaled Steroid Daily | |||
| No | 0.1714 | 0.0801 | 0.0416 |
| Yes | Ref. | -- | |
| Log Nicotine (µg/m3) | −0.0373 | 0.018 | 0.0415 |
In secondary analyses, we attempted to distinguish the contribution of sensitization to each of the four individual allergens separately by replacing the variable, “sensitized to any allergen,” with sensitization to each individual allergen in four separate models. In these analyses, being sensitized to dust mite allergen (p=0.005), cat allergen (p<0.001), or dog allergen (p=0.002) was associated with higher FeNO levels. Cockroach allergen sensitization was not related to FeNO levels (p=0.84).
When the variable, “sensitized to any allergen,” was replaced by all four individual allergen sensitization variables considered together in the same model, sensitization to dust mite allergen (p=0.013) and cat allergen (p=0.003) were associated with higher FeNO levels. Sensitization to dog allergen (p=0.19) and cockroach allergen (p=0.402) were not related to FeNO levels. We also found that the absolute number of allergens to which an individual was sensitized was associated with higher FeNO levels (p<0.001).
Interactions
We tested for biologically plausible interactions (such as allergen sensitization and exposure to settled allergens, allergen sensitization and age, and inhaled steroid use and smoke exposure) in the full multivariable model. There was no significant interaction of sensitization to any of the four settled indoor allergens with exposure to that specific allergen. There was no significant interaction of season of FeNO collection with being sensitized to any allergen, exposure to any of the settled allergens, log of air nicotine level µg per m3, gender, race, income, or time. The interaction of being sensitized to any allergen and baseline age was not statistically significant (p=0.09). Finally, there was no significant interaction of reported use of an inhaled steroid with any of the tobacco smoke exposure measures.
In a secondary analysis we tested for interactions of individual allergen sensitizations with individual allergen exposures and with season. There were no significant interactions of type specific allergen sensitization and allergen exposure. There was an interaction of season of FeNO collection with sensitization to dust mite allergen (p=0.01); sensitization to dust mite allergen was associated with significantly higher levels of FeNO during every season, but it was most pronounced in the fall. The interaction of season of FeNO collection with sensitization to dog allergen was marginally significant (p=0.06); sensitization to dog allergen was associated with higher levels of FeNO during every season, but it was most pronounced in the winter and fall. The interactions of season of FeNO collection with sensitization to cat allergen and cockroach allergen were not statistically significant.
DISCUSSION
We identified several predictors of FeNO levels in children with asthma. FeNO levels were higher among those children with higher baseline FeNO levels, higher in the fall and lower in the summer, higher among children who were sensitized to one or more allergens, and lower among children who were reportedly using inhaled steroids. Our study also provides information on seasonal changes and FeNO trends over time. These findings indicate that FeNO levels respond to many common asthma triggers and mediators. An ideal biomarker for asthma control would reflect pharmacologic intervention, as well as respond to changes in an individual’s exposure to environmental triggers. FeNO appears to fit these characteristics.
We found that baseline FeNO levels were associated with subsequent FeNO levels. This finding is consistent with work by Roberts et al.18 In their study, Roberts et al. used standardized FeNO levels to account for some of the individual differences in FeNO. Our inclusion of baseline visit FeNO levels in the models, which should have adjusted for the individual differences in FeNO levels, indicate that using population standard or “normal” FeNO levels may be insufficient. Thus, it is important to consider an individual’s baseline FeNO levels for managing children with asthma. Indeed, the relationship of age and FeNO was not significant upon inclusion of baseline FeNO levels suggesting that if baseline FeNO is considered, the previously identified age and FeNO relationship is not as important. Ultimately, these differences in FeNO may also help to identify children who have a specific variant of asthma, more severe asthma, or that may benefit from control of environmental triggers.
We found that FeNO levels varied by season among children with atopic and nonatopic asthma. In a study by Koenig et al, the authors reported that mean FeNO levels were higher in the winter than the spring (19.9±12.4 ppb vs. 12.7±6.7 ppb), but the differences were not statistically different.24 In the present study, the highest levels of FeNO occurred in the fall, followed by spring, winter, and summer. The seasonal variation in FeNO levels did not differ when stratified by overall allergen sensitization status or tobacco smoke exposure levels, but there were seasonal differences in FeNO levels among children who were sensitized to dog or dust mite allergen.
Seasonal variation could be due to infectious agents or allergen exposures. We did not find that FeNO levels were associated with parent reported respiratory illness, but FeNO may rise in the presence of select infectious agents.42 The higher levels of FeNO in the fall might also be due to higher levels of exposure to dog or dust mite allergen. Dust mite allergen levels are higher in the fall, for example, and this might explain the elevation of FeNO levels among dust mite sensitized children during that season.43 The seasonal variation in FeNO levels could also be due to seasonal variation of ambient pollution or outdoor allergens, such as pollens and fungal spores.
Consistent with prior research, we found that being sensitized to common allergens was associated with increased FeNO levels.17,25,41 In secondary analyses we found that this relationship was driven by sensitization to cat allergen and dust mite allergen, perhaps because of the higher prevalence of sensitization to cat and dust mite allergen in our cohort. Studies of children with higher prevalence of sensitization to dog or cockroach allergen may find that dog and cockroach allergen sensitization is associated with FeNO levels. Regardless, consistent with other studies, these data indicate that allergen sensitization clearly plays a large role in determining FeNO levels in children.
The finding that inhaled steroid use predicts lower FeNO levels is consistent with previous work.12,44 In fact, the FDA approved the use of FeNO in individuals with asthma to monitor response to anti-inflammatory agents.44 The results of the present study confirm that FeNO may be a valuable biomarker of response to inhaled anti-inflammatory therapy in children with asthma.
The relationship between tobacco smoke exposure and FeNO levels in children who have asthma is unclear.21,22,45 We found a good correlation between the tobacco smoke exposure measures in this study (E table 4, 5, & 6), but the reported number of cigarettes smoked in the home decreased during the study while the biomarkers of exposure did not. This suggests that reported exposure was subject to recall bias. We found that tobacco smoke exposure, as measured by airborne nicotine, was associated with lower FeNO levels. In contrast, neither the biomarkers nor parent reported tobacco smoke exposure was associated with FeNO levels. Since the majority of tobacco smoke measures in this study were not related to FeNO levels, tobacco smoke exposure may not affect FeNO levels in children with asthma. Alternatively, airborne nicotine may be a more direct measure of the fraction of tobacco smoke that affects the lung and therefore affects FeNO levels.
There are several limitations to this study. First, the cohort only included tobacco smoke exposed children with asthma; there was not an unexposed control group. There was, however, a wide range of exposure to tobacco smoke and we included extensive measures of exposure that allowed us to test and quantify for effects of exposure. Moreover, data from nationally representative samples suggests that 85% of children 4 to 16 years of age have measurable levels of tobacco smoke exposure.46 Thus, our findings should be representative of a large proportion of children with asthma. A second potential limitation is that we only had baseline measures of settled dust allergens. Although some investigators have found that a single measurement of settled dust allergens may be a good proxy for one year exposure, this limitation prevented us from evaluating whether changes in settled allergen exposures were associated with changes in FeNO.47 A third potential limitation is that we relied on parent reported medication use. Still, reported medication is a relatively accurate measure of use.48 A fourth limitation is that we were not able to measure flow during our FeNO collection, but we had a restricted range of flows. This limitation means that our FeNO levels while generalizable to other groups may not be comparable to those groups using a specific flow rate beyond the range we used. Our method was replicable, demonstrating a coefficient of variation of 17% in other studies (unpublished data).
This study has shown that FeNO reflects anti-inflammatory therapy and recognized environmental triggers such as allergen sensitization and season. These results also suggest that clinicians and researchers may need to consider an individual’s baseline FeNO levels to manage children with asthma. With these factors in mind, FeNO may help physicians assess airway inflammation to tailor individual asthma management through pharmacologic and environmental interventions. Thus, these results hold promise for the use of FeNO in asthma management.
ACKNOWLEDGEMENTS
We appreciate the insightful comments and review of this manuscript by the Research Section of the Division of General & Community Pediatrics at Cincinnati Children’s Hospital Medical Center as well as the anonymous reviewers.
Sources of Funding: NHLBI R01-HL65731-01, NHLBI 1R21HL083145-01A1, Robert Wood Johnson Generalist Physician Faculty Scholars Award, and the William Cooper Procter Scholar Research Award Cincinnati Children’s Hospital Research Foundation
Abbreviations
- FeNO
exhaled nitric oxide
- GM
geometric mean
- CAP
Cincinnati Asthma Prevention
- HEPA-CPZ
High Efficiency Particulate Air-Carbon, Permanganate, Zeolite
- CI
confidence interval
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 2.O'Connell EJ. The burden of atopy and asthma in children. Allergy. 2004;59(Suppl 78):7–11. doi: 10.1111/j.1398-9995.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;(381):1–24. [PubMed] [Google Scholar]
- 4.Leone FT, Mauger EA, Peters SP, Chinchilli VM, Fish JE, Boushey HA, Cherniack RM, Drazen JM, Fahy JV, Ford J, Israel E, Lazarus SC, Lemanske RF, Martin RJ, McGeady SJ, Sorkness C, Szefler SJ. The utility of peak flow, symptom scores, and beta-agonist use as outcome measures in asthma clinical research. Chest. 2001;119(4):1027–1033. doi: 10.1378/chest.119.4.1027. [DOI] [PubMed] [Google Scholar]
- 5.Apter AJ, ZuWallack RL, Clive J. Common measures of asthma severity lack association for describing its clinical course. J Allergy Clin Immunol. 1994;94(4):732–737. doi: 10.1016/0091-6749(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 6.Zacharasiewicz A, Erin EM, Bush A. Noninvasive monitoring of airway inflammation and steroid reduction in children with asthma. Curr Opin Allergy Clin Immunol. 2006;6(3):155–160. doi: 10.1097/01.all.0000225152.37403.21. [DOI] [PubMed] [Google Scholar]
- 7.Kwok MY, Walsh-Kelly CM, Gorelick MH, Grabowski L, Kelly KJ. National Asthma Education and Prevention Program severity classification as a measure of disease burden in children with acute asthma. Pediatrics. 2006;117(4 Pt 2):S71–S77. doi: 10.1542/peds.2005-2000D. [DOI] [PubMed] [Google Scholar]
- 8.Stout JW, Visness CM, Enright P, Lamm C, Shapiro G, Gan VN, Adams GK, 3rd, Mitchell HE. Classification of asthma severity in children: the contribution of pulmonary function testing. Arch Pediatr Adolesc Med. 2006;160(8):844–850. doi: 10.1001/archpedi.160.8.844. [DOI] [PubMed] [Google Scholar]
- 9.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6(9):1368–1370. [PubMed] [Google Scholar]
- 10.Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, Young DA, Spahn JD. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003;142(5):469–475. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 11.Lanz MJ, Leung DY, White CW. Comparison of exhaled nitric oxide to spirometry during emergency treatment of asthma exacerbations with glucocorticoids in children. Ann Allergy Asthma Immunol. 1999;82(2):161–164. doi: 10.1016/S1081-1206(10)62591-4. [DOI] [PubMed] [Google Scholar]
- 12.Moeller A, Franklin P, Hall GL, Turner S, Straub D, Wildhaber JH, Stick SM. Inhaled fluticasone dipropionate decreases levels of nitric oxide in recurrenty wheezy infants. Pediatr Pulmonol. 2004;38(3):250–255. doi: 10.1002/ppul.20055. [DOI] [PubMed] [Google Scholar]
- 13.Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J. 2002;19(6):1015–1019. doi: 10.1183/09031936.02.01582001. [DOI] [PubMed] [Google Scholar]
- 14.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352(21):2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 15.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, Stout J, Malindzak G, Smartt E, Plaut M, Walter M, Vaughn B, Mitchell H. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 16.Franklin PJ, Turner SW, Le Souef PN, Stick SM. Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax. 2003;58(12):1048–1052. doi: 10.1136/thorax.58.12.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson A, Custovic A, Pipis S, Adisesh A, Faragher B, Woodcock A. Exhaled nitric oxide, sensitization, and exposure to allergens in patients with asthma who are not taking inhaled steroids. Am J Respir Crit Care Med. 1999;160(1):45–49. doi: 10.1164/ajrccm.160.1.9809091. [DOI] [PubMed] [Google Scholar]
- 18.Roberts G, Hurley C, Bush A, Lack G. Longitudinal study of grass pollen exposure, symptoms, and exhaled nitric oxide in childhood seasonal allergic asthma. Thorax. 2004;59(9):752–756. doi: 10.1136/thx.2003.008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verleden GM, Dupont LJ, Verpeut AC, Demedts MG. The effect of cigarette smoking on exhaled nitric oxide in mild steroid-naive asthmatics. Chest. 1999;116(1):59–64. doi: 10.1378/chest.116.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152(2):609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 21.Barreto M, Villa MP, Martella S, Ronchetti F, Darder MT, Falasca C, Pagani J, Massa F, Ronchetti R. Exhaled nitric oxide in asthmatic and non-asthmatic children: influence of type of allergen sensitization and exposure to tobacco smoke. Pediatr Allergy Immunol. 2001;12(5):247–256. doi: 10.1034/j.1399-3038.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- 22.Yates DH, Breen H, Thomas PS. Passive smoke inhalation decreases exhaled nitric oxide in normal subjects. Am J Respir Crit Care Med. 2001;164(6):1043–1046. doi: 10.1164/ajrccm.164.6.2005043. [DOI] [PubMed] [Google Scholar]
- 23.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61(8):722–728. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenig JQ, Jansen K, Mar TF, Lumley T, Kaufman J, Trenga CA, Sullivan J, Liu LJ, Shapiro GG, Larson TV. Measurement of offline exhaled nitric oxide in a study of community exposure to air pollution. Environ Health Perspect. 2003;111(13):1625–1629. doi: 10.1289/ehp.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spanier AJ, Hornung R, Lierl M, Lanphear BP. Environmental exposures and exhaled nitric oxide in children with asthma. J Pediatr. 2006;149(2):220–226. doi: 10.1016/j.jpeds.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect. 2005;113(3):362–367. doi: 10.1289/ehp.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baraldi E, de Jongste JC. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20(1):223–237. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 28.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 1999;160(6):2104–2117. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 29.Sievers Nitric Oxide Analyzer (NOA) 280i Homepage. [Cited 2007 November 1]; Available from: http://www.ionicsinstruments.com/ionics/index.cfm?product_code=NitricOxideAnalyzer&category_code=NOA. [Google Scholar]
- 30.Lanphear BP, Emond M, Jacobs DE, Weitzman M, Tanner M, Winter NL, Yakir B, Eberly S. A side-by-side comparison of dust collection methods for sampling lead-contaminated house dust. Environ Res. 1995;68(2):114–123. doi: 10.1006/enrs.1995.1015. [DOI] [PubMed] [Google Scholar]
- 31.Chapman MD, Aalberse RC, Brown MJ, Platts-Mills TA. Monoclonal antibodies to the major feline allergen Fel d I. II. Single step affinity purification of Fel d I, N-terminal sequence analysis, and development of a sensitive two-site immunoassay to assess Fel d I exposure. J Immunol. 1988;140(3):812–818. [PubMed] [Google Scholar]
- 32.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87(2):505–510. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 33.Luczynska CM, Arruda LK, Platts-Mills TA, Miller JD, Lopez M, Chapman MD. A two-site monoclonal antibody ELISA for the quantification of the major Dermatophagoides spp. allergens, Der p I and Der f I. J Immunol Methods. 1989;118(2):227–235. doi: 10.1016/0022-1759(89)90010-0. [DOI] [PubMed] [Google Scholar]
- 34.Ewan PW, Coote D. Evaluation of a capsulated hydrophilic carrier polymer (the ImmunoCAP) for measurement of specific IgE antibodies. Allergy. 1990;45(1):22–29. doi: 10.1111/j.1398-9995.1990.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 35.Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW, Miller BB, Patterson DG, Jr, Needham LL, Hannon WH, Sampson EJ. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–2291. [PubMed] [Google Scholar]
- 36.Eliopoulos C, Klein J, Koren G. Validation of self-reported smoking by analysis of hair for nicotine and cotinine. Ther Drug Monit. 1996;18(5):532–536. doi: 10.1097/00007691-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24(5):333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- 38.Hammond SK, Sorensen G, Youngstrom R, Ockene JK. Occupational exposure to environmental tobacco smoke. Jama. 1995;274(12):956–960. [PubMed] [Google Scholar]
- 39.Marbury MC, Hammond SK, Haley NJ. Measuring exposure to environmental tobacco smoke in studies of acute health effects. Am J Epidemiol. 1993;137(10):1089–1097. doi: 10.1093/oxfordjournals.aje.a116612. [DOI] [PubMed] [Google Scholar]
- 40.Eisner MD, Katz PP, Yelin EH, Hammond SK, Blanc PD. Measurement of environmental tobacco smoke exposure among adults with asthma. Environ Health Perspect. 2001;109(8):809–814. doi: 10.1289/ehp.01109809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003;112(2):362–368. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 42.de Gouw HW, Grunberg K, Schot R, Kroes AC, Dick EC, Sterk PJ. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J. 1998;11(1):126–132. doi: 10.1183/09031936.98.11010126. [DOI] [PubMed] [Google Scholar]
- 43.van Strien RT, Koopman LP, Kerkhof M, Spithoven J, de Jongste JC, Gerritsen J, Neijens HJ, Aalberse RC, Smit HA, Brunekreef B. Mite and pet allergen levels in homes of children born to allergic and nonallergic parents: the PIAMA study. Environ Health Perspect. 2002;110(11):A693–A698. doi: 10.1289/ehp.021100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silkoff PE, Carlson M, Bourke T, Katial R, Ogren E, Szefler SJ. The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J Allergy Clin Immunol. 2004;114(5):1241–1256. doi: 10.1016/j.jaci.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Dinakar C, Lapuente M, Barnes C, Garg U. Real-life environmental tobacco exposure does not affect exhaled nitric oxide levels in asthmatic children. J Asthma. 2005;42(2):113–118. [PubMed] [Google Scholar]
- 46.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155(1):36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 47.Heinrich J, Holscher B, Douwes J, Richter K, Koch A, Bischof W, Fahlbusch B, Kinne RW, Wichmann HE. Reproducibility of allergen, endotoxin and fungi measurements in the indoor environment. J Expo Anal Environ Epidemiol. 2003;13(2):152–160. doi: 10.1038/sj.jea.7500267. [DOI] [PubMed] [Google Scholar]
- 48.Tisnado DM, Adams JL, Liu H, Damberg CL, Chen WP, Hu FA, Carlisle DM, Mangione CM, Kahn KL. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44(2):132–140. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]